Introduction
Bronchopulmonary dysplasia (BPD) is the most common
complication of preterm children who accept long-term mechanical
ventilation or oxygen therapy. BPD seriously affects the survival
of premature children and quality of life (1). While the etiology and pathogenesis of
BPD remain complex, lines of evidence indicate that oxidative
stress and inflammatory responses play crucial roles in the disease
development (2–4). Clinical trials of antioxidant and
anti-inflammatory agents have been conducted. One of the most
well-investigated types of agent is glucocorticoids.
Glucocorticoids have been shown to reduce the incidence of BPD by
inhibiting the inflammatory response through the production of
antioxidant enzymes, improving pulmonary edema and fibrosis
(5). However, the side-effects of
glucocorticoids, including increased mortality, growth retardation,
cerebral palsy, hyperglycemia, intestinal perforation and
infection, limit their long-term use (6,7).
Therefore, the identification of more efficient and safer
replacements for BPD treatment is required.
Astragalus polysaccharide (APS) is a traditional
Chinese medicine that has been studied in depth and is widely used
in clinics. APS is one of the most important natural active
materials due to its multi-targeting biological activities,
including antioxidant, radical scavenging and anti-inflammatory
activity and immune regulation (8). Gradient and alcohol precipitation,
chromatography, ultrafiltration, microfiltration and other modern
pharmaceutical technology are used in the preparation of APS, so
that the effective ingredients are accurately separated and
purified; the purity is 98%. The antioxidative and
anti-inflammatory effects of APS have been well-recognized in
previous studies (9–11). To the best of our knowledge,
studies concerning APS in BPD are rare. Whether APS prevents or
rescues the development of BPD by antioxidant and/or
anti-inflammatory actions remains unclear due to the lack of
well-built models and clinical trials.
The EA.hy926 cell line is an immortalized human
umbilical vein endothelial cell (HUVEC) line, derived from the
fusion of HUVECs and lung adenocarcinoma cells. The structural
characteristics and functions are similar to lung adenocarcinoma
cells and these alveolar endothelial cells are used to construct
the alveoli in vitro mouse model (12). EA.hy926 cells have been widely
applied in the study of leukocyte adhesion to endothelial cells,
oxidative stress and protein expression (13–18).
In the current study, we aimed to explore the role
of APS in preterm children with BPD and its mechanism of action by
establishing an in vitro cell model of BPD. Our findings
indicated that APS mitigates the cell damage induced by oxidative
stress and inhibits the inflammatory response by downregulating the
production of reactive oxygen species (ROS) and malondialdehyde
(MDA) and the expression of nuclear factor (NF)-κB, as well as
increasing the levels of superoxide dismutase (SOD).
Materials and methods
Reagents and materials
EA.hy926 cells were commercially available from the
Shanghai Institute Cell Bank (Shanghai, China). Fetal bovine serum
(FBS; Cat# 16000-044) and high glucose Dulbecco’s modified Eagle
medium (DMEM; Cat# C11995) were purchased from Gibco (Carlsbad, CA,
USA). APS was purchased from Tianjin Cinorch Pharmaceutical Co.,
Ltd. (Tianjin, China). The triple gas mixture (70% O2,
5% CO2 and 25% N2) was provided by Nanfang Hospital
Oxygen Center (Guangzhou, China). The SOD and MDA test kits were
provided by Nanjing Jiancheng Bioengineering Institute (Nanjing,
China). The ROS kit (C1300) was purchased from Beijing Applygen
Technologies Inc. (Beijing, China). Anti-intercellular adhesion
molecule 1 (ICAM-1; Cat# 3518-1), anti-interleukin (IL)-8 (Cat#
3482-1) and anti-NF-κB p65 (Cat# 2229-1) were purchased from
Epitomics Inc. (Burlingame, CA, USA).
Cell culture
EA.hy926 cells were cultured in high glucose DMEM
containing 10% FBS at 5% CO2 in a 37°C incubator. Then,
EA.hy926 cells were divided into three groups: the air group, the
hyperoxia group and the APS group (2.5 mg/ml). The air group were
cultured for 24, 36 and 48 h in a 5% CO2, 37°C
incubator. The hyperoxia group and the APS group were cultured for
24, 36 and 48 h in the 37°C triple gas mixture.
Assessment of ROS
Following removal of the culture medium, the cells
were washed twice with phosphate-buffered saline (PBS). Then, 1 ml
H2O2 (100 μM) was added to the cells
of the air group as the positive control and 1 ml
2′,7′-dichlorofluorescein-diacetate (DCFH-DA; 10 μM) was
added to the cells of the hyperoxia and APS groups. The cells were
then incubated for 30 min at 37°C. Following pipetting of the
mixture from the petri dish, the cells were washed twice with PBS
and visualized under a fluorescence microscope (IX71; Olympus,
Tokyo, Japan). The fluorescence was measured using an MD5
microplate reader (SpectraMax M5; Molecular Devices Company,
Sunnyvale, CA, USA).
Measurement of SOD and MDA
Cells were sonicated by ultra-sound and mixed
according to the manufacturer’s instructions. The SOD mixture was
incubated in water at 37°C for 40 min and then cooled to room
temperature for 10 min after adding chromogenic reagent. Absorbance
at a wavelength of 550 nm was measured by the MD5 microplate
reader. The MDA mixture was boiled for 80 min and then cooled by
flowing water. Absorbance at a wavelength of 532 nm was measured by
the MD5 microplate reader.
Western blotting
Total protein was extracted from the cells according
to the manufacturer’s instructions (Cat# P0027; Beyotime Institute
of Biotechnology, Jiangsu, China). Then, 20 μg proteins and
ladder were fractionalized using sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and
electrophoretically transferred and blotted onto a nitrocellulose
membrane. Non-specific binding was blocked by 1 h incubation of the
membranes with blocking buffer [(5% non-fat dry milk and 0.05%
Tween-20 in Tris-buffered saline (TBS)]. The blots were then probed
overnight at 4–6°C with primary mouse anti-β-actin (1:5,000; Novus
Biologicals, Littleton, CO, USA), anti-ICAM-1 (1:1,000), anti-IL-8
(1:1,000) or anti-NF-κB p65 (1:100,000) in blocking buffer. After
three washes, the membranes were incubated with horseradish
peroxidase-conjugated goat anti-mouse IgG. The blots were developed
using light-emitting liquid (Cat# WBKLS0100; Millipore, Billerica,
MA, USA) and quantified using an Odyssey instrument (Nanfang
Hospital Clinical Trial Center, Guangzhou, China) (19).
RT-PCR
The total RNA of cells was extracted using TRIzol
reagent (Cat# D9108A; Takara Biotechnology Co., Ltd., Dalian,
China) and cDNA was synthesized according to the instructions from
the PrimeScript® RT reagent kit with gDNA Eraser
(Perfect Real Time; Cat# DRR047S; Takara Biotechnology Co., Ltd.).
RT-PCR primers were as follows: GAPDH, forward:
5′-agaaggctggggctcatttg-3′ and reverse: 5′-aggggccatccacagtcttc-3′;
IL-8, forward: 5′-tagcaaaattga ggccaagg-3′ and reverse:
5′-aaaccaaggcacagtggaac-3′; and ICAM-1, forward:
5′-ggctggagctgtttgagaac-3′ and reverse: 5′-actgtggggttcaacctctg-3′.
The reaction system was prepared according to the instructions of
the SYBR® Premix Ex Taq™ kit (Perfect Real time; Cat#
DRR420A; Takara Biotechnology Co., Ltd.) and then DNA was amplified
as follows: 95°C for 10 min; then 95°C for 15 sec, 61°C for 15 sec
and 72°C for 15 sec for 40 cycles; and finally 95°C for 1 min, 55°C
for 30 sec and 95°C for 30 sec.
Statistical analysis
Data are expressed as mean ± standard deviation and
analyzed using SPSS 13.0 (SPSS, Inc., Chicago, IL, USA).
Statistical comparisons between groups were performed by factorial
analyses. P<0.05 was considered to indicate a statistically
significant difference.
Results
SOD and MDA assessment
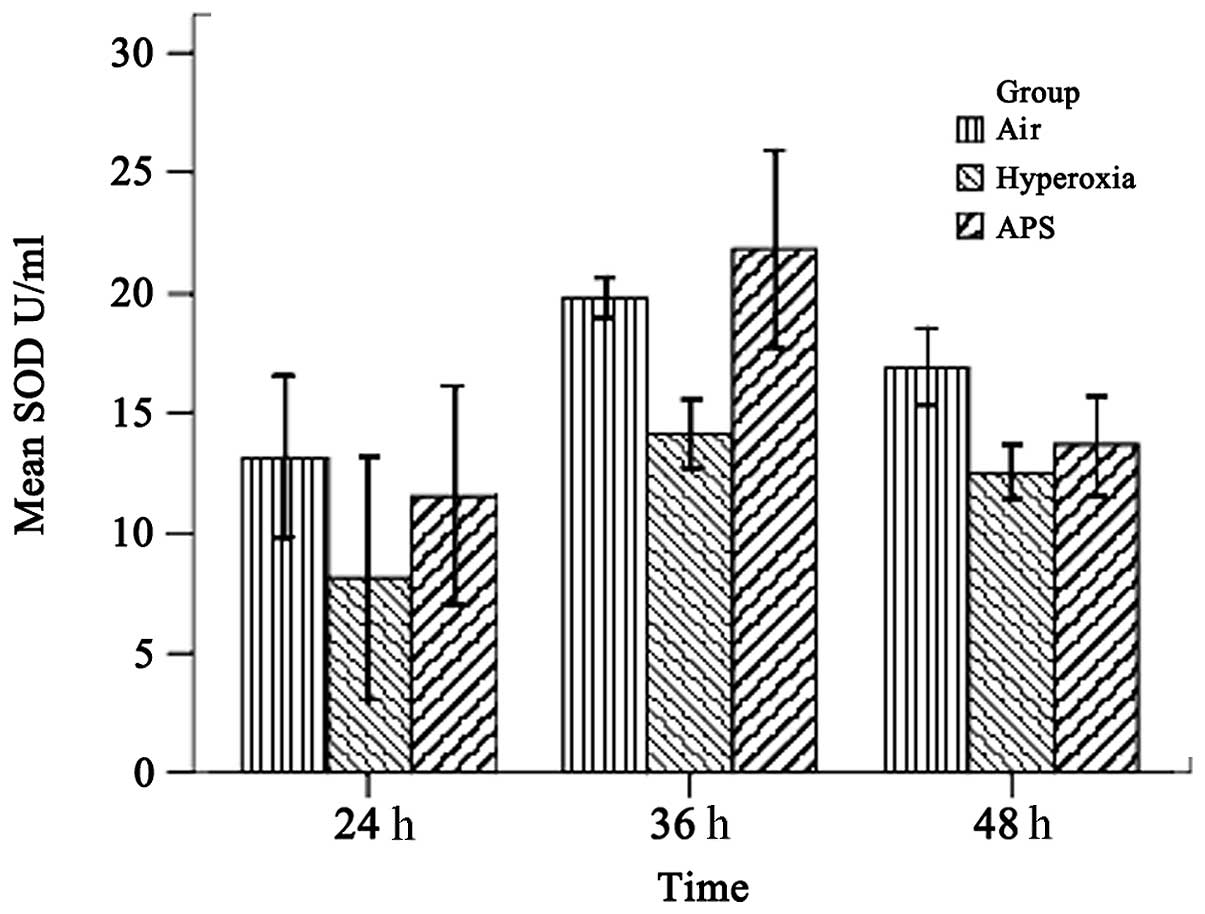
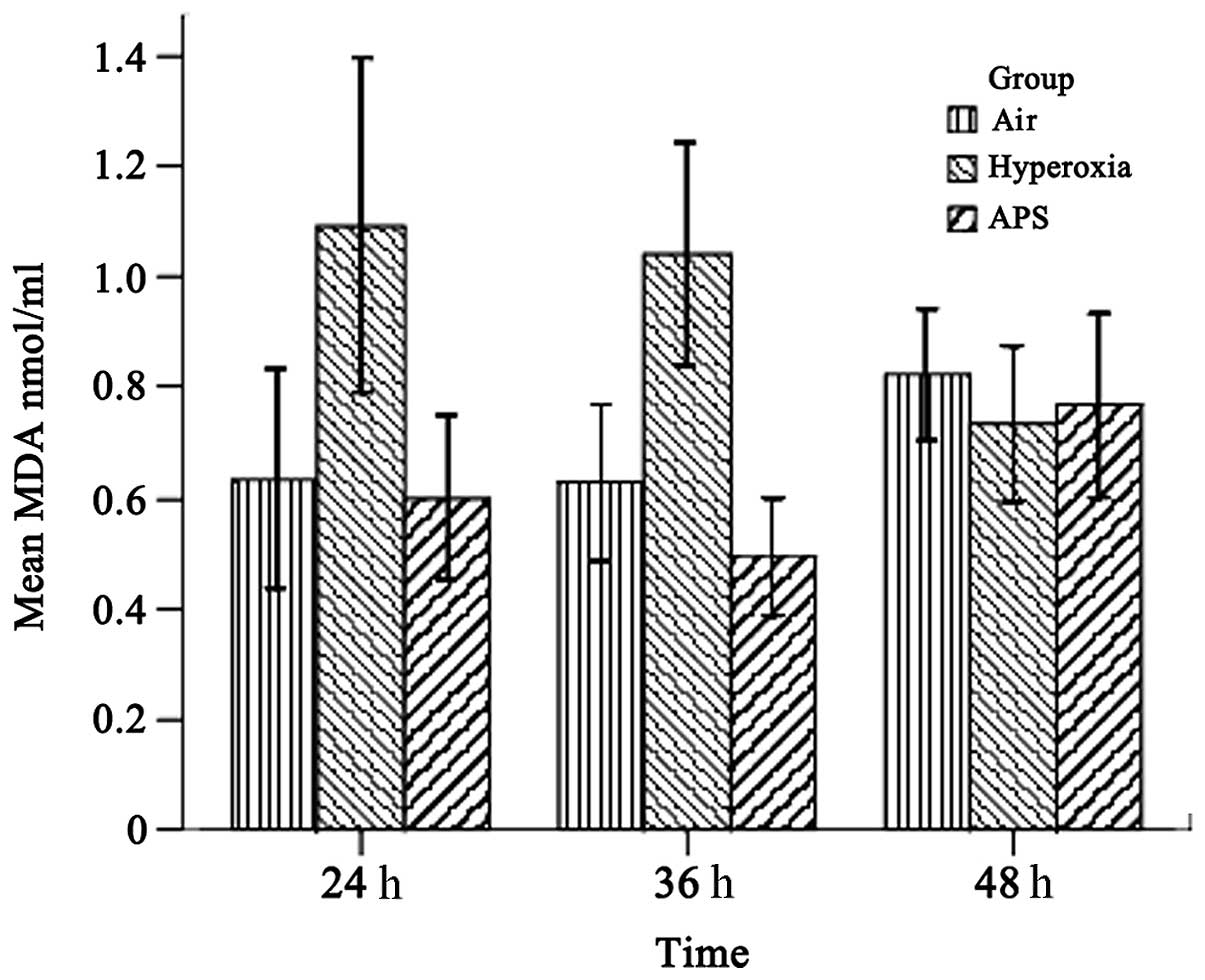
As shown in Figs. 1
and 2, the results demonstrated
that the SOD and MDA levels of the three groups were significantly
different (SOD, F=15.842, P<0.01; MDA, F=21.950, P<0.01). The
average SOD level of the hyperoxia group (11.586±3.955 U/ml) was
lower than those of the air group (16.609±3.472 U/ml) and the APS
group (15.679±5.737 U/ml). By contrast, the MDA level of the
hyperoxia group (0.950±0.270 nmol/ml) was lower than those of the
air group (0.682±0.170 nmol/ml) and the APS group (0.622±0.180
nmol/ml). Furthermore, time course analysis demonstrated
significant differences when the cells were cultured for 24, 36 and
48 h, respectively (F=32.555, P<0.01). It is clear that APS
induces the production of SOD in EA.hy926 cells most significantly
at 36 h (21.818±4.054 U/ml), while there were no significant
differences in the MDA level among the three time points (F=0.662,
P=0.520). These data suggest that APS induces the secretion of SOD
by EA.hy926 cells and blocks MDA generation at the same time.
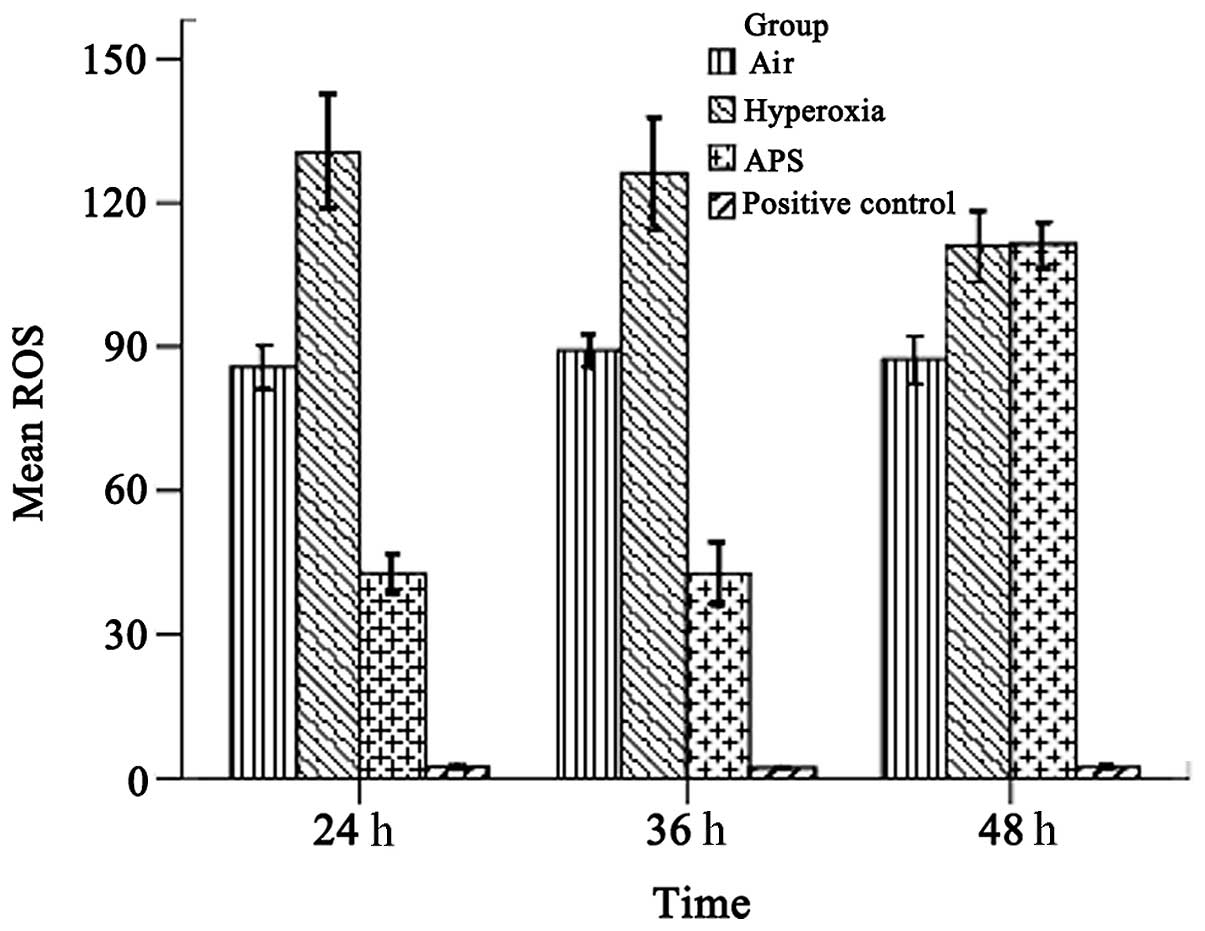
ROS analysis
As shown in Fig. 3,
the average ROS levels were 87.465±4.365, 122.370±7.253, and
65.632±4.046 for the air, hyperoxia and APS groups, respectively;
there were significant differences among the groups (F=955.604,
P<0.01). The results demonstrate that the ROS level of the
EA.hy926 cells was reduced by treatment with APS.
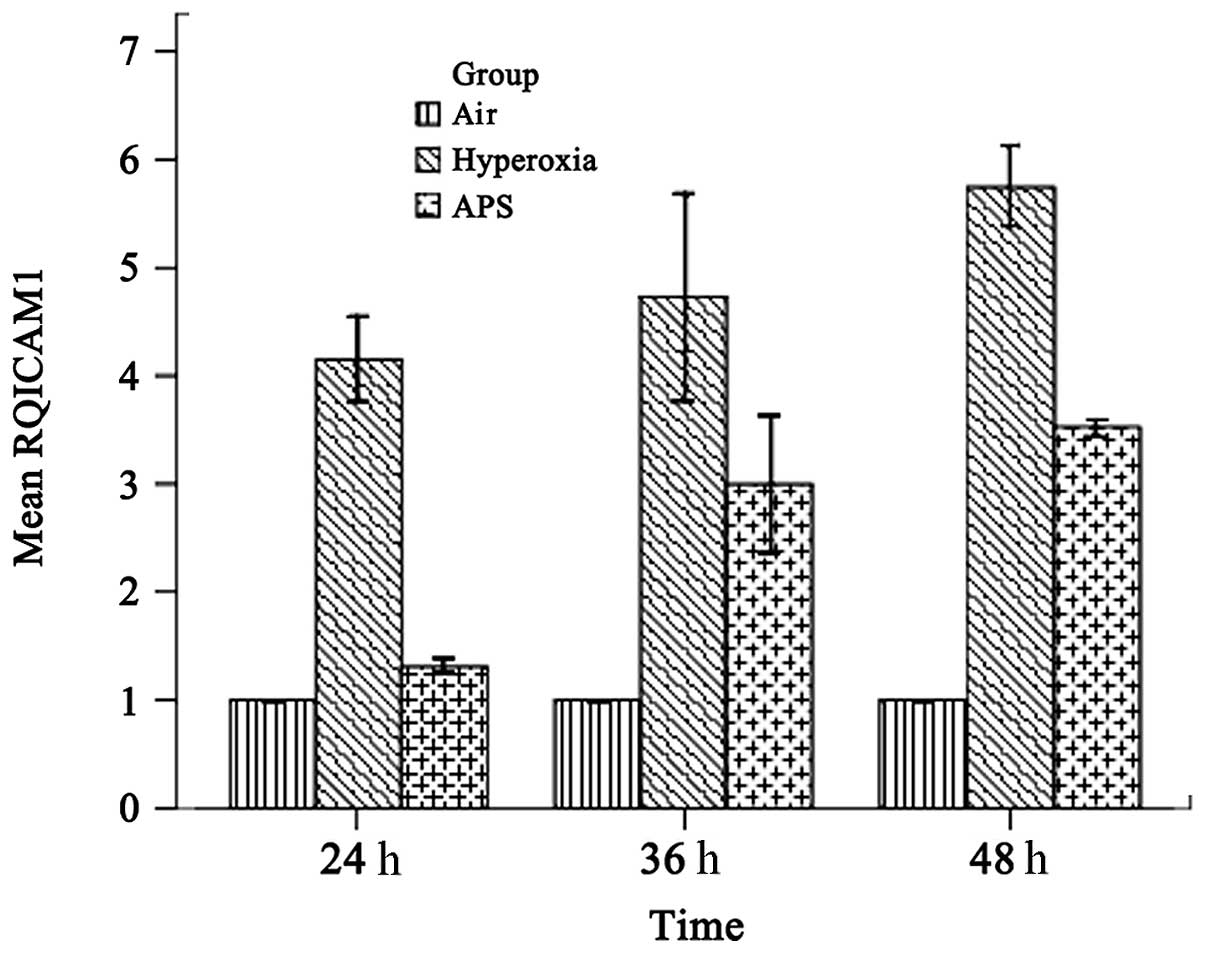
IL-8 and ICAM-1 transcription
As shown in Figs. 4
and 5, the results demonstrated
that IL-8 and ICAM-1 RNA expression was downregulated by APS, at
the average levels of 2.227±0.289 and 2.611±1.052 respectively,
compared with 6.033±0.808 and 4.880±0.891 in the hyperoxia group.
The expression levels of IL-8 and ICAM-1 in the APS group were
significantly lower than the respective levels in the hyperoxia
group (IL-8, F=205.373, P<0.01; ICAM-1, F=158.926,
P<0.01).
Western blotting
The protein expression levels of NF-κB p65, ICAM-1
and IL-8 in the APS group were significantly lower than those in
the hyperoxia group at each time point. However, NF-κB p65 and IL-8
expression levels were not significantly altered over different
time points.
Discussion
Oxidative stress and the inflammatory response are
two important events during BPD development. SOD removes the
superoxide anion radical in order to protect cells from damage
(20). An imbalance between SOD
and ROS directly affects MDA production and oxidative stress
injury. In addition, cytokines play important roles in the lung
inflammatory response. A variety of cytokines are involved in the
occurrence of BPD, including IL-6, IL-8, IL-10, tumor necrosis
factor (TNF)-β and ICAM-1. Therefore, infection leads to or worsens
BPD severity due to the release of inflammatory stimuli. Airway
epithelial cells and pulmonary capillary endothelial cells are
damaged by inflammatory regulators, resulting in the release of
regulatory factors of inflammation and chemokines, as well as
infiltration of inflammatory cells. Neutrophils participate in the
inflammatory response and produce cytokines, including IL-8,
macrophage and inflammatory response protein-1 and TNF-β, which
enhance vascular permeability, resulting in pulmonary interstitial,
alveolar and airway edema (21).
The current study identified that the ROS and MDA levels of the APS
group were significantly reduced compared with those of the
hyperoxia group (P<0.05), while the SOD content was increased
compared with that in the hyperoxia group (P<0.05). The
expression of IL-8, ICAM-1 and NF-κB p65 in the APS group was lower
than in the hyperoxia group. These assays indicate that APS acts
against oxidative stress injury and reduces the inflammatory
response.
The oxidative stress response is regulated by an
imbalance between ROS generation and antioxidant enzyme degradation
of ROS (22). Under physiological
circumstances, there is a delicate balance between human ROS
generation and the antioxidant defense system. The balance is
likely to be broken when the overexpression or inadequate clearance
of ROS is out of the metabolic control of cells, resulting in
oxidative stress injury (23,24).
It is known that SOD contributes to scavenging ROS. The primary
role of SOD is to translate highly reactive superoxide free
radicals into hydrogen peroxide and water; hydrogen peroxide is
then transformed into water by catalase, glutathione peroxidase and
glutathione reductase. Oxidative stress injury results in lipid
peroxidation and produces MDA. SOD scavenges oxygen free radicals
to protect cells from damage; the level of its activity indirectly
reflects the ability of the body to scavenge oxygen free
radicals.
APS, as a potent antioxidant, effectively inhibits
the generation of oxygen free radicals, preventing membrane lipid
peroxidation and reduction of biofilm injury. APS ameliorates
hypoxia and prevents reperfusion lung injury (25). In vitro tissue culture
experiments indicate that APS stimulates the total antioxidant
capacity of cells, as well as reduces the generation of oxygen free
radicals and thus prevents rat lung epithelial cell injury. APS
also protects the intestinal mucosa of rats with obstructive
jaundice from oxidative stress damage, which may relate to
increases in SOD levels and reductions in MDA levels (26). Li XT et al identified that
APS promotes the wound healing of chronic ulcers, by a mechanism
related to the reduced expression of inflammatory stimuli, wound
lipid peroxidation and enhancement of SOD expression (27). The activity of SOD in dogs’ blood
may be significantly improved by APS. APS increases the activity of
superoxide dismutase, as well as reduces the content of lipid
peroxide and the cell damage caused by free radicals (28). Our study identified that APS is
able to prevent the production of ROS due to intracellular
oxidative stress by increasing the expression of SOD in EA.hy926
cells and reducing the peroxidation of the cytoplasm, which
protects cells from oxidative stress injury.
The inflammatory response plays a crucial role in
the development of BPD. A variety of cytokines, including IL-6,
IL-8, IL-10 and ICAM-1, are considered to participate in lung
inflammation, which is closely related to the development of BPD.
NF-κB is one of the transcription factors regulating the expression
of inflammatory cytokines. NF-κB upregulates the expression of a
variety of inflammatory cytokines, which induce inflammation and
then promote the development of BPD. NF-κB is a heterodimer
complex, composed of p65/p50. NF-κB binds to the inhibitory-type
IκB protein in the cytoplasm. When endothelial cell damage occurs,
IκB is phosphorylated and degraded. Phosphorylated NF-κB
translocates to the nucleus, binds to the nucleotide sequence of
the κB domain and regulates the transcription of a variety of
inflammatory cytokines and adhesion molecules (29,30).
The activation of NF-κB leads to the overexpression of
inflammation-related factors and causes a significant inflammatory
response. In return, the increased inflammatory mediators and
cytokines further activate NF-κB, amplifying the initial
inflammatory signals. The activation of NF-κB stimulates
endothelial cells to release IL-8. IL-8 is a potent neutrophil
chemotactic factor that promotes and prolongs the inflammatory
response (31). Wu et al
identified that NF-κB also induces the production and release of
ICAM-1 (32), which dilates blood
vessels, causes the migration of inflammatory cells and induces the
release of cytokines and chemokines into adherent tissues. A
previous study demonstrated that the expression of VCAM-1, ICAM-1
and NF-κB mRNA in cardiac microvascular endothelial cells in
reperfusion injury is suppressed by APS (33). In a lipopolysaccharide
(LPS)-induced inflammatory response, the generation of TNF-α and
IL-8 was inhibited by APS, which may play a role in preventing
inflammation (34). In
osteoarthritis, APS is reported to reduce the inflammatory response
of synovial cells and the generation of apoptosis (35). The current
study identified that APS inhibits the activation of NF-κB p65,
thereby reducing the expression of ICAM-1 and IL-8 and the
inflammatory response.
Oxidative stress and inflammatory responses affect
and promote each other. Inflammatory cells release ROS and are
involved in oxidative stress. However, ROS-induced oxidative stress
activates NF-κB p65 signaling and promotes the expression of
pro-inflammatory cytokine genes and the chemotaxis of inflammatory
cells, including neutrophils (36). Additional oxidative stress may
enhance the chemotaxis of neutrophils and macrophages and stimulate
cell adhesion molecule expression, inducing the inflammatory
response. The mechanisms by which APS inhibits the activation of
NF-κB p65 may be: i) APS has a direct anti-inflammatory role by
inhibiting the expression of NF-κB p65 directly; and ii) APS
eliminates oxygen free radicals, which is the main stimulant of
NF-κB p65 activation. Higher concentrations of APS have an improved
scavenging effect on superoxide anion free radicals and lipid free
radicals, with the concentration increasing as the scavenging
effect increases.
In summary, oxidative stress and inflammation are
two important pathological events of BPD, independently or
interactively. Lipid peroxidation of EA.hy926 cells may be reduced
by APS, which removes the oxygen free radicals within the cells as
an antioxidant. In addition, the activation of NF-κB p65 may be
effectively blocked and the expression of cytokines, including IL-8
and ICAM-1, may be reduced by APS, which inhibits the inflammatory
response. This study was limited to in vitro experiments.
The mechanisms of the antioxidant and anti-inflammatory effects of
APS in the protection against BPD pathogenesis require further
validation in vivo.
Acknowledgements
This study was completed in the
Clinical Trials Center of Nanfang Hospital, Guangzhou, China.
Financial support was obtained from the Presidential Foundation of
Nanfang Hospital (2010A001). The manuscript was reviewed by Dr Yan
Hua Liang.
References
|
1.
|
Jobe AH: The new bronchopulmonary
dysplasia. Curr Opin Pediatr. 23:167–172. 2011. View Article : Google Scholar
|
|
2.
|
Joung KE, Kim HS, Lee J, et al:
Correlation of urinary inflammatory and oxidative stress markers in
very low birth weight infants with subsequent development of
bronchopulmonary dysplasia. Free Radic Res. 45:1024–1032. 2011.
View Article : Google Scholar
|
|
3.
|
Gien J and Kinsella JP: Pathogenesis and
treatment of bronchopulmonary dysplasia. Curr Opin Pediatr.
23:305–313. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Fernandez-Gonzalez A, Alex Mitsialis S,
Liu X and Kourembanas S: Vasculoprotective effects of heme
oxygenase-1 in a murine model of hyperoxia-induced bronchopulmonary
dysplasia. Am J Physiol Lung Cell Mol Physiol. 302:L775–L784. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Doyle LW, Ehrenkranz RA and Halliday HL:
Postnatal hydrocortisone for preventing or treating
bronchopulmonary dysplasia in preterm infants: a systematic review.
Neonatology. 98:111–117. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Needelman H, Evans M, Roberts H, Sweney M
and Bodensteiner JB: Effects of postnatal dexamethasone exposure on
the developmental outcome of premature infants. J Child Neurol.
23:421–424. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Jobe AH: Postnatal corticosteroids for
bronchopulmonary dysplasia. Clin Perinatol. 36:177–188. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Shao BM, Xu W, Dai H, Tu P, Li Z and Gao
XM: A study on the immune receptors for polysaccharides from the
roots of Astragalus membranaceus, a Chinese medicinal herb.
Biochem Biophys Res Commun. 320:1103–1111. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Zhang BQ, Hu SJ, Qiu LH, et al: Effects of
Astragalus membranaceus and its main components on the acute
phase endothelial dysfunction induced by homocysteine. Vascul
Pharmacol. 46:278–285. 2007.
|
|
10.
|
Yang M, Qian XH, Zhao DH and Fu SZ:
Effects of Astragalus polysaccharide on the erythroid lineage and
microarray analysis in K562 cells. J Ethnopharmacol. 127:242–250.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Gao XH, Xu XX, Pan R, et al: Saponin
fraction from Astragalus membranaceus roots protects mice
against polymicrobial sepsis induced by cecal ligation and puncture
by inhibiting inflammation and upregulating protein C pathway. J
Nat Med. 63:421–429. 2009.
|
|
12.
|
Xu D, Perez RE, Ekekezie II, Navarro A and
Truog WE: Epidermal growth factor-like domain 7 protects
endothelial cells from hyperoxia-induced cell death. Am J Physiol
Lung Cell Mol Physiol. 294:L17–L23. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Song YH, Neumeister MW, Mowlavi A and
Suchy H: Tumor necrosis factor-alpha and lipopolysaccharides induce
differentially interleukin 8 and growth related oncogene-alpha
expression in human endothelial cell line EA.hy926. Ann Plast Surg.
45:681–683. 2000. View Article : Google Scholar
|
|
14.
|
Thornhill MH, Li J and Haskard DO:
Leucocyte endothelial cell adhesion: a study comparing human
umbilical vein endothelial cells and the endothelial cell line
EA-hy-926. Scand J Immunol. 38:279–286. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Jones MK, Sarfeh IJ and Tarnawski AS:
Induction of in vitro angiogenesis in the endothelial-derived cell
line, EA.hy926, by ethanol is mediated through PKC and MAPK.
Biochem Biophys Res Commun. 249:118–123. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Huang J, de Paulis T and May JM:
Antioxidant effects of dihydrocaffeic acid in human EA.hy926
endothelial cells. J Nutr Biochem. 15:722–729. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Aranda E and Owen GI: A semi-quantitative
assay to screen for angiogenic compounds and compounds with
angiogenic potential using the EA.hy926 endothelial cell line. Biol
Res. 42:377–389. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Yu C, Liu X and Zhang Y: Effects of fluid
shear stress on the expression of IL-8 receptor mRNA in EA.Hy926
cells. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 24:303–307. 2007.(In
Chinese).
|
|
19.
|
Kunnumakkara AB, Diagaradjane P, Anand P,
et al: Curcumin sensitizes human colorectal cancer to capecitabine
by modulation of cyclin D1, COX-2, MMP-9, VEGF and CXCR4 expression
in an orthotopic mouse model. Int J Cancer. 125:2187–2197. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Li J-T: Effect of astragalus
polysaccharides on bleomycin-induced pulmonary fibrosis rats. China
Journal of Traditional Chinese Medicine and Pharmacy. 10:2360–2363.
2011.(In Chinese).
|
|
21.
|
Hou Y: Progress in association between
antenatal infection, inflammation and broncho pulmonary dysplasia
of newborn. International Journal of Padiatrics. 36:143–146.
2009.(In Chinese).
|
|
22.
|
Lee JW and Davis JM: Future applications
of antioxidants in premature infants. Curr Opin Pediatr.
23:161–166. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Welty SE: Is there a role for antioxidant
therapy in bronchopulmonary dysplasia? J Nutr. 131:947S–950S.
2001.PubMed/NCBI
|
|
24.
|
Martindale JL and Holbrook NJ: Cellular
response to oxidative stress: signaling for suicide and survival. J
Cell Physiol. 192:1–15. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Chen R, Shao H, Lin S, Zhang JJ and Xu KQ:
Treatment with Astragalus membranaceus produces
antioxidative effects and attenuates intestinal mucosa injury
induced by intestinal ischemia-reperfusion in rats. Am J Chin Med.
39:879–887. 2011.
|
|
26.
|
Xiping Z, Ke W, Yaping Y, Hongchan Z and
Qihui C: Protective effect and mechanisms of radix astragali
injection on the intestinal mucosa of rats with obstructive
jaundice. Mediators Inflamm. 2010:7571912010.PubMed/NCBI
|
|
27.
|
Li XT, Zhang YK, Kuang HX, et al:
Mitochondrial protection and anti-aging activity of astragalus
polysaccharides and their potential mechanism. Int J Mol Sci.
13:1747–1761. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Li X, He DL, Cheng XF, Zhang LL, Yu LH and
Li JJ: Effects of components isolated from Astragalus
mongholicus on expression of p-selectin in shock wave induced
kidney injury in rabbit model. Zhongguo Zhong Yao Za Zhi.
30:1606–1609. 2005.(In Chinese).
|
|
29.
|
Hall G, Hasday JD and Rogers TB:
Regulating the regulator: NF-kappaB signaling in heart. J Mol Cell
Cardiol. 41:580–591. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Zhong C, Zhou Y and Liu H: Nuclear factor
kappaB and anesthetic preconditioning during myocardial
ischemia-reperfusion. Anesthesiology. 100:540–546. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Van Zee KJ, Fischer E, Hawes AS, et al:
Effects of intravenous IL-8 administration in nonhuman primates. J
Immunol. 148:1746–1752. 1992.PubMed/NCBI
|
|
32.
|
Wu Y, Zhang R, Zhou C, et al: Enhanced
expression of vascular cell adhesion molecule-1 by
corticotrophin-releasing hormone contributes to progression of
atherosclerosis in LDL receptor-deficient mice. Atherosclerosis.
203:360–370. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Hao Y, Qiu QY and Wu J: Effect of
Astragalus polysaccharides in promoting neutrophil-vascular
endothelial cell adhesion and expression of related adhesive
molecules. Zhongguo Zhong Xi Yi Jie He Za Zhi. 24:427–430. 2004.(In
Chinese).
|
|
34.
|
Yuan Y, Sun M and Li KS: Astragalus
mongholicus polysaccharide inhibits lipopolysaccharide-induced
production of TNF-alpha and interleukin-8. World J Gastroenterol.
15:3676–3680. 2009. View Article : Google Scholar : PubMed/NCBI
|