Introduction
Budd-Chiari syndrome (BCS) is a series of disorders
defined as obstruction of the hepatic veins and/or the proximal
inferior vena cava (IVC). In western countries, BCS usually results
from hypercoagulability (including myeloproliferative disorders,
oral contraceptives and pregnancy)-induced thrombosis in one to
three hepatic veins, or even in the IVC, which is characterized by
an acute onset (1). It has been
demonstrated that these patients may be treated with
anticoagulation and thrombolytic (streptokinase) therapies to
dissolve the thrombi in the hepatic veins (2,3).
However, in eastern countries, membranous obstruction of the IVC
and/or the hepatic veins is the most frequently occurring
underlying cause of BCS (4), and
this is most likely induced by organized thrombi or congenital
factors. In eastern countries, BCS is a chronic condition and
patients are treated with balloon dilatation or stent placement
(5). In addition, a transjugular
intrahepatic portosystemic shunt (TIPS) or alternative surgical
treatment (including shunting procedures and liver transplantation)
may be performed in patients with diffuse hepatic venous occlusion
(6,7).
The incidence of BCS complicated by IVC thrombosis
is 5% (8); however, BCS
complicated by hepatic vein thrombosis, which classically manifests
as acute or chronic episodes of ascites and liver failure, is
relatively rare and has a poor prognosis. It has been demonstrated
that, for simple hepatic vein membranous or segmental occlusion in
BCS without thrombosis, balloon dilatation or stenting treatment
may result in a satisfactory therapeutic efficacy (9,10).
Anticoagulant and systemic thrombolytic therapy may be conducted
for hepatic vein thrombosis in BCS without membranous or segmental
occlusion (2,3). In contrast with the previously
mentioned lesions, membranous or segmental occlusion of the hepatic
vein and hepatic vein thrombosis co-exist in patients with BCS in
which the hepatic vein obstruction is complicated by thrombosis.
Therefore, there is no blood flow in the hepatic veins, due to
hepatic vein obstruction and thrombosis, which presents a challenge
with regard to the use of systemic thrombolysis to dissolve the
clots in the hepatic veins. According to previous studies, a TIPS
and liver transplantation are able to successfully treat BCS
complicated by hepatic vein thrombosis (11,12).
However, to date, there have been relatively few reports regarding
the application of catheter-directed local thrombolysis combined
with balloon dilatation or stent placement in the hepatic veins in
the treatment of hepatic vein obstruction in BCS complicated by
thrombosis. From February 2006 to April 2012, we treated 14
patients with BCS complicated by hepatic vein thrombosis by
performing a catheter-directed local injection of urokinase into
the hepatic veins, combined with balloon dilatation or stent
placement. Certain patients underwent a predilatation of the
occluded hepatic veins prior to the catheter-directed thrombolysis.
The aim of the study was to assess the efficacy and safety of this
method in the treatment of BSC-hepatic vein obstruction complicated
by thrombosis.
Materials and methods
Clinical information
A total of 14 patients, including six males and
eight females, with an average age of 34±10.3 years (range, 15–55
years), were recruited for this study. The course of the disease in
the patients ranged between ten days and eight years. With regard
to the clinical manifestations, there were 14 cases of abdominal
distension and stubborn ascites; one case of upper gastrointestinal
hemorrhage; five cases of varicose abdominal veins; two cases of
leg swelling; one case of varicose veins in the lower limbs and one
case of pigmentation and ulceration in the lower limbs. All the
cases were diagnosed as BCS complicated by hepatic vein thrombosis,
as indicated by color Doppler ultrasound, computed tomography
angiography (CTA), magnetic resonance angiography (MRA) or digital
subtraction angiography (DSA). Among the 14 cases, there were eight
and six cases of acute and subacute thrombosis, respectively
(Table I). One patient (case 1)
had undergone an IVC stent placement seven years previously and
another patient had received a mesoatrial shunt four years
previously. This study was conducted in accordance with the
Declaration of Helsinki and with approval from the Ethics Committee
of the Affiliated Hospital of Xuzhou Medical College (Xuzhou,
China). Written informed consent was obtained from all
participants.
 | Table I.General patient data. |
Table I.
General patient data.
| Case no. | Gender (M/F) | Age (years) | HV with proximal
occlusion and combined thrombosis | HV with diffuse
occlusion | Thrombosis
property | IVC status |
|---|
| 1 | F | 37 | RHV, MHV, LHV,
AHV | None | Acute | Stenosis |
| 2 | F | 42 | RHV, LHV | MHV | Acute | Clear |
| 3 | F | 55 | RHV, MHV, LHV | None | Acute | Clear |
| 4 | M | 42 | RHV, MHV, LHV | None | Subacute | Stenosis |
| 5 | F | 28 | RHV | MHV, LHV | Acute | Clear |
| 6 | F | 42 | RHV, MHV, LHV | None | Subacute | Clear |
| 7 | M | 27 | MHV, LHV | RHV | Acute | Clear |
| 8 | F | 25 | RHV, LHV | MHV | Subacute | Clear |
| 9 | M | 34 | RHV, MHV, LHV | None | Acute | Clear |
| 10 | M | 28 | RHV | MHV, LHV | Acute | Stenosis |
| 11 | F | 31 | RHV, MHV, LHV | None | Subacute | Clear |
| 12 | F | 40 | RHV, MHV, LHV | None | Acute | Clear |
| 13 | M | 15 | RHV | MHV, LHV | Subacute | Stenosis |
| 14 | M | 23 | RHV, MHV | LHV | Subacute | Clear |
IVC and hepatic vein angiography
Following the puncture of the femoral vein and/or
the internal jugular vein, the IVC angiography was performed by
inserting a 5F pigtail catheter to examine whether the IVC was
clear and whether thrombosis was apparent. Subsequent to this, a 5F
angiographic catheter (Tempo®-4; Cordis Corporation,
Miami, FL, USA) was inserted into the hepatic vein or the accessory
hepatic vein to replace the pigtail catheter, using the soft end of
a smooth guide wire. If the insertion was not successful, then a
steel needle with an arc-shaped head-end was utilized to puncture
and open the occluded hepatic vein, followed by the insertion of
the 5F angiographic catheter into the hepatic vein or the accessory
hepatic vein to perform the angiography. Through the angiography,
it was possible to determine the size of the thrombus, the
collateral circulation status within the hepatic vein or the
accessory hepatic vein and the occlusion status at the proximal end
of the vein.
Catheter-directed urokinase
thrombolysis
In all the cases, a 5F pigtail or infusion catheter
(cat. no. N5.0-35-100-P-16S-0-RIS-8.0/16.0; Cook Medical, Inc.,
Bloomington, IN, USA) was inserted into the hepatic vein via the
jugular vein. A balloon with a diameter of 8 mm was used to
predilate the occluded hepatic vein, prior to the thrombolysis by
the indwelling catheter, in the six patients with subacute thrombus
in the hepatic vein. In one case, a 5F infusion catheter was
inserted into the accessory hepatic vein through the femoral vein.
A total of 100,000 units urokinase in 20 ml saline was pulse-spray
injected through the catheter once every 3–6 h. During the
thrombolytic therapy, 5,000 units low-molecular-weight heparin
sodium was injected subcutaneously every 12 h. Based on the
angiography reviews performed every two days, the position of the
catheter was adjusted according to the thrombolytic status to
ensure that the sidehole region of the catheter was always in
contact with the thrombus. The criteria for ending the therapy
were: i) the thrombus was completely dissolved; ii) the thrombus
was consistent in two consecutive reviews; and iii) internal
bleeding was observed or other complications occurred.
Balloon dilation and/or stent
placement
Following the thrombolytic therapy, balloons with a
diameter ranging between 10 and 14 mm were inserted into the
hepatic or accessory hepatic veins of the patients. Five patients
were fitted with a stent measuring 10–14 mm in diameter (Bard
International, Inc., Murray Hill, NJ, USA) due to the balloon
dilation resulting in an unsatisfactory reduction of the hepatic
vein pressure or a retraction rate of the vein dilation of >50%.
In one patient with a thrombus in the accessory hepatic vein, the
balloon dilation and stent placement were performed under the
protection of an Antheor™ temporary vena cava filter (Boston
Scientific, Natick, MA, USA). A balloon with a diameter of 26 mm
was used to dilate the narrow section of the IVC in two
patients.
Pressure monitoring
A piezometric tube was utilized to monitor the
pressure of the hepatic vein, IVC and right atrium prior to and
following the interventional therapy.
Follow-up studies
The hepatic vein and IVC angiography were performed
immediately subsequent to the operation. An ultrasound examination
of the liver was conducted 1 week and 1, 3, 6 and 12 months
following the operation, as well as each year subsequently, in
order to evaluate the condition of the hepatic vein and IVC. The
patients were prescribed oral warfarin for anticoagulation from the
second day following operation for 24 months and the dose of
warfarin was adjusted to maintain the international normalized
ratio (INR) between two and three. In addition, a second
angioplasty was performed in the patients with hepatic vein and IVC
restenosis.
Statistical analysis
The paired Student’s t-test was utilized to compare
the pressure gradient between the hepatic vein and the right atrium
prior to and following the interventional therapy. P<0.05 was
considered to indicate a statistically significant difference.
Results
Treatment results
There were 13 cases in which the treatment had a
100% success rate, with the time required for thrombolysis ranging
from three to eight days (average, 5.2±1.7 days). The thrombi were
completely and partially dissolved in nine and four cases,
respectively; one hepatic vein was recanalized in 12 patients
(right hepatic vein, seven cases; left hepatic vein, three cases;
middle hepatic vein, one case and accessory hepatic vein, one case)
(Fig. 1, case 1 and Fig. 2A–G, case 13), while two hepatic
veins (right and left) were recanalized in one patient. The
pressure gradient between the hepatic vein and the right atrium
prior to interventional therapy was 18–41 cmH2O
(average, 31.1±7.3 cmH2O; 1 cmH2O= 0.098kPa),
which decreased to 3–11 cmH2O (average 6.8±2.7
cmH2O; t=15.1, P<0.01) following the procedure. There
were no serious complications, such as bleeding or pulmonary
embolism. The IVC was patent following balloon dilatation in two
patients with IVC stenosis. Balloon dilatation was not performed in
two patients with enlargement of the liver caudate lobe inducing
IVC stenosis. The treatment was unsuccessful in one case, where a
fatal liver tissue fracture-induced bleeding occurred during the
catheter-directed thrombolysis treatment.
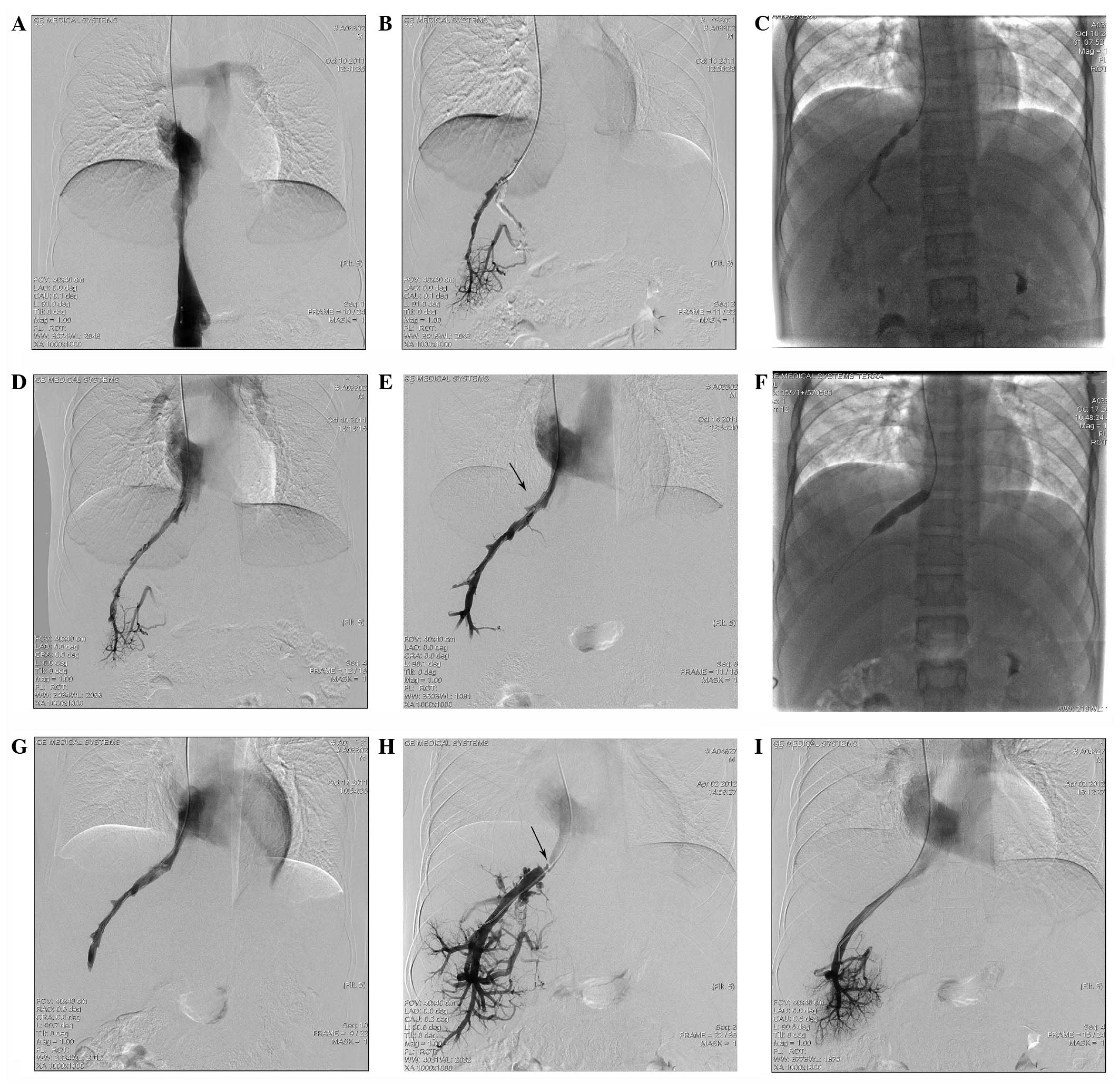
 | Figure 1.Case 1: Female, 37 years old, with
Budd-Chiari syndrome combined with acute thrombosis in the
accessory hepatic vein (AHV). Stent placement had been performed in
the inferior vena cava (IVC) seven years previously by another
hospital. (A) Stent region shown by IVC angiography: The blood
stream was unobstructed within the stent, while stenosis occurred
at the distal end of the vessel (indicated by the arrow). (B) In
the AHV angiography performed through the femoral vein, a
perforated membranous occlusion was shown at the opening of AHV,
and a filling defect was generally observed in the vessel cavity.
(C) Four days following the indwelling catheter thrombolytic
therapy, the thrombus had predominantly dissolved, and only a small
quantity of the embolus had shifted to the opening of AHV and
blocked it (indicated by the arrow). (D) Four days following the
thrombolytic therapy, a temporary filter was inserted through the
jugular vein and a balloon catheter with a diameter of 10 mm was
inserted through the femoral vein, by which the opening of the AHV
was dilated. (E) Following the balloon dilation, the opening of the
AHV remained narrow (indicated by the arrow). (F) Following the
placement of a 12–40 mm stent, the vein cavity became
unobstructed. |
Follow-up results
A total of 13 patients were followed up for 2–60
months (average, 24.8±19.6 months), among which one patient
presented with hepatic vein restenosis six months subsequent to the
interventional procedure. The patient was successfully treated with
a second balloon dilatation (Fig. 2H
and I, case 13). There was no recurrence of restenosis or
thrombosis in the remaining 12 patients (Table II).
 | Table II.Patient status following
treatment. |
Table II.
Patient status following
treatment.
| Case no. | Thrombolysis period
(days) | Urokinase dosage
(units) | HV intervention
| Treated HV | Follow-up
(months) | Outcome |
|---|
| Balloon (mm) | Stent (mm) |
|---|
| 1 | 7 |
280×104 | 10–40 | 12–40 | AHV | 60 | No recurrence |
| 2 | 7 |
210×104 | 15–40 | 14–40 | RHV | 55 | No recurrence |
| 3 | 3 |
180×104 | 10–40 | - | LHV | 48 | No recurrence |
| 4 | 5 |
200×104 | 12–40 | - | RHV, LHV | 18 | No recurrence |
| 5 | 4 |
160×104 | 14–40 | 12–40 | RHV | 38 | No recurrence |
| 6 | 8 |
320×104 | 12–40 | - | LHV | 12 | No recurrence |
| 7 | 4 |
160×104 | 12–40 | - | RHV | 9 | No recurrence |
| 8 | 6 |
260×104 | 14–40 | 16–40 | MHV | 6 | No recurrence |
| 9 | 5 |
200×104 | 12–40 | - | LHV | 25 | No recurrence |
| 10 | 3 |
120×104 | 14–40 | 14–40 | RHV | 26 | No recurrence |
| 11 | 5 |
180×104 | 16–40 | - | RHV | 17 | No recurrence |
| 12 | 3 |
150×104 | - | - | - | - | Fatality |
| 13 | 7 |
280×104 | 14–40 | - | RHV | 6 | Restenosis, second
PTA |
| 14 | 6 |
240×104 | 16–40 | - | RHV | 2 | No recurrence |
Discussion
BCS complicated by thrombosis is relatively rare and
the corresponding treatment includes systemic thrombolysis,
agitation thrombolysis, TIPS and liver transplantation (10–15).
In the present study, hepatic vein thrombosis was present in 14
patients with proximal hepatic vein obstruction. Catheter-directed
thrombolysis was performed to remove the thrombi, followed by
balloon dilatation and/or stent placement to recanalize the
obstructed hepatic veins. The treatment was considered to have
achieved technical and clinical success in 13 of the 14 patients.
The hepatic veins were patent without serious complications, such
as bleeding or pulmonary embolism, following the first and second
interventional therapies.
The pathogenesis of BCS complicated by hepatic vein
thrombosis has not yet been elucidated. Numerous factors, including
malignancy, myeloproliferative disease, rheumatological disorders,
hypercoagulability, infection and ulcerative colitis are potential
etiological factors of thrombosis. However, the causes remain
unidentified in 16–35% cases (1–3,16).
Kuo et al (17) studied
three cases of BCS complicated by hepatic vein thrombosis, in which
the hepatic veins were recanalized without stenosis or occlusion
following the removal of the thrombus by thrombolysis. In the
present study, a residual membranous or segmental hepatic vein
obstruction was apparent in 13 patients following the successful
removal of the thrombus by thrombolysis, indicating that the
hepatic vein thrombosis was secondary to hepatic vein obstruction.
This finding differed from that in the study by Kuo et al
(17). Following the obstruction
of the hepatic veins, a number of factors, including slow blood
flow, eddy formation and blood flow reversal, act in combination to
promote thrombosis.
There are various treatments for BCS complicated by
hepatic vein thrombosis. Dacha et al (1) described one case of BCS with hepatic
vein thrombosis, the clots in the hepatic veins were partially
dissolved with anticoagulation therapy. This case of BCS occurred
due to thrombosis in the hepatic vein, without membranous or
segmental occlusion; therefore the patient was different from the
study. Thrombolytic therapy is important in the treatment of BCS
with thrombosis. The drugs for thrombolysis consist of urokinase,
streptokinase and recombinant tissue plasminogen activator (rt-PA).
The methods utilized to deliver these drugs include systemic
administration, injection through the hepatic artery and portal
vein and transcatheter insertion into the hepatic vein (1,17,18).
Sharma et al (18)
performed thrombolytic therapy 12 times in 10 patients with acute
BCS; of these 10 patients, three were treated with the thrombolytic
drugs systemically, one was injected through the hepatic artery,
four received the drugs by a local injection in the hepatic veins
and two were locally treated with the drugs through the portal
veins/TIPS. Among the patients who were administered with the drugs
systemically or through the hepatic artery, the treatment was
considered to be a partial success in only one patient; however,
treatment was successful in five patients who received the
injection locally through a TIPS or through the hepatic veins. In
the present study, local thrombolysis was performed in the hepatic
veins in 13 patients, and the clots were dissolved completely and
partially in nine and four cases, respectively. Similar to the
treatment of deep venous thrombosis in the lower limbs, the
efficacy of thrombolysis was correlated with the time of the
thrombosis (acute or subacute) and the application of a sidehole
catheter for contiguous thrombolysis (19,20).
There are certain advantages to utilizing a sidehole catheter for
contiguous thrombolysis in hepatic veins: i) The presence of side
holes on the catheter increases the contact area of the
thrombolytic drug with the clots; and ii) the thrombolytic drugs
are directly injected into the clots, which enhances the drug
concentration and decreases the drug dose required, thereby
reducing the risk of complications, such as bleeding.
Predilatation is important in the treatment of BCS
complicated by IVC or hepatic vein thrombosis. Ding et al
(21) performed predilatation
(balloon diameter, 12–16 mm) in 13 cases of BCS with IVC
obstruction complicated by thrombosis prior to the thrombolytic
therapy: All the treatments were successful and no complications
were observed. In the present study, predilatation was performed in
the occluded hepatic veins of six patients using a balloon with a
diameter of 8 mm, prior to the catheter-directed thrombolysis.
These six patients were diagnosed with subacute thrombosis through
color Doppler ultrasound. The purpose of predilatation is to
partially recanalize the obstructed hepatic veins and restore the
blood flow in the hepatic veins with thrombosis, which helps to
improve the efficacy of the thrombolytic therapy. One potential
complication of predilatation is pulmonary embolism, caused by the
dislodging of a thrombus. There were no complications, such as
pulmonary embolism, in the present study due to the following
factors: i) Predilatation was only performed in patients with
subacute thrombosis, in which the thrombi were unlikely to be
dislodged due to their adhesion to the walls of the hepatic veins;
ii) following the dilatation of the 8 mm-diameter balloon, an
apparent lumen retraction (>50%) was observed at the proximal
end of the hepatic veins, which made it more difficult for large
clots to travel to the pulmonary arteries; iii) the following
catheter-directed thrombolysis was able to gradually dissolve any
clots in a wide radius around the side holes of the catheter in the
hepatic veins.
In the current study, one patient presented with a
perforated membrane occlusion of the accessory hepatic vein
complicated by acute thrombosis. During the thrombolytic treatment,
the emboli moved to the opening of the accessory hepatic vein, and
therefore the balloon dilatation and stent placement in the
accessory hepatic vein were performed under the protection of a
temporary filter, which avoided pulmonary embolism.
There was one fatality during the catheter-directed
thrombolytic treatment due to liver tissue fracture-induced
bleeding. It was difficult to distinguish the liver parenchyma from
extensive thrombosis in the hepatic vein during the detection of
the hepatic vein by the catheter, and, as a result, the catheter
went too deep and punctured the distal hepatic vein end through to
the liver parenchyma. The catheter was subsequently retained in the
hepatic vein for urokinase thrombolysis, which led to liver
parenchyma fracture and bleeding.
Several methods, including TIPS (11), surgical shunt and liver
transplantation (12) may be
utilized to treat BCS complicated by hepatic vein thrombosis. In
contrast with the previously mentioned studies, the present study
successfully applied local thrombolysis, balloon dilatation and/or
stent placement in 13 patients. This therapy restored the blood
flow to the hepatic veins through thrombolysis and percutaneous
transluminal angioplasty (PTA), which was consistent with the
physiological status in the treatment of BCS.
There were a number of limitations to the present
investigation, including the fact that it was a single center
retrospective study with few patients. Furthermore, the study did
not include a long-term follow-up. To provide more conclusive
results, it may be necessary to increase the number of patients and
perform randomized controlled studies, in addition to observing the
long-term follow-up results of catheter-directed thrombolysis
combined with angioplasty in the treatment of hepatic vein
obstruction in BCS complicated by thrombosis.
In conclusion, catheter-directed thrombolysis was
performed to remove thrombi, followed by balloon dilatation and/or
stent placement to recanalize the hepatic veins, which corresponded
well with the physiological status. Predilatation and multiple
sidehole catheters were able to promote the efficacy of the
thrombolysis. The preliminary results indicated that
catheter-directed thrombolysis combined with angioplasty was an
effective and safe method for the treatment of hepatic vein
obstruction in BCS complicated by thrombosis and that the short- or
mid-term efficacy of the treatment was reliable. However, the
long-term efficacy of the treatment requires further
observation.
Acknowledgements
This study was supported by the ‘333
Project’ of Jiangsu Province (grant no. BRA2011221).
References
|
1.
|
Darwish Murad S, Plessier A,
Hernandez-Guerra M, et al: EN-Vie (European Network for Vascular
Disorders of the Liver): Etiology, management, and outcome of the
Budd-Chiari syndrome. Ann Intern Med. 151:167–175. 2009.PubMed/NCBI
|
|
2.
|
Dacha S, Devidi M and Osmundson E:
Budd-Chiari syndrome in a patient with ulcerative colitis and no
inherited coagulopathy. World J Hepatol. 3:164–169. 2011.PubMed/NCBI
|
|
3.
|
Reza F, Naser DE, Hossein G and Mehrdad Z:
Combination of thrombolytic therapy and angioplastic stent
insertion in a patient with Budd-Chiari syndrome. World J
Gastroenterol. 13:3767–3769. 2007.PubMed/NCBI
|
|
4.
|
Valla DC: Primary Budd-Chiari syndrome. J
Hepatol. 50:195–203. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Cura M, Haskal Z and Lopera J: Diagnostic
and interventional radiology for Budd-Chiari syndrome.
Radiographics. 29:669–681. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Molmenti EP, Segev DL, Arepally A, et al:
The utility of TIPS in the management of Budd-Chiari syndrome. Ann
Surg. 241:978–983. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Mentha G, Giostra E, Majno PE, et al:
Liver transplantation for Budd-Chiari syndrome: A European study on
248 patients from 51 centres. J Hepatol. 44:520–528. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Li T, Zhang WW, Bai W, Zhai S and Pang Z:
Warfarin anticoagulation before angioplasty relieves thrombus
burden in Budd-Chiari syndrome caused by inferior vena cava
anatomic obstruction. J Vasc Surg. 52:1242–1245. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Keshava SN, Moses V and Surendrababu NR:
Cannula-assisted and transabdominal ultrasound-guided hepatic
venous recanalization in Budd Chiari syndrome: a novel technique to
avoid percutaneous transabdominal access. Cardiovasc Intervent
Radiol. 32:1257–1259. 2009. View Article : Google Scholar
|
|
10.
|
Li T, Zhai S, Pang Z, et al: Feasibility
and midterm outcomes of percutaneous transhepatic balloon
angioplasty for symptomatic Budd-Chiari syndrome secondary to
hepatic venous obstruction. J Vasc Surg. 50:1079–1084. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Carnevale FC, Santos AC, Tannuri U and
Cerri GG: Hepatic veins and inferior vena cava thrombosis in a
child treated by transjugular intrahepatic portosystemic shunt.
Cardiovasc Intervent Radiol. 33:627–630. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Iwasaki T, Kawai H, Oseki K, et al:
Japanese case of Budd-Chiari syndrome due to hepatic vein
thrombosis successfully treated with liver transplantation. Hepatol
Res. 42:213–218. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Clark PJ, Slaughter RE and Radford DJ:
Systemic thrombolysis for acute, severe Budd-Chiari syndrome. J
Thromb Thrombolysis. 34:410–415. 2012. View Article : Google Scholar
|
|
14.
|
Soyama A, Eguchi S, Yanaga K, Takatsuki M,
Hidaka M and Kanematsu T: Living donor liver transplantation with
extensive caval thrombectomy for acute-on-chronic Budd-Chiari
syndrome. Surg Today. 41:1026–1028. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Ding PX, Li YD, Han XW and Wu G: Agitation
thrombolysis for fresh iatrogenic IVC thrombosis in patients with
Budd-Chiari syndrome. J Vasc Surg. 52:782–784. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Moug SJ, Craig SR, Roditi G and Horgan PG:
Images of interest. Hepatobiliary and pancreatic: extensive
thrombosis in Budd-Chiari syndrome. J Gastroenterol Hepatol.
20:13022005. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Kuo GP, Brodsky RA and Kim HS:
Catheter-directed thrombolysis and thrombectomy for the Budd-Chiari
syndrome in paroxysmal nocturnal hemoglobinuria in three patients.
J Vasc Interv Radiol. 17:383–387. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Sharma S, Texeira A, Texeira P, Elias E,
Wilde J and Olliff SP: Pharmacological thrombolysis in Budd-Chiari
syndrome: a single centre experience and review of the literature.
J Hepatol. 40:172–180. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Enden T, Haig Y, Kløw NE, et al CaVenT
Study Group: Long-term outcome after additional catheter-directed
thrombolysis versus standard treatment for acute iliofemoral deep
vein thrombosis (the CaVenT study): a randomised controlled trial.
Lancet. 379:31–38. 2012. View Article : Google Scholar
|
|
20.
|
Manninen H, Juutilainen A, Kaukanen E and
Lehto S: Catheter-directed thrombolysis of proximal lower extremity
deep vein thrombosis: a prospective trial with venographic and
clinical follow-up. Eur J Radiol. 81:1197–1202. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Ding PX, Li YD, Han XW, Wu G, Shui SF and
Wang YL: Treatment of Budd-Chiari syndrome with urokinase following
predilation in patients with old inferior vena cava thrombosis.
Radiol Med. 116:56–60. 2011. View Article : Google Scholar : PubMed/NCBI
|