Introduction
Lung cancer is one of the most common malignancies
and the leading cause of cancer-related mortality every year
worldwide (1). Multiple risk
factors, including environmental deterioration, smoking, exposure
to radon and the overexpression or mutation of certain key proteins
(for example, epidermal growth factor receptor) may contribute to
the occurrence and development of the disease (2–4). Due to
the heterogeneity of the disease, the lack of early diagnostic
markers and the high recurrence rate (~50% of cases), the
therapeutic efficacy of current anti-lung cancer therapies remains
unsatisfactory (5), the therapeutic
efficacy of current anti-lung cancer therapies, including surgical
resection, radiotherapy and chemotherapy remain unsatisfactory, and
the prognosis for patients with lung cancer is poor (5-year
survival rate, <16%) (6,7). Although lung cancer is divided into
various subtypes, non-small cell lung cancer (NSCLC) is the most
common, accounting for >85% of cases (8) NSCLC can be classified into three
subtypes, including adenocarcinoma, squamous cell carcinoma and
large cell carcinoma. In recent years, efforts have been made to
explore the pathogenesis of NSCLC (9–12);
however, the mechanism underlying the carcinogenesis and
development of NSCLC remains poorly understood. Elucidating the
molecular mechanisms involved in NSCLC may help researchers to
identify effective therapeutic targets, and also provide novel
biomarkers for the risk assessment and early diagnosis of NSCLC
(13).
In recent years, the roles of non-coding RNA in
various diseases have become a hot topic among scientists and
physicians. MicroRNA (miR) are a group of small, non-coding RNA
that may negatively regulate the expression of their target genes
(14). Abnormal behaviors of miR in
various types of cancer have been observed, and the roles of miR as
tumor suppressors or oncomiR have been reported previously
(15–17). miR-210 has been demonstrated to serve
important roles in a variety of cancer types. For example, it was
revealed that the deletion of miR-210-3p may promote the
carcinogenesis of renal cell carcinoma (18), while miR-210 is increased in
osteosarcoma and may contribute to the malignant progression of the
disease (19). Furthermore, in
breast cancer, miR-210 may interact with F-box only protein 31 and
regulate the proliferation and migration of human breast cancer
cells (20).
In NSCLC, the upregulation of miR-210-3p has been
demonstrated in several previous studies (21–23);
however, the role of miR-210-3p in the pathogenesis NSCLC requires
further investigation. The aim of the present study was to
investigate the expression and biological functions of miR-210-3p
in NSCLC, and to elucidate the underlying molecular mechanisms in
NSCLC. The expression of miR-210-3p in NSCLC tumor tissues and cell
lines was examined, and the associations between the expression of
miR-210-3p and the clinical features of patients with NSCLC were
investigated. Furthermore, the effect of miR-210-3p on cell
proliferation and apoptosis was explored.
Materials and methods
Patients and clinical information
The present study enrolled 30 patients (Average age
62.1±9.8 years, Age range, 35–79 years; 18 males and 12 females)
who had been diagnosed with NSCLC between August 2010 and December
2015 at Handan First Hospital (Handan, China). For each patient,
paired cancer tissues and adjacent normal tissues were collected
during surgery and immediately stored in liquid nitrogen until use.
Patients who received chemotherapy or radiotherapy were excluded
from the study. The clinical information of the patients is
summarized in Table I. The Research
Ethics Committee of Handan First Hospital approved the present
study, and each patient signed an informed consent form.
 | Table I.Univariate analysis for correlation
between the expression levels of miR-210-3p and the clinical
characteristics of the patients. |
Table I.
Univariate analysis for correlation
between the expression levels of miR-210-3p and the clinical
characteristics of the patients.
|
Characteristics | n | Relative miR-210-3p
expression level | P-value |
|---|
| Age, years |
|
| 0.214 |
|
<60 | 11 | 1.29±0.83 |
|
|
≥60 | 19 | 1.37±1.07 |
|
| Sex |
|
| 0.187 |
|
Male | 18 | 1.39±0.76 |
|
|
Female | 12 | 1.21±0.98 |
|
| Tumor size, cm |
|
| 0.005 |
| ≥5 | 9 | 1.52±1.29 |
|
|
<5 | 21 | 1.10±0.82 |
|
| Histological grade
(differentiation) |
|
| 0.024 |
|
Well-intermediate | 14 | 1.31±0.97 |
|
|
Poor | 16 | 1.12±1.12 |
|
| Metastasis |
|
| 0.011 |
|
Yes | 8 | 1.49±1.05 |
|
| No | 22 | 1.14±0.74 |
|
Cell culture
The human NSCLC cell lines A549, H358, H1650 and
H1299, and normal human lung epithelial cell line BEAS-2B, were
purchased from the Shanghai Institute of Cell Biology, Chinese
Academy of Sciences (Shanghai, China). Cells were maintained in
Dulbecco's modified Eagle's medium (DMEM) supplemented with 10%
fetal bovine serum (FBS) (both Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) and 1% penicillin/streptomycin solution
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) in an incubator at
37°C with 5% CO2.
Cell transfection
The miR-210-3p inhibitors
(5′-UCAGCCGCUGUCACACGCACAG-3′; 50 nM), miR-210-3p inhibitor
negative control (NC; 5′-CAGUACUUUUGUGUAGUACAA-3′; 50 nM) and SIN3A
small interfering (si)RNA (20 nM; forward,
5′-CUACGUCUCAAGGAACCUTT-3′ and reverse 5′-UAGGUUCCUUGAGACGUAGTT-3′)
were synthesized by Shanghai GenePharma Co., Ltd., (Shanghai,
China). The sequences of the SIN3A siRNA were: Cell transfection
was performed using Lipofectamine® RNAi Max (Invitrogen;
Thermo Fisher Scientific, Inc.). Cells were divided into five
groups: Control group (untreated cells), NC group (miR-210-3p
inhibitor NC-transfected group), inhibitor group (miR-210-3p
inhibitor-transfected group), inhibitor + SIN3A siRNA group
(miR-210-3p inhibitor + SIN3A siRNA-transfected group), NC + SIN3A
siRNA group (miR-210-3p inhibitor NC + SIN3A siRNA-transfected
group). The effects of the miR-210-3p inhibitor on A549 or H1299
cells were examined using various assays at 48 h after
transfection.
Cell proliferation analysis
The effects of the miR-210-3p inhibitor on the
proliferation of A549 and H1299 cells was determined by using an
MTT assay (the purple formazan was dissolved in DMSO) at 12, 24 and
48 h, as well as a Cell Proliferation kit I (MTT; Sigma-Aldrich;
Merck KGaA), according to the manufacturer's protocol. Viability
was determined by the optical density values at 490 nm.
Cell apoptosis analysis
After transfection for 48 h, A549 and H1299 cells
were double-stained with annexin V-fluorescein isothiocyanate
(FITC) at 4°C for 15 min and propidium iodide (PI) and at 4°C for 5
min using a PI/Annexin V-FITC Apoptosis Detection kit
(Sigma-Aldrich; Merck KGaA). The rates of apoptosis of the cells in
different groups were examined with a BD FACSCalibur™ flow
cytometer (BD Biosciences, San Jose, CA, USA). The data was
analyzed using FlowJo (version 7.6.5; Tree Star, Inc., Ashland, OR,
USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from cells and the tissue
samples using TRIzol® (Invitrogen; Thermo Fisher
Scientific, Inc.) and RT-qPCR was performed. The expression of
miR-210-3p was examined using a Hairpin-it™ MicroRNA Quantitation
kit (Shanghai GenePharma Co., Ltd.), with U6 (RNU6B; Shanghai
GenePharma Co., Ltd.) used for normalization. The thermocycling
profiles were as follow: 95°C for 3 min; followed by 40 cycles of
95°C for 12 sec and 62°C for 40 sec. The data were analyzed using
the 2−ΔΔCq method (24).
The mRNA expression levels of SIN3A were examined by performing
reverse transcription with a PrimeScript™ RT Master Mix (Takara
Biotechnology Co., Ltd., Dalian, China) at 37°C for 15 min. PCR was
performed using a SYBR® Fast qPCR Mix (Takara
Biotechnology Co., Ltd.), with GAPDH for normalization. RT-qPCR was
conducted on an ABI 7500 Real-Time PCR System (Applied Biosystems;
Thermo Fisher Scientific, Inc.), according to the manufacturer's
protocol. The primer sequences were as follows: miR-210-3p forward,
5′-GTGCAGGGTCCGAGGT-3′ and reverse, 5′-TATCTGTGCGTGTGACAGCGGCT-3′;
U6 forward, 5′-CTCGCTTCGGCAGCACA-3′ and reverse,
5′-AACGCTTCACGAATTTGCGT-3′; SIN3A forward,
5′-TTAAATCTCAGAGCATCGACAC-3′ and reverse,
5′-AGGAGTTGTCACATTCACCA-3′; GAPDH forward,
5′-CATTTCCTGGTATGACAACGA-3′ and reverse,
5′-GTCTACATGGCAACTGTGAG-3′. The thermocycling profiles were as
follows: 95°C for 30 sec; followed by 40 cycles of 95°C for 5 sec
and 60°C for 30 sec. The data were analyzed using the
2−ΔΔCq method.
Western blot analysis
A549 cells were harvested at 48 h after transfection
and lysed with radioimmunoprecipitation assay buffer (Beyotime
Institute of Biotechnology, Shanghai, China). The concentration of
the total protein was determined using a BCA Protein Assay kit
(Beyotime Institute of Biotechnology). Subsequently, 8% SDS-PAGE
gel was used to separate the proteins, which were then transferred
to polyvinylidene difluoride membranes and blocked with 5% non-fat
milk at room temperature for 1 h. Subsequently, the membranes were
incubated overnight at 4°C with the following primary antibodies
(1:1,000) obtained from Wuhan Boster Biological Technology, Ltd.
(Wuhan, China): anti-SIN3A (cat. no. BM5270), anti-B-cell lymphoma
2 (Bcl-2; cat. no. A00040-1), anti-Caspase-3 (cat. no. BM3957) and
anti-GAPDH (cat. no. A00227). The following day, the membranes were
incubated with horseradish peroxidase-conjugated secondary
antibodies (cat. no. A0208, 1:1,000; Beyotime Institute of
Biotechnology) at room temperature for 45 min and treated with
BeyoECL Plus enhanced chemiluminescent reagent (Beyotime Institute
of Biotechnology). The signals were detected and imaged using a
ChemiDoc™ XRS+ system (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) and analyzed by using ImageJ software (version
1.47; NIH, Bethesda, MD, USA).
Dual-luciferase reporter assay
The prediction of the targets of miR-210-3p was
performed using bioinformatics tools (TargetScan) as previously
described (25). The wild-type SIN3A
3′ untranslated region (UTR; SIN3A-3′UTR), which contains the
miR-210-3p binding site, and a mutant SIN3A 3′UTR sequence
(SIN3A-MUT) were cloned into p-MIR-reporter plasmids (Thermo Fisher
Scientific, Inc.) and transfected into A549 cells with miR-210-3p
mimics (synthesized by Shanghai GenePharma Co., Ltd.; 50 nM; sense,
5′-CUGUGCGUGUGACAGCGGCUGA-3′; antisense,
5′-AGCCGCUGUCACACGCACAGUU-3′) or a mimic NC (50 nM; sense,
5′-UUCUCCGAACGUGUCACGUTT-3′; antisense,
5′-ACGUGACACGUUCGGAGAATT-3′) using Lipofectamine® RNAi
Max. Cells were collected 48 h after transfection, and the
activities of the luciferases were detected using a Dual-Luciferase
Reporter Assay kit (Beyotime Institute of Biotechnology). The
activity of firefly luciferase was normalized to that of renilla
luciferase.
Statistical analysis
All statistical analysis was performed using SPSS v.
22.0 (IBM Corp., Armonk, NY, USA). Data were presented as the mean
± standard deviation of three repeated experiments. The differences
between the expression levels of miR-210-3p and SINA3 in the paired
tumor tissues and adjacent tissues were analyzed using a paired
Student's t-test, while the differences among multiple groups for
in vitro studies were analyzed using one-way analysis of
variance followed by a Turkey's post hoc test. Pearson's
correlation coefficient was used for correlation analysis. Cox
regression model was applied for univariate analysis. P<0.05 was
considered to indicate a statistically significant difference.
Results
Comparison of the expression of
miR-210-3p in tissue samples and cell lines
Initially, paired lung cancer tissues and adjacent
normal tissues were collected from 30 patients with NSCLC, and the
levels of miR-210-3p in the different tissues were examined using
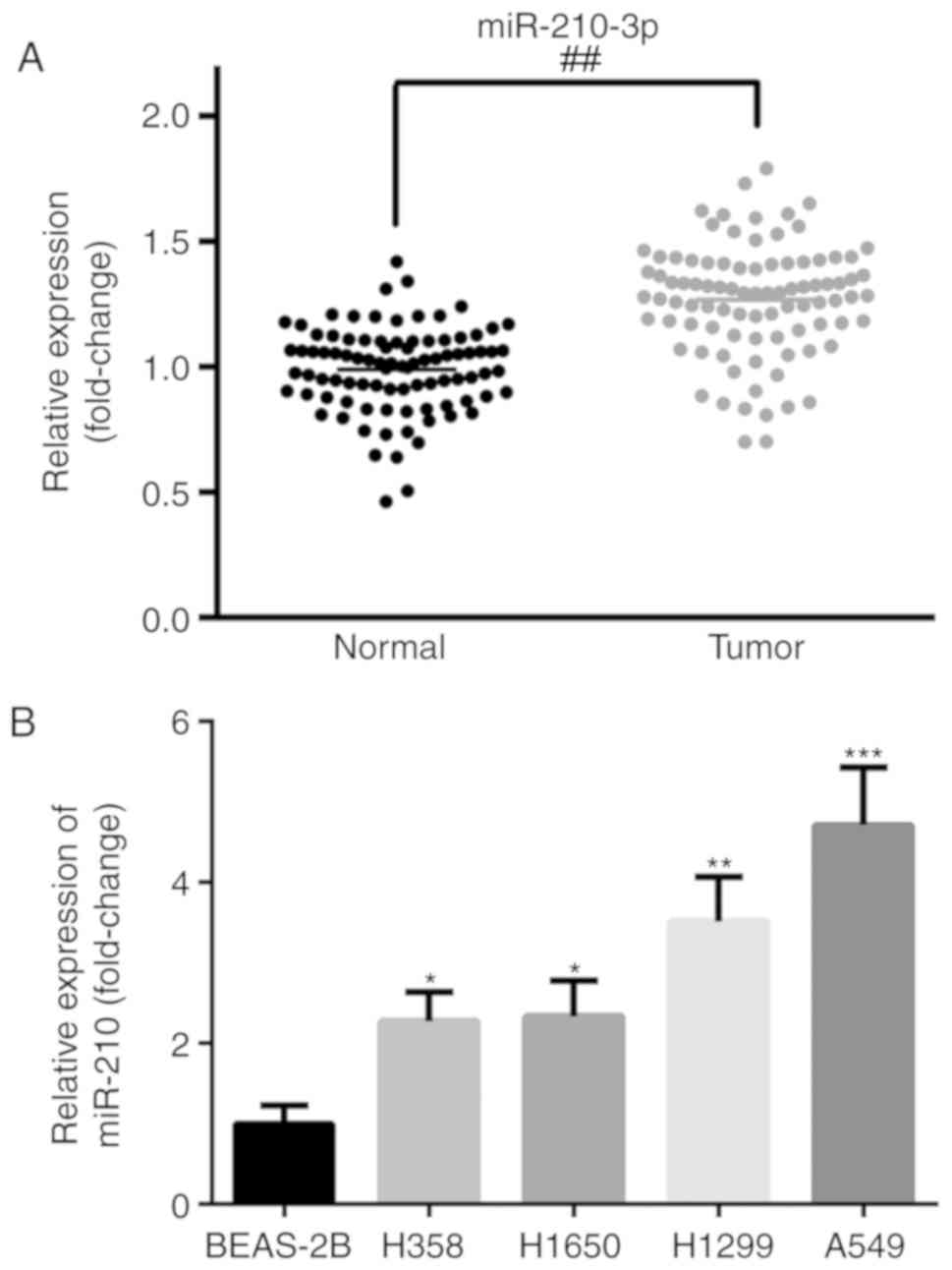
RT-qPCR (Fig. 1). The expression of
miR-210-3p was significantly increased in the cancer tissues
compared with the level in the adjacent tissues (P<0.01;
Fig. 1A). The expression level of
miR-210-3p was positively correlated with tumor size (P=0.005),
histological grade (P=0.024) and metastasis (P=0.011) (Table I). Furthermore, the expression levels
of miR-210-3p in four NSCLC cell lines (A549, H358, H1650, H1299)
and a normal human lung epithelial cell line (BEAS-2B) were also
examined. As demonstrated in Fig.
1B, the expression of miR-210-3p was significantly increased in
NSCLC cell lines compared with the level in the BEAS-2B cell line
(P<0.05). A549 and H1299 exhibited the highest expression of
miR-210-3p and as such were utilized for further analysis.
Effect of transient miR-210-3p
knockdown on the proliferation and apoptosis of A549 and H1299
cells in vitro
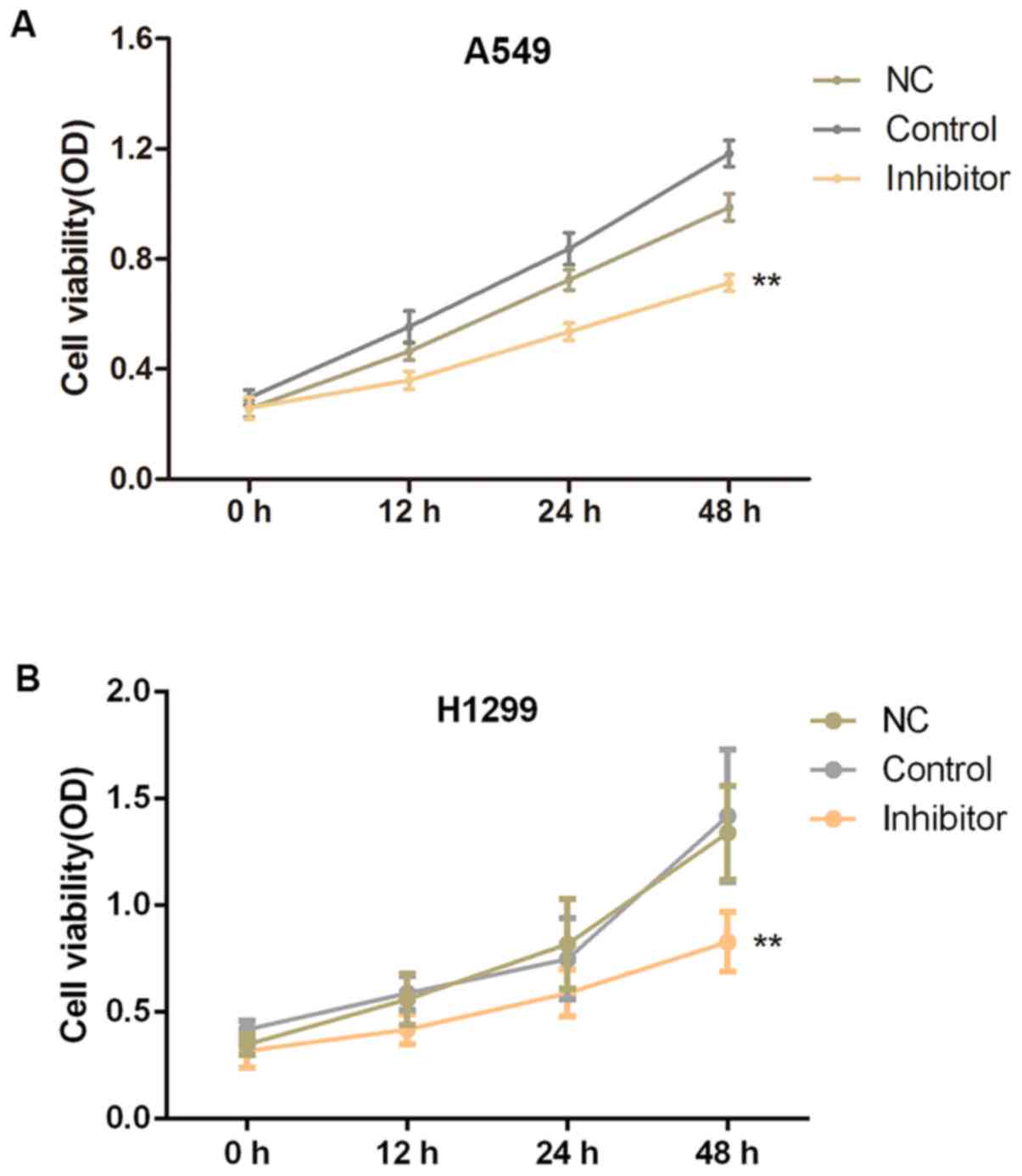
To further explore the roles of miR-210-3p in the
pathogenesis of NSCLC, A549 and H1299 cells were cultured and
transfected with miR-210-3p inhibitors or inhibitor NCs, and the
effects of miR-210-3p on the proliferation and apoptosis of A549
cells were examined using an MTT assay and flow cytometry,
respectively. The transient downregulation of miR-210-3p in the
inhibitor group significantly suppressed the cell growth at 24 and
48 h after transfection, as compared with the control group
(P<0.01; Fig. 2). Furthermore,
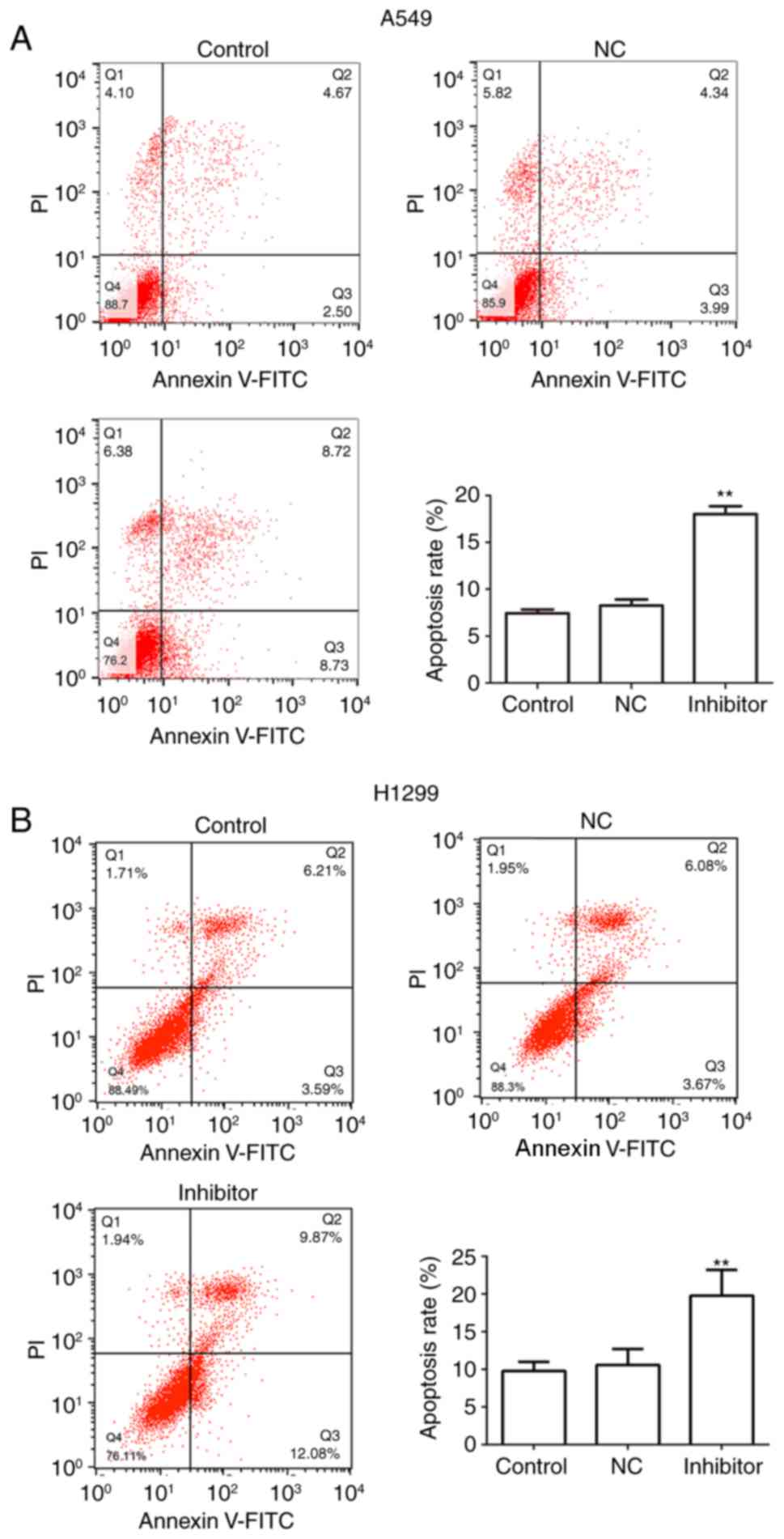
the results of the flow cytometry analysis indicated that
transfection of A549 and H1299 cells with miR-210-3p inhibitors
induced a marked increase in the rate of cell apoptosis in
vitro, as compared with the level in the control group
(P<0.01; Fig. 3).
SIN3A is a direct target of miR-210-3p
in NSCLC
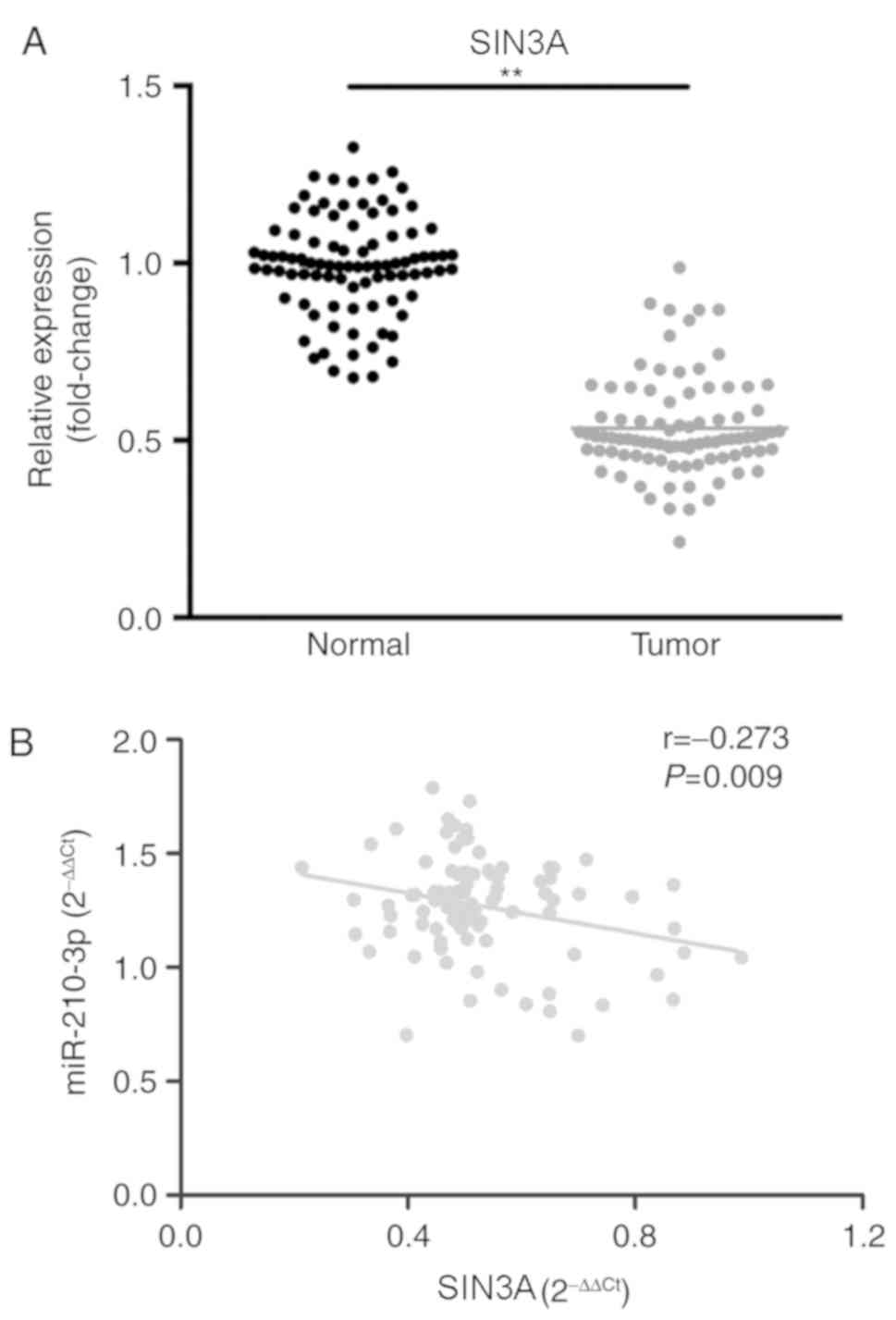
Using bioinformatics tools (TargetScan (25). SIN3A was predicted as a target of
miR-210-3p. Therefore, the association between miR-210-3p and SIN3A
in the pathogenesis of NSCLC was explored. The expression level of
SIN3A was demonstrated to be significantly decreased in lung cancer
tissues compared with the level in the paired adjacent tissues
(P<0.01; Fig. 4A), and the
expression level of miR-210-3p was negatively correlated with that
of SIN3A (r=−0.273; P=0.009; Fig.
4B).
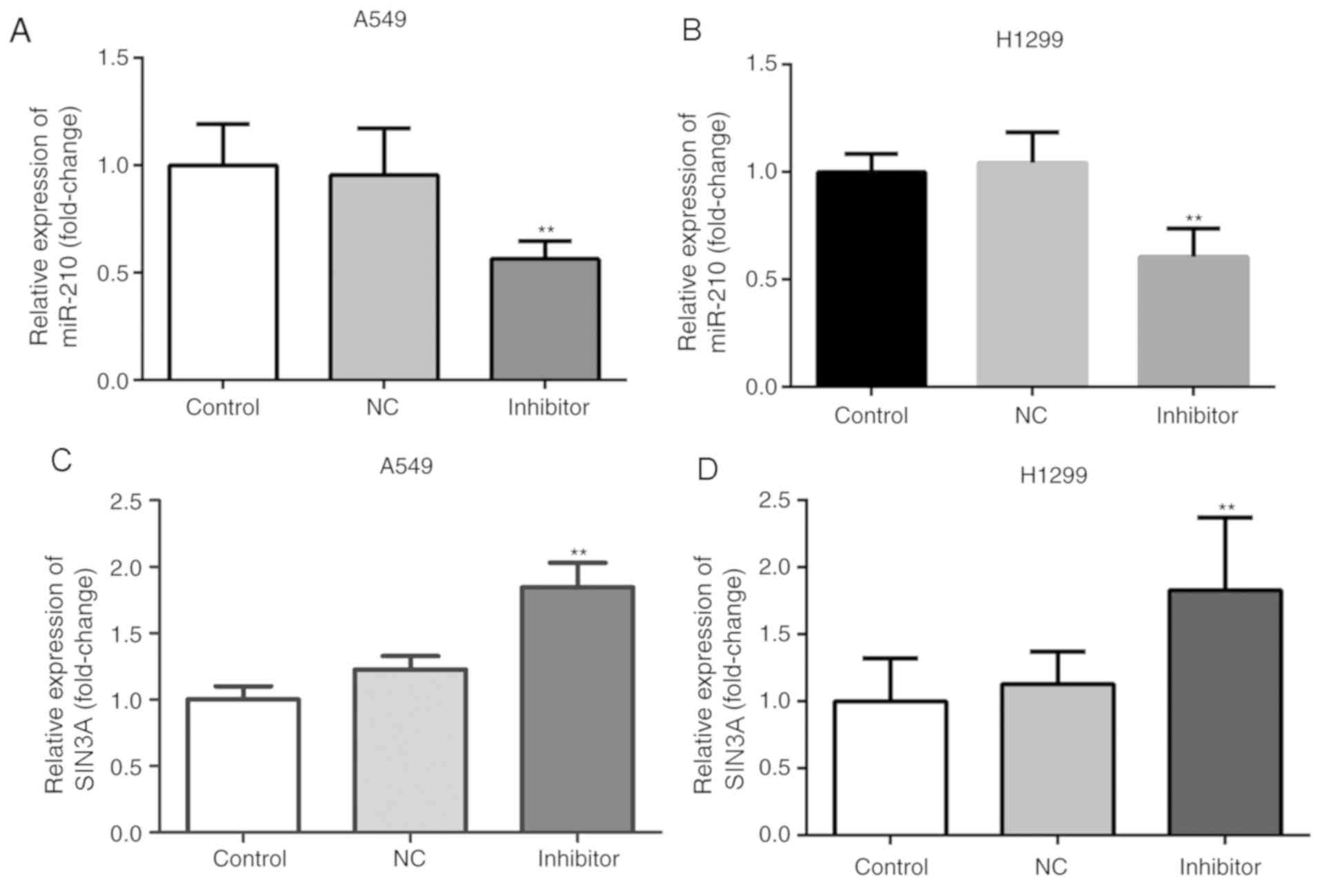
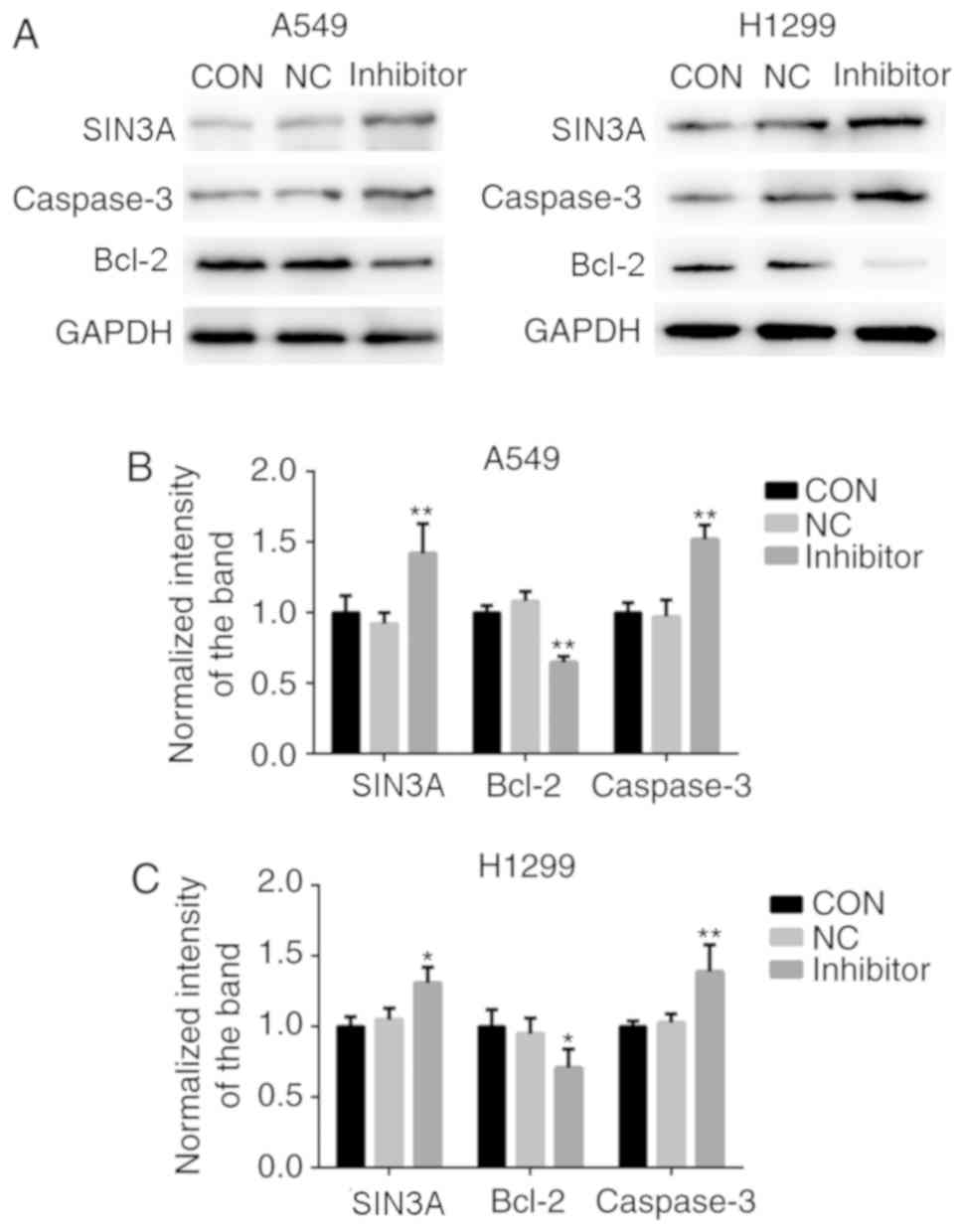
Knockdown of miR-210-3p induced a significant
decrease in the expression level of miR-210-3p and a significant
increase in the expression of SIN3A in A549 and H1299 cells at the
mRNA and protein levels, when compared with the control group
(P<0.05; Figs. 5 and 6). Furthermore, compared with the control
group, knockdown of miR-210-3p significantly decreased the
expression level of the anti-apoptotic factor Bcl-2 and increased
the expression of the pro-apoptotic factor Caspase-3 (P<0.05;
Fig. 6B and C). On the other hand,
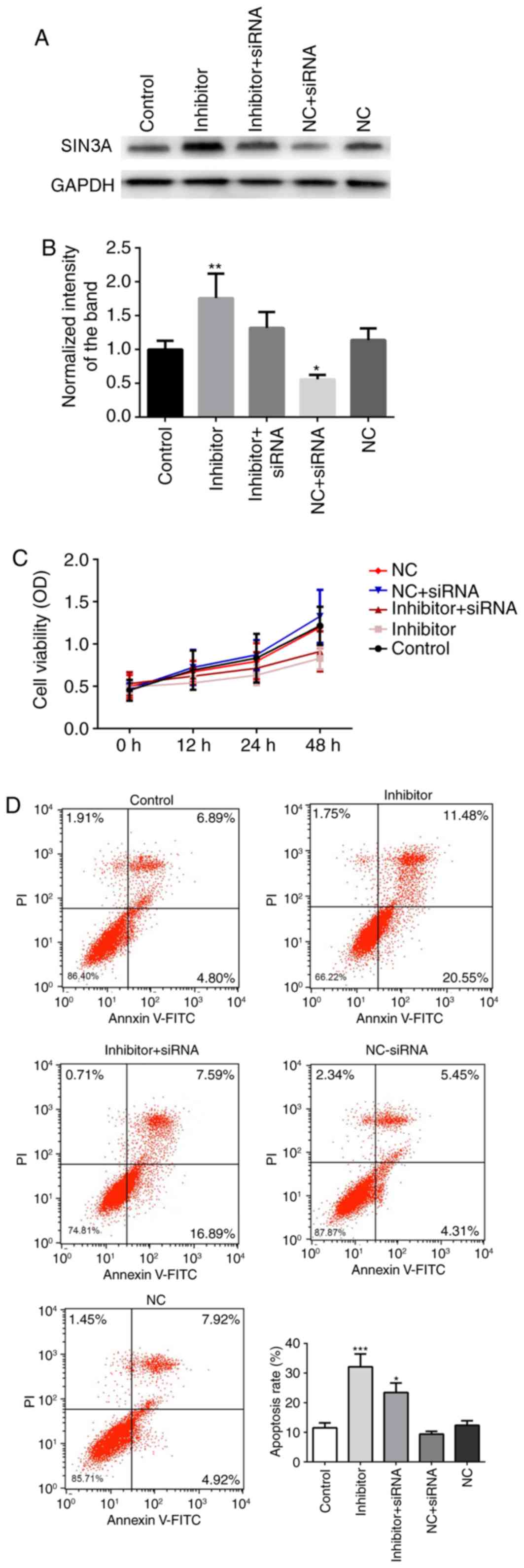
co-transfection with miR-210-3p inhibitor and SIN3A siRNA partially
blocked the miR-210-3p inhibitor-induced pro-apoptotic effects
(Fig. 7). Co-transfection with
miR-210-3p NC and SIN3A siRNA further reduced the expression of
SIN3A (P<0.05), when compared with the NC group, increased the
proliferation and inhibited the apoptosis of A549 cells (but with
no significant difference compared with control; P>0.05).
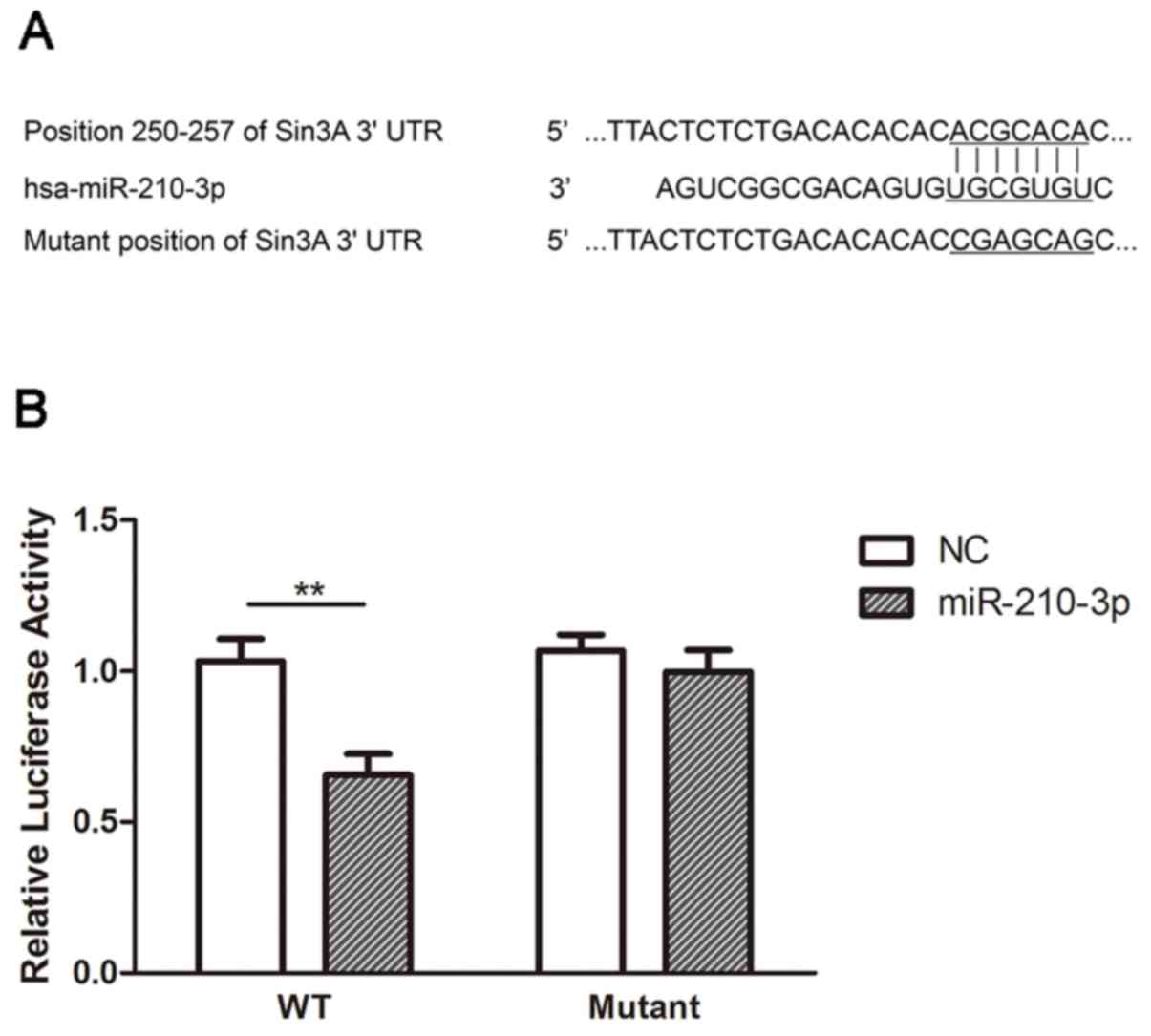
Finally, a dual-luciferase reporter assay was used
to investigate whether miR-210-3p directly targets SIN3A. It was
revealed that transfection of the cells with miR-210-3p mimics
significantly suppressed the luciferase activity of the SIN3A-3′UTR
reporter compared with the activity in the NC (P<0.01); whereas
miR-210-3p mimics had no significant effect on cells transfected
with the SIN3A-MUT reporter (Fig.
8). These results suggest that miR-210-3p directly targets the
3′UTR of SIN3A.
Discussion
The roles of miR-210 in various cancer types have
been discussed in numerous previous studies. miR-210 has been
demonstrated to be upregulated in the majority of cancer types,
including pancreatic cancer (26,27),
colorectal cancer (28,29), breast cancer (30–32) and
renal cell carcinoma (33–35), suggesting that it may act as an
oncomiR. The roles of miR-210 in lung cancer have also been
discussed previously. Zhang et al (36) reported that miR-210 was upregulated
in the plasma of patients with early-stage NSCLC, suggesting that
miR-210 has the potential to be a biomarker for the early diagnosis
of NSCLC that may be detected by non-invasive techniques. Eilertsen
et al (21) demonstrated that
the expression of miR-210 in stromal cells and cancer cells may
serve as a prognostic marker in NSCLC, and Li et al
(37) suggested that the serum
levels of miR-210 may serve as a diagnostic and prognostic marker
for NSCLC. However, the majority of these studies have focused on
the diagnostic and prognostic value of miR-210, and investigations
into the effects of miR-210 on lung cancer cell behavior, including
cell growth, apoptosis and migration, as well as the specific
mechanisms underlying the role of miR-210 in the pathogenesis of
NSCLC, have been limited. In the present study, increased
miR-210-3p expression in cancer tissues and different NSCLC cell
lines was observed, which was consistent with previous findings.
Furthermore, knockdown of miR-210-3p in the A549 and H1299 lung
cancer cell lines led to the significant suppression of cell
proliferation and increase of cell apoptosis. These results suggest
that miR-210-3p is upregulated in NSCLC and that it may regulate
the proliferation and apoptosis of lung cancer cells.
Using bioinformatics tools, SIN3A was predicted as a
target gene of miR-210-3p; however, to the best of our knowledge,
the association between miR-210-3p and SIN3A in NSCLC has not yet
been discussed. SIN3A is a transcriptional regulator that contains
a number of protein-interaction domains (38). Previous studies have indicated that
SIN3A is involved in the processes of cell proliferation,
apoptosis, differentiation and migration, as well as the regulation
of the cell cycle and embryonic development, via interacting with
certain proteins, including Myc, Myc-associated factor X, Max
dimerization protein and methyl-CpG binding protein 2 (39,40).
Previous studies have identified SIN3A as a tumor suppressor in
NSCLC. Suzuki et al (41)
observed decreased expression of SIN3A in NSCLC, and Das et
al (42) demonstrated that
downregulation of SIN3A could increase the invasive behavior of
A549 cells.
In the present study, a series of experiments were
performed to explore the association between miR-210-3p and SIN3A
in NSCLC. First, the expression levels of SIN3A in NSCLC tissues
and adjacent normal tissues were compared, and it was revealed that
SIN3A was downregulated in NSCLC, which was consistent with the
results described by Suzuki et al (41). A subsequent correlation analysis
indicated that the expression of SIN3A was significantly negatively
correlated with the expression of miR-210-3p, suggesting that the
upregulation of miR-210-3p may lead to inhibited expression of
SIN3A in lung cancer tissues. The transfection of A549 and H1299
cells with an miR-210-3p inhibitor induced a significant increase
in the expression of SIN3A, and also led to a significant decrease
in the expression of the downstream anti-apoptotic protein, Bcl-2,
and an increase in the expression of the pro-apoptotic protein,
Caspase-3. Furthermore, co-transfection with miR-210-3p inhibitor
and SIN3A siRNA partially blocked miR-210-3p inhibitor-induced
pro-apoptotic effects. Finally, a dual-luciferase reporter assay
indicated that SIN3A is a direct target of miR-210-3p. As
discussed, miR-210-3p may regulate the proliferation and apoptosis
of A549 cells, and SIN3A is a key regulator of cell proliferation
and apoptosis; thus, miR-210-3p may promote the proliferation and
inhibit the apoptosis of lung cancer cells, at least partially,
through targeting SIN3A.
The present study has limitations. First, due to
ethical issues, only 30 clinical samples were included; therefore,
the results should be verified with a larger sample size. Second,
the present study includes only a clinical study and in
vitro cell studies, and in vivo animal studies should
also be performed to confirm the roles of miR-210-3p and SIN3A in
NSCLC in the future.
In conclusion, the results of the present study
indicate that miR-210-3p is upregulated in NSCLC and may regulate
the proliferation and apoptosis of lung cancer cells by targeting
SIN3A. These results may provide a novel therapeutic target for the
treatment of NSCLC.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XL and HD analyzed the patient data. LS performed
histological examination of samples. JuZ and LZ performed some of
the cell experiments. JR performed most of the cell experiments and
he was a major contributor in writing the manuscript. JiZ designed
the study and wrote part of the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The Research Ethics Committee of Handan First
Hospital approved the present study. Each patient signed an
informed consent form
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Li Z, Zhu W, Xiong L, Yu X, Chen X and Lin
Q: Role of high expression levels of STK39 in the growth, migration
and invasion of non-small cell type lung cancer cells. Oncotarget.
7:61366–61377. 2016.PubMed/NCBI
|
|
2
|
Bernatsky S, Ramsey-Goldman R, Petri M,
Urowitz MB, Gladman DD, Fortin PR, Yelin EH, Ginzler E, Hanly JG,
Peschken C, et al: Smoking is the most significant modifiable lung
cancer risk factor in systemic lupus erythematosus. J Rheumatol.
45:393–396. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fan J, Zhang W, Lei C, Qiao B, Liu Q, Chen
Q, Jiao H, Jiang L, Cui S and Chen J: Vascular endothelial growth
factor polymorphisms and lung cancer risk. Int J Clin Exp Med.
8:6406–6411. 2015.PubMed/NCBI
|
|
4
|
Feng X, Qin JJ, Zheng BS, Huang LL, Xie XY
and Zhou HF: Association of epidermal growth factor receptor (EGFR)
gene polymorphism with lung cancer risk: A systematic review. J
Recept Signal Transduct Res. 34:333–334. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Higashi K, Ueda Y, Arisaka Y, Sakuma T,
Nambu Y, Oguchi M, Seki H, Taki S, Tonami H and Yamamoto I: 18F-FDG
uptake as a biologic prognostic factor for recurrence in patients
with surgically resected non-small cell lung cancer. J Nucl Med.
43:39–45. 2002.PubMed/NCBI
|
|
6
|
Wang J, Sheng Z, Yang W and Cai Y:
Elevated serum concentration of chitinase 3-like 1 is an
independent prognostic biomarker for poor survival in lung cancer
patients. Cell Physiol Biochem. 38:461–468. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang Y, Li H, Han J and Zhang Y:
Down-regulation of microRNA-124 is correlated with tumor metastasis
and poor prognosis in patients with lung cancer. Int J Clin Exp
Pathol. 8:1967–1972. 2015.PubMed/NCBI
|
|
8
|
Hou J, Meng F, Chan LW, Cho WC and Wong
SC: Circulating plasma MicroRNAs as diagnostic markers for NSCLC.
Front Genet. 7:1932016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kuribayashi K, Funaguchi N and Nakano T:
Chemotherapy for advanced non-small cell lung cancer with a focus
on squamous cell carcinoma. J Cancer Res Ther. 12:528–534. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Costa DF and Torchilin VP: Micelle-like
nanoparticles as siRNA and miRNA carriers for cancer therapy.
Biomed Microdevices. 20:592018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Marwitz S, Heinbockel L, Scheufele S,
Kugler C, Reck M, Rabe KF, Perner S, Goldmann T and Ammerpohl O:
Fountain of youth for squamous cell carcinomas? On the epigenetic
age of non-small cell lung cancer and corresponding tumor-free lung
tissues. Int J Cancer. 143:3061–3070. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zheng CH, Chen XM, Zhang FB, Zhao C and Tu
SS: Inhibition of CXCR4 regulates epithelial mesenchymal transition
of NSCLC via the Hippo-YAP signaling pathway. Cell Biol Int.
42:1386–1394. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Takahashi Y, Sakaguchi K, Horio H,
Hiramatsu K, Moriya S, Takahashi K and Kawakita M: Urinary N1,
N12-diacetylspermine is a non-invasive marker for the diagnosis and
prognosis of non-small-cell lung cancer. Br J Cancer.
113:1493–1501. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Luo C, Tetteh PW, Merz PR, Dickes E,
Abukiwan A, Hotz-Wagenblatt A, Holland-Cunz S, Sinnberg T, Schittek
B, Schadendorf D, et al: miR-137 inhibits the invasion of melanoma
cells through downregulation of multiple oncogenic target genes. J
Invest Dermatol. 133:768–775. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jing XG, Chen TF, Huang C, Wang H, An L,
Cheng Z and Zhang GJ: MiR-15a expression analysis in non-small cell
lung cancer A549 cells under local hypoxia microenvironment. Eur
Rev Med Pharmacol Sci. 21:2069–2074. 2017.PubMed/NCBI
|
|
16
|
Ning T, Peng Z, Li S, Qu Y, Zhang H, Duan
J, Wang X, Yang H, Liu R, Deng T, et al: miR-455 inhibits cell
proliferation and migration via negative regulation of EGFR in
human gastric cancer. Oncol Rep. 38:175–182. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ning T, Zhang H, Wang X, Li S, Zhang L,
Deng T, Zhou L, Liu R, Wang X, Bai M, et al: miR-370 regulates cell
proliferation and migration by targeting EGFR in gastric cancer.
Oncol Rep. 38:384–392. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yoshino H, Yonemori M, Miyamoto K,
Tatarano S, Kofuji S, Nohata N, Nakagawa M and Enokida H:
microRNA-210-3p depletion by CRISPR/Cas9 promoted tumorigenesis
through revival of TWIST1 in renal cell carcinoma. Oncotarget.
8:20881–20894. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang H, Mai Q and Chen J: MicroRNA-210 is
increased and it is required for dedifferentiation of osteosarcoma
cell line. Cell Biol Int. 41:267–275. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu D, Xia H, Wang F, Chen C and Long J:
MicroRNA-210 interacts with FBXO31 to regulate cancer proliferation
cell cycle and migration in human breast cancer. Onco Targets Ther.
9:5245–5255. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Eilertsen M, Andersen S, Al-Saad S,
Richardsen E, Stenvold H, Hald SM, Al-Shibli K, Donnem T, Busund LT
and Bremnes RM: Positive prognostic impact of miR-210 in non-small
cell lung cancer. Lung Cancer. 83:272–278. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Puisségur MP, Mazure NM, Bertero T,
Pradelli L, Grosso S, Robbe-Sermesant K, Maurin T, Lebrigand K,
Cardinaud B, Hofman V, et al: miR-210 is overexpressed in late
stages of lung cancer and mediates mitochondrial alterations
associated with modulation of HIF-1 activity. Cell Death Differ.
18:465–478. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhu W, Zhou K, Zha Y, Chen D, He J, Ma H,
Liu X, Le H and Zhang Y: Diagnostic value of serum miR-182,
miR-183, miR-210, and miR-126 levels in patients with early-stage
non-small cell lung cancer. PLoS One. 11:e01530462016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Agarwal V, Bell GW, Nam JW and Bartel DP:
Predicting effective microRNA target sites in mammalian mRNAs.
Elife. 4:2015. View Article : Google Scholar
|
|
26
|
Takikawa T, Masamune A, Hamada S, Nakano
E, Yoshida N and Shimosegawa T: miR-210 regulates the interaction
between pancreatic cancer cells and stellate cells. Biochem Biophys
Res Commun. 437:433–439. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yu Q, Xu C, Yuan W, Wang C, Zhao P, Chen L
and Ma J: Evaluation of plasma MicroRNAs as diagnostic and
prognostic biomarkers in pancreatic adenocarcinoma: miR-196a and
miR-210 could be negative and positive prognostic markers,
respectively. Biomed Res Int 2017. 64958672017.
|
|
28
|
Qu A, Du L, Yang Y, Liu H, Li J, Wang L,
Liu Y, Dong Z, Zhang X, Jiang X, et al: Hypoxia-inducible MiR-210
is an independent prognostic factor and contributes to metastasis
in colorectal cancer. PLoS One. 9:e909522014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang W, Qu A, Liu W, Liu Y, Zheng G, Du L,
Zhang X, Yang Y, Wang C and Chen X: Circulating miR-210 as a
diagnostic and prognostic biomarker for colorectal cancer. Eur J
Cancer Care (Engl). 26:2017. View Article : Google Scholar
|
|
30
|
Bar I, Merhi A, Abdel-Sater F, Ben Addi A,
Sollennita S, Canon JL and Delrée P: The MicroRNA miR-210 is
expressed by cancer cells but also by the tumor microenvironment in
triple-negative breast cancer. J Histochem Cytochem. 65:335–346.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shidfar A, Costa FF, Scholtens D, Bischof
JM, Sullivan ME, Ivancic DZ, Vanin EF, Soares MB, Wang J and Khan
SA: Expression of miR-18a and miR-210 in normal breast tissue as
candidate biomarkers of breast cancer risk. Cancer Prev Res
(Phila). 10:89–97. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tang Y, Zhou X, Ji J, Chen L, Cao J, Luo J
and Zhang S: High expression levels of miR-21 and miR-210 predict
unfavorable survival in breast cancer: a systemic review and
meta-analysis. Int J Biol Markers. 30:e347–e358. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fedorko M, Stanik M, Iliev R,
Redova-Lojova M, Machackova T, Svoboda M, Pacik D, Dolezel J and
Slaby O: Combination of MiR-378 and MiR-210 serum levels enables
sensitive detection of renal cell carcinoma. Int J Mol Sci.
16:23382–23389. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Redova M, Poprach A, Besse A, Iliev R,
Nekvindova J, Lakomy R, Radova L, Svoboda M, Dolezel J, Vyzula R
and Slaby O: MiR-210 expression in tumor tissue and in vitro
effects of its silencing in renal cell carcinoma. Tumour Biol.
34:481–491. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Samaan S, Khella HW, Girgis A, Scorilas A,
Lianidou E, Gabril M, Krylov SN, Jewett M, Bjarnason GA, El-said H
and Yousef GM: miR-210 is a prognostic marker in clear cell renal
cell carcinoma. J Mol Diagn. 17:136–144. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang H, Mao F, Shen T, Luo Q, Ding Z,
Qian L and Huang J: Plasma miR-145, miR-20a, miR-21 and miR-223 as
novel biomarkers for screening early-stage non-small cell lung
cancer. Oncol Lett. 13:669–676. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li ZH, Zhang H, Yang ZG, Wen GQ, Cui YB
and Shao GG: Prognostic significance of serum microRNA-210 levels
in nonsmall-cell lung cancer. J Int Med Res. 41:1437–1444. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bansal N, Bosch A, Leibovitch B, Pereira
L, Cubedo E, Yu J, Pierzchalski K, Jones JW, Fishel M, Kane M, et
al: Blocking the PAH2 domain of Sin3A inhibits tumorigenesis and
confers retinoid sensitivity in triple negative breast cancer.
Oncotarget. 7:43689–43702. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lewis MJ, Liu J, Libby EF, Lee M, Crawford
NP and Hurst DR: SIN3A and SIN3B differentially regulate breast
cancer metastasis. Oncotarget. 7:78713–78725. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Solaimani P, Wang F and Hankinson O:
SIN3A, generally regarded as a transcriptional repressor, is
required for induction of gene transcription by the aryl
hydrocarbon receptor. J Biol Chem. 289:33655–33662. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Suzuki H, Ouchida M, Yamamoto H, Yano M,
Toyooka S, Aoe M, Shimizu N, Date H and Shimizu K: Decreased
expression of the SIN3A gene, a candidate tumor suppressor located
at the prevalent allelic loss region 15q23 in non-small cell lung
cancer. Lung Cancer. 59:24–31. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Das TK, Sangodkar J, Negre N, Narla G and
Cagan RL: Sin3a acts through a multi-gene module to regulate
invasion in Drosophila and human tumors. Oncogene. 32:3184–3197.
2013. View Article : Google Scholar : PubMed/NCBI
|