Introduction
Activin A, a member of the transforming growth
factor-β superfamily, mimics Nodal, binds Activin receptors and
phosphorylates Smad2, thus activating it (1). Once activated, Smad2 associates with
Smad4, translocates to the nucleus and regulates gene expression in
conjuction with other transcription factors. Nodal/Activin A
signals play a crucial role in inducing the development of the
mesoderm and endoderm in the Xenopus embryo (2,3).
Activin A (100 ng/ml) promotes the differentiation
of human embryonic stem (ES) cells into pancreatic β cells
(4), and also into endoderm
(5). By contrast, 5 ng/ml of
Activin A was found to maintain self-renewal and to support the
long-term feeder-free culture of human ES cells (6). However, it has not been fully
established whether Activin A induces the differentiation of human
ES cells into endoderm or maintains them in an undifferentiated
state. Curiously, 100 ng/ml of Activin A, FGF-2 and BMP-4 was found
to induce the expression of endoderm markers, while still
maintaining pluripotency markers such as Oct3/4 and Nanog (7). These data suggest that a mixture of
other growth factors or the specific concentration of Activin A may
determine whether human ES cells differentiate or whether their
pluripotency is maintained. To develop a feeder-free medium of
human ES or induced pluripotent stem (iPS) cells, it is necessary
to determine the appropriate concentration of Activin A.
Materials and methods
Cell culture and embryoid body
formation
The human iPS cell line 201B7 (Riken Cell Bank,
Tsukuba, Japan) was cultured in ReproFF media (Reprocell, Yokohama,
Japan) for feeder-free culture (Reprocell, Tokyo, Japan) on dishes
(Asahi Techno Glass, Funabashi, Japan) coated with Matrigel (Becton
Dickinson, Franklin Lakes, NJ, USA) in 5% carbon dioxide at 37°C in
a humidified chamber, and harvested with Accutase (Innovative Cell
Technologies, Inc., San Diego, CA, USA) for subsequent experiments.
Dissociated 201B7 cells were cultured in hanging drops at a density
of 1,000 cells per 30 μl of medium composed of Dulbecco’s minimum
essential medium-F12 medium (Sigma Aldrich Japan K.K., Tokyo,
Japan) supplemented with 20% knockout serum replacement (KSR)
(Invitrogen Japan K.K., Tokyo, Japan), 10% minimum essential amino
acids (Invitrogen Japan K.K.), 2 mM L-glutamine (Invitrogen Japan
K.K.) and 1 mM 2-mecaptoethanol (Sigma Aldrich Japan K.K.)
[iPSm(-)] (8). After 4 days of
hanging drop culture, the resulting embryoid bodies (EBs) were
plated onto plastic dishes coated with Matrigel. Cells were
observed under an Olympus IMT-2 microscope (Olympus, Tokyo,
Japan).
Alkaline phosphatase staining
Alkaline phosphatase (ALP) staining was carried out
in cells cultured on a 6-well plate (Asahi Techno Glass) coated
with Matrigel. Alkaline phosphatase staining was performed with
leukocyte alkaline phosphatase (Sigma Aldrich Japan K.K.) according
to the manufacturer’s instructions.
Immunostaining
Cells cultured on 4-well chamber slides (Becton
Dickinson) were fixed in 4% paraformaldehyde (Sigma Aldrich Japan
K.K.) and incubated with hydrogen oxide in 100% methanol for 30 min
at 4°C. Specimens were incubated with 2% fetal bovine serum in PBS
(wash buffer) for 30 min at 4°C. For the anti-Oct3/4 (Becton
Dickinson) and anti-Nanog (Reprocell) antibodies, specimens were
incubated in 0.1% sodium citrate (Wako Pure Chemicals, Osaka,
Japan) and 0.1% Triton X-100 (Wako Pure Chemicals) in distilled
water. Diluted (1:500) anti-Oct3/4, anti-Nanog, anti-SSEA-4 and
anti-TRA-1-60 antibodies (all from Nihon Millipore K.K., Tokyo,
Japan) were incubated in wash buffer overnight at 4°C. After
washing three times with PBS, diluted 1:500 horseradish
peroxidase-labeled anti-mouse (GE Healthcare Japan, Tokyo, Japan)
or anti-rabbit (GE Healthcare Japan) antibodies were incubated in
wash buffer for 3 h at 4°C. Diaminobenzidine (Dako Japan, Tokyo,
Japan) was applied, and the nuclei were stained with hematoxylin
(Muto Pure Chemicals Co., Ltd., Tokyo, Japan) for 15 sec. Specimens
were observed and photographed under an Olympus AX80 microscope
(Olympus).
Cell proliferation assay
Cells were seeded onto a 96-well plate (Asahi Techno
Glass) coated with Matrigel at a density of 1,000 cells/well.
Twenty-four hours later, the medium was replaced with Activin A
(R&D Systems Inc., Minneapolis, MN, USA). Seventy-two hours
later, the 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-
2-(4-sulfophenyl)-2H-tetrazolium inner salt (MTS) assay was
performed according to the manufacturer’s instructions (Promega
Corporation, Tokyo, Japan). MTS was bio-reduced by cells into a
colored formazan product that reduces absorbance at 490 nm. The
absorbance was analyzed at a wavelength of 490 nm with a Bio-Rad
iMark microplate reader (Bio-Rad, Hercules, CA, USA).
Results
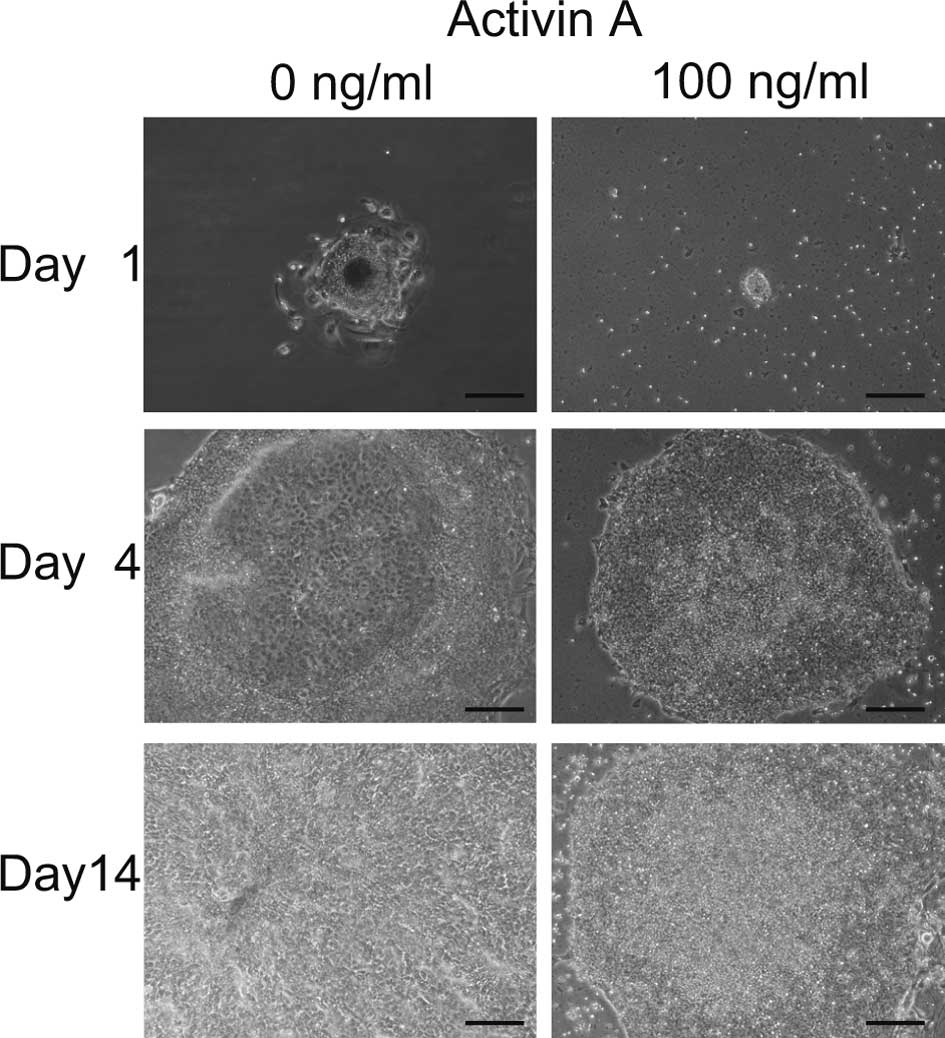
Initially, we aimed to promote the differentiation
of iPS cells to hepatocytes with EB formation using 100 ng/ml of
Activin A (9). Four days after the
transfer of EBs to 6-well plates, differentiated cells appeared in
the center of the colonies cultured with 0 ng/ml of Activin A,
while no morphological change was noted in cells cultured with 100
ng/ml of Activin A (Fig. 1).
Fourteen days later, all cells cultured with 0 ng/ml of Activin A
were differentiated, while cells cultured with 100 ng/ml remained
undifferentiated. These data indicate that Activin A maintained iPS
cells in an undifferentiated state. It appeared that colonies grew
more slowly when cultured with 100 ng/ml than with 0 ng/ml Activin
A.
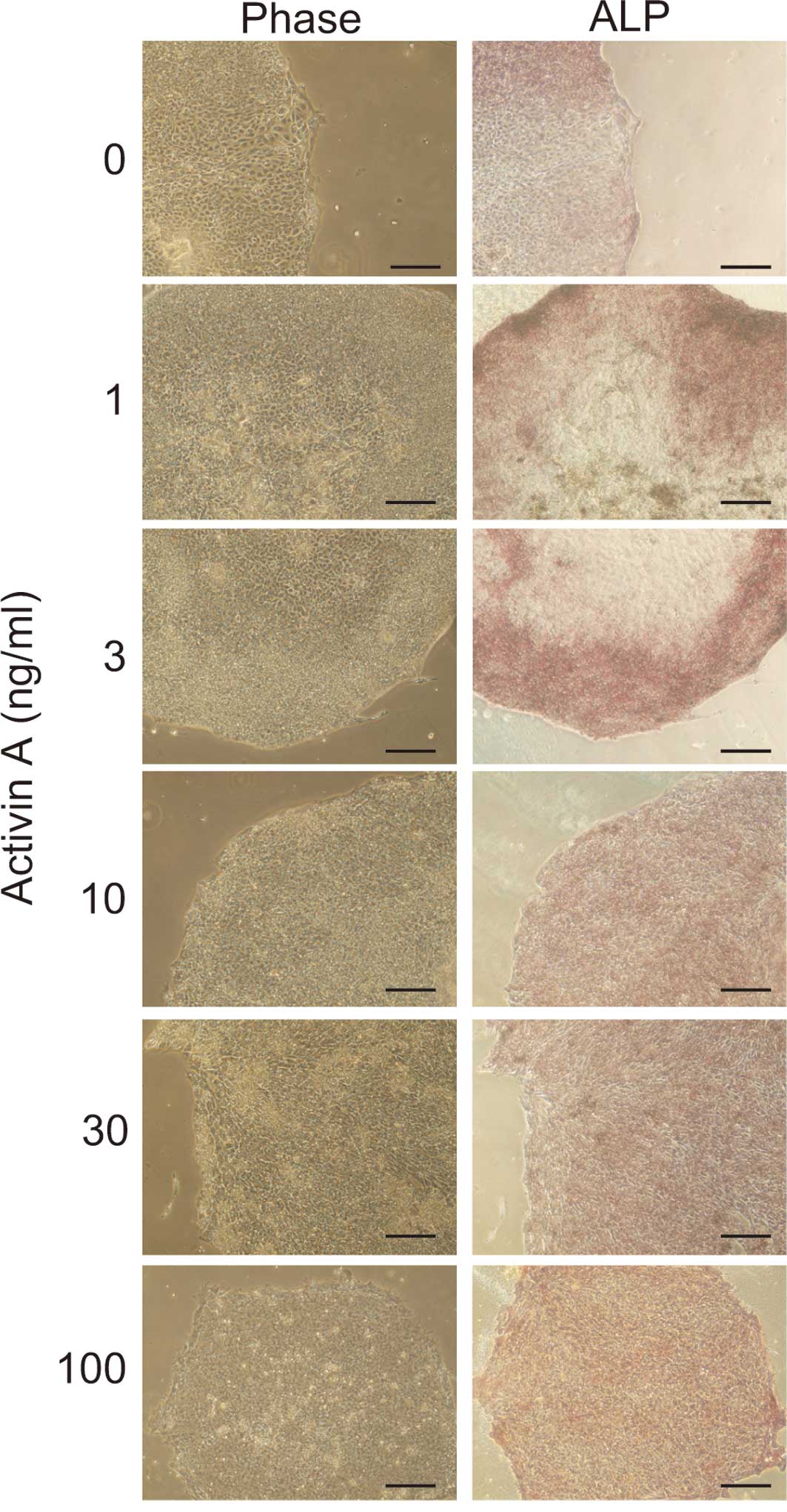
To determine whether the cells maintained an
undifferentiated state after 14 days of culture in Activin A, ALP
staining was performed. Cell cultured in >10 ng/ml of Activin A
were positive for ALP staining (Fig.
2). In contrast, most of the cells cultured in <3 ng/ml of
Activin A were negative for ALP staining. This suggests that 10
ng/ml of Activin A was sufficient to maintain the cells in an
undifferentiated state.
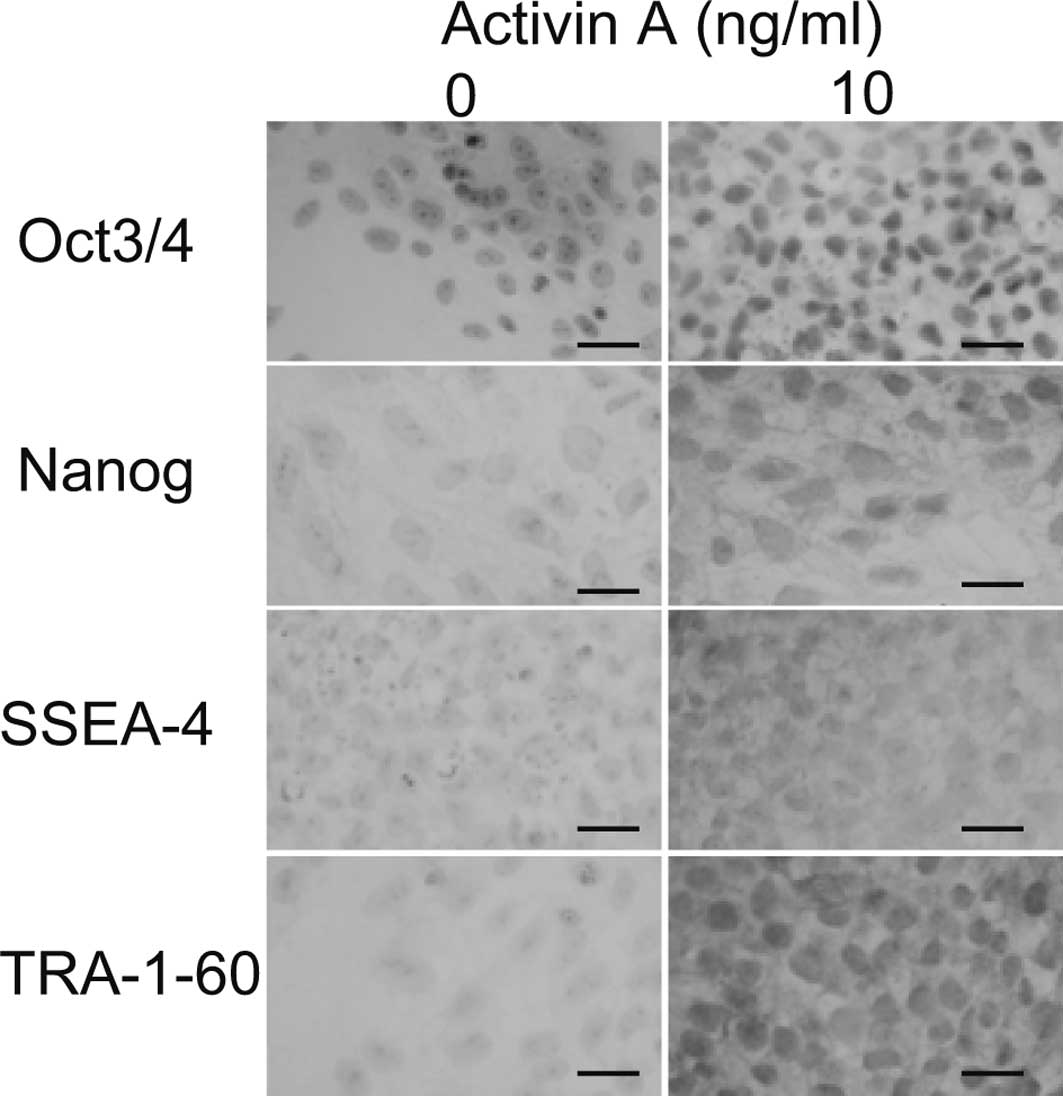
To confirm that the cells remained undifferentiated
at 10 ng/ml of Activin A, immunostaining was performed. All cells
cultured in 10 ng/ml of Activin A demonstrated positive nuclear
staining for Oct3/4 and Nanog (Fig.
3). None of the cells in the absence of Activin A were positive
for Oct3/4 or Nanog. The cell surfaces of all of the cells cultured
in 10 ng/ml Activin A were positive for SSEA-4 and TRA-1-60, while
none were positive in the absence of Activin A. These results
suggest that 10 ng/ml of Activin A maintains cells in an
undifferentiated state.
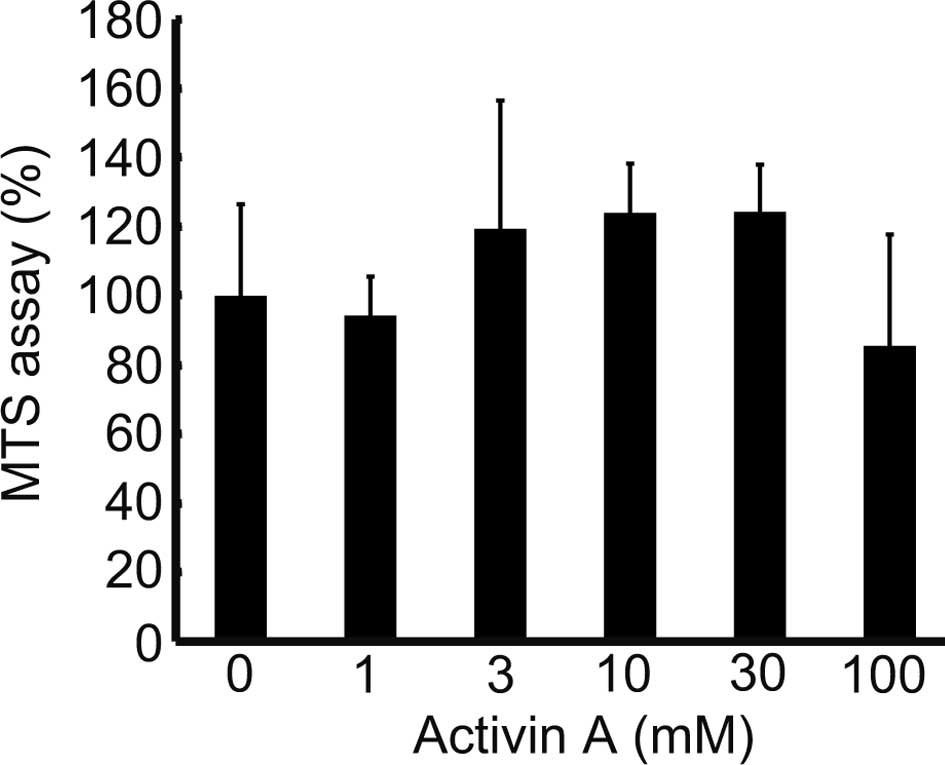
Fig. 1 indicates
that cells cultured with 100 ng/ml of Activin A exhibited weaker
proliferation than those cultured in 0 ng/ml. The MTS assay was
performed to analyze the effects of Activin A on the proliferative
potential of the cells, and revealed a proliferation of >100% in
cells cultured with 3–30 ng/ml of Activin A, and of 85.5% at 100
ng/ml, compared to cells treated with 0 ng/ml (Fig. 4). This demonstrated that Activin A
promotes cell proliferation at a concentration of 3–30 ng/ml, while
a concentration of 100 ng/ml suppresses cell proliferation.
Discussion
Activin A (10 ng/ml) and FGF2 (12 ng/ml) maintain
the pluripotency of human iPS cells in a feeder-free condition
(10). Cultured human iPS cells
form teratoma when transplanted into the testis capsule of severe
combined immunodeficientbeife mice after 20 passages. In our
experiments, 100 ng/ml of Activin A alone was added to the medium,
since our initial goal was to develop a method of differentiating
human iPS cells to hepatocytes. Unexpectedly, the cultured cells
via EBs showed no morphological changes after 14 days of EB
formation. Beattie et al obtained similar results when
culturing human ES cells (HSF6) in media with several cocktails of
growth factors (11). They found
that a combination of Activin A (50 ng/ml), nicotinamide (10 mM)
and keratinocyte growth factor (50 ng/ml) was sufficient to
preserve the pluripotency of human ES cells. Our data clearly
indicate that Activin A alone is capable of maintaining
pluripotency markers in human iPS cells, as evidenced by ALP
staining and immunostaining. Previously, the proliferation of human
ES cells was found to be reduced and the passage of cells was
halted by Actin A (11). Xiao
et al succeeded in the long-term feeder-free culture of
human ES cells (H1) for a time period longer than 150 days and 20
passages with 5 ng/ml of Activin A (6). The differences in results between the
two previous reports may be attributed to the cell lines and
concentrations of Activin A used. In certain cell lines, activation
of another pathway may be necessary, since the Wnt pathway
preserves the pluripotency of human ES cells (12). Xiao et al applied a lower
concentration of Activin A for a successful long-term passage. Our
MTS assay showed that human iPS cells exhibited a weaker
proliferative potential when cultured in 100 ng/ml of Activin A
compared to 0 ng/ml. Moreover, 3–30 ng/ml of Activin A was suitable
for human iPS cell proliferation compared to 0 ng/ml. Our data and
previous reports suggest that a lower concentration of Activin A is
appropriate for human ES and iPS cells to maintain not only
pluripotency markers, but also proliferative potential.
Activin A (25 ng/ml) was found to increase the
expression of Oct3/4 and Nanog in human ES cells with pluripotency
when added in chemically defined medium (13). When the medium was deprived of
Activin A, the expression of Nanog was down-regulated (14). These reports verify that Activin A
controls the expression of Oct3/4 and Nanog to maintain
pluripotency.
Sulzbacher et al hypothesized that the
pluripotency or differentiation of cells is dependent and occurs in
a concentration- and stage-dependent manner (1). Activin A (5–50 ng/ml) was applied to
maintain the pluripotency of human ES and iPS cells alone or with
other growth factors (6,10,11,15).
In the present study, as in the study of Xiao et al, 5 and
10 ng/ml of Activin A, respectively, were applied as a sole growth
factor to maintain pluripotency. These data suggest that a lower
concentration of Activin A maintains the pluripotency of human ES
or iPS cells. Conversely, human ES (H9) cells were found to
differentiate with FGF2 or Activin A (10 ng/ml) alone after three
passages, and a combination of Activin A + FGF2 or Nodal + FGF2 was
found to be optimal for the long-term expression of pluripotency
markers (15). One may speculate
that H9 cells require FGF2 in addition to Activin A. Whether
Activin A is enough to maintain pluripotency or requires other
growth factors may depend on the cell line.
To induce mesoendodermal differentiation, the
optimal combination was found to consist of BMP4 (10 ng/ml), FGF2
(20 ng/ml), LY294002 (10 μM) and a higher concentration of Activin
A (100 ng/ml) (10). These data
indicate that a combination of growth factors and a higher
concentration of Activin A is necessary for human ES or iPS cells
to differentiate. Notably, Activin A (100 ng/ml), FGF-2 and BMP-4
induce the expression of endoderm markers, while still maintaining
pluripotency markers such as Oct3/4 and Nanog (7). This may explain our finding that 100
ng/ml of Activin A maintained pluripotency markers, while other
researchers noted differentiation at this high concentration.
Our next study will carry out the passage and
long-term culture of human iPS cells in Activin A while analyzing
the undifferentiated state.
Acknowledgements
This study was supported in part by a
Grant-in-Aid for the Encouragement of Scientists from the Japan
Society for the Promotion of Science (JSPS) (no. 22931047). The
authors thank Dr Masaki Takiguchi and Dr Katsuro Iwase for the
technical assistance and fruitful discussions.
References
|
1.
|
Sulzbacher S, Schroeder IS, Truong TT and
Wobus AM: Activin A-induced differentiation of embryonic stem cells
into endoderm and pancreatic progenitors – the influence of
differentiation factors and culture conditions. Stem Cell Rev.
5:159–173. 2009.
|
|
2.
|
Smith JC, Price BM, van Nimmen K and
Huylebroeck D: Identification of a potent Xenopus mesoderm-inducing
factor as a homologue of Activin A. Nature. 345:729–731. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Thomsen G, Woolf T, Whitman M, et al:
Activins are expressed early in Xenopus embryogenesis and can
induce axial mesoderm and anterior structures. Cell. 63:485–493.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
D’Amour KA, Agulnick AD, Eliazer S, Kelly
OG, Kroon E and Baetge EE: Efficient differentiation of human
embryonic stem cells to definitive endoderm. Nat Biotechnol.
23:1534–1541. 2005.
|
|
5.
|
Yao S, Chen S, Clark J, et al: Long-term
self-renewal and directed differentiation of human embryonic stem
cells in chemically defined conditions. Proc Natl Acad Sci USA.
103:6907–6912. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Xiao L, Yuan X and Sharkis SJ: Activin A
maintains self-renewal and regulates fibroblast growth factor, Wnt,
and bone morphogenic protein pathways in human embryonic stem
cells. Stem Cells. 24:1476–1486. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Touboul T, Hannan NR, Corbineau S, et al:
Generation of functional hepatocytes from human embryonic stem
cells under chemically defined conditions that recapitulate liver
development. Hepatology. 51:1754–1765. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Tomizawa M, Toyama Y, Ito C, et al:
Hepatoblast-like cells enriched from mouse embryonic stem cells in
medium without glucose, pyruvate, arginine, and tyrosine. Cell
Tissue Res. 333:17–27. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Basma H, Soto-Gutierrez A, Yannam GR, et
al: Differentiation and transplantation of human embryonic stem
cell-derived hepatocytes. Gastroenterology. 136:990–999. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Vallier L, Touboul T, Brown S, et al:
Signaling pathways controlling pluripotency and early cell fate
decisions of human induced pluripotent stem cells. Stem Cells.
27:2655–2666. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Beattie GM, Lopez AD, Bucay N, et al:
Activin A maintains pluripotency of human embryonic stem cells in
the absence of feeder layers. Stem Cells. 23:489–495. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Sato N, Meijer L, Skaltsounis L, Greengard
P and Hemmati-Brivanlou A: Maintenance of pluripotency in human and
mouse embryonic stem cells through activation of Wnt signaling by a
pharmacological GSK-3-specific inhibitor. Nat Med. 10:55–63. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
James D, Levine AJ, Besser D and
Hemmati-Brivanlou A: TGFbeta/activin/nodal signaling is necessary
for the maintenance of pluripotency in human embryonic stem cells.
Development. 132:1273–1282. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Vallier L, Mendjan S, Brown S, et al:
Activin/Nodal signalling maintains pluripotency by controlling
Nanog expression. Development. 136:1339–1349. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Vallier L, Alexander M and Pedersen RA:
Activin/Nodal and FGF pathways cooperate to maintain pluripotency
of human embryonic stem cells. J Cell Sci. 118:4495–4509. 2005.
View Article : Google Scholar : PubMed/NCBI
|