Introduction
Yellow tea is a rare type of tea, which is slowly
gaining recognition in Western countries. It is produced only in
China and has a long history of use (1). Yellow tea usually implies a special
tea processed similarly to green tea; however, it has a slower
drying phase, where the damp tea leaves are allowed to sit and
become yellow. This color is acquired by adding an extra step
during production, called ‘sealed yellowing’, which is a slow
oxidation process of tea polyphenols, including catechin (2). This unique step causes the tea to
mellow and become bright yellow in color, without the grassy taste
of green tea.
Antioxidants are substances that protect cells from
damage caused by free radicals. Free radicals are molecules that
have lost an electron and are therefore unstable (3). These free radicals take electrons
from other molecules in order to stabilize themselves, ultimately
creating new free radicals in the process. The removal of electrons
may cause damage to DNA and lead to the possible development of
inflammation (4).
Gastric injury is an injury to the stomach. It may
be blunt or penetrating and may involve damage to the stomach
organs. Ethanol promotes the rapid formation of injuries in the
stomach, which occurs mainly due to an inflammatory reaction
(5). Ethanol-induced gastric
injury is characterized by epithelial cellular loss, mucosal edema
and subepithelial hemorrhage (6).
Cytokines, including interleukin (IL)-6 and tumor necrosis factor
(TNF)-α, are small proteins that are produced and released from a
number of cells under physiological and pathological conditions
(7). IL-6 is increasingly
recognized as an almost ubiquitous participant in numerous types of
inflammatory processes (8). TNF-α
is a macrophage-derived cytokine with chemotactic potency, which
has been implicated in the acute phase reaction under various
inflammatory conditions (9).
In the current study, the antioxidant activity of
yellow tea and its preventive effect on gastric injury were
examined. 1,1-Diphenyl-2-picryhydrazyl (DPPH) free radical- and
hydroxyl (OH) radical-scavenging assays were conducted to evaluate
the antioxidant activity of yellow tea. Additionally, measurements
of the levels of the inflammation-related cytokines IL-6 and TNF-α
were used to determine the preventive effects of yellow tea on
HCl/ethanol-induced gastric injury in Sprague-Dawley rats.
Materials and methods
Preparations of yellow tea
Yellow tea was purchased from Sichuan Mengding
Huangcha Tea Industry Co., Ltd., China. The yellow tea was stored
at −80°C and freeze-dried to produce a powder. A twenty-fold volume
of boiling water was added to the powdered sample and extraction
was conducted twice. The aqueous extract was evaporated using a
rotary evaporator (Eyela N-1100, Tokyo, Japan), concentrated and
then dissolved in dimethylsulfoxide (DMSO; Amresco, Solon, OH, USA)
to adjust to the stock concentration (20%, w/v).
DPPH free radical assay
The DPPH free radical-scavenging activity was
determined according to the method of Blois (10). Four milliliters of 100, 200 and 500
μg/ml concentrations of the sample solution were added to
1.0 ml DPPH methanol solution (1.5×10−4 M). After
storing at room temperature for 30 mins, the absorbance of the
solution was determined at 520 nm using a spectrophotometer and the
remaining DPPH was quantified. The results expressed are the means
of triplicate values (11).
OH radical assay
The OH radical-scavenging activity was assessed as
described by Banerijee et al (12). The reaction system (1.4 ml),
contained yellow tea extract, deoxyribose (6 mM, 0.2 ml), 0.2 ml
sodium phosphate buffer solution (20 mM, pH 7.4), 0.2 ml anhydrous
iron chloride (FeCl3; 400 μM), 0.2 ml
FeSO4-ethylenediaminetetraacetic acid (EDTA; 400
μM), 0.2 ml H2O2 (3 mM), 0.2 ml
ascorbic acid (400 μM) and 0.2 ml yellow tea extract
solution (50, 100 and 200 μg/ml). After incubation at 37°C
in a water bath for 60 min, the reaction was stopped by adding 1 ml
trichloroacetic acid and 1 ml 2-thiobarbituric acid in a 1.4-ml
reaction system. The solution was boiled for 20–25 min at 90°C in a
water bath. The absorbance was measured at 532 nm. All analyses
were run in triplicate and averaged.
Animals
Male Sprague-Dawley rats (n=50, 7-weeks-old) were
purchased from the Experimental Animal Center of Chongqing Medical
University (Chongqing, China). The rats were maintained in a
temperature-controlled facility (temperature, 25±2°C; relative
humidity, 50±5%) with a 12-h light/dark cycle and free access to a
standard rat chow diet and water.
Gastric injury assay
The experimental design was as follows: the normal
and control groups received 14-day repeated oral administration of
distilled water and a single dose of the vehicle (2 ml/kg b.w.
olive oil, p.o.); the sample groups received 14-day repeated oral
administration of 250, 500 or 1,000 mg/kg yellow tea extract. Then,
the control and sample group rats were administered 1 ml
HCl/ethanol (60% in 150 mM HCl) p.o. through esophageal intubation
and were then sacrificed 1 h later under deep ether anesthesia. The
stomachs were removed, inflated by injecting 10 ml 1% formalin for
10 min to fix the tissue walls and opened along the greater
curvature. The area (mm2) of hemorrhagic lesions
developed in the stomach was measured using a digital camera (D550;
Canon, Tokyo, Japan) with a square grid and the images were
analyzed by ImageJ software (National Institutes of Health,
Bethesda, MD, USA). These experiments followed a protocol approved
by the Animal Ethics Committee of Chongqing Medical University
(Chongqing, China).
Analysis of inflammation-related
cytokines in serum by enzyme-linked immunosorbent assay
(ELISA)
For the serum cytokine assay, blood from the
inferior vena cava was collected in a tube and centrifuged (1,370 ×
g for 10 min at 4°C). The serum was aspirated and assayed as
follows: Concentrations of the inflammatory-related cytokines IL-6
and TNF-α in serum were measured by ELISA according to the kit
manufacturer’s instructions (BioLegend, San Diego, CA, USA).
Briefly, after the biotinylated antibody reagent was added to
96-well plates, supernatants of homogenized serum were incubated at
37°C in CO2 for 2 h. After washing with
phosphate-buffered saline (PBS), horseradish peroxidase
(HRP)-conjugated streptavidin peroxidase solution was added and the
plate was incubated for 30 min at room temperature. The absorbance
was then measured at 450 nm using a microplate reader (13).
Statistical analysis
Data are presented as mean ± standard deviation
(SD). Differences between the mean values for individual groups
were assessed with one-way analysis of variance (ANOVA) with
Duncan’s multiple range test. P<0.05 was considered to indicate
a statistically significant difference. SAS version 9.1 (SAS
Institute Inc., Cary, NC, USA) was used for statistical
analyses.
Results
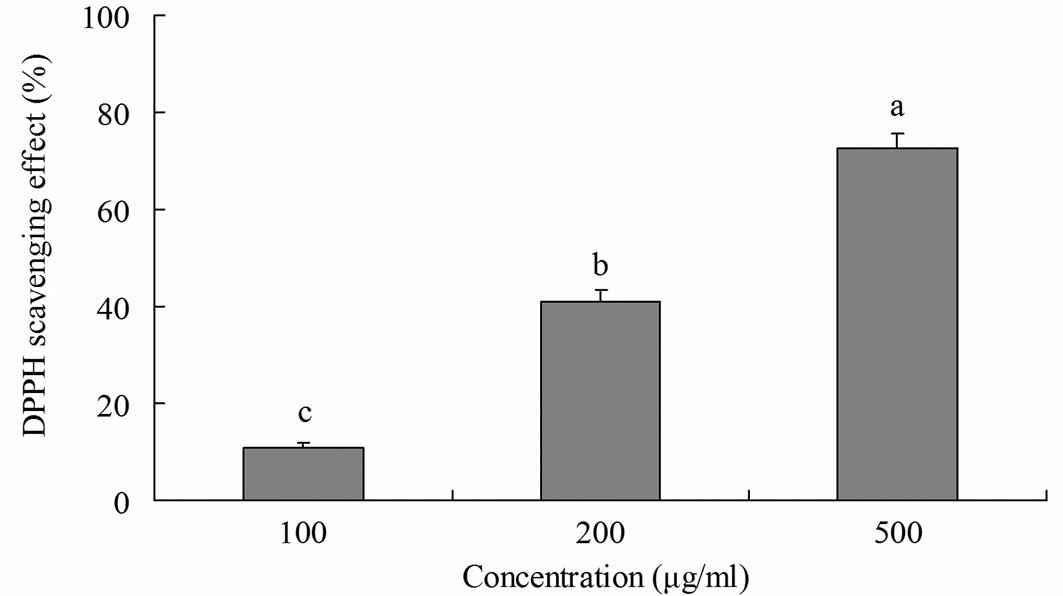
DPPH radical-scavenging activity
The radical-scavenging activity of yellow tea was
investigated using DPPH radicals (Fig.
1). Yellow tea showed scavenging activity on DPPH radicals at
various concentrations. At 100, 200 and 500 μg/ml, the
radical-scavenging activities were 10.8, 41.0 and 72.6%,
respectively. This indicates that the radical-scavenging activity
of yellow tea increased as the concentration of its extract
increased.
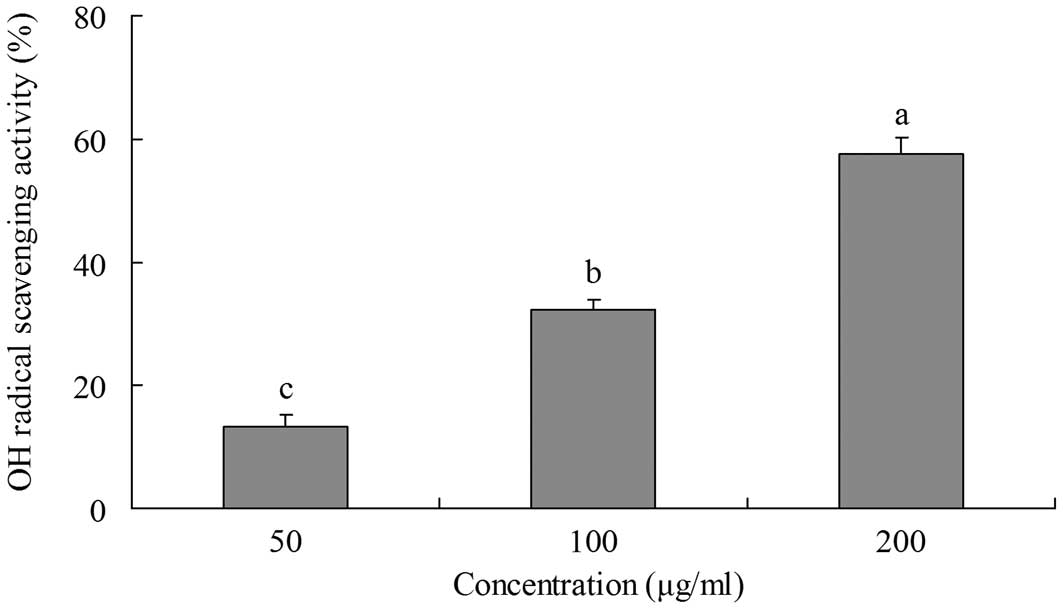
OH radical-scavenging activity
The ability of yellow tea to scavange OH radicals
was evaluated by measuring the deoxyribose damage induced by the
Fe3+/ascorbate/EDTA/H2O2 system
using the thiobarbituric acid (TBA) method (14). Deoxyribose degrades into fragments
that react with TBA upon heating at a low pH to form a pink color.
The inhibitory effects of yellow tea on deoxyribose damage are
shown in Fig. 2. The inhibitory
rate of a 200 μg/ml extract was 57.6%, which is higher than
those of 100 μg/ml (32.2%) and 50 μg/ml (13.2%)
extracts.
Inflammation-related cytokine levels in
serum
The IL-6 level of normal rats was 52.1±4.5 pg/ml;
however, that of control rats was significantly increased to
271.2±10.2 pg/ml following the induction of gastric injury. The
levels of IL-6 in rats fed with 250, 500 and 1,000 mg/kg yellow tea
were 250.7±11.2, 206.3±9.8 and 144.3±12.2 pg/ml, respectively
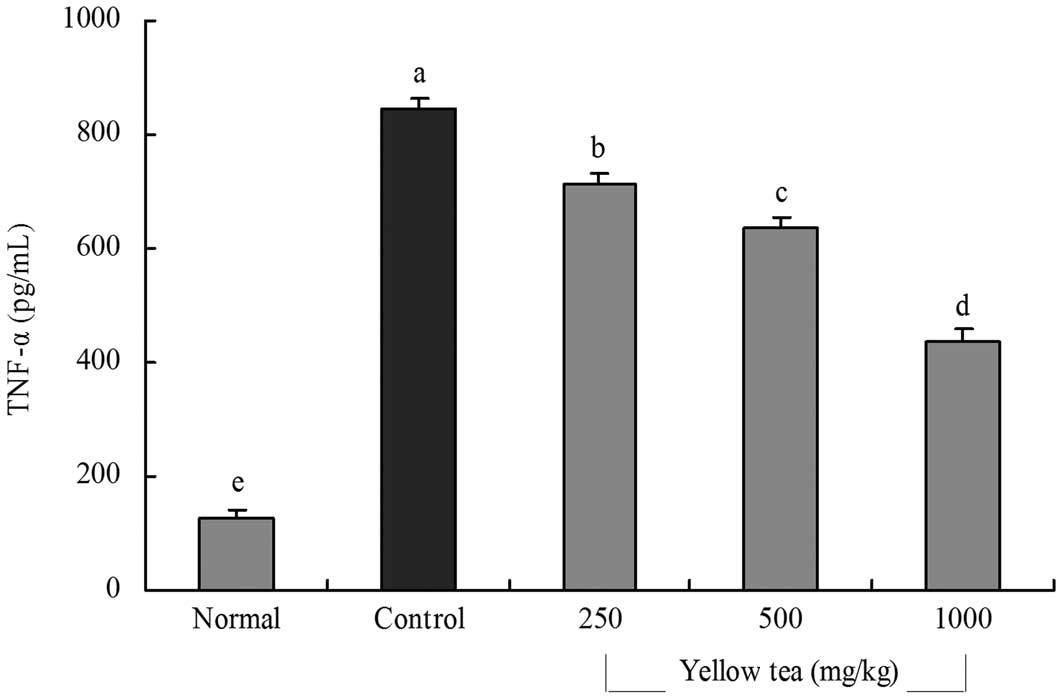
(Fig. 3). The TNF-α levels in
normal, control and 250, 500 and 1,000 mg/kg yellow tea-treated
rats were 127.5±14.6, 843.3±22.2, 715.3±18.5, 635.4±20.6 and
438.4±19.4 pg/ml, respectively (Fig.
4). The serum IL-6 and TNF-α levels in the rats in the yellow
tea-treated groups were significantly lower than those in the
control group.
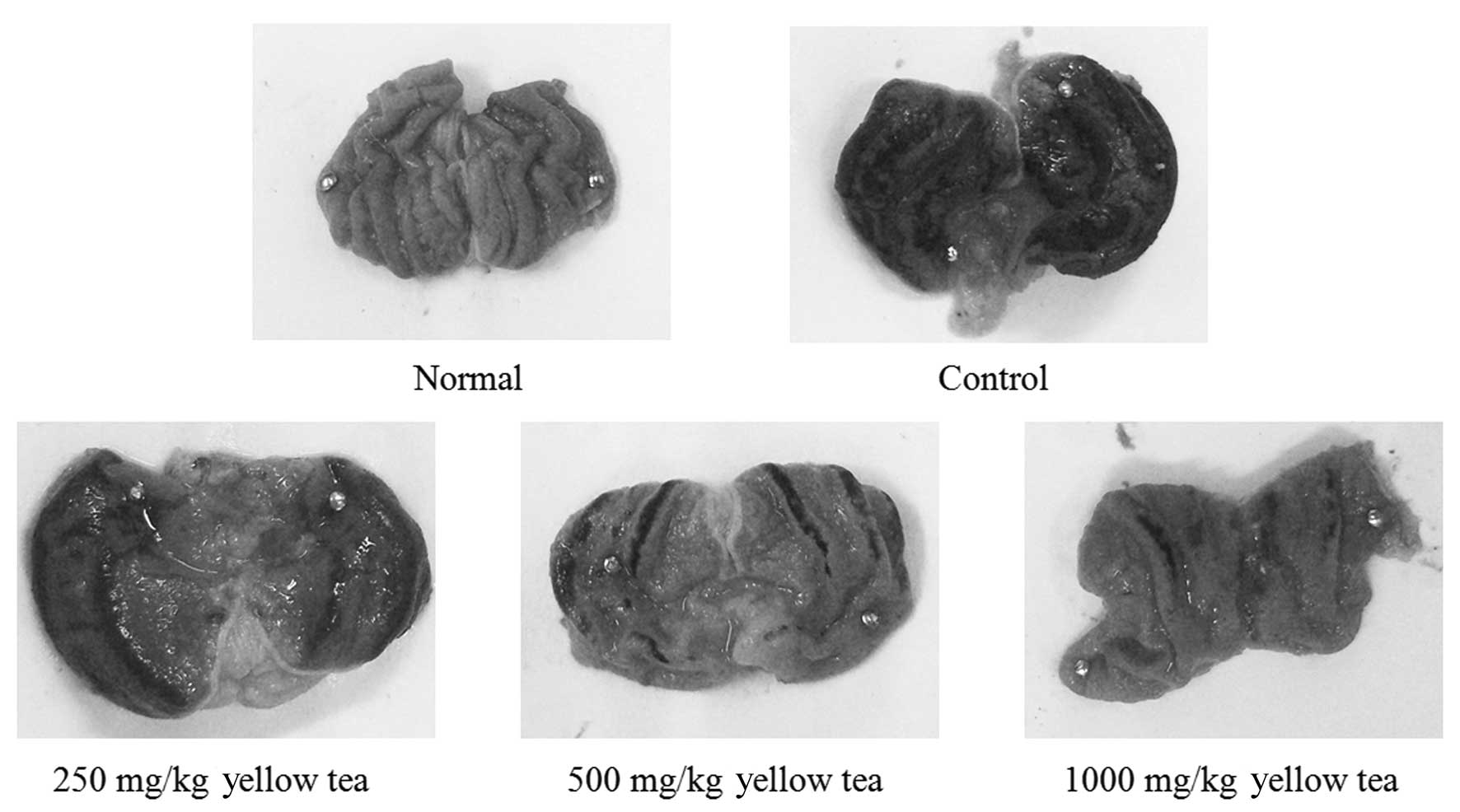
Gastric injury levels
The administration of yellow tea to rats prior to
the induction of gastritis led to reduced gastric injury. The rats
of the control group demonstrated a gastric injury area of 14.2±3.4
mm2. Treatment with 250 and 500 mg/kg yellow tea
resulted in gastric injury inhibition rates of 11.3% (gastric
injury area, 12.6±2.2 mm2) and 56.3% (gastric injury
area, 6.2±1.6 mm2), respectively, while 1,000 mg/kg
yellow tea (inhibition rate, 74.6%; gastric injury area, 3.6±1.1
mm2) demonstrated the best gastritis preventive effect
(Table I and Fig. 5). The results suggest that yellow
tea has a strong preventive effect on gastric injury.
 | Table I.Preventive effect of yellow tea
treatment against HCl/ethanol-induced gastric injury. |
Table I.
Preventive effect of yellow tea
treatment against HCl/ethanol-induced gastric injury.
| Gastric injury data
|
|---|
| Group | Gastric injury
(mm2) | Inhibition rate
(%) |
|---|
| Normal | 0.0±0.0c | 100.0 |
| Control | 14.2±3.4a | 0.0 |
| Yellow tea
(mg/kg) | | |
| 250 | 12.6±2.2c | 11.3 |
| 500 | 6.2±1.6c | 56.3 |
| 1000 | 3.6±1.1c | 74.6 |
Discussion
Although yellow tea is a traditional drink, little
scientific data on its effects are available. Yellow tea is a type
of fermented tea. Since a large number of digestive enzymes are
generated during its smothering process, a slow oxidation process,
yellow tea is beneficial for the spleen and stomach. A previous
study demonstrated that yellow tea, which is rich in tea
polyphenols, polysaccharides, vitamins and amino acids, has special
effects for preventing and curing esophageal cancer (2).
The DPPH assay is based on the capacity of a
substance to scavenge stable radicals. It has been widely used to
test the ability of compounds or plant extracts to act as free
radical-scavengers or hydrogen donors (11). DPPH is a stable free radical
(purple in color) which may accept an electron or hydrogen radical
to become a stable yellow diamagnetic molecule. It is widely used
to predict the potential antioxidative capability of foods and
plant extracts in vitro. The highly reactive OH radical
causes oxidative damage to DNA, proteins and lipids, which
contribute to inflammation, mutagenesis and cytotoxicity (15).
The serum levels of cytokines, including IL-6,
TNF-α, IL-1β and interferon (IFN)-γ, in patients with inflammatory
diseases are higher compared with those in healthy individuals
(16). Thus, lower levels of IL-6
and TNF-α are indicative of anti-inflammatory effects and yellow
tea demonstrates a good protective effect against gastric damage.
Hepatocytes bear a variety of cytokine receptors. IL-6 is an
interleukin that acts as a pro-inflammatory and anti-inflammatory
cytokine. In humans, it is encoded by the IL-6 gene (17). IL-6 is secreted by T cells and
macrophages to stimulate the immune response, particularly in the
process of tissue damage leading to inflammation. IL-6 also plays a
role in fighting infection (18).
TNF-α is a cytokine involved in systemic inflammation and is a
member of a group of cytokines that stimulate the acute phase
reaction. The primary role of TNF-α is in the regulation of immune
cells. TNF, as an endogenous pyrogen, is able to induce fever,
induce apoptotic cell death, sepsis (through IL-1 and IL-6
production), cachexia and inflammation, as well as inhibit
tumorigenesis and viral replication (19). The inflammatory cytokines IL-6 and
TNF-α play pathogenic roles in diseases of the stomach (20). Although systemic IL-6 levels are
elevated following traumatic hemorrhage, hepatocellular function is
impaired and gastric injury occurs (21). TNF-α is also a key mediator in a
number of experimental models of stomach injury (22).
The current study demonstrates that yellow tea has
antioxidant activity and is effective in the prevention of
HCl/ethanol-induced gastric injury in Spraque-Dawley rats. Our
results demonstrate that the protective effects of yellow tea may
be due to its antioxidant activity and reductions in the levels of
pro-inflammatory cytokines, including IL-6 and TNF-α. The
appearance of the stomach also indicated that yellow tea is able to
prevent HCl/ethanol-induced gastric injury. These results suggest
that yellow tea has in vitro anti-oxidant effects and is
potentially useful in the treatment or prevention of
chemical-induced gastric injury in vivo.
Acknowledgements
This study was supported by the
Natural Science Foundation Project of CQ CSTC (No.
CSTC2012jjA80002) and Supported by the Science and Technology
Research Project of Chongqing Municipal Education Commission (No.
KJ121504).
References
|
1.
|
Zhou JR, Ni DJ, Chen YQ, Zhan XP and Yuan
FT: Study on quality variation during the yellow tea processing.
Hubei Nong Ye Ke Xue. 43(1): 93–95. 2004.(In Chinese).
|
|
2.
|
Zhao X: In vitro anticancer effect of
yellow tea in HT-29 human colon cancer cells. Journal of Beijing
Union University (Natural Sciences). 23(3): 11–13. 2009.(In
Chinese).
|
|
3.
|
Valko M, Rhodes CJ, Moncol J, Izakovic M
and Mazur M: Free radicals, metals and antioxidants in oxidative
stress-induced cancer. Chem Biol Interact. 160:1–40. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Collins AR: Oxidative DNA damage,
antioxidants, and cancer. Bioessays. 21:238–246. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Szabo S, Trier JS, Brown A and Schnoor J:
Early vascular injury and increased vascular permeability in
gastric mucosal injury caused by ethanol in the rat.
Gastroenterology. 88:228–236. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Medeiros JV, Gadelha GG, Lima SJ, Garcia
JA, Soares PM, Santos AA, Brito GA, Ribeiro RA and Souza MH: Role
of the NO/cGMP/KATP pathway in the protective effects of sildenafil
against ethanol-induced gastric damage in rats. Br J Pharmacol.
153:721–727. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Ramadori G and Armbrust T: Cytokines in
the liver. Eur J Gastroenterol Hepatol. 13:777–784. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
McCurry KR, Campbell DA Jr, Scales WE,
Warren JS and Remick DG: Tumor necrosis factor, interleukin 6, and
the acute phase response following hepatic ischemia/reperfusion. J
Surg Res. 55:49–54. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Ming WJ, Bersani L and Mantovani A: Tumor
necrosis factor is chemotactic for monocytes and polymorphonuclear
leukocytes. J Immunol. 138:1469–1472. 1987.PubMed/NCBI
|
|
10.
|
Blois MS: Antioxidant determinations by
the use of a stable free radical. Nature. 181:1199–1200. 1958.
View Article : Google Scholar
|
|
11.
|
Kang HS, Chung HY, Jung JH, Kang SS and
Choi JS: Antioxidant effect of Salvia miltiorrhiza. Arch
Pharm Res. 20:496–500. 1997. View Article : Google Scholar
|
|
12.
|
Banerijee A, Dasgupta N and Bratati De: In
vitro study of antioxidant activity of Syzygium cumini
fruit. Food chem. 90:727–733. 2005. View Article : Google Scholar
|
|
13.
|
Park HS, Park JY and Yu R: Relationship of
obesity and visceral adiposity with serum concentrations of CRP,
TNF-α and IL-6. Diabetes Res Clin Pract. 69:29–35. 2005.
|
|
14.
|
Gutteridge JM, Rowley DA, Griffiths E and
Halliwell B: Low-molecular-weight iron complexes and oxygen radical
reactions in idiopathic haemochromatosis. Clin Sci (Lond).
68:463–467. 1985.PubMed/NCBI
|
|
15.
|
Uauy R, Hoffman DR, Peirano P, Birch DG
and Birch EE: Essential fatty acid in visual and brain development.
Lipids. 36:885–895. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Gratacós J, Collado A, Filella X, Sanmartí
R, Cañete J, Llena J, Molina R, Ballesta A and Muñoz-Gómez J: Serum
cytokines (IL-6, TNF-α, IL-1β and IFN-γ) in ankylosing spondylitis:
a close correlation between serum IL-6 and disease activity and
severity. Rheumatology. 33:927–931. 1994.
|
|
17.
|
Ferguson-Smith AC, Chen YF, Newman MS, May
LT, Sehgal PB and Ruddle FH: Regional localization of the
interferon-beta 2/B-cell stimulatory factor 2/hepatocyte
stimulating factor gene to human chromosome 7p15–p21. Genomics.
2:203–208. 1988.PubMed/NCBI
|
|
18.
|
van der Poll T, Keogh CV, Guirao X,
Buurman WA, Kopf M and Lowry SF: Interleukin-6 gene-deficient mice
show impaired defense against pneumococcal pneumonia. J Infect Dis.
176:439–444. 1997.
|
|
19.
|
Gosselin D and Rivest S: Role of IL-1 and
TNF in the brain: twenty years of progress on a Dr. Jekyll/Mr. Hyde
duality of the innate immune system. Brain Behav Immun. 21:281–289.
2007.PubMed/NCBI
|
|
20.
|
Abdollahi H, Shams S, Zahedi MJ, Moghadam
SD, Hayatbakhsh MM and Jafarzadeh A: IL-10, TNF-α and IFN-γ levels
in serum and stomach mucosa of Helicobacter pylori-infected
patients. Iran J Allergy Asthma Immunol. 10:267–271. 2011.
|
|
21.
|
Gislason H, Røkke O and Svanes K: Release
of cytokines associated with gastric mucosal injury. Eur Surg Res.
28:278–286. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Kuzuhara T, Suganuma M, Oka K and Fujiki
H: DNA-binding activity of TNF-inducing protein from
Helicobacter pylori. Biochem Biophys Res Commun.
362:805–810. 2007. View Article : Google Scholar : PubMed/NCBI
|