Introduction
Spinal cord injury (SCI) is a severe worldwide
health problem resulting from different etiological factors such as
traffic accidents. SCI can be divided into two stages: the acute
phase which is caused by directly tissue compression and
hemorrhage, and the secondary injury phase, in which secondary cell
apoptosis occurs at the lesion site, affecting both neurons and
astrocytes (1,2) It has long been believed that the
central nervous system (CNS) is incapable of regenerating itself
after injury and during the repair process after SCI. Thus, an
ideal treatment would be one administered systemically but without
significant side effects. Erythropoietin (EPO), a potent inhibitor
of apoptosis and a promising therapeutic agent, has been used in a
variety of neurological insults, including traumatic brain injury,
brain stroke and spinal cord injury (3,4).
Therefore, identifying the specific molecular pathway mediating the
EPO neuronal protective effects of EPO after SCI is of great value
to the patients concerned.
Platelet-derived growth factor (PDGF) is synthesized
and secreted by multiple types of cells and can bind to its
specific receptor (PDGFR) in the CNS (5). Due to the reported neurotrophic and
neuroprotective effects of exogenously administered PDGFs. PDGFs
are speculated to play a role in CNS development, maintenance, and
response to CNS injury (5). The
PDGF family has four gene products (PDGF-A-D) that act via two
classical receptor tyrosine kinases, PDGF-αR and PDGF-βR. Members
of the PDGF family have multiple roles during embryogenesis and in
a variety of pathological situations in the adult (6). In addition, PDGFs can maintain the
reactivity of the neuron and facilitate the growth of the axon
(7). PDGF-B, a member of the PDGF
family, has the capacity to promote the mitosis of glial cells and
exhibits a neuroprotective effect in the rat cerebrum both during
development and in the mature stage. These results indicate the
neuroprotective effects of PDGF-B in the function of both the
developing and developed brain. Recently scientists have found that
EPO accelerates smooth muscle cell-rich vascular lesion formation
in mice through endothelial cell activation involving enhanced PDGF
release (8). Thus, our working
hypothesis is that PDGF-B levels change in spinal cord injury rats
after EPO treatment. This study may pave the way for the future
treatment of SCI patients.
Materials and methods
Animal model induction and locomotive
function exam
Adult male Sprague-Dawley (SD) rats, weighing
∼180–200 g, were purchased from the Experimental Animal Centre of
Zhejiang University (Hangzhou, China). Rabbit anti-rat PDGF-B
antibody, biotinylated goat anti-rabbit secondary antibody and
avidin-HRP complex were purchased from Boster Co. (Wuhan, China).
3,3′-Diaminobenzidine hydrochloride was from Sigma Co. (St. Louis,
MO, USA). Recombinant human EPO was purchased from the China
Shenyang Sunshine Pharmaceutical Company Limited (Shenyang, China).
All experiments and procedures were approved by the Committee on
Animal Care and Use of Zhejiang University. Protocol regarding the
animal experiments was approved by the Ethics Committee of the
School of Medicine, Zhejiang University, in accordance with NIH
guidelines for the ethical care of experimental animals.
Thirty adult male SD rats were randomly divided into
three groups (n=10): i) sham operation control group, ii) SCI group
and iii) EPO treatment SCI group. Under sodium pentobarbital (40
mg/kg, i.p.) anesthesia, the vertebral column of the rats was
exposed, and a laminectomy was carried out at the T10 level. A
contusion injury was then performed using a weight-drop device in
the SCI group and EPO treatment SCI group rats. A weight of 10 g
was dropped from a height of 50 mm on the exposed spinal cord, and
the impounder was left for 20 sec before being withdrawn to produce
a moderate contusion. EPO treatment SCI group rats received single
doses of EPO (1,000 U/kg i.p.) immediately after the incision was
closed while the SCI animals received normal saline (via i.p.
injection). Sham operation control group animals received the same
surgical procedure but sustained no impact injury, and their spinal
cord was left exposed for 5 min. They received normal saline (via
i.p. injection) immediately after the incision was closed. Seven
days after the surgery, rats were laid on the floor and observed
for their crawling ability for 5 min. Basso, Beattie and Bresnahan
locomotor rating scale (BBB) was used for the evaluation of their
locomotive ability.
Immunohistochemical assay and hematoxylin
and eosin (H&E) staining
Seven days after the surgery, five rats in each
group were anesthetized with a lethal dose of Nembutal. Their
thoracic cavities were opened and perfused intracardially with
normal saline. Following saline perfusion, the animals were
perfused with 300–400 ml fixative containing 4% paraformaldehyde in
0.1 M PBS (pH 7.4). After perfusion, the T6–14 segment of the
spinal cord of each rat was extracted. The tissues were fixed in
the same fixative for 4 h and then placed in 30% phosphate-buffered
sucrose until the tissue sank. Tissues were routinely processed for
paraffin embedding and mounted onto 0.02% poly-L-lysine-coated
slides. The avidin-biotin-peroxidase complex system was used with
3,3′-diaminobenzidine hydrochloride (DAB) as the chromagen.
Briefly, tissue sections were washed in PBS, incubated in 1% bovine
serum albumin (BSA) for 30 min, and then incubated overnight at 4°C
in the primary antibody (1:100 of PDGF-B primary antibody) plus 1%
BSA in PBS. The control sections were incubated in PBS. The next
day, the sections were incubated in a biotinylated goat anti-rabbit
secondary antibody (diluted to 1:200 in PBS), and subsequently in
an avidin-horseradish peroxidase (HRP) solution. Immunolabelling
was visualized with 0.05 DAB plus 0.3% hydrogen peroxide in PBS.
The sections were then dehydrated through ethanol and xylene before
mounting under coverslips with Permount™. H&E staining was also
applied to determine the pathological changes in the three
groups.
Real-time qPCR assay of the PDGF-B mRNA
expression
Seven days after the surgery, the remaining five
animals per group were anesthetized with a lethal dose of Nembutal.
The T6–14 segment of the spinal cord of each rat was extracted
immediately. For real-time RT-qPCR analysis, total-RNA was isolated
from the spinal cord segment using the TRIzol® reagent
kit according to the manufacturer's instructions. The RNA
concentration was measured spectrophotometrically, and 2 μg of
total-RNA was added to the complementary DNA synthesis reaction
system (20 μl). The reaction mixture comprised 4 μl of 5X RT
buffer, 2.5 μmol/l oligodeoxythymidylic acid oligo(dt), 5 mmol/l
deoxyribonucleotide triphosphate (dNTP) and 20 U of RNase
inhibitor. The hexamers were annealed by incubating the samples to
70°C for 5 min. M-MLV reverse transcriptase (200 U) was added, and
the mixture was incubated at 42°C for 60 min. The reaction was
stopped by heating to 72°C for 10 min. For real-time RT-qPCR, the
reaction mixture (40 μl) consisted of 4 μl complementary DNA, 35.2
μl SYBR®-Green qPCR mix, 0.5 μl of 5 U TaqDNA polymerase
and 0.3 μl of 20 pmol/μl PDGF primer. The complementary DNA was
denatured by heating to 94°C for 3 min. The template was amplified
by 40 rounds of PCR (denaturation at 94°C for 10 sec, annealing at
60°C for 30 sec, extension at 72°C for 30 sec) before collecting
the fluorescence at 72°C. Meanwhile, primers were used for the
housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH)
in the real-time RT-qPCR to amplify GAPDH as an internal control
for quantifying PDGF-B expression. The primers for PDGF-B were as
follows: forward, 5-CTCCATCCGCTCCTTTGATGACCTT-3 and reverse,
5-CAGCTCAGCCCCATCTTCGTCTA-3. The primers for GAPDH were as follows:
forward, 5-GGTGGACCTCATGGCCTACAT-3 and reverse,
5-GCCTCTCTCTTGCTCTCAGTATCCT-3. The PCR product length of PDGF-B was
73 base pairs (bp).
Statistical analysis
Slides were examined at x400 magnification and
analyzed with UTHSCSA Image Tool 3.0 (University of Texas Medical
School at San Antonio, TX, USA). The number of PDGF-B-positive
cells was measured. The mean ± SD for all data was calculated.
Statistical analysis was performed using SPSS® version
12.0 statistical software for Windows®. The significance
of any differences between the three groups was evaluated using the
t-test. P-values were two tailed, and a P<0.05 was considered
statistically significant. Relative quantitation was carried out
using the comparative CT method. The ΔCT
value was determined by subtracting the average GAPDH CT
value from the average PDGF CT value. The calculation of
ΔΔCT involves diabetic or EPO treatment group =
ΔCT control - ΔCT. The PDGF mRNA
level in the SCI and EPO treatment SCI group relative to the
control was determined using the formula:
2−(ΔΔCT). The PDGF mRNA level in the sham
operation group was set to 1.
Results
BBB scales and H&E staining
The sham operation control group animals walked
normally after recovery from the anesthesia while rats in the SCI
and EPO treatment SCI group exhibited dramatic and bilateral hind
limb paralysis with no movement or only slight movements of a joint
after injury. The animals were followed up for 7 days post-surgery
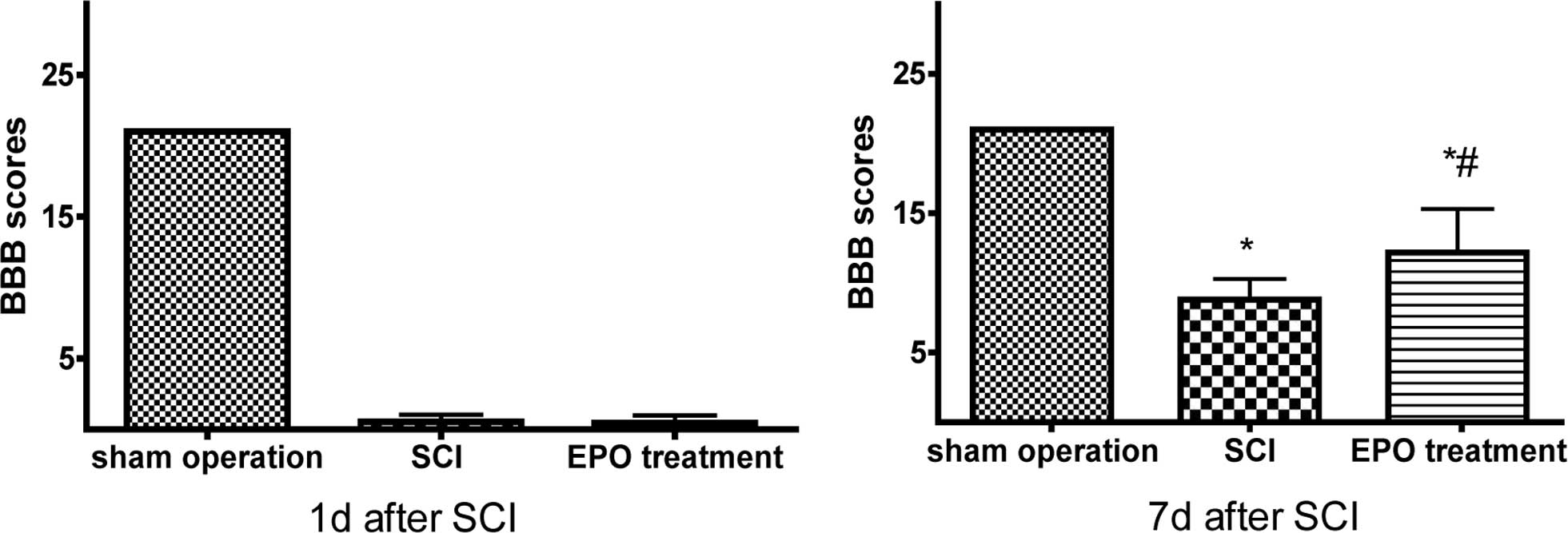
to score their locomotive activity according to the BBB scale. The
locomotive dysfunction was found reproducible, and the BBB score in
the SCI rats recovered during 7 days to a plateau below the range
of 10 points while EPO treatment SCI rats scored above 12 points.
This indicated that EPO helped to moderate the severity of the
injury and facilitated fast recovered after spinal cord injury
(P<0.01) (Fig. 1).
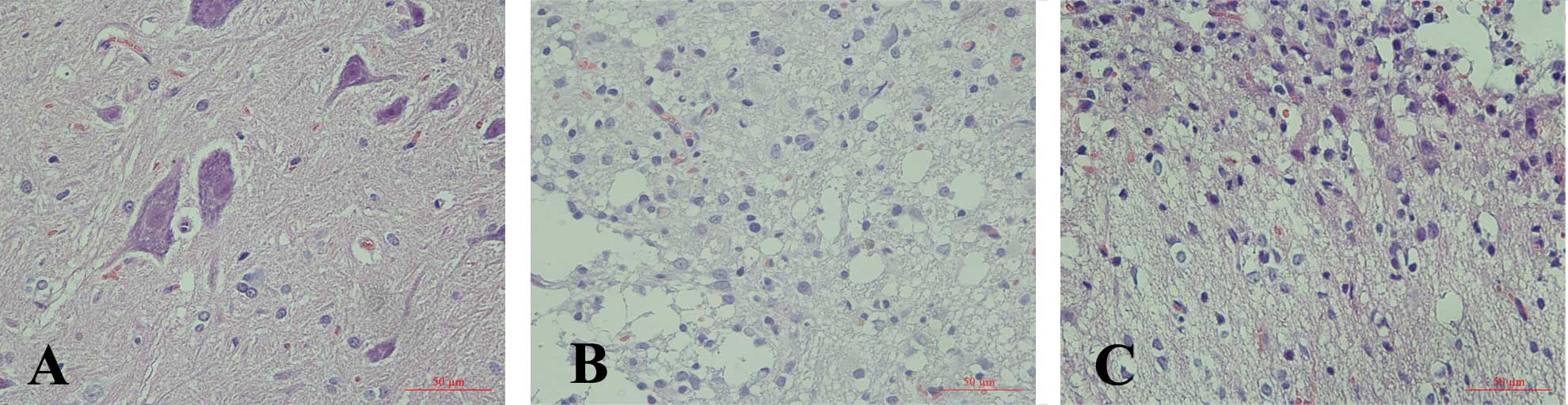
Consistently, H&E staining of the lesioned
thoracic spinal cord revealed the formation of a large cavity
involving the lateral funiculi, dorsal and central gray matter.
Seven days after injury we noted some neuronal apoptosis and the
numbers of gliocytes increased. A progressive disruption of the
dorsal white and central gray matter tissue architecture was noted
and gliocytes filled the injury area. Compared with the SCI group,
less neuronal degenerating was noted and more gliocytes were
observed to fill the injured spinal cord of the EPO treatment SCI
rats. The structure of the T10 segment in the sham operation
control group was intact and microscopic structures such as neurons
and astrocytes in the grey matter were clear (Fig. 2).
Immunohistochemistry and RT-qPCR assays
of the expression of PDGF-B
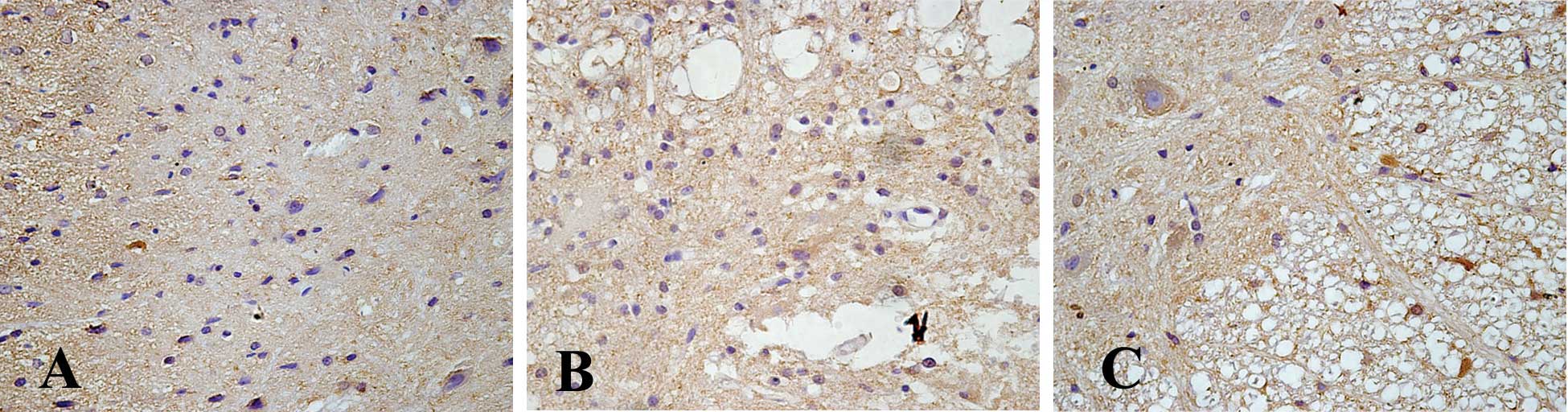
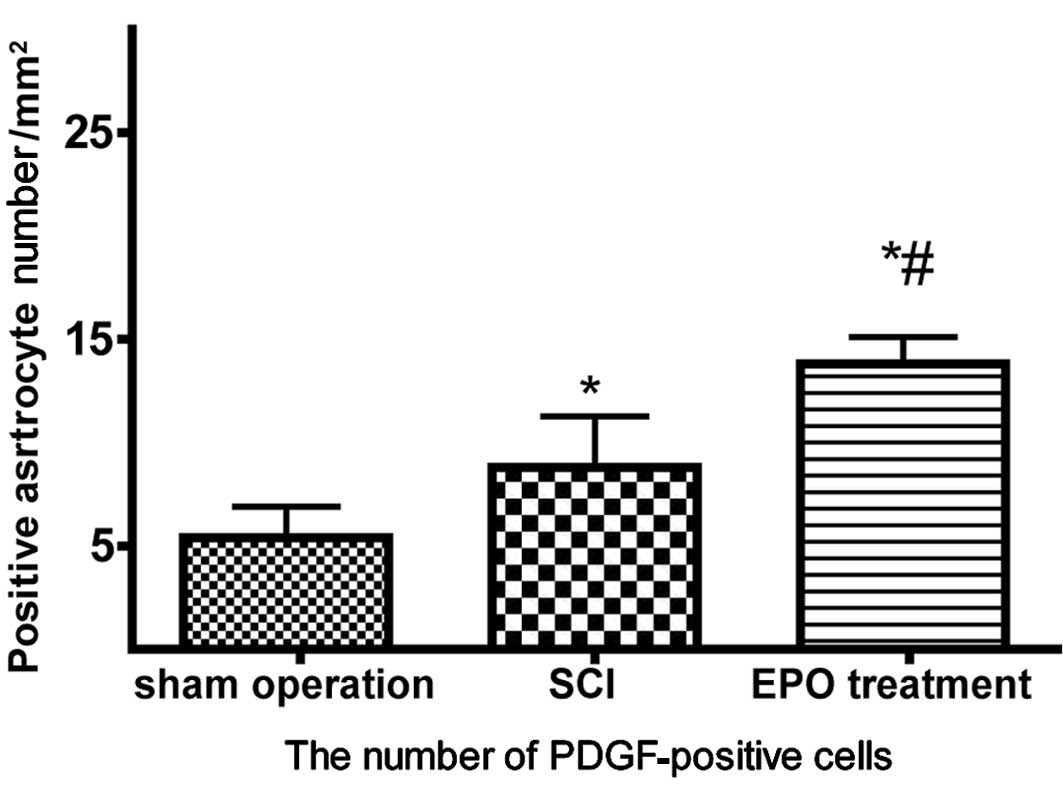
Cells that stained positive for PDGF-B showed
buff-colored granules with DAB staining. There were few
PDGF-B-positive cells in the sham operation control group. However,
7 days after SCI, further migration of PDGF-B-positive cells
occurred, and the size of the spinal cord porosis resulting from
the trauma was reduced in the EPO treatment SCI group. The
PDGF-B-positive cell number was significantly higher in the EPO
group compared to that of the SCI group (P<0.05) (Figs. 3 and 4).
Similar results were noted in the real-time RT-qPCR
assay. The PDGF-B mRNA level was up-regulated in the EPO treatment
SCI group compared to the SCI group, as indicated by the decreased
ΔCT values (comparative CT method).
Specifically, the PDGF-B mRNA level in the SCI group was 3.53 times
that of the control group, and this value in the EPO treatment SCI
group was 4.65 times that of the control group (Table I).
 | Table I.Relative quantification of PDGF mRNA
levels. |
Table I.
Relative quantification of PDGF mRNA
levels.
| Group | CT
PDGF | CT
GAPDH | ΔCT | ΔΔCT | Fold difference |
|---|
| Sham operation | 25.73 | 21.76 | 3.97 | 0 | 1 |
| Spinal cord
injury | 23.14 | 20.99 | 2.15 | −1.82 | 3.53 |
| EPO treatment | 22.31 | 20.56 | 1.75 | −2.22 | 4.65 |
Discussion
EPO exhibits neuroprotective effects in a variety of
models of central and peripheral nervous system injury (9,10).
The local EPO and EPO-R system is markedly engaged in the early
stages after compressive spinal cord injury. The reduction in EPO
immune-expression and the increase in EPO-R staining strongly
support the possible usefulness of a therapeutic approach based on
exogenous EPO administration (11). Moreover, EPO may influence the
release of neurotransmitters, playing an important role in synaptic
plasticity in the adult brain (12). EPO was also found to increase
Schwann cell infiltration into the lesion site, although neither
compound had any effect on macrophage infiltration either within
the lesion site itself or in the surrounding intact tissue
(13). Recently, scientists
revealed that the binding of EPO to its receptor induces the
activation of JAK2, leading to phosphorylation of the inhibitor of
nuclear factor-κB. The functional analysis of different receptor
forms revealed a correlation of the abilities to induce NF-κB
activity and to generate anti-apoptotic signals (14). Zhang et al found that a high
dose of EPO attenuates the increase in iNOS expression in the
facial nucleus after facial nerve transection and thus may enhance
the survival of facial motor neurons (15). The present results showed a more
rapid recovery as indicated by higher BBB scores, less disruption
and more neuronal regeneration of the spinal cord in the EPO
treatment SCI group than that in the SCI group. This demonstrated
that EPO helps to improve the function and pathological changes
after SCI in rats.
Members of the PDGF family have multiple roles
during embryogenesis and in a variety of pathological situations in
the adult. PDGF-B is compatible with normal CNS development and
astroglial response to injury (16). To our knowledge, exogenous PDGF was
neuroprotective against toxicity induced by HIV-1 Tat in primary
midbrain neurons. Administration of PDGF was able to rescue the
dopaminergic neurons in the substantia nigra from Tat-induced
neurotoxicity. EPO was found to induce expression of PDGF-B, which
was at least partially responsible for the induction of Stat5
phosphorylation in SMCs by HUVEC-conditioned medium (8). However, in this study, the expression
of PDGF-B in the EPO treatment group was constantly high,
exhibiting rapid ability to repair the injured tissue and regain
neurological function.
After SCI, the PDGF-positive cell numbers were found
to be significantly higher in the spinal cord (17,18).
Recent studies indicate that PDGF-B protects neurons by suppressing
the NMDA-evoked current and translocating the glutamate transporter
to the cell membrane. The balance between the expression of NMDAR
and PDGF-B partly determines the ontogenic susceptibility to brain
injury. Enhancement of the PDGF-B/receptor signal pathway may
rescue neonatal brains at risk of hypoxic-ischemic injury (19).
These results provide strong evidence for the
ability of EPO to repair injured tissue and recover neurological
function via increased expression of PDGF-B. The results also
provide the first evidence for the involvement of PDGF-B in the
neuroprotective effect of EPO on SCI. However, side effects of EPO
and the underlying mechanism of its neuroprotective function still
require further investigation.
Abbreviations:
|
EPO,
|
erythropoietin;
|
|
SCI,
|
spinal cord injury;
|
|
PDGF-B,
|
platelet-derived growth factor-B;
|
|
CNS,
|
central nervous system;
|
|
SD rat,
|
Sprague-Dawley rat;
|
|
BBB scale,
|
Basso, Beattie and Bresnahan locomotor
rating scale
|
Acknowledgements
This project was supported by the
Health Bureau of Zhejiang Province, China (no. 2009A222).
References
|
1.
|
Schmitt C, Miranpuri GS, Dhodda VK,
Isaacson J, Vemuganti R and Resnick DK: Changes in spinal cord
injury-induced gene expression in rat are strain-dependent. Spine
J. 6:113–119. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Hong Z, Chen H, Hong H, Lin L and Wang Z:
TSP-1 expression changes in diabetic rats with spinal cord injury.
Neurol Res. 31:878–882. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Grasso G, Sfacteria A, Meli F, Fodale V,
Buemi M and Iacopino DG: Neuroprotection by erythropoietin
administration after experimental traumatic brain injury. Brain
Res. 1182:99–105. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Hasselblatt M, Ehrenreich H and Siren AL:
The brain erythropoietin system and its potential for therapeutic
exploitation in brain disease. J Neurosurg Anesthesiol. 18:132–138.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Ishii Y, Oya T, Zheng L, et al: Mouse
brains deficient in neuronal PDGF receptor-beta develop normally
but are vulnerable to injury. J Neurochem. 98:588–600. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Bergsten E, Uutela M, Li X, et al: PDGF-D
is a specific, protease-activated ligand for the PDGF
beta-receptor. Nat Cell Biol. 3:512–516. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Wu QH, Chen WS, Chen QX, Wang JH and Zhang
XM: Changes in the expression of platelet-derived growth factor in
astrocytes in diabetic rats with spinal cord injury. Chin Med J
(Engl). 123:1577–1581. 2010.PubMed/NCBI
|
|
8.
|
Janmaat ML, Heerkens JL, de Bruin AM,
Klous A, de Waard V and de Vries CJ: Erythropoietin accelerates
smooth muscle cell-rich vascular lesion formation in mice through
endothelial cell activation involving enhanced PDGF-BB release.
Blood. 115:1453–1460. 2010. View Article : Google Scholar
|
|
9.
|
Montero M, Poulsen FR, Noraberg J, et al:
Comparison of neuroprotective effects of erythropoietin (EPO) and
carbamylerythropoietin (CEPO) against ischemia-like oxygen-glucose
deprivation (OGD) and NMDA excitotoxicity in mouse hippocampal
slice cultures. Exp Neurol. 204:106–117. 2007. View Article : Google Scholar
|
|
10.
|
Okutan O, Solaroglu I, Beskonakli E and
Taskin Y: Recombinant human erythropoietin decreases
myeloperoxidase and caspase-3 activity and improves early
functional results after spinal cord injury in rats. J Clin
Neurosci. 14:364–368. 2007. View Article : Google Scholar
|
|
11.
|
Grasso G, Sfacteria A, Passalacqua M, et
al: Erythropoietin and erythropoietin receptor expression after
experimental spinal cord injury encourages therapy by exogenous
erythropoietin. Neurosurgery. 56:821–827. 2005. View Article : Google Scholar
|
|
12.
|
Adamcio B, Sargin D, Stradomska A, et al:
Erythropoietin enhances hippocampal long-term potentiation and
memory. BMC Biol. 6:372008. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
King VR, Averill SA, Hewazy D, Priestley
JV, Torup L and Michael-Titus AT: Erythropoietin and carbamylated
erythropoietin are neuroprotective following spinal cord
hemisection in the rat. Eur J Neurosci. 26:90–100. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Bittorf T, Buchse T, Sasse T, Jaster R and
Brock J: Activation of the transcription factor NF-kappaB by the
erythropoietin receptor: structural requirements and biological
significance. Cell Signal. 13:673–681. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Zhang W, Sun B, Wang X, Liu J, Zhang Z and
Geng S: Erythropoietin enhances survival of facial motor neurons by
inhibiting expression of inducible nitric oxide synthase after
axotomy. J Clin Neurosci. 17:368–371. 2010. View Article : Google Scholar
|
|
16.
|
Enge M, Wilhelmsson U, Abramsson A, et al:
Neuron-specific ablation of PDGF-B is compatible with normal
central nervous system development and astroglial response to
injury. Neurochem Res. 28:271–279. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Yang C, Li B, Liu TS, Zhao DM and Hu FA:
Effect of electracupuncture on proliferation of astrocytes after
spinal cord injury. Zhongguo Zhen Jiu. 25:569–572. 2005.(In
Chinese).
|
|
18.
|
Huang X, Kim JM, Kong TH, et al: GM-CSF
inhibits glial scar formation and shows long-term protective effect
after spinal cord injury. J Neurol Sci. 277:87–97. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Egawa-Tsuzuki T, Ohno M, Tanaka N, et al:
The PDGF B-chain is involved in the ontogenic susceptibility of the
developing rat brain to NMDA toxicity. Exp Neurol. 186:89–98. 2004.
View Article : Google Scholar : PubMed/NCBI
|