Introduction
Breast cancer is one of the most common malignancies
and it has a serious impact on female health. Morbidity and
mortality are high in female malignant tumors (1). According to the World Health
Organization, ~1.3 million women are diagnosed with breast cancer
each year (2). The incidence rate
of breast cancer ranks first among all malignant tumors in women
and breast cancer mortality is only lower than that of lung cancer
(3,4).
Despite the development of surgical techniques and
meticulously designed chemotherapy regimens, relapse remains almost
inevitable in patients with advanced cases of the disease. Although
there are many chemical therapeutic drugs for the treatment of
breast cancer that are able to kill or inhibit the growth of
tumors, they usually are associated with a number of side-effects.
Therefore, the exploration and development of novel antitumor
chemotherapeutics is critical to the improvement of integrated
treatment plans.
Quercetin is a polyphenolic compound widely
distributed in a number of fruits, vegetables and plants. Previous
studies have indicated that quercetin has promising applications in
cancer therapy (5–7). It has been reported that quercetin is
capable of inhibiting the growth of cancer cells through the
induction of apoptosis in a variety of cancer cell lines. Previous
studies have demonstrated that quercetin is able to inhibit the
proliferation of gastric, esophageal and ovarian cancers (8–10).
However, the effects of quercetin on breast cancer have rarely been
investigated. Therefore, the aim of this study was to investigate
the anticancer effect and mechanism of action of quercetin on
breast cancer.
Materials and methods
Materials
The MCF-7 human breast cancer cell line was provided
by the Tumor Cell Library of the Chinese Academy of Medical
Sciences (Beijing, China). Quercetin (Sigma, St. Louis, MO, USA)
was suspended in dimethylsulfoxide (DMSO) and stored at −20°C.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
and DMSO were purchased from Sigma. Dulbecco’s modified Eagle’s
medium (DMEM), fetal calf serum (FCS) and TRIzol were bought from
Invitrogen Life Technologies (Carlsbad, CA, USA). Hoechst 33258 was
provided by Biyuntian Biotechnology (Haimen, China).
Methods
Cell culture
MCF-7 cells were cultured in 10% DMEM (containing
10% FCS) at 37°C in 5% CO2 and under saturated humidity
conditions. The medium was replaced every two or three days.
Cell viability and proliferation
MCF-7 cells were harvested during the logarithmic
growth phase and digested with trypsin. To study the effects of
quercetin on cell proliferation and viability, MCF-7 cells
(5×103/well) were plated in 96-well plates and incubated
in DMEM supplemented with 10% FCS. After 24 h, cells were washed
once with medium and treated with 0, 2.5, 5, 10, 20 and 40 mg/ml
quercetin in the medium. Control wells contained MCF-7 cells
without quercetin and blank wells contained only culture medium.
Cell proliferation and cell viability were determined after 24 or
48 h of treatment by incubation in DMEM, supplemented with 10% FCS
containing 20 μl MTT (5 mg/ml), for 4 h. The culture
solution was swilled, 150 μl DMSO was added to each well and
the solution was subsequently shaken to completely dissolve the
blue-purple precipitate obtained from MTT. A microplate reader
(OPTImax; Molecular Dynamics, Sunnyvale, CA, USA) was used to test
the absorbance (A) of each well at 540 nm and average values were
obtained. Experiments were repeated ≥3 times and data are presented
as the mean ± SD.
Analysis of cell apoptosis
In order to investigate the apoptosis-inducing
effect of quercetin, morphological analysis was carried out
following staining with Hoechst 33258. Apoptosis of MCF-7 cells was
detected by flow cytometry (Axiovert 200; Carl Zeiss SMT GmbH,
Oberkochen, Germany). The cells were collected following treatment
with 0, 2.5, 5, 10, 20 and 40 mg/ml quercetin. Subsequently, the
cells (1×106) were centrifuged at 700 × g for 5 min and
the supernatants were discarded. The cells were washed twice with
phosphate-buffered saline (PBS), 70% alcohol was added and the
mixture was centrifuged after 30 min at 700 × g for 5 min. After
washing with PBS twice, 1 ml propidium iodide (PI) was added, and
the cells were incubated for 30 min in the dark at 25°C. Cell
apoptosis was then detected using a flow cytometer (FCM: BD
FACSCalibur; BD Biosciences, San Jose, CA, USA).
Measurement of survivin mRNA
expression
The expression of survivin mRNA was detected by
reverse transcription-polymerase chain reaction (RT-PCR). The
survivin and H-GAPDH primers were designed using Primer 5.0
(Premier Biosoft International, Palo Alto, CA, USA), with H-GAPDH
as the internal reference. Total RNA was extracted and purified
using the TRIzol kit and subsequently synthesized by two-step
RT-PCR. The PCR parameters were as follows: 33 cycles of
denaturation at 95°C for 30 sec, annealing at 54°C for 45 sec, and
extension at 72°C for 1 min.
Detection of survivin protein
expression
Survivin protein was detected by western blot
analysis. MCF-7 cells were treated with different concentrations of
quercetin for 24 h and subsequently cultured for 6 h following a
change of medium. Different groups of cells were collected in order
to extract and quantify the protein. The protein was separated by
sodium dodecyl sulfate-polyacrylamide gel electrophoresis
(SDS-PAGE) and then transferred to a blocked nitrocellulose (NC)
membrane for 1 h with 5% skimmed dry milk. The membrane was
incubated with rabbit anti-human survivin antibody, diluted
(1:1,000) in blocking buffer overnight at 4°C and then washed with
Tris-buffered saline Tween (TBST, 3×10 min). Subsequently, the
membrane was incubated with TBST for 3×10 min. An ECL Western
Blotting kit (Suzhou JiShi biological technology company, Suzhou,
China) was used for detection.
Statistical analysis
Data are presented as the mean ± SD. Statistical
analysis was performed with a one-way analysis of variance (ANOVA)
using SPSS software (SPSS, Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Inhibitory effect of quercetin on the
proliferation of MCF-7 cells
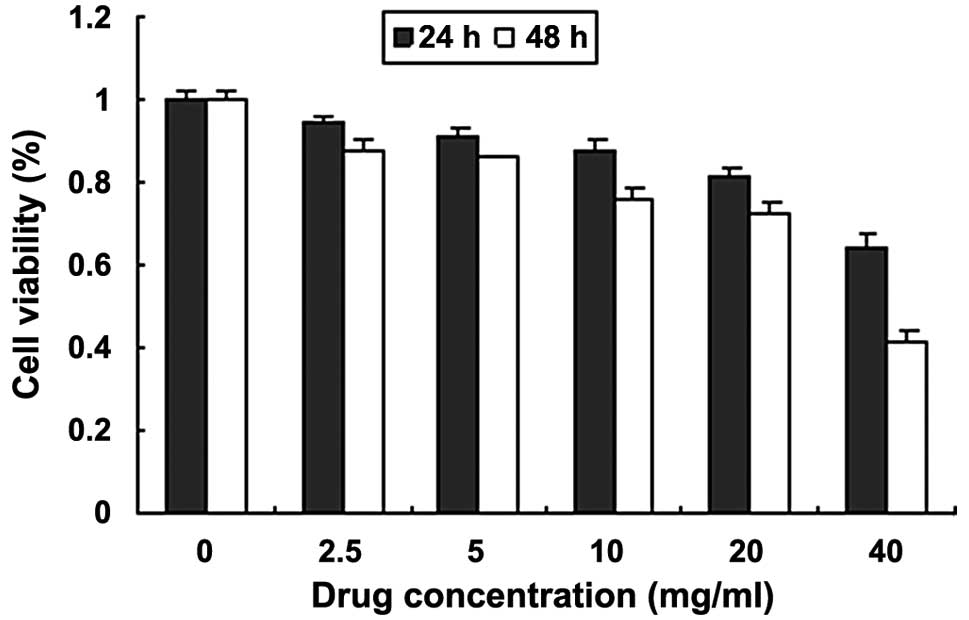
Fig. 1 demonstrates
the inhibitory effect of quercetin on the proliferation of MCF-7
cells. The results showed that cell growth activity was reduced and
proliferation was inhibited. The viability of the cells was reduced
when the incubation time was extended for the same dose. The
minimum cell activity was observed following treatment with 40
mg/ml quercetin for 48 h. The highest inhibition rate was 58.72%
and the rate of inhibition was concentration- and
time-dependent.
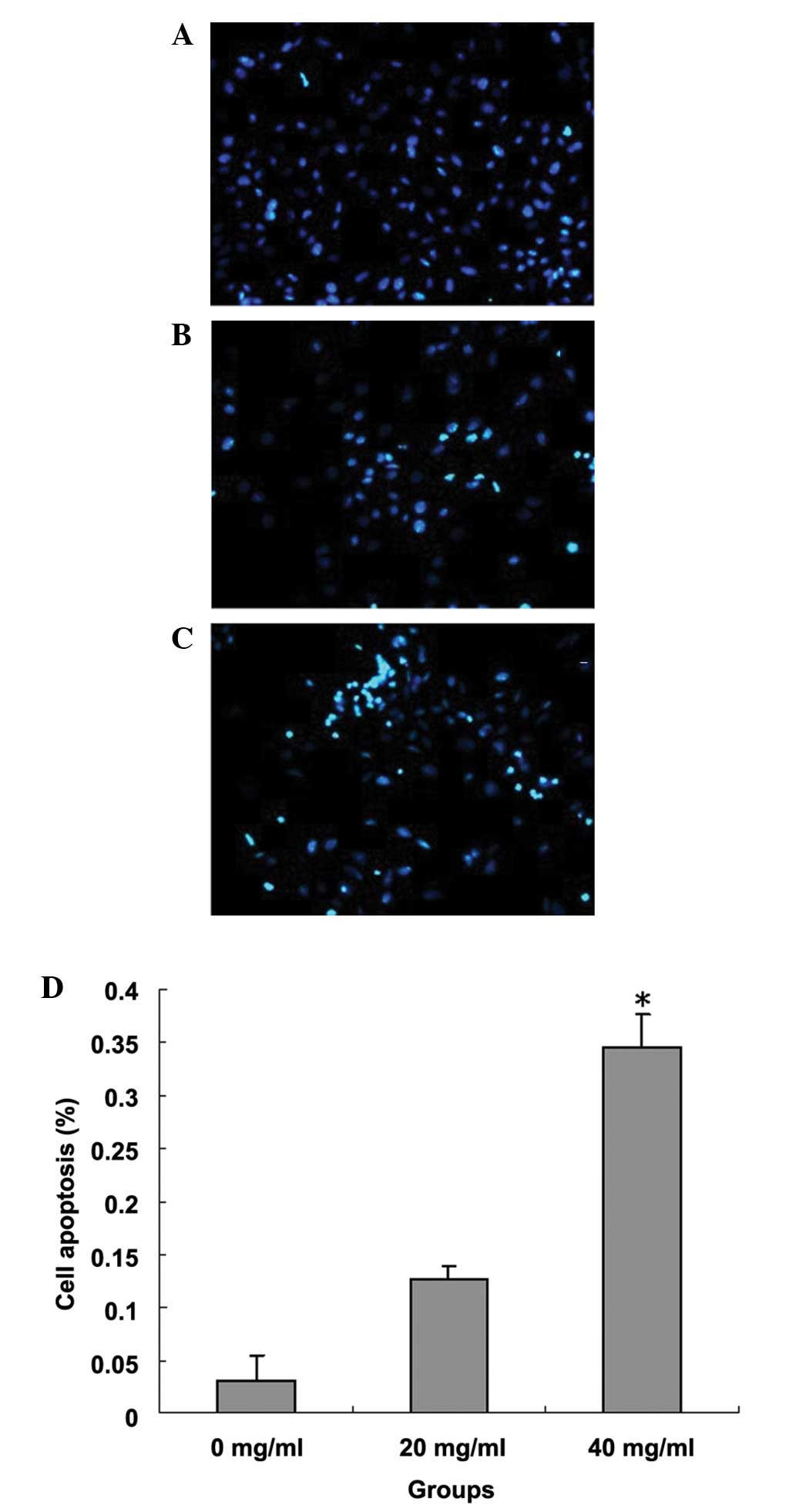
Effect of quercetin on apoptosis of
MCF-7 cells
Table I shows that
quercetin induced the apoptosis of breast cancer cells in a
concentration-dependent manner. The number of cells (40.24 and
59.71%) in the G0/G1 phase significantly increased in the
quercetin-treated cells compared with that of the control group.
The apoptosis rate of the quercetin 40 mg/ml group was 37.81%,
which was significantly higher than the apoptosis rates in the low
concentration (20 mg/ml) and control groups. Fig. 2 shows that when cells were stained
with Hoechst 33258 and observed under a fluorescence microscope,
increased levels of nuclear fragmentation and apoptotic bodies
(bright-blue) were detected in the cells treated with
quercetin.
 | Table I.Effect of quercetin on the cell cycle
and apoptosis of MCF-7 cells (mean ± SD). |
Table I.
Effect of quercetin on the cell cycle
and apoptosis of MCF-7 cells (mean ± SD).
| Group | Cell cycle (%)
| Apoptosis rate
(%) |
|---|
| G0/G1 | S | G2/M |
|---|
| 0 mg/ml | 37.81±0.51 | 31.24±0.14 | 30.95±0.34 | 1.03±0.14 |
| 20 mg/ml | 40.24±0.33 | 27.15±0.05 | 32.61±0.24 | 7.31±0.21 |
| 40 mg/ml | 59.71±0.16 | 16.27±0.21 | 24.02±0.29 |
27.11±0.27a,b |
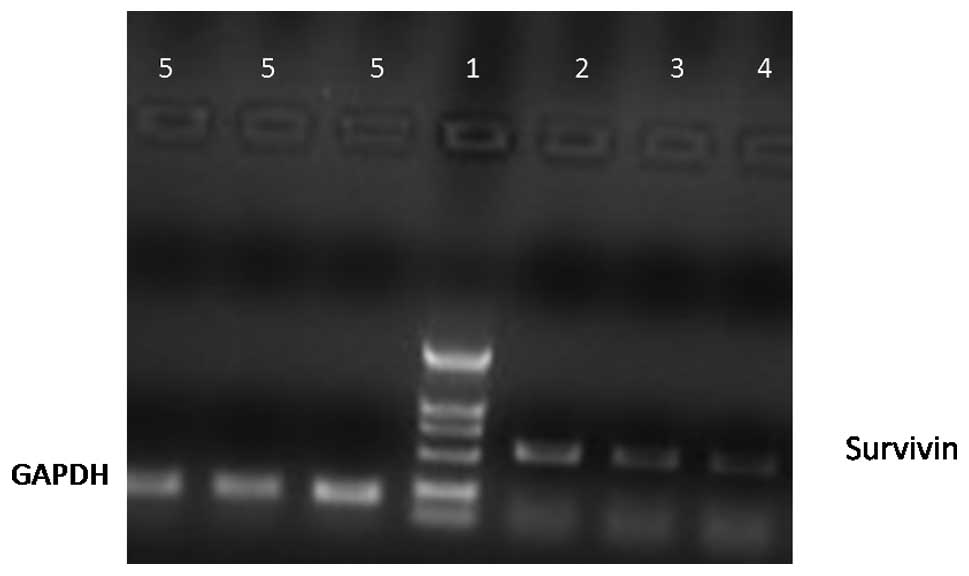
Expression of survivin mRNA in MCF-7
cells
Fig. 3 shows that
the survivin mRNA levels were reduced when the concentration of
quercetin increased.
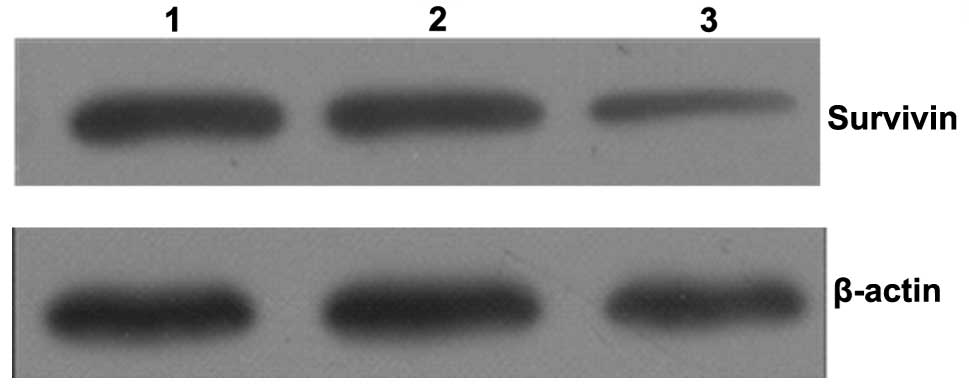
Expression of survivin protein
Survivin expression levels were examined by western
blot analysis of MCF-7 cells following quercetin treatment for 48
h. As shown in Fig. 4, reductions
in survivin protein levels were observed following quercetin
treatment. This corresponds with the changes observed in the levels
of survivin mRNA.
Discussion
Due to the high incidence and high fatality rates of
breast cancer in recent years, the development of an effective
method of treatment is urgently required. Bio-flavonoids extracted
from fruits and vegetables have a variety of biological properties,
including antioxidant, antibacterial, anti-inflammatory, antiviral,
anticancer and cancer preventing activities (11,12).
It has been demonstrated that the flavonoid quercetin inhibits the
proliferation of colon, pancreas, stomach, bladder and ovarian
cancers, as well as the proliferation of other types of tumor
cells, and induces tumor cell apoptosis (13–15).
The antitumor effect of quercetin on breast cancer
cells in vitro was detected using an MTT assay and flow
cytometry. It was observed that quercetin treatment led to cells
remaining in the G0/G1 phase and significantly reduced the
proportion of cells in the G2 phase. This effectively inhibited
breast cancer cell proliferation and prompted cells apoptosis. The
inhibitory effect of quercetin on cancer cell proliferation
increased as the drug concentration and length of action time
increased. Quercetin caused a concentration- and time-dependent
reduction in the viability of MCF-7 human breast cancer cells.
Survivin is a member of a protein family responsible
for apoptosis inhibition (inhibitor of apoptosis protein, IAP). It
has a simple and unique molecular structure, and has been
identified to be the IAP with the strongest ability to inhibit
apoptosis (16,17). Survivin exhibits high expression
levels in a number of tumors and is not expressed at all in normal
adult tissues (with the exception of placenta and thymus tissue).
Ryan et al (18)
demonstrated that the degree of survivin expression correlated with
tumor progression, the degree of malignancy and pathology
classification, and that survivin may be used in relation to breast
cancer diagnosis and in indicating prognosis.
The present study investigated the effects of
quercetin as an inhibitor of the proliferation and apoptosis of
MCF-7 breast cancer cells. Furthermore, the potential antitumor
effect of quercetin in breast cancer treatment was evaluated by
measuring the expression level of survivin mRNA. RT-PCR
demonstrated that the expression level of survivin mRNA was reduced
in MCF-7 human breast cancer cells following treatment with
quercetin. Western blot analysis revealed that the level of
survivin protein also decreased. The survivin mRNA and protein
levels were negatively correlated with quercetin concentration. The
results indicate that quercetin may be capable of improving the
sensitivity of breast cancer cells to chemotherapy by decreasing
the expression level of survivin mRNA in breast cancer cells.
Furthermore, during the breast cancer treatment process, the
detection of survivin expression levels in tumor tissue may be used
to determine the effectiveness of treatment. Survivin expression
may not only provide a basis for clinical diagnosis of breast
cancer, but also may be used as an indicator to estimate the
prognosis of breast cancer.
Acknowledgements
This study was supported by a grant to
Xinxiang Medical University from the National Basic Research
Program of China (31200897) and with assistance from Key Laboratory
for Medical Tissue Regeneration of Henan Province (Xinxiang, China)
and the Tumor and Signal Transduction Laboratory of Xinxiang
Medical University.
References
|
1.
|
Huang X, Wong MK, Yi H, et al: Combined
therapy of local and metastatic 4T1 breast tumor in mice using
SU6668, an inhibitor of angiogenic receptor tyrosine kinases, and
the immunostimulator B7.2-IgG fusion protein. Cancer Res.
62:5727–5735. 2002.PubMed/NCBI
|
|
2.
|
Duo J, Ying GG, Wang GW and Zhang L:
Quercetin inhibits human breast cancer cell proliferation and
induces apoptosis via Bcl-2 and Bax regulation. Mol Med Rep.
5:1453–1456. 2012.PubMed/NCBI
|
|
3.
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
4.
|
Mallin K, Palis BE, Watroba N, et al:
Completeness of American Cancer Registry Treatment Data:
implications for quality of care research. J Am Coll Surg.
216:428–437. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Slusarz A, Shenouda NS, Sakla MS, et al:
Common botanical compounds inhibit the hedgehog signaling pathway
in prostate cancer. Cancer Res. 70:3382–3390. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Cheng S, Gao N, Zhang Z, et al: Quercetin
induces tumor-selective apoptosis through downregulation of Mcl-1
and activation of Bax. Clin Cancer Res. 16:5679–5691. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Psahoulia FH, Drosopoulos KG, Doubravska
L, Andera L and Pintzas A: Quercetin enhances TRAIL-mediated
apoptosis in colon cancer cells by inducing the accumulation of
death receptors in lipid rafts. Mol Cancer Ther. 6:2591–2599. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Qin Y, He LY, Chen Y, Wang WY, Zhao XH and
Wu MY: Quercetin affects leptin and its receptor in human gastric
cancer MGC-803 cells and JAK-STAT pathway. Xi Bao Yu Fen Zi Mian Yi
Xue Za Zhi. 28:12–16. 2012.(In Chinese).
|
|
9.
|
Neibert KD and Maysinger D: Mechanisms of
cellular adaptation to quantum dots - the role of glutathione and
transcription factor EB. Nanotoxicology. 6:249–262. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Chen SS, Corteling R, Stevanato L and
Sinden J: Polyphenols inhibit indoleamine 3,5-dioxygenase-1
enzymatic activity - a role of immunomodulation in chemoprevention.
Discov Med. 14:327–333. 2012.PubMed/NCBI
|
|
11.
|
Heinritz W, Paasch U, Sticherling M, et
al: Evidence for a founder effect of the germline fumarate
hydratase gene mutation R58P causing hereditary leiomyomatosis and
renal cell cancer (HLRCC). Ann Hum Genet. 72(Pt 1): 35–40.
2008.PubMed/NCBI
|
|
12.
|
Comalada M, Camuesco D, Sierra S, et al:
In vivo quercitrin anti-inflammatory effect involves release of
quercetin, which inhibits inflammation through down-regulation of
the NF-kappaB pathway. Eur J Immunol. 35:584–592. 2005. View Article : Google Scholar
|
|
13.
|
Nguyen TT, Tran E, Nguyen TH, Do PT, Huynh
TH and Huynh H: The role of activated MEK-ERK pathway in
quercetin-induced growth inhibition and apoptosis in A549 lung
cancer cells. Carcinogenesis. 25:647–659. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Kuo PC, Liu HF and Chao JI: Survivin and
p53 modulate quercetin-induced cell growth inhibition and apoptosis
in human lung carcinoma cells. J Biol Chem. 279:55875–55885. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Kim WK, Bang MH, Kim ES, et al: Quercetin
decreases the expression of ErbB2 and ErbB3 proteins in HT-29 human
colon cancer cells. J Nutr Biochem. 16:155–162. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Li F and Altieri DC: The cancer
antiapoptosis mouse survivin gene: characterization of locus and
transcriptional requirements of basal and cell cycle-dependent
expression. Cancer Res. 59:3143–3151. 1999.
|
|
17.
|
Yoshida M, Yamamoto M and Nikaido T:
Quercetin arrests human leukemic T-cells in late G1 phase of the
cell cycle. Cancer Res. 52:6676–6681. 1992.PubMed/NCBI
|
|
18.
|
Ryan BM, O’Donovan N and Duffy MJ:
Survivin: a new target for anti-cancer therapy. Cancer Treat Rev.
35:553–562. 2009. View Article : Google Scholar : PubMed/NCBI
|