Introduction
Pulmonary fibrosis (PF) is a heterogeneous disorder
of lung interstitial tissue (1). The
process of PF is complex and following lung injury, inflammatory
cells migrate to the injured site via chemokine gradients (2). Recruited leukocytes release cytokines
and chemokines, which increase levels of local profibrotic
interleukin (IL)-13, IL-4 and transforming growth factor (TGF)-β
(2). Subsequently, bone
marrow-derived fibrocytes or resident fibroblasts proliferate and
differentiate into myofibroblasts, leading to excessive
extracellular matrix (ECM) synthesis, tissue reshaping and fibrotic
lesions (3). Etiologies of PF vary
and include chemicals (airborne contaminants and toxic components
from the smoking), organic or inorganic dust, radiation and trauma
(4). With persisting stimulation or
failure to regulate wound repair, fibrosis can develop at any stage
(5). Currently, idiopathic PF (IPF)
is thought to be one of the most common form of PF, with median
survival times of 3–5 years following diagnosis (6). Exploring novel and effective treatments
for this condition is of great interest.
Molecular pathways engaged in PF pathogenesis have
not been fully elucidated and are potentially multifactorial
(7). Matrix metalloproteinase (MMP)2
and MMP9, also known as gelatinases, are secreted by various types
of cells in the lungs (4). MMP2/9
are vital in digesting collagens IV/V and gelatin in the basement
membrane, leading to ECM breakdown and migration of cells adherent
to the interstitial ECM (4). In
addition, imbalance of synthesis and degradation of ECM components
contributes to airway remodeling in the context of PF (4). Furthermore, chemokine gradients are
required for trafficking of circulating leukocytes and fibrocytes
to the lung tissue. CXC chemokine receptor 4 (CXCR4) and its ligand
14 (CXCL14), serve a supporting role in PF development and are
involved in therapeutic treatment regiments (8,9). TGF-β1
is essential in promoting fibroblast-myofibroblast transformation
with enhancing ECM synthesis that contributes to PF (10). TGF-β1 downstream signaling effects
are executed by Smad2/3 (11).
Therapeutic interventions based on these endogenous regulatory
mechanisms may provide potential antifibrotic drugs or strategies
to block PF.
Combination therapy is an attractive and promising
method to prevent PF (8).
Dexamethasone (Dxs) is widely used as an antifibrotic agent due to
its protection of the lungs against fibrosis by inhibiting the
production of inflammatory mediators (9). Berberine (BBR), a known non-toxic
natural agent, inhibits nuclear factor-κB proinflammatory and
profibrotic mediators in bleomycin (BLM)-induced PF and is used as
an alternative treatment counteracting PF (10). Reports demonstrated that Dxs plus
penehyclidine hydrochloride (12) or
alfacalcidol (13) attenuate PF more
effectively compared with either single treatment. However, whether
Dxs plus BBR has a better therapeutic effect in PF compared with
the single treatments remains unknown. In the present study, a
BLM-induced rat PF model was successfully constructed. The effect
of Dxs plus BBR treatment on preventing lung damage and collagen
deposition at histological levels was evaluated. In addition, the
combination effects of Dxs plus BBR on hydroxyproline (Hyp),
CXCL14, MMP2/9 and α-smooth muscle actin (α-SMA) expression and on
activation of the Smad2/3 signaling pathway were assessed. The
present study suggested that the combination of Dxs with BBR
presents an effective approach to prevent PF.
Materials and methods
Preparation of PF rat model and
treatments
A total of 30 male Wistar albino rats, aged 8–10
week old, weighing 180–220 g were purchased from Shanghai SIPPR-Bk
Laboratory Animal Co. Ltd. The rats were acclimatized at 25°C with
12-h light/dark cycles with relative humidity between 40–70% for a
week, and had free access to food and water as previously reported
(11). Following acclimation, rats
were randomly divided into control, BLM, Dxs, BBR and Dxs plus BBR
groups (n=6/group). PF was established through a single
endotracheal injection of BLM (5 mg/kg; Zhejiang Hisun
Pharmaceutical Co., Ltd.) in all groups except the control
(11). Following 24 h, Dxs, BBR and
Dxs plus BBR preventive groups were administered Dxs (3 mg/kg/day,
Sigma Chemical Co.; Merck KGaA) (12), BBR (200 mg/kg/day, Aladdin Reagent
Co., Ltd.) (11) or Dxs (3
mg/kg/day) plus BBR (200 mg/kg/day), respectively, by
intraperitoneal injection for 14 successive days. The control and
BLM groups were simultaneously treated with saline (2
ml/kg/day).
To study the involvement of the hedgehog (Hh)
signaling pathway in the effect of Dxs and BBR treatment, another
three groups of rats were randomly divided into BLM, Dxs plus BBR
and Dxs+BBR+purmorphamine groups (n=6/group) without a separate
untreated control or a purmorphamine control. The BLM and Dxs plus
BBR groups were established as mentioned above. Animals of the
Dxs+BBR+purmorphamine group were BLM-induced and then
intraperitoneally injected with Dxs (3 mg/kg/day) (12), BBR (200 mg/kg/day) (11) and purmorphamine (0.69 mg/kg/day,
Aladdin Reagent Co., Ltd.) (14) for
14 consecutive days. Rats were euthanized with an overdose of
pentobarbital (200 mg/kg) and lung tissues were collected, prepared
for histology evaluation and/or stored at −80°C for western blot
analysis.
Histology evaluation
The lung tissues from all rats were collected,
routinely fixed in 10% buffered formalin at 4°C for 48 h,
dehydrated in a graded ethanol series (50, 70, 85, 95 and 100%),
cleared in xylene and embedded in paraffin. Sections of 4-µm
thickness were cut then deparaffinized. Hematoxylin and eosin
(H&E) staining was performed to observe histological changes.
Briefly, the lung sections were stained with eosin (cat. no.
714094; BASO Diagnostic, Inc.) for 1 min at room temperature,
rinsed with running water for 15 min and then dyed with hematoxylin
(cat. no. 714094; BASO Diagnostic, Inc.) for 5 min at room
temperature eosin another. Masson's trichrome staining was
performed using Masson's trichrome kits (Beijing Leagene
Biotechnology Co., Ltd.) to measure the density of collagen fibers
according to the manufacturer' instruction. The degree of lung
fibrosis was assessed by infiltration of inflammatory cells,
thickness of the alveolar walls and severity of the collagen
deposition. Histology evaluation and Masson's trichrome staining
were performed with the same tissue positioning. Images of H&E
and Masson's trichrome staining were obtained in the same field of
vision and obtained using a light microscope (Eclipse Ni-E; Nikon
Corporation) under ×200 magnification. For each lung tissue
section, three fields were randomly selected for imaging.
Hydroxyproline (Hyp) assessment
Lung tissue samples (30–100 mg wet weight) were
lysed in radioimmunoprecipitation assay lysis buffer (JRDUN Bio.
Co., Ltd.) at 4°C for 30 min. The lysates were centrifuged at 3,500
× g at 4°C for 10 min, and total protein levels in the supernatants
were quantified using the BCA Protein assay kit (cat. no.
PICPI23223; Thermo Fisher Scientific, Inc.). Hyp content in lung
tissue extract was evaluated using a hydroxyproline assay kit (cat.
no. A030-2; Nanjing Jiancheng Bioengineering Institute) according
to the manufacturer's instructions and calculated using a
microtiter plate reader (BioTek Instruments, Inc.) at a wavelength
of 550 nm.
Enzyme-linked immunosorbent (ELISA)
analysis
On days 3, 7 and 14, rats were anesthetized with 10%
of chloral hydrate (400 mg/kg body weight; intraperitoneal
injection; Sinopharm Chemical Reagent Co., Ltd.). No signs of
peritonitis were observed. Blood samples were extracted from the
eyes then centrifuged at 3,000 × g at 4°C for 5 min to separate the
serum. ELISA was conducted to assess serum CXCL14, collagen I,
collagen III, MMP2 and MMP9 content according to the manufacturer's
protocols (Xin-Yu Biotechnology Pharmaceutical Co., Ltd.). The
absorbance value (at 450 nm) was recorded using a microplate reader
(Bio-Rad Laboratories, Inc.). The catalogue numbers of ELISA kits
used to quantify CXCL14, collagen I, collagen III, MMP2 and MMP9
were EK-3394, xyH142, xyH144, xy-1706E and bsk00125,
respectively.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA from lung tissues was obtained using
TRIzol reagent (cat. no. 1596-026; Invitrogen; Thermo Fisher
Scientific, Inc.). cDNA was obtained using the RevertAid First
Stand cDNA Synthesis kit (cat. no. #K1622; Fermentas; Thermo Fisher
Scientific, Inc.) with following conditions for 60 min, 85 for 5
min then 4°C for 5 min. The prepared cDNA was stored at −20°C and
used for the next step. qPCR was conducted using the SYBR Green mix
(cat. no. #K0223; Thermo Fisher Scientific, Inc.). GAPDH was used
as endogenous control. Primer sequences were as follows: CXCL14 (83
bp), forward, 5′-AGTGTAAGTGTTCCCGGAAGG-3′ and reverse,
5′-GCAGTGTGGGTACTTTGGCTT-3′; CXCR4 (125 bp), forward,
5′-GAAGTGGGGTCTGGAGACTAT-3′ and reverse,
5′-TTGCCGACTATGCCAGTCAAG-3′; collagen I (178 bp), forward,
5′-GCTGACCTTCCTGCGCCTAATG-3′ and reverse,
5′-GGTGCTGTAGGTGAAGCGACTG-3′; collagen III (234 bp), forward,
5′-ATGCCCACAGCCTTCTAC-3′ and reverse, 5′-CCCACTCCAGACTTGACATC-3′;
α-SMA (104 bp), forward, 5′-CCCAGACATCAGGGAGTAATGG-3′ and reverse,
5′-TCTATCGGATACTTCAGCGTCA-3′; MMP2 (117 bp), forward,
5′-ATGCCATCCCTGATAACC-3′ and reverse, 5′-ACTTCACGCTCTTGAGAC-3′;
MMP9 (203 bp), forward, 5′-AGGGAGATGCCCATTTCG-3′ and reverse,
5′-GCCGTCCTTATCGTAGTCAG-3′; and GAPDH (197 bp), forward,
5′-CTGCCCAGAACATCATCC-3′ and reverse, 5′-CTCAGATGCCTGCTTCAC-3′. The
amplification condition were as follows: 95°C for 10 min; 40 cycles
of 95 °C for 15 sec, 60°C for 45 sec and then 95°C for 15 sec. The
2−∆∆Cq method was used for relative quantification
(15).
Western blot analysis
Lung tissue samples were homogenized using
radioimmunoprecipitation assay buffer (cat. no. R0010; Beijing
Solarbio Science & Technology Co., Ltd.) and centrifuged
(12,000 × g; 10 min; 4°C). Total protein contents were quantified
using a bicinchoninic acid protein assay kit (cat. no. PICPI23223;
Thermo Fisher Scientific, Inc.). Western blotting was performed as
previously described (11). Briefly,
25 µg of total protein were loaded per lane and isolated on 15%
SDS-PAGE, then transferred onto nitrocellulose (NC) membranes (cat.
no. HATF00010; EMD Millipore). Following blocking with 5% nonfat
milk in TBST buffer (50 mM Tris [pH 7.4], 100 mM NaCl, 0.1%
Tween-20) for 1 h at 25°C, NC membranes were incubated with the
primary antibodies. Antibodies were diluted in blocking solution
(5% nonfat milk) prior to use. The following antibodies were used:
Anti-CXCR4 (1:2,000; cat. no. Ab181020), anti-CXCL14 (1:1,000; cat.
no. Ab137541), anti-MMP2 (1:2,000; cat. no. Ab37150), anti-MMP9
(1:2,000; cat. no. Ab38898), anti-collagen I (1:1,000; cat. no.
Ab6308), anti-collagen III (1:1,000; cat. no. Ab7778), anti-α-SMA
(1:300; cat. no. Ab5694) and anti-phosphorylated (p)-Smad2/3
(1:500; cat. no. Ab63399; all Abcam); and anti-Smad2/3 (1:1,000;
cat. no. #8685) and anti-GAPDH (1:2,000; cat. no. #5174; all Cell
Signaling Technology, Inc.) at 4°C overnight. Next membranes were
incubated with horseradish peroxidase-conjugated secondary
antibodies (1:1,000; cat. nos. A0208, A0181 and A0216; Beyotime
Institute for Biotechnology) for 1 h at 25°C. Bands were visualized
and quantified using an enhanced chemiluminescence system
(Amersham; GE Healthcare). GAPDH served as internal control and
Smad2/3 was used in the evaluation of the phosphorylation of
Smad2/3. Predicted band sizes for collagen I, collagen III, CXCR4,
CXCL14, MMP2, MMP9, α-SMA, p-Smad2/3, Smad2/3 and GAPDH were 130,
138, 39, 13, 72, 89, 42, 48, 53 and 37 kDa, respectively and the
molecular weights were 133, 140, 44, 14, 72, 92, 41, 47, 54 and 36
kDa, respectively. Band density was quantified with ImageJ software
version 1.7 (National Institutes of Health).
Statistical analysis
Each experiment was independently performed ≥3
times. Data are presented as the mean ± standard error of the mean.
Student's t-test was used to compare two groups and one-way
analysis of variance with post-hoc Tukey's test was used to compare
multiple groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
Dxs plus BBR attenuates BLM-induced
histological changes
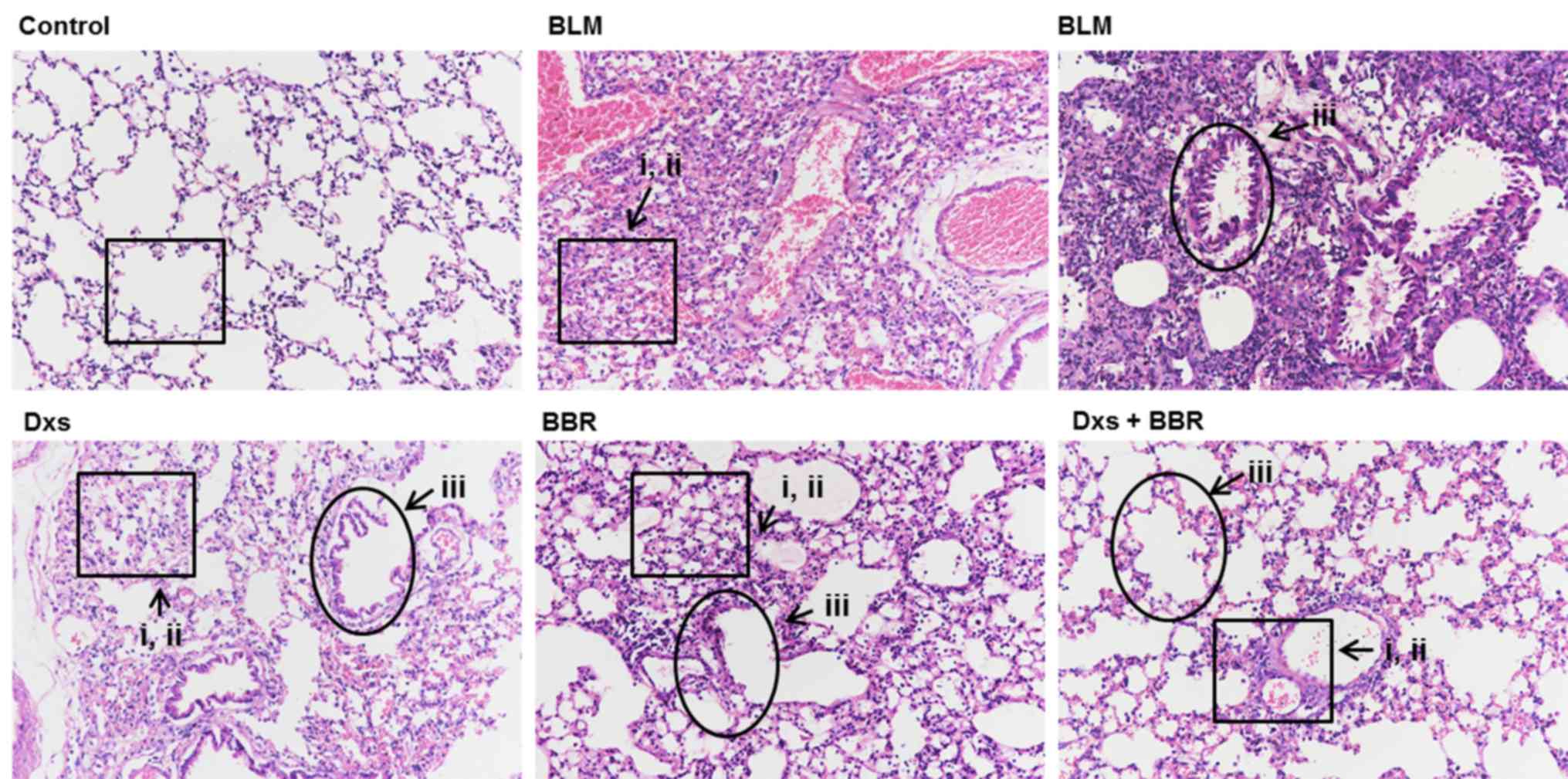
Histological changes in lung tissues were analyzed
by H&E staining. As presented in Fig. 1, the BLM-treated group exhibited
marked morphologic changes compared with the control, including: i)
Serious inflammatory infiltration, with fibroblasts in the lung
interstitium; ii) extensive collapsed alveoli, a disappearing
alveolar space and abundant cord-type fibrous tissues; and iii) a
thickened alveolar interval and increased pulmonary interstitial
substances. Dxs or BBR treatment decreased BLM-induced lung damage
as follows: i) Decreased phagocytic infiltration and inflammatory
cells, and proliferated fibroblasts observed in the lung
interstitium; ii) fewer damaged alveoli; and iii) slightly
thickened alveolar spacing and less pulmonary interstitial
accumulation. Effects of Dxs plus BBR were better compared with Dxs
or BBR treatment alone. Pulmonary damage was markedly reduced to
normal levels, demonstrating that the combination of Dxs and BBR
presented more effective in inhibiting PF at a histological
level.
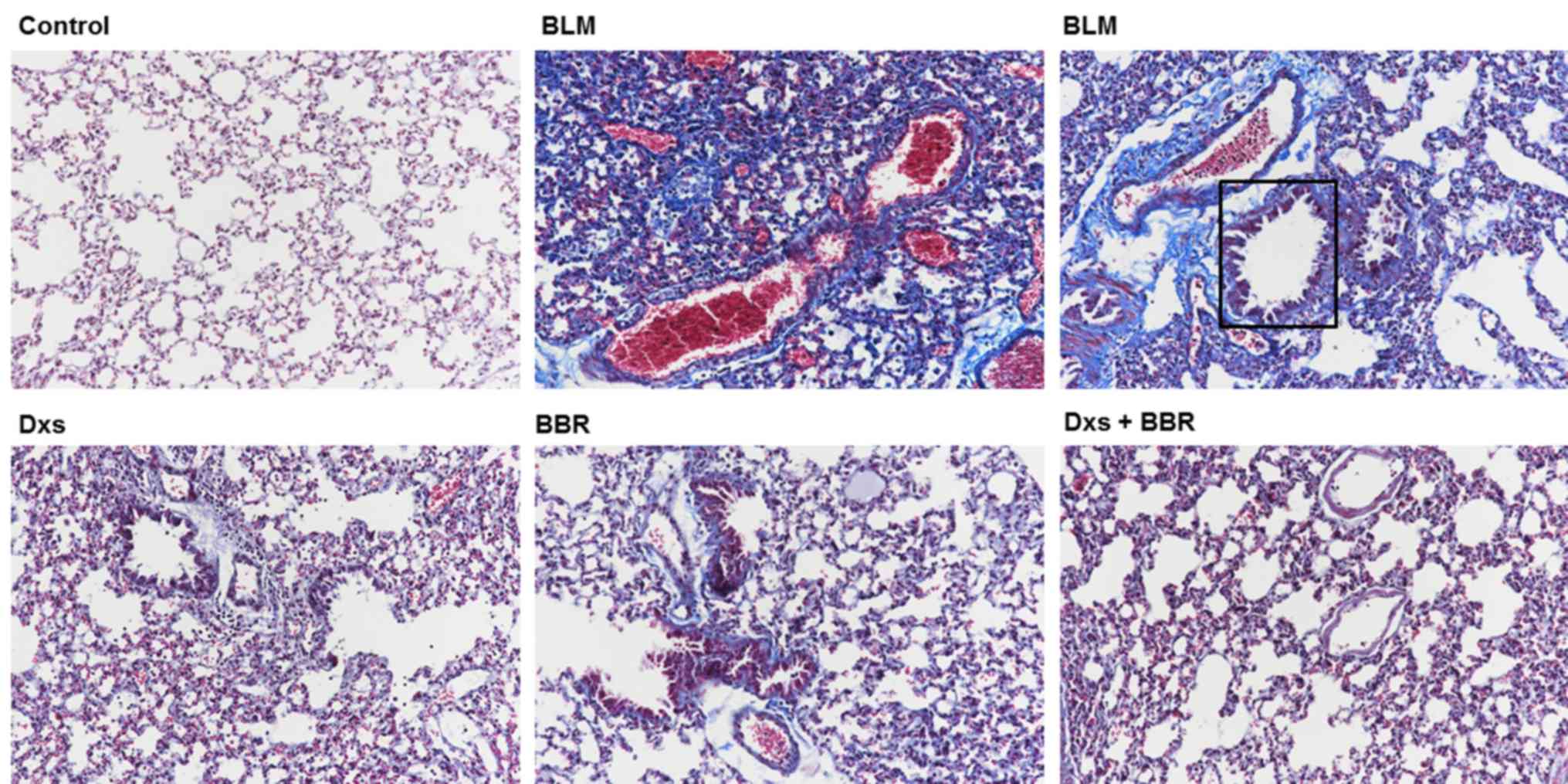
Dxs plus BBR reduces collagen
deposition and Hyp content
Using Masson's staining (Fig. 2), it was evident that BLM
(highlighted by square area) induced severe collagen deposition and
alveolar thickening in lung tissues compared with the control. Dxs
or BBR treatment markedly inhibited collagen deposition when
compared with the BLM group. No differences in collagen deposition
were observed between the control and the Dxs plus BBR groups.
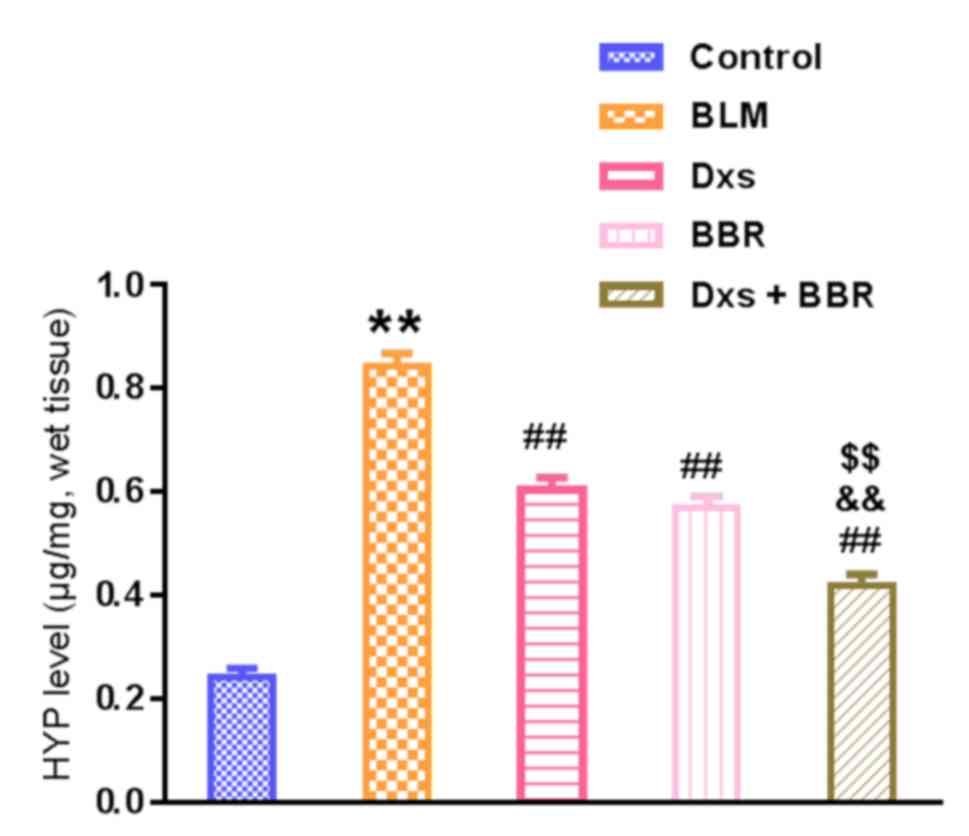
Hyp content in the lung tissues, a representative
marker for collagen deposition, was further measured. As summarized
in Fig. 3, BLM significantly
increased Hyp levels compared with the control (P<0.01). Dxs or
BBR treatment had a beneficial outcome, significantly reducing the
Hyp levels compared with the BLM group (P<0.01). These
observations are in line with the Masson's analysis. Combination of
Dxs and BBR induced a significantly greater inhibitory effect on
collagen accumulation compared with Dxs or BBR single treatment
(P<0.01).
Dxs plus BBR reduces levels of
fibrogenesis-associated makers
To assess the antifibrotic effect of Dxs plus BBR
treatment at molecular level, collagen I/III and
differentiation-dependent α-SMA levels in serum and lung tissues
were measured. p-Smad2/3 was further evaluated via measuring the
ratio of p-Smad2/3: Total Smad2/3 levels. As presented in Fig. 4, BLM significantly increased collagen
I/III, α-SMA and p-Smad2/3 levels compared with the control
(P<0.01). This effect was significantly alleviated by Dxs or BBR
single treatment (P<0.01). No significant differences in
decreasing fibrogenesis-associated markers were observed between
Dxs and BBR single treatment groups. Dxs plus BBR treatment
significantly decreased collagen I/III (all P<0.05, except for
serum collagen I at day 3), α-SMA (P<0.05) and p-Smad2/3
(P<0.01) levels compared with the Dxs or BBR groups. The data
suggest that Dxs plus BBR was effective in preventing BLM-induced
PF at molecular levels and Smad2/3 signaling pathway activation
served a role in this process.
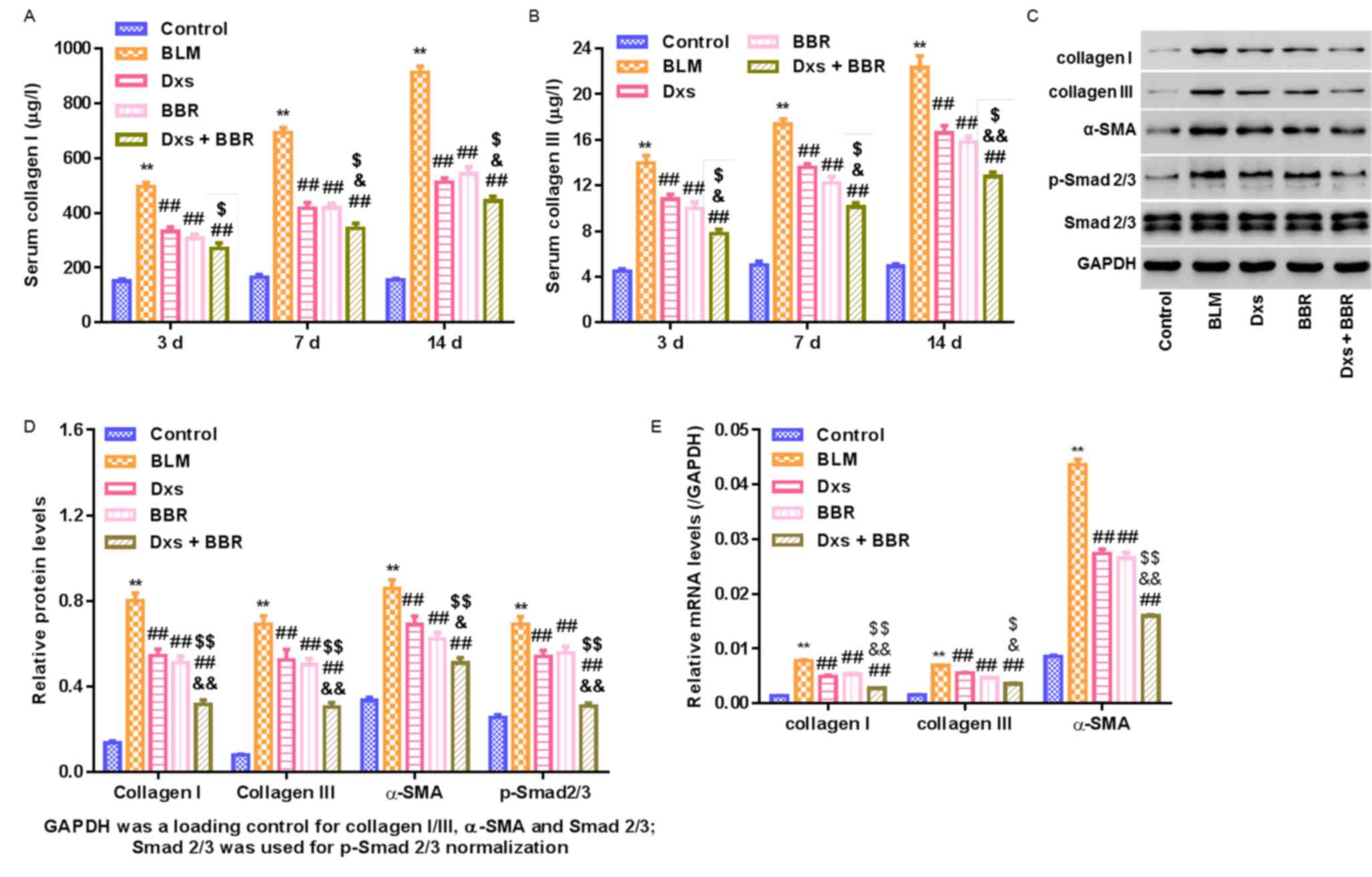 | Figure 4.Regulation of fibrogenesis-associated
markers in a rat model with pulmonary fibrosis. A rat model was
established, including control animals, BLM-induced PF rats and
BLM-induced animals treated with Dxs, BBR or Dxs plus BBR. (A)
Collagen I and (B) collagen III serum levels assessed by ELISA on
days 3, 7 and 14 of treatment. Western blot (C) images and (D)
quantitative analysis of lung tissues on day 14 evaluating collagen
I/III, α-SMA, p-Smad2/3, Smad2/3 and GAPDH. (E) Reverse
transcription-qualitative polymerase chain reaction analysis of
collagen I/III and α-SMA in lung tissues on day 14. GAPDH served as
internal control for collagen I/III, α-SMA and Smd2/3. Smd2/3
served as internal control for p-Smad2/3. **P<0.01 vs. Control;
##P<0.01 vs. BLM; $P<0.05 and
$$P<0.01 vs. Dxs; and &P<0.05 and
&&P<0.01 vs. BBR. BLM, bleomycin; Dxs,
dexamethasone; BBR, berberine; SMA, smooth muscle actin; p-,
phosphorylated. |
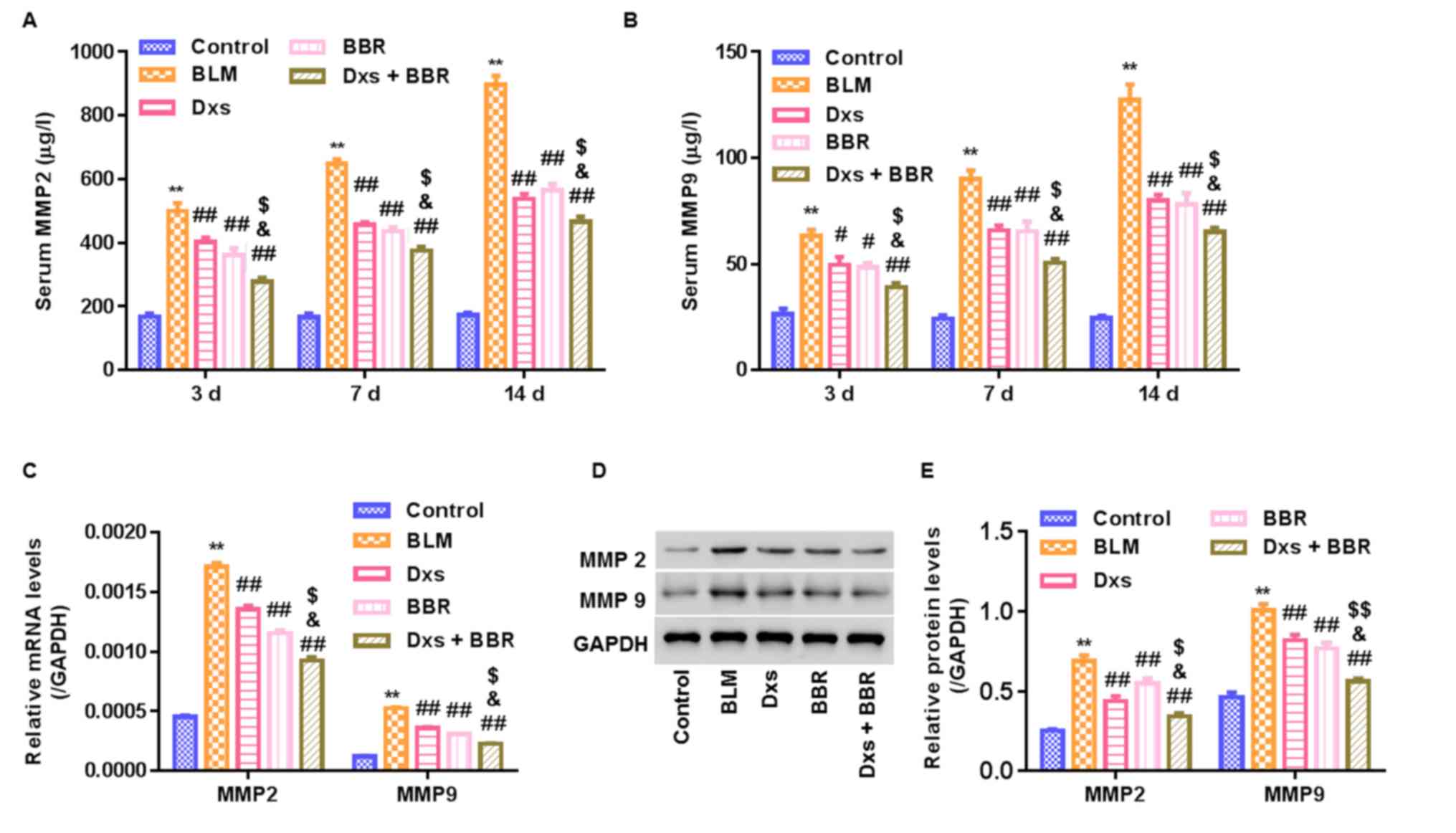
Dxs plus BBR inhibits MMP2/9
expression
To study whether MMP2/9 are involved in the
antifibrotic effect of Dxs plus BBR in BLM-treated rats, MMP2/9
expression was measured. ELISA assessment revealed that BLM
significantly increased serum MMP2/9 levels in a time-dependent
manner, with the highest increase observed on day 14 (P<0.01;
Fig. 5A and B). RT-qPCR and western
blot analyses were conducted to assess MMP2/9 mRNA and protein
expression in lung tissues on day 14, respectively. It was observed
that Dxs or BBR treatment significantly decreased MMP2/9 mRNA and
protein levels compared with the BLM group (P<0.01). Dxs plus
BBR treatment revealed to further significantly reduce MMP2/9
levels in serum or lung tissues compared with the Dxs and BBR
single treatment groups (Fig.
5C-E).
Dxs plus BBR inhibits CXCL14
expression
To study whether CXCL14 was involved in the
antifibrotic effect of Dxs plus BBR treatment, serum protein and
lung tissue mRNA and protein levels of CXCL14 and CXCR4 were
measured. As presented in Fig. 6A, a
time-dependent increase in serum CXCL14 and CXCR4 levels was
observed for the BLM group compared with the control (P<0.01),
with the highest increase measured on day 14. BLM further
significantly elevated CXCL14 and CXCR4 mRNA and protein levels in
lung tissues on day 14 compared with the control group (P<0.01;
Fig. 6B-D). Dxs or BBR treatment
significantly decreased CXCL14 and CXCR4 levels in all samples
compared with the BLM group (P<0.05). No marked differences were
observed between these groups. In the Dxs plus BBR group, CXCL14
and CXCR4 levels in the serum and lung tissue were significantly
lower compared with the Dxs and BBR single treatment groups
(P<0.05).
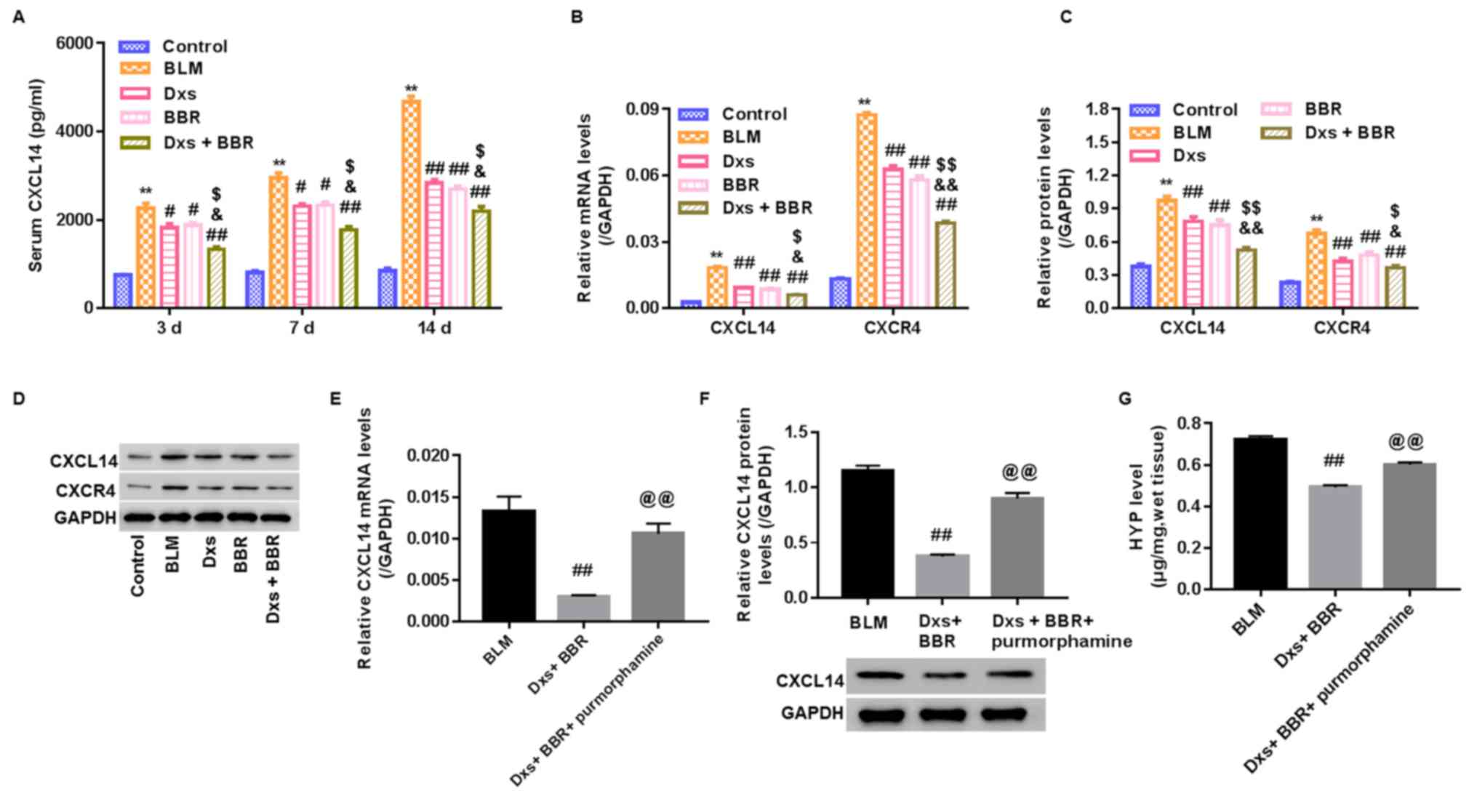 | Figure 6.Regulation of CXCL14 in a rat model
with pulmonary fibrosis. A rat model was established, including
control animals, BLM-induced PF rats and BLM-induced animals
treated with Dxs, BBR or Dxs plus BBR. (A) CXCL14 serum levels
assessed by ELISA on days 3, 7 and 14. (B) Reverse
transcription-qualitative polymerase chain reaction analysis, (C
and D) western blot bands and quantification for CXCL14 and CXCR4
in lung tissues on day 14. For the assessment of hedgehog signaling
pathway activation, BLM-induced PF rats were treated with Dxs plus
BBR or Dxs+BBR+purmorphamine. (E) Reverse transcription-qualitative
polymerase chain reaction analysis, (F) western blot images and
quantification, and (G) Hyp levels in lung tissues on day 14.
**P<0.01 vs. Control; #P<0.05 and
##P<0.01 vs. BLM; $P<0.05 and
$$P<0.01 vs. Dxs; &P<0.05 and
&&P<0.01 vs. BBR; @@P<0.01 vs.
Dxs+BBR. BLM, bleomycin; Dxs, dexamethasone; BBR, berberine; CXC,
C-X-C motif; R, receptor; L, ligand; Hyp, hydroxyproline. |
Dxs plus BBR affects the Hh signaling
pathway
To study whether the Hh signaling pathway was
involved in the effect of Dxs plus BBR treatment, BLM-induced rats
with PF were intraperitoneally injected with Dxs+BBR+purmorphamine
and on day 14, CXCL14 mRNA and protein expression and Hyp content
in lung tissues were assessed. Purmorphamine is used in Hh
signaling pathway activation (14).
It was observed that purmorphamine significantly enhanced CXCL14
mRNA, CXCL14 protein and Hyp levels in lung tissues compared with
the Dxs plus BBR group (P<0.01; Fig.
6E-G). The data suggested that inhibiting the Hh signaling
pathway may be the primary mechanism by which Dxs plus BBR affect
BLM-induced PF in rats.
Discussion
The BLM-induced PF model is widely used for
diagnosing and treating PF and helping to explore underlying
mechanisms (16). Treating rats with
BLM via endotracheal injection caused a gradual increase of
alveolar inflammation. On day 7, inflammation started to increase,
proliferation of fibroblasts was promoted and collagen production
was increased. On day 14, the alveolar structure disappeared and
collagen was widely deposited, exhibiting first pathological
indicators of PF. Hyp serves as one of the main components in
collagen (17). Hyp is used to
assess presence and extent of collagen deposition and is an
accepted indicator for predicting drug efficacy in PF treatment
(18). In the present study, a
histological examination, Masson's trichrome staining and Hyp
content in lung tissues were used to assess the rat PF model and
its response to Dxs and BBR treatment on day 14. With Dxs or BBR
intervention, lung damage and increases in Hyp content induced by
BLM were markedly alleviated, confirming antifibrotic effects of
BBR and Dxs at a histological level, which was in agreement with
previous studies (10,19). Combination of BBR and Dxs
intervention exhibited stronger effects compared with the single
intervention groups and lung damage and collagen deposition were
reduced to a level similar to the control group. The results
demonstrated that BBR plus Dxs treatment was an effective strategy
to prevent PF.
The degree of PF was further assessed at molecular
levels. Dxs or BBR were reported to prevent the Smad2/3 signaling
pathway activation, which is a major determinant mechanism in
promoting PF (11).
Myofibroblast-derived collagen I/III and α-SMA account for the
provisional ECM accumulation (2). In
the present study, it was assessed whether collagen I/III and α-SMA
were involved in the cooperative effect of BBR and Dxs treatment in
regulating collagen deposition in the BLM-induced rat PF model and
Smad2/3 signaling pathway activation was determined. A successful
construction of a PF model was confirmed by severe collagen
deposition and Smad2/3 signaling pathway activation in the BLM
group, demonstrated by significant increases of collagen I/III,
α-SMA and p-Smad2/3 levels. The present study further confirmed
inhibitory effect of BBR or Dxs single treatment on the expression
of these fibrotic makers. However, with BBR plus Dxs treatment,
collagen I/III, α-SMA and p-Smad2/3 levels significantly decreased
further compared with the single treatments. The results
demonstrated that the anti-fibrotic effect of BBR plus Dxs on
BLM-induced PF were significantly enhanced when compared with BBR
or Dxs alone with inhibition of Smad2/3 signaling pathway
activation potentially the underlying mechanism.
In a literature reported BLM-induced PF model,
overall MMP2/9 expression in lung tissue determined by
immunohistochemistry increases, with a peak on day 4 and decreases
following day 7, with distinctly higher levels observed until day
14 (20). In the present study, the
levels of MMPs in serum and lung tissue was measured on day 3, 7
and 14 using ELISA and western bolt. By contrast, data demonstrated
a time-dependent increase in MMPs in both serum and lung tissue of
BLM-induced PF (Fig. 5). Dxs
inhibits MMP2/9 expression in human lung cancer cells (21). Berberine (BBR) has further been
reported to exhibit inhibitory effects on MMP2/9 in human airway
smooth muscle HASMCs cells (22),
breast cancer cells (23) and lung
cancer A549 cells (23). However,
data on the roles of BBR, Dxs or BBR plus Dxs in regulating MMP2/9
expression in BLM-treated rats are scarce. Findings of the current
study suggested that with BBR or Dxs treatment, MMP2/9 levels were
significantly reduced compared with the BLM group; however, the
inhibitory effect was further significantly increased using
combination treatment of BBR and Dxs. The results demonstrated an
involvement of MMP2/9 in the prevention of PF. To further verify
the activity of MMP2/9, a gelatin zymography assay may be performed
in the future to measure levels of active and latent MMP2/9.
CXCL14 is upregulated during PF (24). Our previous study revealed that
eliminating CXCL14 expression provides a therapeutic strategy for
preventing mouse L929 fibroblasts from undergoing fibrogenesis
(25). However, whether CXCL14 was
involved in the anti-fibrotic effect of BBR or Dxs treatment,
remains unknown. Data from the present study revealed that Dxs or
BBR single treatment significantly reduced the levels of CXCL14 and
its receptor CXCR4 compared with the BLM group. The combined used
of BBR and Dxs induced significant decreases of CXCL14 and CXCR4
compared with the single treatment groups. This demonstrated an
involvement of CXCL14 in the effectiveness of the combination
treatment.
Activation of the Hh signaling pathway accelerates
PF development and circulating CXCL14 has been used as a biomarker
in assessing Hh signaling pathway activation (26). The current study further evaluated
whether the Hh signaling pathway was involved in the antifibrotic
function of Dxs plus BBR in the BLM-induced rat PF model. Data
suggested that purmorphamine, an Hh signaling pathway activator,
enhanced CXCL14 expression in the Dxs plus BBR group, indicating an
activation of the Hh signaling pathway in the treatment process. In
addition, it was observed that purmorphamine worsened rat PF in the
BBR plus Dxs treatment group, as demonstrated by significantly
increased Hyp content in lung tissues. The results suggested that
Dxs plus BBR acted via the Hh signaling pathway to exert its
antifibrotic effects in BLM-induced rat PF.
In conclusion, the results of the present study
suggested that the combination of BBR and Dxs may have a
cooperative effect on preventing BLM-induced rat PF. The
antifibrotic mechanisms of BBR plus Dxs included downregulating
MMP2/9 and CXCL14 levels and interrupting Smad2/3 and Hh signaling
pathway activation. Dxs combined with BBR may represent an
effective therapy to treat human PF.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Shanghai
Municipal Health and Family Planning Commission 2016 (grant no.
20164Y0241).
Availability of data and materials
The datasets used and/or analyzed in the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
HPL and YCQ designed the study. LL, QHL, FYL and YHS
conducted experiments and performed data entry. LW, ZFW, WM, XLZ
and SFZ were responsible for statistical analysis and data
interpretation. LL, HPL, YCQ and XLZ prepared the manuscript. All
authors read and approved the final manuscript.
Ethical approval and consent to
participate
Experimental procedures and the Animal Use and Care
protocols were approved by the Committee for Ethical Use of Animals
of Baoshan District Hospital of Integrated Traditional Chinese and
Western Medicine (Shanghai, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Datta A, Scotton CJ and Chambers RC: Novel
therapeutic approaches for pulmonary fibrosis. Br J Pharmacol.
163:141–172. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wilson MS and Wynn TA: Pulmonary fibrosis:
Pathogenesis, etiology and regulation. Mucosal Immunol. 2:103–121.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wynn TA: Integrating mechanisms of
pulmonary fibrosis. J Exp Med. 208:1339–1350. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chakrabarti S and Patel KD: Matrix
metalloproteinase-2 (MMP-2) and MMP-9 in pulmonary pathology. Exp
Lung Res. 31:599–621. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Corbel M, Belleguic C, Boichot E and
Lagente V: Involvement of gelatinases (MMP-2 and MMP-9) in the
development of airway inflammation and pulmonary fibrosis. Cell
Biol Toxicol. 18:51–61. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu YM, Nepali K and Liou JP: Idiopathic
pulmonary fibrosis: Current status, recent progress, and emerging
targets. J Med Chem. 60:527–553. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Keane MP, Strieter RM, Lynch JP III and
Belperio JA: Inflammation and angiogenesis in fibrotic lung
disease. Semin Respir Crit Care Med. 27:589–599. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wuyts WA, Antoniou KM, Borensztajn K,
Costabel U, Cottin V, Crestani B, Grutters JC, Maher TM, Poletti V,
Richeldi L, et al: Combination therapy: The future of management
for idiopathic pulmonary fibrosis? Lancet Respir Med. 2:933–942.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li HP, Li X, He GJ, Yi XH and Kaplan AP:
The influence of dexamethasone on the proliferation and apoptosis
of pulmonary inflammatory cells in bleomycin-induced pulmonary
fibrosis in rats. Respirology. 9:25–32. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chitra P, Saiprasad G, Manikandan R and
Sudhandiran G: Berberine attenuates bleomycin induced pulmonary
toxicity and fibrosis via suppressing NF-κB dependant TGF-β
activation: A biphasic experimental study. Toxicol Lett.
219:178–193. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chitra P, Saiprasad G, Manikandan R and
Sudhandiran G: Berberine inhibits Smad and non-Smad signaling
cascades and enhances autophagy against pulmonary fibrosis. J Mol
Med (Berl). 93:1015–1031. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhai L, Yan X, Wang H, Zhao N, Liang G and
Tie XU: Experimental study on the therapeutic effect of
penehyclidine hydrochloride plus dexamethasone on pulmonary
fibrosis induced by paraquat in rats. Acta Academiae Med Xuzhou.
30:516–519. 2010.
|
|
13
|
Yang X, Wu L, Li G, Ran Q and Zhang L:
Alphacalcidol combined with dexamethasone for reducing pulmonary
fibrosis in mice and its mechanism. Xi Bao Yu Fen Zi Mian Yi Xue Za
Zhi. 33:488–491. 2017.(In Chinese). PubMed/NCBI
|
|
14
|
Chen MM, Bai HY, Zeng ZL and Neurology DO:
The activation of the SHH pathway affects the cytoskeletal
proteinα-tubulin and MAP-2 in stroke rat model. Zhong Feng Yu Shen
Jing Ji Bing Za Zhi. 32:577–582. 2013.(In Chinese).
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tashiro J, Rubio GA, Limper AH, Williams
K, Elliot SJ, Ninou I, Aidinis V, Tzouvelekis A and Glassberg MK:
Exploring animal models that resemble idiopathic pulmonary
fibrosis. Front Med (Lausanne). 4:1182017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li P and Wu G: Roles of dietary glycine,
proline, and hydroxyproline in collagen synthesis and animal
growth. Amino Acids. 50:29–38. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
El-Kashef DH: Nicorandil ameliorates
pulmonary inflammation and fibrosis in a rat model of silicosis.
Int Immunopharmacol. 64:289–297. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen XL, Xiao QM, Liu JJ, Xia-Hong HE and
Ouyang B: PPARγ agonist and dexamethasone alleviate the pulmonary
fibrosis induced by bleomycin in rats through upregulating
glucocorticoid receptors. Xian Dai Sheng Wu Yi Xue Jin Zhan.
23:4609–4613. 2011.(In Chinese).
|
|
20
|
Kim JY, Choeng HC, Ahn C and Cho SH: Early
and late changes of MMP-2 and MMP-9 in bleomycin-induced pulmonary
fibrosis. Yonsei Med J. 50:68–77. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Roomi MW, Monterrey JC, Kalinovsky T,
Niedzwiecki A and Rath M: Modulation of MMP-2 and MMP-9 by
cytokines, mitogens and inhibitors in lung cancer and malignant
mesothelioma cell lines. Oncol Rep. 22:1283–1291. 2009.PubMed/NCBI
|
|
22
|
Liu SJ, Yin CX, Ding MC, Xia SY, Shen QM
and Wu JD: Berberine suppresses in vitro migration of human aortic
smooth muscle cells through the inhibitions of MMP-2/9, u-PA, AP-1,
and NF-κB. BMB Rep. 47:388–392. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kalaiarasi A, Anusha C, Sankar R,
Rajasekaran S, John Marshal J, Muthusamy K and Ravikumar V: Plant
Isoquinoline Alkaloid berberine exhibits chromatin remodeling by
modulation of histone deacetylase to induce growth arrest and
apoptosis in the A549 cell line. J Agric Food Chem. 64:9542–9550.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ishii T, Nureki SI, Miyazaki E, Masuda D,
Nishio S, Yamasue M, Fujisaki KH, Takenaka R, Takeo I, Masaru A and
Kumamoto T: Elevated levels of BRAK/CXCL14 from patients with
idiopathic pulmonary fibrosis. Am J Respiratory Crit Care Med.
185:A51782012.
|
|
25
|
Li L, Li Q, Wei L, Wang Z, Ma W, Liu F,
Shen Y, Zhang S, Zhang X, Li H and Qian Y: Chemokine (C-X-C motif)
ligand 14 contributes to lipopolysaccharide-induced fibrogenesis in
mouse L929 fibroblasts via modulating PPM1A. J Cell Biochem.
120:13372–13381. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jia G, Chandriani S, Abbas AR, DePianto
DJ, N'Diaye EN, Yaylaoglu MB, Moore HM, Peng I, DeVoss J, Collard
HR, et al: CXCL14 is a candidate biomarker for Hedgehog signalling
in idiopathic pulmonary fibrosis. Thorax. 72:780–787. 2017.
View Article : Google Scholar : PubMed/NCBI
|