Introduction
Myocardial fibrosis (MF) occurs with hypertension,
myocardial infarction and heart failure (1). It results from disruption of the
equilibrium between synthesis and degradation of collagen and this
unbalance leads to an excessive accumulation of collagen fibers
within the myocardium (2). MF has
an extremely complicated process, which involves inflammatory
cytokines and signaling pathways (3).
Rho-associated coiled coil-forming protein kinase
(Rock), a member of the serine/threonine kinase family, is the most
studied Rho downstream effector molecule. It participates in a
variety of cell regulation behaviors and functions, including
contraction, transmigration adhesion, growth and splitting,
existence, endotheliocyte transformation and fibroblastic
hyperplasia (4). A number of
abnormal activations of the Rho/Rock pathway are present in
diseases (5). The Rho/Rock pathway
is activated by multiple cytokines and inflammatory mediators,
including platelet-derived growth factor, transforming growth
factor-β, endothelin-1 and angiotensin II (6). These cytokines and vascular active
materials mediate a number of inflammatory lesions and fibre
hyperplasia diseases. Therefore, the Rho/Rock pathway may be
involved in the after effects of inflammatory mediators and
therefore may participate in the pathophysiological process of
these diseases.
Previous studies have suggested that the Rho/Rock
pathway also plays a significant role in bowel fibrosis, liver
fibrosis and renal fibrosis. Hudson (7) reported that in the development
process of bowel fibrosis, the function of connective tissue growth
factor (CTGF) was associated with the activation of Smads,
Rho/Rock, mitogen-activated protein kinase (MAPK) and protein
kinase C (PKC). Murata et al (8) reported that the specific Rock blocker
Y-27632 prevents the activation of hepatic stellate cells (HSC) and
rat liver fibrosis. Tian and Kaufman (9) identified that Rho exists in the
cytoplasm in the non-active form combined with guanosine
diphosphate (GDP); however, it has an effect on intracellular
effective factors in the active form combined with guanosine
triphosphate (GTP). Nagatoya et al (10) identified that the development of
renal tubular interstitial fibrosis is prevented by blocking the
Rho/Rock pathway. However, the function of the Rho/Rock pathway in
ischemic MF remains unclear.
The present study focused on the expression and
function of the Rho/Rock pathway in ischemic MF of rats.
Additionally, the pathogenesis of MF was explored, in order to
provide valuable data for clinical practice.
Materials and methods
Materials
Streptomycin avidin peroxidase immunohistochemistry
(SP-IHC) kits of RhoA, Rock I, vimentin and CD31 were purchased
from Zhongshan Biotechnology Co., Ltd. (Beijing, China). All other
chemicals used were of analytical grade from commercial suppliers
in China.
Animals and MF modeling
Fifty male Wistar rats (SCXKJ2007-0003; 180–220 g)
were purchased from Jilin University Laboratory Animal Center and
were randomized into ten groups: control and model groups at 2 h,
12 h, 7 days and 21 days (n=5, respectively). The MF rat model was
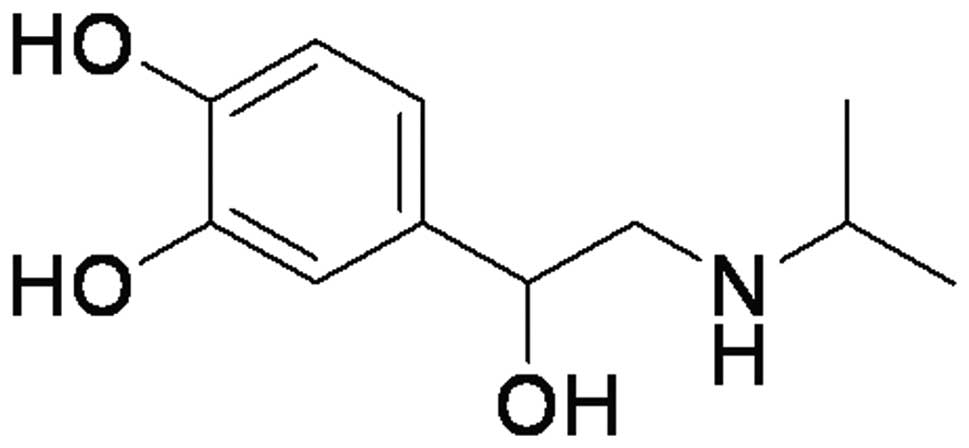
established by peritoneal injection of ISO (Fig. 1) on 25th June 2011 in the College
of Pharmacy, Jilin University and the control group was injected
with the same volume of 0.9% NaCl. MF and control rats were
sacrificed at 2 h, 12 h, 7 days and 21 days, respectively. At the
end of the experiments, rats were sacrificed and their hearts were
removed. A portion of the heart was fixed in 10% phosphate-buffered
formalin for histological studies. Another portion was snap-frozen
in liquid nitrogen and stored at −80°C for extractions. The study
was approved by the ethics committee of the institution.
Analysis of serum enzymes
Serum aspartate aminotransferase (AST), lactic
dehydrogenase (LDH), creatine kinase (CK) and creatine kinase
isozyme (CK-MB) activities were measured using the MD-100
Multifunctional Automatic Biochemistry Analyzer (Sanhe Medical
Equipment Co., Ltd., Dandong, China) according to the
manufacturer’s instructions.
Hematoxylin and eosin (H&E) and
Masson’s staining
Renal histology was assessed by light microscopy
with H&E staining and Masson’s trichrome staining. Ten
high-power microscopic fields were randomly selected. Fibrosis was
quantified and compared between the MF and control groups.
Reverse transcription-polymerase chain
reaction (RT-PCR)
Total RNA was extracted from the heart tissues.
Primers for RhoA, Rock I and glyceraldehyde 3-phosphate
dehydrogenase (GAPDH) were designed and synthesized by Shanghai
Sangon Biological Engineering Technology and Services Co., Ltd.,
(Shanghai, China). The sequences for these primers are presented in
Table I. Total RNA (0.5 μg)
was amplified using the Titan™ One Tube RT-PCR kit
(Boehringer-Mannheim, Shanghai, China). The amplification consisted
of 30 cycles. The products were separated by agarose gel
electrophoresis and visualized by ethidium bromide staining. Bands
were digitized using a Tanon-1000 Gel Image System (Shanghai,
China). The ratios of RhoA and Rock I band grayscales to GAPDH band
grayscales in the various groups were measured.
 | Table IUpstream and downstream primer
sequences for GAPDH, RhoA and Rock I. |
Table I
Upstream and downstream primer
sequences for GAPDH, RhoA and Rock I.
| Primer | Sequence | Length (bp) | Temperature (°C) |
|---|
| GAPDH |
5′-ACCACAGTCCATGCCATCAC-3′ | | |
|
5′-TCCACCACCCTGTTGCTGTA-3′ | 452 | 55 |
| RhoA |
5′-GATGGAGCTTGTGGTAAGA-3′ | | |
|
5′-AAACTATCAGGGCTGTCG-3′ | 239 | 55 |
| Rock I |
5′-GCACACTGGCAATGTAATGC-3′ | | |
|
5′-GTTGAACAGAACAAGTGACC-3′ | 302 | 55 |
Western blotting
Western blotting was performed as previously
described (11). Briefly, heart
tissues were homogenized in protein lysis buffer and 50 g proteins
were separated on 10% sodium dodecyl sulfate (SDS) gels and
electroblotted to polyvinylidine fluoride membranes. The membranes
were blocked with skimmed milk powder solution for 2 h. Blots were
incubated using goat anti-mouse anti-Rock I monoclonal antibody
(1:400 dilution) at 4°C overnight, followed by
peroxidase-conjugated rabbit anti-goat antibody. Color was
developed with enhanced chemiluminescence (ECL) in a dark room. The
grayscales were analysed using the Tanon-1000 Gel Image System.
Immunofluorescent and immunohistochemical
staining
The paraffin-embedded tissue sections (0.2
μm) were deparaffinized with xylene and rehydrated with
graded washes of ethanol to phosphate-buffered saline (PBS). Then,
each slice was treated with 30 μl 3%
H2O2 (reagent A), incubated at
room temperature for 20 min and washed twice with PBS. Then, 30
μl goat serum (reagent B) was added, followed by incubation
at room temperature for 20 min and two washes with PBS. Each slice
was incubated in 30 μl primary antibody (mouse anti-rat Rock
I monoclonal antibody, 1:200 dilution) and placed in the wet box at
4°C overnight. After washing with PBS, the slices were incubated in
30 μl biotinylated polyclonal secondary antibody (reagent C)
at room temperature for 30 min, followed by washing with PBS. The
diaminobenzidine (DAB) method was used for color development,
followed by washing with tap water. Slices were restained with
hematoxylin, incubated in ammonia, dehydrated with gradient
ethanol, transparentized with xylene and finally sealed with
neutral gum. The cells with brown particles in their cytoplasm and
nucleus were denoted positive under a light microscope.
Statistical analysis
All experiments were performed at least three times.
Data were presented as mean ± standard error of the mean (SEM). All
statistical analyses were performed using SPSS 11.5 for Windows
(SPSS Inc., Chicago, IL, USA). Comparisons between multiple groups
were performed by one-way analysis of variance (ANOVA). P<0.05
was considered to indicate a statistically significant
difference.
Results
Diagnostic serum enzymes
Two hours after ISO injection, the activity of serum
AST, LDH, CK and CK-MB increased when compared with the control
group; however, only changes in AST and CK-MB expression were
statistically significant (P<0.01, P<0.05, respectively).
Four hours after ISO injection, AST continuously increased, LDH, CK
and CK-MB expression peaked at 24 h after ISO injection, and LDH,
CK and CK-MB became less active. For three weeks, the activities of
these substances became steady and remained significantly higher
than the control. AST reached the peak 6 h after ISO injection
(P<0.01) and gradually decreased 12 h later, still significantly
higher than the control. These results demonstrate that ISO (15
mg/kg) successfully causes myocardial ischemia in rats (Table II).
 | Table IIDiagnostic serum enzymes (U/l). |
Table II
Diagnostic serum enzymes (U/l).
| Group | AST | LDH | CK | CK-MB |
|---|
| Control | 83.4±7.8 | 251±90.4 | 588.2±228.3 | 575.1±118.6 |
| ISO | | | | |
| 2 h | 125.0±14.8a | 271.2±53.6 | 646.2±116.9 | 761.5±64.4b |
| 4 h | 167.8±37.6a | 586±323.2a |
1283.2±258.1a | 762.9±62.6b |
| 6 h | 183±27.6a | 368.2±128.6 | 581.0±222.6 | 745.6±130.0 b |
| 12 h | 144.4±32.4a |
425.6±140.0a | 575.0±134.3 | 672.4±105.9 |
| 24 h | 96.4±8.1 | 305.2±71.0 | 528.6±131.9 | 652.7±78.5 |
| 48 h | 123.4±39.0a | 391.4±147.4 | 549.8±248.6 | 469.4±109.0 |
| 72 h | 96.2±6.4 | 466.0±87.0a | 722.2±95.6 | 727.4±202.0b |
| 7 days | 100.0±10.4 | 327.4±73.1 | 633.2±134.1 | 727.6±10.0 |
| 21 days | 100.6±9.7 | 335.6±90.7 | 644±211.9 | 644.1±99.6 |
Light microscope examination
A morphological assessment, as the golden standard
for disease diagnosis, is the most reliable method for diagnosing
MF and its development (12).
Examination of the rat heart tissue slices revealed that that there
was clear denaturation and dropsy in the apex cordis and
endocardium. Sporadic spotty necrosis was observed in the
endocardium 2 h after ISO injection. Four hours after ISO
injection, increased denaturation, appearance of small necrotic
foci and broken myofibril structure were observed at the apex
cordis, endocardium and around blood vessels. Additionally, there
was phagocyte infiltration and coagulative myolysis. Forty-eight
hours after ISO injection, widespread, multiple and sporadic
necrotic foci with clear boundaries were observed. Fibroblasts were
rich in these foci and the extent of necrosis became gradually
aggravated. One week after ISO injection, multiple and sporadic
necrotic foci with clear boundaries were observed around coronary
artery branching. In the foci, there was cellular infiltration in
monocytes, lymphocytes and neutrophils; the fibroblasts
proliferated and a certain amount of collagen fiber was observed.
At three weeks, the level of necrosis peaked and a large amount of
fibrosis was observed. This indicates that ISO induced MF in rats,
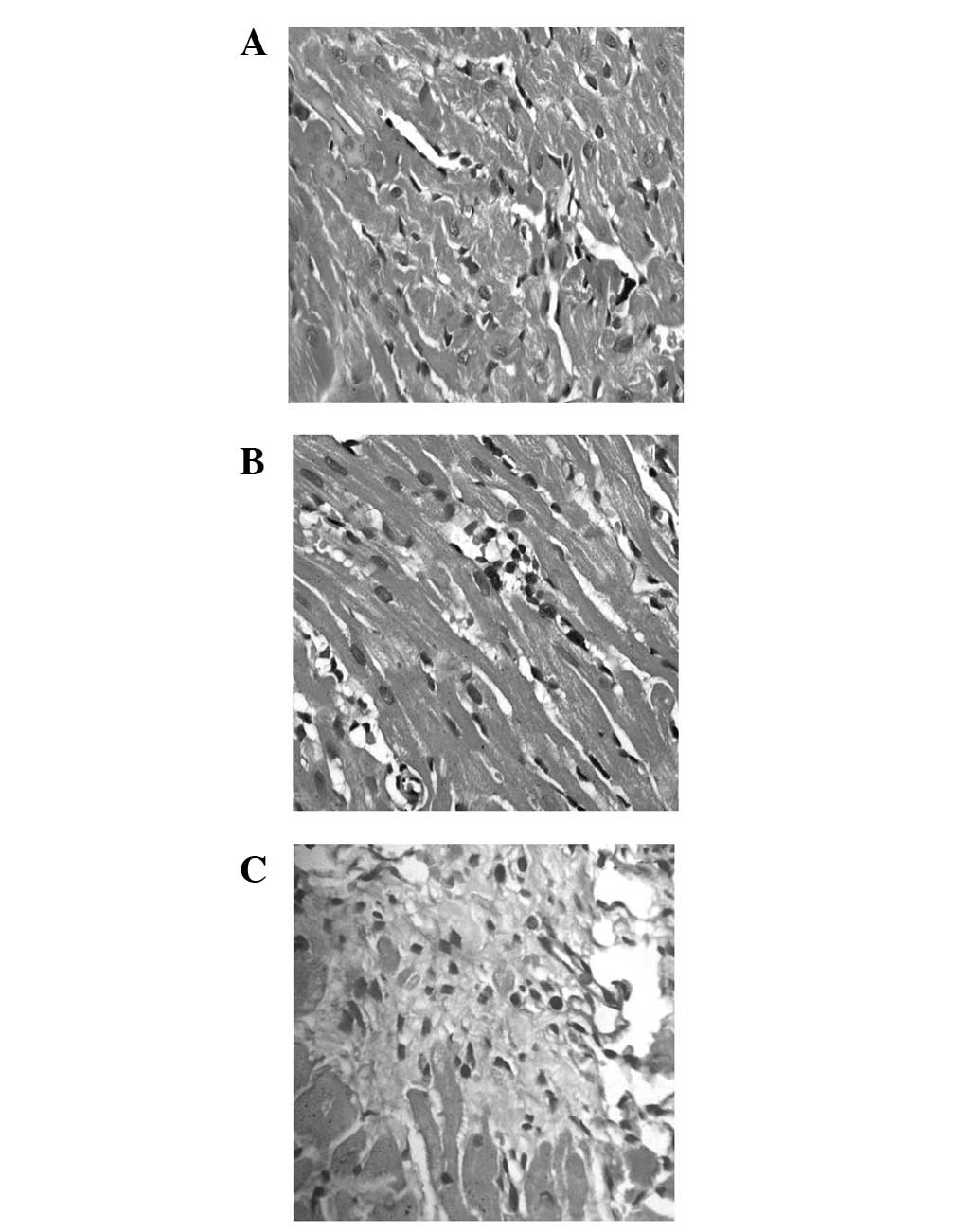
as shown in Fig. 2, including
prominent interstitial expansion, inflammatory cell infiltration
and interstitial collagen accumulation (12 h-21 days). In addition,
the myocardium of rats presented a significant degree of fibrosis
at 21 days (Fig. 2C). These
results show that we succeeded in creating an MF rat model.
Masson’s trichrome staining
The pathological features with Masson’s trichrome
staining of the ISO-treated groups included girdle-shaped collagen
in the interstitium of the heart, particularly on day 12 in the
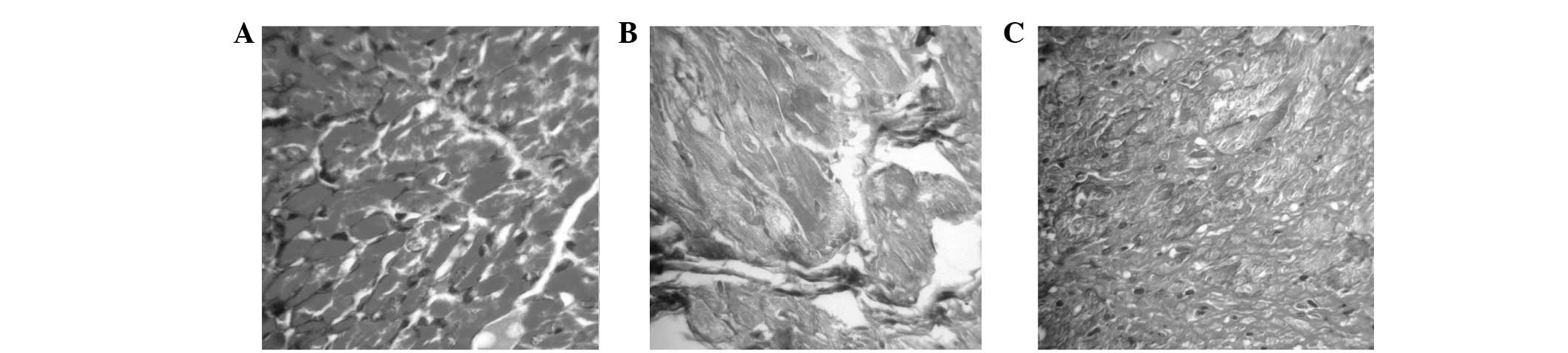
ISO-treated rats (Fig. 3A–C).
Masson’s staining presented collagen fibers as green, muscle fibers
as red and red blood cells as jacinth. Green collagen fibers were
mainly observed at the apex of the heart, subendocardial necrosis
foci and around larger vessels among the muscle bundles. In
addition, only a small portion of collagen expression was
identified at the myocardial tissue of the control group. Two hours
after ISO injection, the number of collagen fibers was greater than
the control group. For three weeks, the number of collagen fibers
significantly increased. These results further confirm the results
presented in serology and H&E staining (Fig. 3)
Expression of RhoA and Rock I
Semi-quantitative RT-PCR analysis was performed on
the heart tissues. The products of RhoA and Rock I are presented in
Fig. 4B. The bands were analyzed
by densitometry and the target transcript levels were normalized
against GAPDH (Fig. 4A). Two hours
after ISO injection, the expression of RhoA and Rock I in the
myocardium had gradually increased compared to that in the control
group. No significant difference was identified between the control
and ISO-treated groups. Twelve hours after ISO injection, RhoA and
Rock I mRNA expression had markedly increased (Fig. 5; P<0.01 and P<0.05,
respectively). The mRNA expression of RhoA reached the maximum
value 12 h after ISO injection (P<0.01). The mRNA expression of
Rock I reached a maximum value at 7 days (P<0.01). These results
indicate that the Rho/Rock pathway plays an important role in MF
formation (Fig. 4).
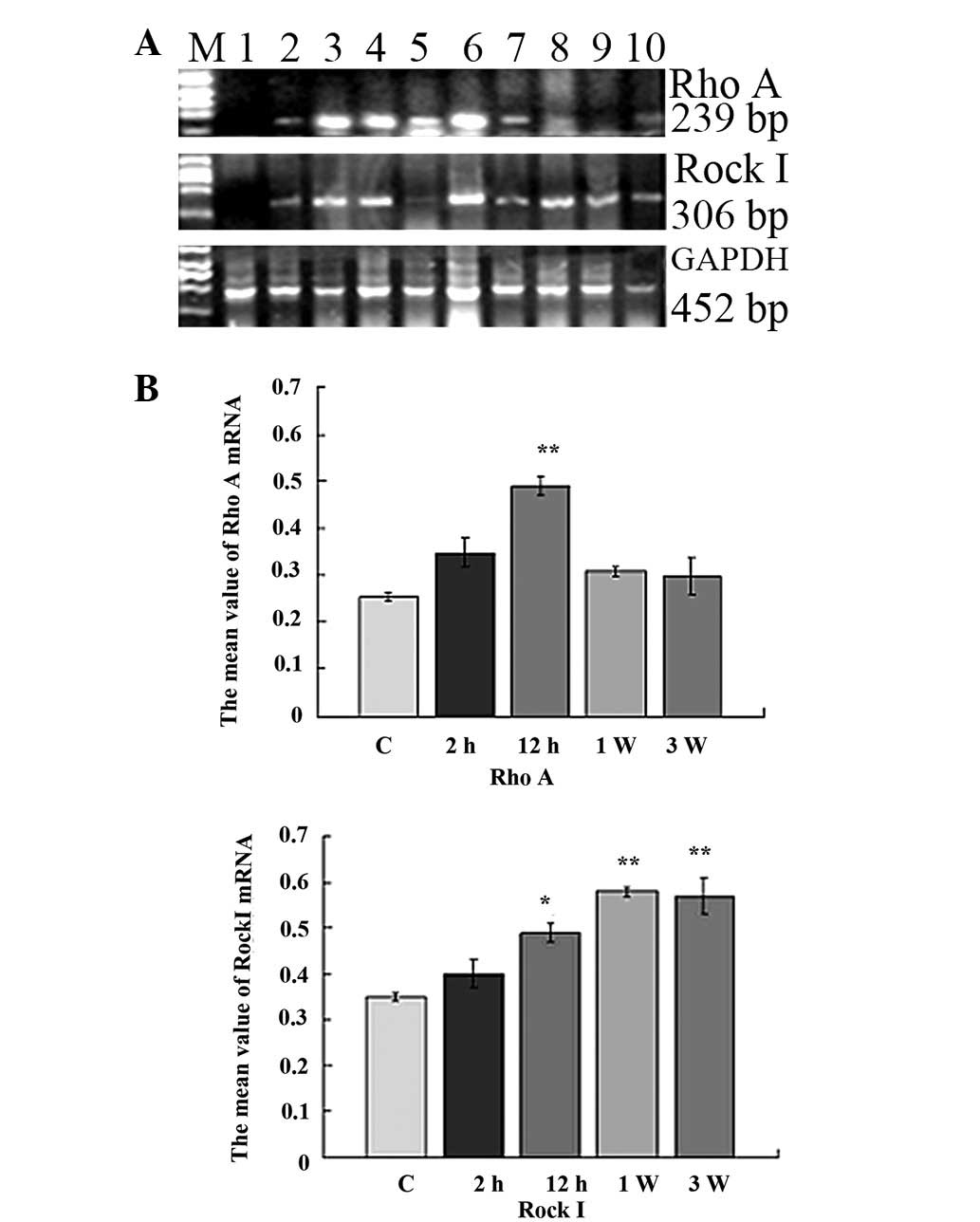 | Figure 4Expression of RhoA and Rock I in
myocardial tissue detected by RT-PCR. (A) M, DNA marker 2000; lane
1, Control group; lanes 2–10, 2 h, 4 h, 6 h, 12 h, 24 h, 48 h, 72
h, 7 days and 21 days after ISO injection. (B) Grayscale values.
*P<0.05, **P<0.01 vs. the control
group. Rock, Rho-associated coiled coil-forming protein kinase;
RT-PCR, reverse transcription-polymerase chain reaction; ISO,
isoprenaline hydrochloride; GAPDH, glyceraldehyde 3-phosphate
dehydrogenase. |
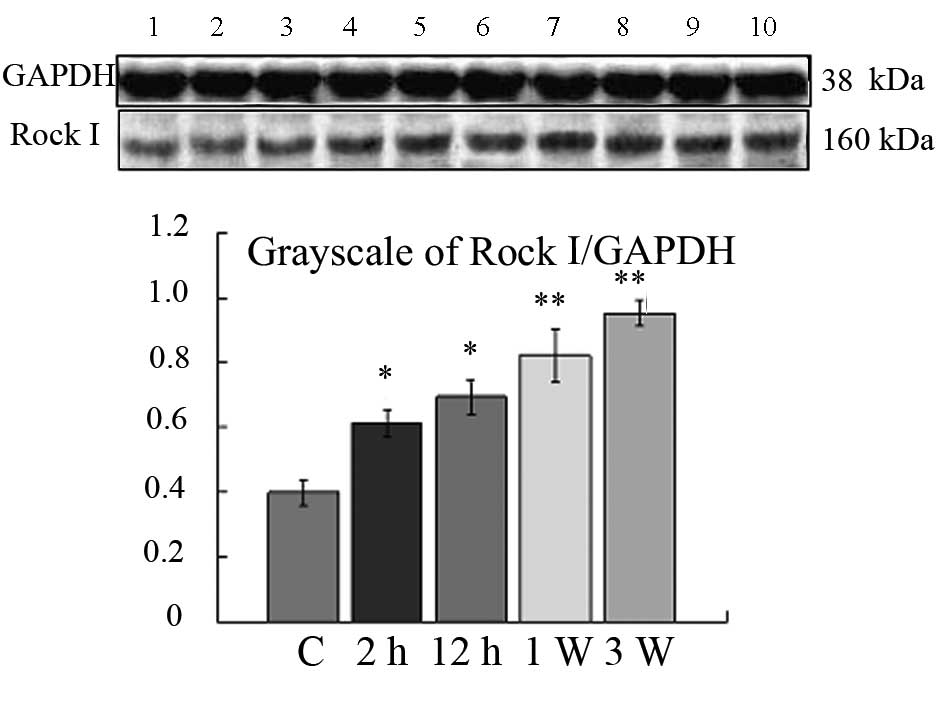 | Figure 5Western blotting of Rock I. Lane 1,
control group; lanes 2–9, 2 h, 4 h, 6 h, 12 h, 24 h, 48 h, 72 h, 7
days and 21 days1 days after ISO injection. *P<0.05,
**P<0.01 vs. the control group. Rock, Rho-associated
coiled coil-forming protein kinase; ISO, isoprenaline
hydrochloride; GAPDH, glyceraldehyde 3-phosphate dehydrogenase. |
Western blotting was used to investigate the
expression of Rock I. The results were analyzed semi-quantitatively
according to the grayscale value. As shown in Fig. 5, the expression level of Rock I in
the control group was undetectable in SDS-polyacrylamide gel
electrophoresis (PAGE). The expression of Rock I in the ISO-treated
group from 2 h to 21 days increased significantly when compared to
the control group (Fig. 5;
P<0.05 and P<0.01, respectively). These results validate the
mRNA expression of Rock I.
Immunofluorescence of CD31
expression
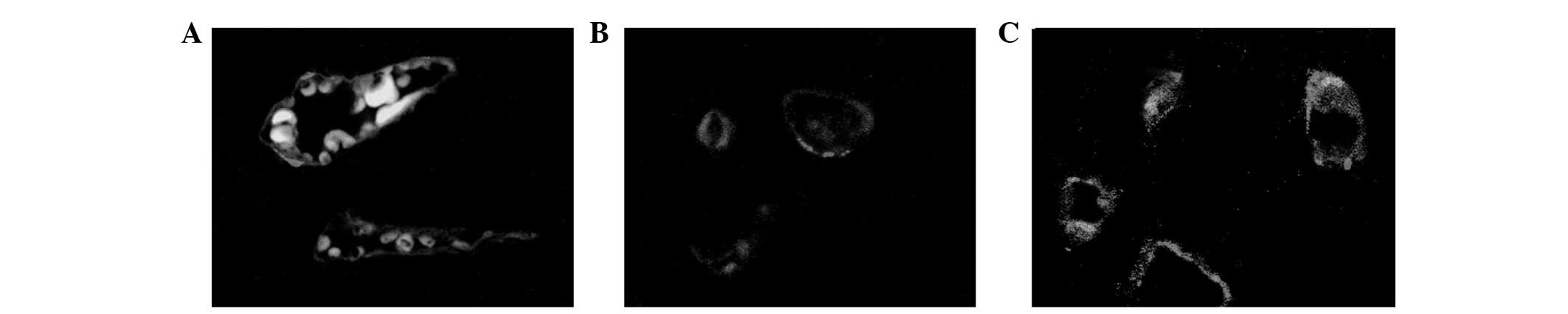
As shown in Fig. 6,
CD31 was highly specific and sensitive for vascular
endotheliocytes. Fibrosis triggered the CD31 expression of
endotheliocytes.
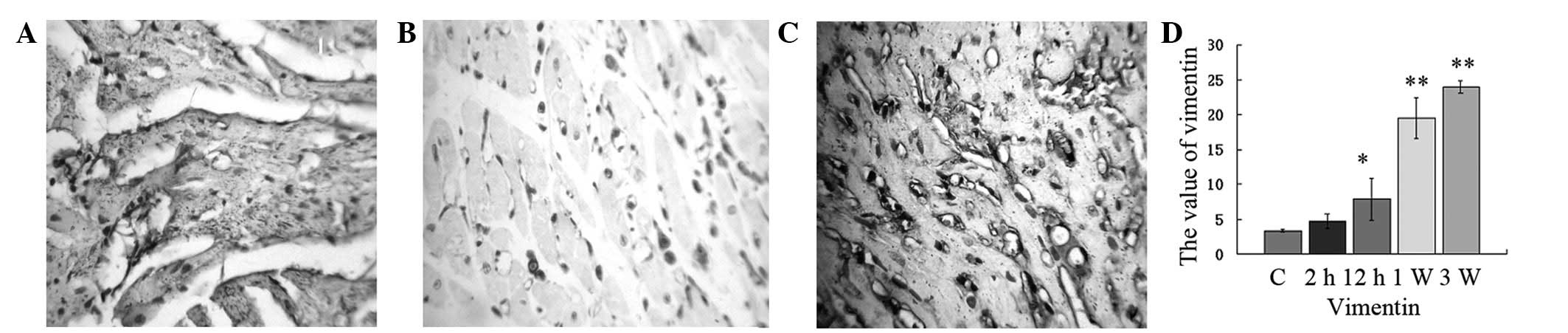
Expression of vimentin
We examined the change in vimentin protein
expression following ISO injection. Staining revealed that the
cytoplasm around the nucleus was brown and granular and the nucleus
was not stained. A few vimentin-positive cells were observed among
the myocardial myofibrils of the control group. Two hours after ISO
injection, the fraction of vimentin-positive cells did not
increase. From 12 h to 21 days after ISO injection, a large number
of vimentin-positive cells were observed in necrosis foci, which
increased gradually. The expression of vimentin significantly
increased from 2 h to 21 days and reached the maximum when compared
with the control group at 21 days (Fig. 7; P<0.01). In addition, the cells
became fibroblasts. These results indicate that fibroblasts play an
important role in MF.
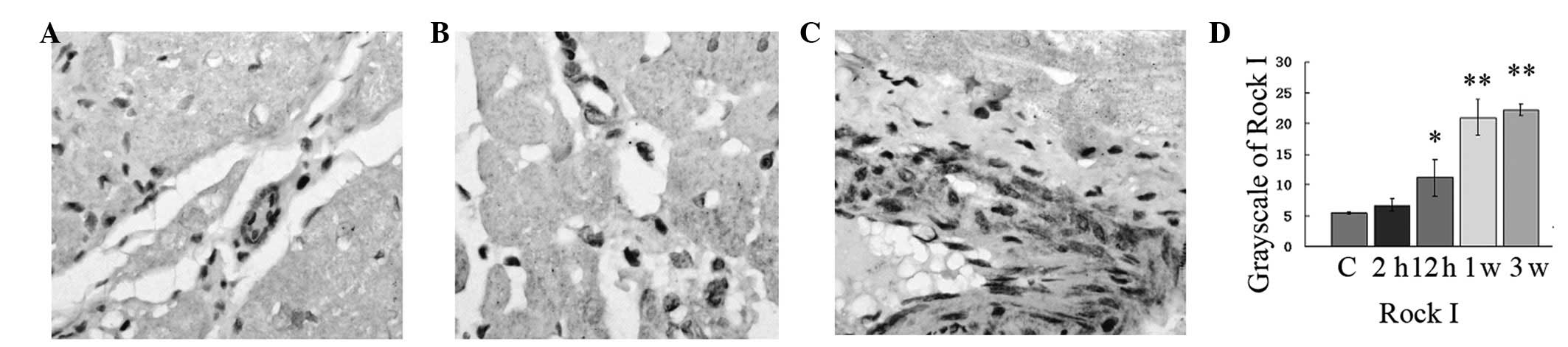
Expression of Rock I
Histopathological examination of the myocardium of
the control group revealed clear integrity of the myocardial cell
membrane and normal cardiac fibers without any infarction or
fibrosis. No inflammatory cell infiltration was observed (Fig. 8). In the ISO-treated group,
widespread loss of myofibers, focal myonecrosis and marked edema
with moderate infiltration of lymphocytes and macrophages were
observed (P<0.01; Fig. 8). The
protein expression of Rock I reached the maximum value at 21 days
(P<0.01). In addition, the expressional cell type was
endotheliocytes and fibroblasts (Fig.
8). Rock I played an important role in MF formation.
Simultaneously, the expression of CD31 and vimentin validated the
expression of Rock I.
Discussion
The pathogenesis of acute MF is not fully
understood. Studies on ISO-induced MF provide a good insight into
the pathology of MF and clearly indicate the involvement of
oxidative stress (13). The
myocardium contains various enzyme systems. When the myocardium is
traumatized, enzymes are released into the blood and increase the
activities of these enzymes in the serum. These enzymes are called
myocardial enzymes (14). The
combination of several enzymes that are closely related to the
myocardium is called myocardial enzyme spectrum. Although none of
the enzyme spectrum is specific to the myocardium, they have
specificity for the diagnosis of myocardium trauma (15). Clinically, the level of cardiac
enzymes is used indirectly to measure the trauma of cardiocytes. In
the present study, serum enzymes and H&E staining in myocardial
tissue in the ISO-treated group were significantly higher than in
the control group. Excessive collagen accumulation in the
myocardium was observed in the ISO-treated group. These findings
demonstrate that the MF rat model was successfully induced by
injection of 15 mg/kg ISO and this model mimics ischemic MF in
humans.
CD31 is a 130 kDa integral membrane protein, a
member of the immunoglobulin superfamily that mediates cell-to-cell
adhesion (16). CD31 is expressed
constitutively on the surface of adult and embryonic endothelial
cells and is weakly expressed on numerous peripheral leukocytes and
platelets. In the present study, when there is MF, CD31 was
expressed in endothelial cells.
Vimentin is expressed in a wide variety of
mesenchymal cell types, including fibroblasts and endothelial cells
(16). Rho/Rock controls a wide
variety of signal transduction pathways (17). Rock exists as two isoforms, Rock I
and Rock II. They share an overall homology of 92% in their kinase
domains. Two major families of Rock inhibitors, fasudil and
Y-27632, are extensively used. Rock not only regulates the actin
cytoskeleton, but also the expression of genes associated with
tissue fibrosis (18). Data from
one study suggests that diabetes impairs cardiac function through
upregulation of RhoA (19). The
increase in RhoA expression and activity in diabetic hearts results
in increased phosphorylation of Rock targets (19). In the present study, the mRNA and
protein expression of RhoA and Rock I were significantly increased.
The biological effector cells of RhoA and Rock I were assessed by
specific staining. The results revealed a large number of active
fibroblasts and endotheliocytes around the deposits in MF rats.
These findings show that the Rho/Rock signaling pathway plays an
important role in MF formation. Inhibition of Rho/Rock may be a
novel therapeutic target for prevention of ischemic MF. Further
research is required to clarify the exact molecular signaling
pathways.
Acknowledgements
This study was supported by the
Science and Technology Department of Jilin Province, China (nos.
201101067 and 3D511B873432) and the Basic Research Foundation of
Jilin University (no. 450060481127).
References
|
1
|
Porter KE and Turner NA: Cardiac
fibroblasts: at the heart of myocardial remodeling. Pharmacol Ther.
123:255–278. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Díez J: Mechanisms of cardiac fibrosis in
hypertension. J Clin Hypertens (Greenwich). 9:546–550. 2007.
|
|
3
|
Dreesen O and Brivanlou AH: Signaling
pathways in cancer and embryonic stem cells. Stem Cell Rev. 3:7–17.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bove PF and van der Vliet A: Nitric oxide
and reactive nitrogen species in airway epithelial signaling and
inflammation. Free Radic Biol Med. 41:515–527. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tapia PC: RhoA, Rho kinase, JAK2 and STAT3
may be the intracellular determinants of longevity implicated in
the progeric influence of obesity: Insulin, IGF-1 and leptin may
all conspire to promote stem cell exhaustion. Med Hypotheses.
66:570–576. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Burch ML, Zheng W and Little PJ: Smad
linker region phosphorylation in the regulation of extracellular
matrix synthesis. Cell Mol Life Sci. 68:97–107. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hudson VM: Rethinking cystic fibrosis
pathology: the critical role of abnormal reduced glutathione (GSH)
transport caused by CFTR mutation. Free Radic Biol Med.
30:1440–1461. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Murata I, Takenaka K, Yoshinoya S, Kikuchi
K, Kiuchi T, Tanigawa T and Ito K: Clinical evaluation of pulmonary
and hypertension in systemic scleros related disorders: A Doppler
echocardiographic study of 135 Japanese patients. Chest. 111:36–43.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tian B and Kaufman PL: Effects of the Rho
kinase inhibitor Y-27632 and the phosphatase inhibitor Calyculin A
on outflow facility in monkeys. Exp Eye Res. 80:215–225. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nagatoya K, Moriyama T, Kawada N, Takeji
M, Oseto S, Murozono T, Ando A, Imai E and Hori M: Y-27632 prevents
tubulointerstitial fibrosis in mouse kidneys with unilateral
ureteral obstruction. Kidney Int. 61:1684–1695. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gao X, He X, Luo B, Peng L, Lin J and Zuo
Z: Angiotensin II increases collagen I expression via transforming
growth factor-beta1 and extracellular signal-regulated kinase in
cardiac fibroblasts. Eur J Pharmacol. 606:115–120. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Stange EF, Travis SP, Vermeire S,
Beglinger C, et al: European evidence based consensus on the
diagnosis and management of Crohn’s disease: definitions and
diagnosis. Gut. 55(Suppl 1): i1–i15. 2006.
|
|
13
|
Kell DB: Iron behaving badly:
inappropriate iron chelation as a major contributor to the
aetiology of vascular and other progressive inflammatory and
degenerative diseases. BMC Med Genomics. 2:1–79. 2009.PubMed/NCBI
|
|
14
|
Velders M, Schleipen B, Fritzemeier KH,
Zierau O and Diel P: Selective estrogen receptor-β
activation stimulates skeletal muscle growth and regeneration.
FASEB J. 26:1909–1920. 2012.
|
|
15
|
Cheng XW, Shi GP, Kuzuya M, Sasaki T,
Okumura K and Murohara T: Role for cysteine protease cathepsins in
heart disease focus on biology and mechanisms with clinical
implication. Circulation. 125:1551–1562. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Strieter RM, Keeley EC, Burdick MD and
Mehrad B: The role of circulating mesenchymal progenitor cells,
fibrocytes, in promoting pulmonary fibrosis. Trans Am Clin Climatol
Assoc. 120:49–59. 2009.PubMed/NCBI
|
|
17
|
Kolavennu V, Zeng L, Peng H, Wang Y and
Danesh FR: Targeting of RhoA/ROCK signaling ameliorates progression
of diabetic nephropathy independent of glucose control. Diabetes.
57:714–723. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Loirand G, Guérin P and Pacaud P: Rho
Kinases in cardiovascular physiology and pathophysiology. Circ Res.
98:322–334. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhou H, Li YJ, Wang M, Zhang LH, Guo BY,
Zhao ZS, Meng FL, Deng YG and Wang RY: Involvement of RhoA/ROCK in
myocardial fibrosis in a rat model of type 2 diabetes. Acta
Pharmacol Sin. 32:999–1008. 2011. View Article : Google Scholar : PubMed/NCBI
|