Introduction
Anthracycline antibiotics, such as doxorubicin, are
potent antitumor agents used in a wide spectrum of malignancies
(1,2). However, the use of anthracyclines is
limited by the risk of developing life-threatening congestive heart
failure (3,4). The early detection of
anthracycline-induced cardiotoxicity is crucial for the prevention
of heart failure.
Although myocardial biopsy is the ‘gold standard’
for evaluating anthracycline-induced cardiotoxicity, the
invasiveness of this procedure excludes it from routine clinical
use (5). Recently, many studies
concerning early biochemical markers for detecting
doxorubicin-induced cardiotoxicity have been performed (6,7).
The major hypothesis regarding the pathophysiology
of anthracycline-induced cardiotoxicity is that cardiac damage is
caused by oxidative stress through the generation of reactive
oxygen species (ROS) (8,9). The reduction of several
NADPH-dependent microsomal enzymes by doxorubicin results in ROS
generation. Mitochondria are a primary target of
doxorubicin-induced cardiotoxicity mediated by the induction of
ROS. This increase in ROS leads to several damaging events in the
mitochondrion that drastically diminish mitochondrial function. The
enzymatic antioxidant system consists of superoxide dismutase (SOD)
and glutathione peroxidase (GPx), which inactivate ROS. These
enzymes indirectly reflect ROS activity. Few studies have been
carried out on oxidative stress markers for the early detection of
doxorubicin-induced cardiotoxicity (10,11).
Thus, the aim of the present study was to evaluate whether
oxidative stress markers may be early markers of
doxorubicin-induced cardiotoxicity.
Materials and methods
Model of doxorubicin-induced
cardiomyopathy
Forty-four male rabbits used in this study were
maintained in an air-conditioned room, fed with commercial standard
chow and allowed free access to tap water ad libitum in the
Laboratory Animal Center of Sun Yat-Sen University. All
experimental procedures were approved by the Center for Drug
Evaluation and Research Institutional Animal Care and Use Committee
and were in compliance with the Guidelines for the Use and Care of
Laboratory Animals. Rabbits were randomly divided into one control
group (8 rabbits) and four doxorubicin groups. The rabbits in the
control group received weekly intravenous injections of saline
comparable to the volume of doxorubicin received by rabbits in the
doxorubicin groups. Rabbits in the doxorubicin groups received
intravenous injections of 2 mg/ kg doxorubicin (Shenzhen Main Luck
Pharmaceuticals, Inc., Shenzhen, China) via an ear marginal vein at
weekly intervals for 1 (group 1, 8 rabbits), 2 (group 2, 8
rabbits), 4 (group 3, 9 rabbits) or 8 (group 4, 11 rabbits) weeks.
Animals were observed daily and weighed weekly throughout the
duration of the study.
Pathological evaluation of the heart
Two weeks after the designated number of doses had
been given, the rabbits were euthanized with 3% pentobarbital
sodium administered into a marginal ear vein. As soon as ethically
possible, the chest cavity was opened and the left ventricle
harvested and processed as described below, according to a
previously reported protocol (12). Animals that died spontaneously
during the study were not included in the data analysis.
One portion of the harvested left ventricle was
fixed in phosphate-buffered 10% formalin, embedded in paraffin and
sectioned at a thickness of 5 μm. Sections were stained with
H&E. The frequency and severity of doxorubicin-induced
myocardial lesions were assessed semi-quantitatively by light
microscopy.
Cardiac samples from 4 rabbits in each group were
processed for electron microscopy. In these rabbits, a portion of
the left ventricle was rapidly (within 1 min) cut into
1-mm3 pieces, fixed with 2.5% glutaraldehyde and stored
at 4°C. These portions were embedded in glycol methacrylate resin,
sectioned at a thickness of 1 μm and stained with alkaline
toluidine blue for electron microscope analysis. Changes were
graded based on the number of myocytes showing myofibrillar loss
and cytoplasmic vacuolization [score of 0–3 according to Billingham
(13)] in the toluidine
blue-stained sections.
Serum GPx and SOD concentrations
To monitor serum GPx and SOD concentrations, blood
samples were collected before dosing and 2 weeks after the
administration of 1, 2, 4 or 8 weekly doses of doxorubicin. Blood
samples were collected from the marginal artery of either ear.
Blood samples were centrifuged at 2,000 rpm for 10 min at 4°C, and
the sera was frozen at −80°C until assayed. The blood samples from
the rabbits that died were excluded from the analysis. Serum GPx
and SOD concentrations were assayed using a rabbit NT-proBNP
specific enzyme-linked immunosorbent (ELISA) assay.
Statistical analysis
Data were reported as the means ± standard deviation
(SD). One-way analysis of variance (ANOVA) was used to determine
significant differences in the rabbits’ weight. The Mann-Whitney
test for non-parametric data was used to determine significant
differences in cardiomyopathy scores. The Tukey-Kramer multiple
comparisons test was used to assess significant differences in GPx
or SOD concentrations among the groups. A p-value <0.05 was
considered statistically significant.
Results
General toxicity
Four doxorubicin-treated rabbits died during the
9-week experimental period (1 after a cumulative dose of 8 mg/kg,
and 3 after a cumulative dose of 16 mg/kg). No arterial blood
samples for GPx and SOD analysis were available from these animals,
and they were excluded from the study. Thus, a total of 8 rabbits
were analyzed in group 3, and 8 rabbits in group 4. Compared to the
control group, the rabbits in group 4 experienced a loss in body
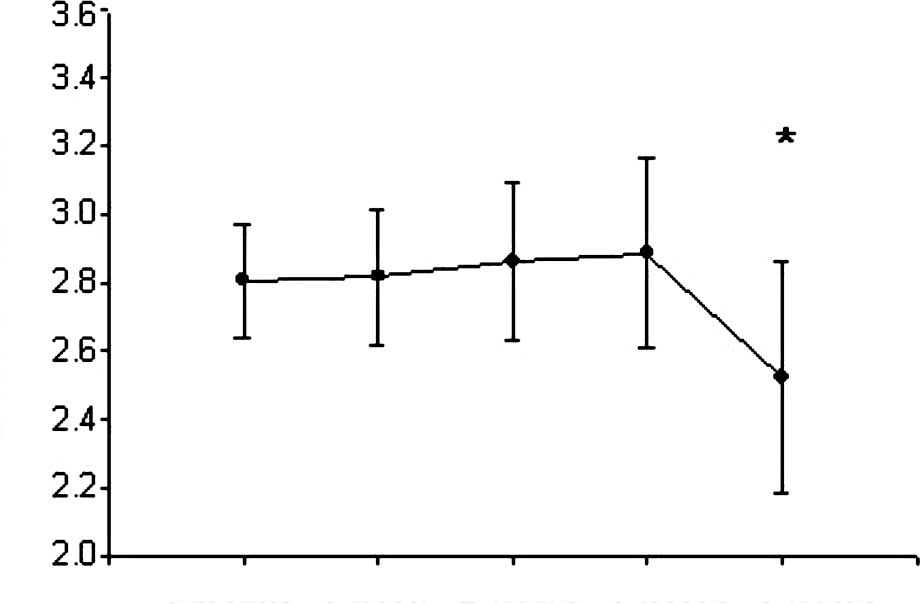
weight (p<0.05, Fig. 1).
Myocardial pathology
Doxorubicin caused myocardial damage that was
visible by light microscopy as cytoplasmic vacuolization and loss
of myofibrils at all dose levels. Both changes were frequently
noted in the same cell, and the frequency of affected cells
increased as lesions became more severe. Data on the incidence and
severity of these lesions at the various cumulative doses of
doxorubicin are summarized in Table
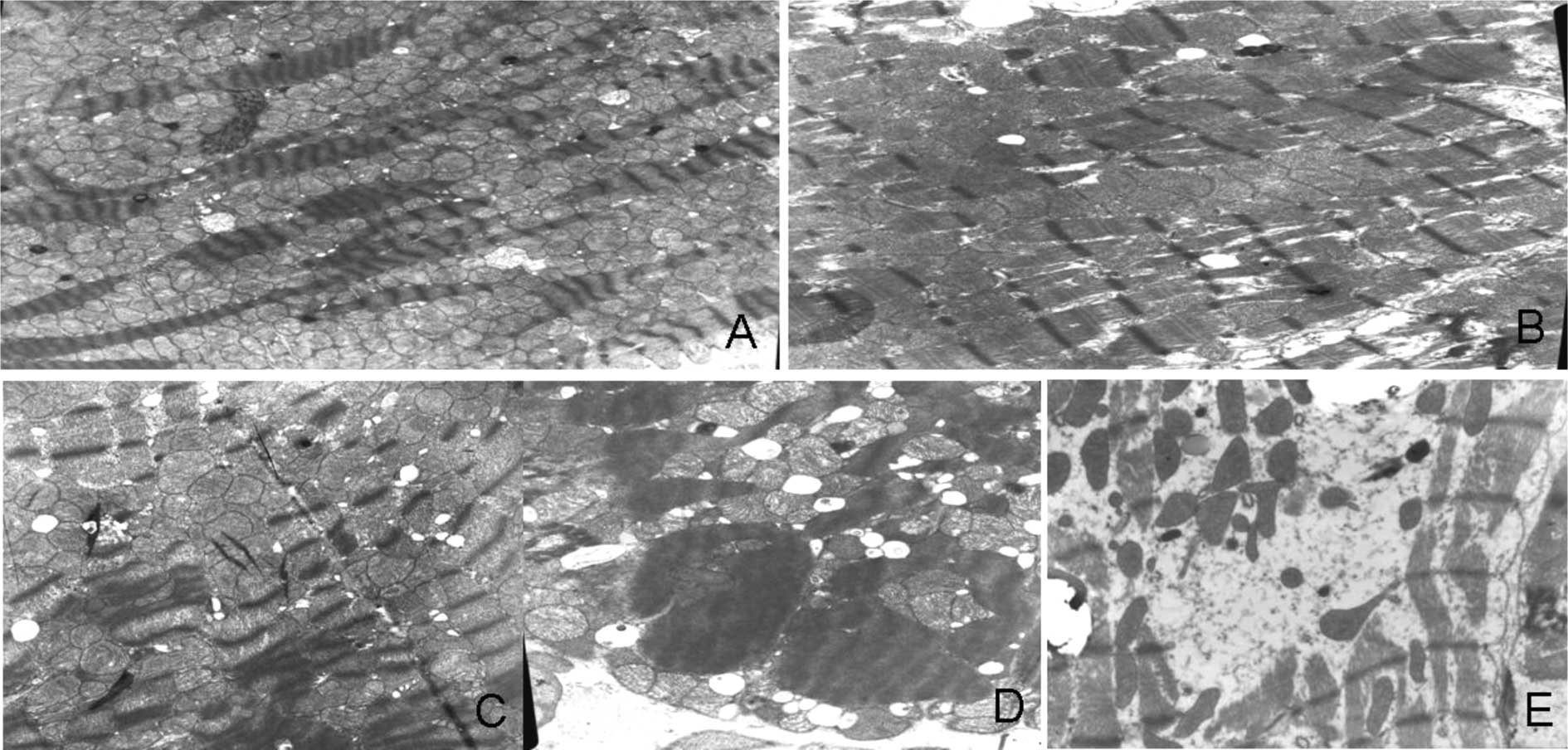
I. Electron microscopy revealed three types of myocardial
damage: sarcoplasmic vacuolization, mitochondrial swelling and
disruption, and myofibrillar lysis. Mitochondrial swelling and
myofibrillar lysis occurred in groups 2, 3 and 4. As the cumulative
dose of doxorubicin increased, the mitochondrial disruption and
lysis of myofibrils also became more serious (Fig. 2).
 | Table I.Cardiomyopathy scores. |
Table I.
Cardiomyopathy scores.
| No. of animals | Cardiomyopathy score
|
|---|
| 0 | 1 | 1.5 | 2 | 2.5 | 3.0 |
|---|
| Control group | 8 | 8 | 0 | 0 | 0 | 0 | 0 |
| Group 1 | 8 | 7 | 1 | 0 | 0 | 0 | 0 |
| Group 2 | 8 | 4 | 3 | 1 | 0 | 0 | 0 |
| Group 3a | 8 | 0 | 1 | 4 | 3 | 0 | 0 |
| Group 4a,b | 8 | 0 | 0 | 0 | 1 | 2 | 5 |
Serum GPx concentration
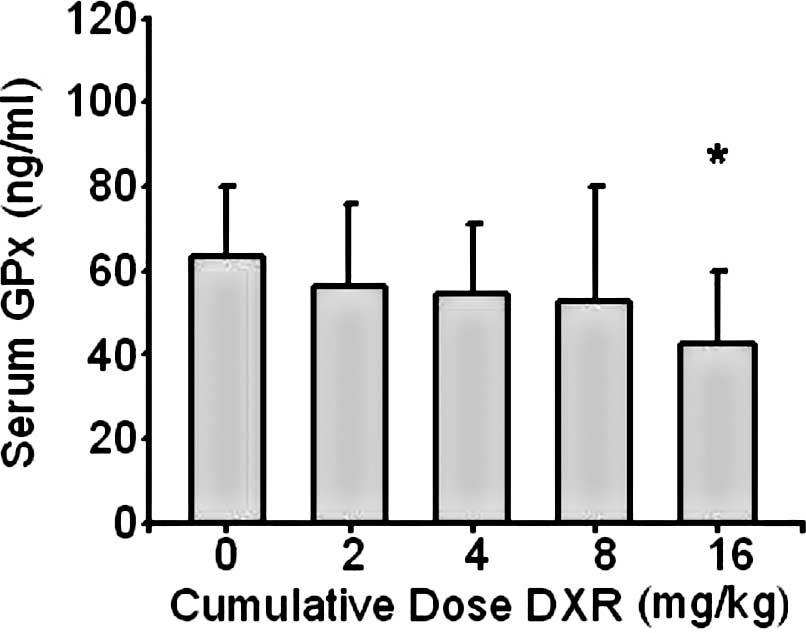
The mean serum GPx concentration in the control
group was 63±17 ng/ml. Compared to the control group, serum GPx
concentrations decreased in group 4 (p<0.05) (Fig. 3).
Serum SOD concentration
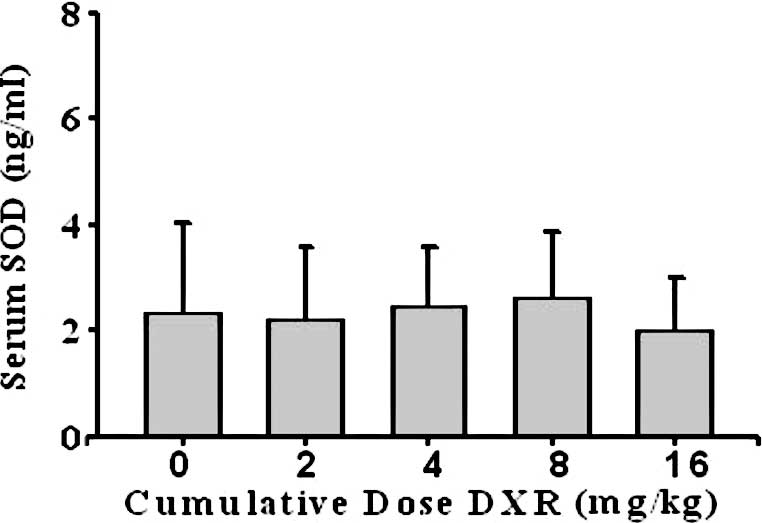
The mean serum SOD concentration in the control
group was 2.4±1.7 ng/ml. No significant differences were found
between the control group and groups 1, 2, 3 or 4 (p>0.05)
(Fig. 4).
Discussion
Anthracyclines, particularly doxorubicin and
epirubicin, are among the most commonly used cytotoxic drugs
because of their potent anticancer activity. However, the use of
anthracyclines is limited due to the risk of irreversible
congestive heart failure. Due to poor prognosis, the early
detection of doxorubicin-induced cardiotoxicity is very important.
Pre-clinical anthracycline cardiomyopathy remains disputed. Many
studies have shown that mitochondrial swelling or disruption and
myofilament lysis is the result of anthracycline-induced
cardiotoxicity (14,15), but no definitive electron
microscope grading scheme has been developed. In this study, we
found that rabbits receiving cumulative doxorubicin dosages of 4
mg/ kg, despite a normal appearance under the light microscope, had
mitochondrial swelling or disruption and myofibril lysis visible
with electron microscopy.
Several studies support the notion of mitochondria
being the main source of doxorubicin-induced ROS.
Doxorubicin-induced free radicals would lead to lipid peroxidation
and membrane damage (16). Changes
in membrane permeability and subsequent cardiomyocyte destruction
are the final steps of cell damage. The cardiotoxic effect of
doxorubicin-induced cardiotoxicity has been attributed to
irreversible damage of mitochondria in heart cells, which express a
unique enzyme on the inner membrane that is able to reduce
anthracyclines to their semiquinone derivatives. Increased
oxidative stress and an antioxidant deficit have been suggested to
play a major role in doxorubicin-induced cardiomyopathy and
congestive heart failure (17).
ROS, mainly superoxide anions, hydrogen peroxide and hydroxyl
radical, are too unstable to measure in the serum. The enzymatic
antioxidant system consists of SOD and GPx, which indirectly
reflect ROS activity. In this study, cardiac injury was
demonstrable by electron microscopy at the cumulative dose of 4
mg/kg doxorubicin. However, no changes in serum GPx or SOD
concentration were found at the cumulative dose of 4 mg/kg
doxorubicin. Mercuro et al (10) found that oxidative stress
parameters may play a role as useful markers of early
cardiotoxicity, but they did not obtain a myocardial biopsy. This
finding may explain the different results to some extent.
Classical long-term models have been applied by
several researchers (18). The
rabbit model is considered the basis for comparing new
anthracyclines and new cardioprotective compounds, generally over
periods of 10–18 weeks, with cumulative doses of 20–30 mg/kg. Thus,
the classical long-term rabbit model was used for early and rapid
evaluation of anthracycline cardiotoxicity. In this study, the
rabbits became weak or died after 8 weeks of 2 mg/kg doxorubicin
weekly. Thus, the duration was limited to only 8 weeks.
This study had several limitations. First, the mean
follow-up period of the different groups was very short, so the
possibility that a longer-term study may find greater damage in the
higher dose groups and result in higher GPx or SOD concentrations
cannot be excluded. Second, the sample size was small. A larger
study with a longer follow-up period after high cumulative doses of
anthracycline is required to address these issues.
In conclusion, increased cumulative anthracycline
doses are not associated with an early and significant increase in
oxidative stress. The predictive value of serum GPx and SOD
concentrations for the development of cardiomyopathy is not yet
known and should be evaluated in a prospective follow-up study.
References
|
1.
|
Torrisi R, Cardillo A, Cancello G, et al:
Phase II trial of combination of pegylated liposomal doxorubicin,
cisplatin, and infusional 5-fluorouracil (CCF) plus trastuzumab as
preoperative treatment for locally advanced and inflammatory breast
cancer. Clin Breast Cancer. 10:483–488. 2010. View Article : Google Scholar
|
|
2.
|
Krischer JP, Epstein S, Cuthbertson DD,
Goorin AM, Epstein ML and Lipshultz SE: Clinical cardiotoxicity
following anthracycline treatment for childhood cancer: the
Pediatric Oncology Group experience. J Clin Oncol. 15:1544–1552.
1997.
|
|
3.
|
Alvarez JA, Scully RE, Miller TL,
Armstrong FD, Constine LS, Friedman DL and Lipshultz SE: Long-term
effects of treatments for childhood cancers. Curr Opin Pediatr.
19:23–31. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Li K, Sung RYZ, Huang WZ, et al:
Thrombopoietin protects against in vitro and in vivo cardiotoxicity
induced by doxorubicin. Circulation. 113:2211–2220. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Jemal A, Siegel R, Ward E, Murray T, Xu J,
Smigal C and Thun MJ: Cancer statistics, 2006. CA Cancer J Clin.
5:106–130. 2006. View Article : Google Scholar
|
|
6.
|
Horacek JM, Vasatova M, Ticky M, Pudil R,
Jebavy L and Maly J: The use of cardiac biomarkers in detection of
cardiotoxicity associated with conventional and high-dose
chemotherapy for acute leukemia. Exp Oncol. 32:97–99.
2010.PubMed/NCBI
|
|
7.
|
Horacek JM, Ticky M, Jebavy L, Pudil R,
Ulrychova M and Maly J: Use of multiple biomarkers for evaluation
of anthracycline-induced cardiotoxicity in patients with acute
myeloid leukemia. Exp Oncol. 30:157–159. 2008.PubMed/NCBI
|
|
8.
|
Faber M, Coudray C, Hida H, Mousseau M and
Favier A: Lipid peroxidation products, and vitamin and trace
element status in patients with cancer before and after
chemotherapy, including adriamycin. A preliminary study. Biol Trace
Elem Res. 47:117–123. 1995. View Article : Google Scholar
|
|
9.
|
Kaya E, Keskin L, Aydogdu I, Kuku I,
Bayraktar N and Erkut MA: Oxidant/antioxidant parameters and their
relationship with chemotherapy in Hodgkin’s lymphoma. J Int Med
Res. 33:687–692. 2005.PubMed/NCBI
|
|
10.
|
Mercuro G, Cadeddu C, Piras A, et al:
Early epirubicin-induced myocardial dysfunction revealed by serial
tissue Doppler echocardiography: correlation with inflammatory and
oxidative stress markers. Oncologist. 12:1124–1133. 2007.
View Article : Google Scholar
|
|
11.
|
Horino N, Kobayashi Y and Usui T:
Elevation of lipid peroxide in children treated with a combination
of chemotherapeutic agents including doxorubicin. Acta Paediatr
Scand. 72:549–551. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Lai RC, Wang XD, Zhang X, Lin WQ and Rong
TH: Heart fatty acid-binding protein may not be an early biomarker
for anthracycline-induced cardiotoxicity in rabbits. Med Oncol.
Feb. 10–2011, (E-pub ahead of print).
|
|
13.
|
Billingham ME, Mason JW, Bristow MR and
Daniels JR: Anthracycline cardiomyopathy monitored by morphologic
changes. Cancer Treat Rep. 62:865–872. 1978.PubMed/NCBI
|
|
14.
|
Wallace KB: Doxorubicin-induced cardiac
mitochondrio-nopathy. Pharmacol Toxicol. 93:105–115. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Berthiaume JM and Wallace KB:
Adriamycin-induced oxidative mitochondrial cardiotoxicity. Cell
Biol Toxicol. 23:15–25. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Milei J, Boveris A, Llesuy S, Molina HA,
Storino R, Ortega D and Milei SE: Amelioration of
adriamycin-induced cardiotoxicity in rabbits by prenylamine and
vitamins A and E. Am Heart J. 111:95–102. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Sun X, Zhou Z and Kang YJ: Attenuation of
doxorubicin chronic toxicity in metallothionein-overexpressing
transgenic mouse heart. Cancer Res. 61:3382–3387. 2001.PubMed/NCBI
|
|
18.
|
Van Vleet JF and Ferrans VJ: Clinical and
pathologic features of chronic adriamycin toxicosis in rabbits. Am
J Vet Res. 41:1462–1469. 1980.PubMed/NCBI
|