Introduction
Renal ischemia/reperfusion injury (IRI) is one of
the underlying causes of acute renal failure (1,2).
Inflammation substantially contributes to the pathogenesis of IRI
with a central role for certain cells, adhesion molecules and
cytokines (3). Neutrophils are
inflammatory cells that are potent sources of reactive oxygen
species (ROS), which are extensively generated during IRI and
mediate cellular damage. Oxidative stress is caused by an imbalance
between ROS production and anti-oxidant capacity. The formation of
ROS and disturbances in the balance between oxidants and
antioxidants play key roles in the mechanism by which renal IR
causes tissue injury (4,5).
Propofol (2,6-diisopropylphenol) is a venous
anesthetic extensively used in clinical practice and characterized
by rapid induction and quick patient recovery from its effects. In
addition, propofol is widely used as a sedative for intensive care
patients. Propofol has a myocardial protective effect through a
range of mechanisms, including oxygen free radical scavenging
(6–8), blocking of the calcium channel by
inhibiting the L-type calcium channel (9,10)
and inhibiting neutrophil activation (11,12).
The myocardial protective effect of propofol has been identified by
a variety of methods using an IRI model (6–8,12,13).
However, the potential benefits of propofol in ameliorating renal
IRI and its mechanism of action remain unknown.
Bone morphogenetic protein 2 (BMP2) is a member of
the multifunctional BMP family, which is part of the transforming
growth factor β1 (TGF-β1) superfamily, originally identified as
substances that induce bone and cartilage formation at ectopic
extraskeletal sites in vivo. BMP2 in particular is heavily
glycosylated and is involved in osteoblastogenesis (14). It not only plays a significant role
in the regulation of cell proliferation and apoptosis, but also in
the development and repair of various organs, including bone,
nerve, heart and kidney (15,16).
In the current study, a regional renal IRI rat model
was used to determine the effects of a clinically relevant propofol
concentration during the peri-ischemic period of anesthesia. After
72 h of reperfusion, alterations in levels of the
O2− scavenger superoxide dismutase (SOD) and
the lipid peroxidation by-product malondialdehyde (MDA) and the
involvement of BMP2 in this process were investigated to determine
whether propofol has a protective effect against renal IRI that
involves these parameters of redox status.
Materials and methods
Animals
Male Wistar rats were obtained from the Animal
Resource Center, Shenyang Pharmaceutical University (Shenyang,
China). The rats were housed in cages with free access to water and
food. Thirty-two male rats weighing 180–230 g were used. The Animal
Ethics Committee of Liaoning Cancer Hospital and Institute approved
this study and the experiments complied with established guidelines
for animal care.
Induction of kidney IRI
Rats were anesthetized with intraperitoneal
injections of chloral hydrate (Tianjin Ruijinte Chemical Co., Ltd.,
Tianjin, China). Using a midline abdominal incision, the two renal
pedicles were clamped for 50 min with microaneurysm clamps. During
the period of ischemia, body temperature was maintained by placing
rats on a 37°C heating pad. Following removal of the clamps, the
kidneys were inspected for 1 min for restoration of blood flow, as
noted by a return to their original color and then the abdomen was
closed. Sham-operated rats received identical surgical procedures
with the exception that microaneurysm clamps were not applied. To
maintain fluid balance, all rats were supplemented with 5 ml saline
administered via the femoral vein. Propofol was purchased from
Qingyuan Jiabo Pharmaceutical Co. (Shenyang, China). Rats treated
with propofol received identical surgical procedures with the
exception that they were supplemented with 5 ml saline containing
either 5 or 10 mg/kg propofol. The administration of saline or
propofol was performed immediately prior to surgery. Rats were
sacrificed 3 days after reperfusion. Blood was collected and kidney
tissues were divided to be either snap frozen for subsequent mRNA
extraction, fixed in 2% glutaraldehyde solution for electron
microscopy or fixed in 10% neutral buffered formalin for paraffin
embedding.
Assessment of kidney function and
oxidative stress
Serum creatinine (SCr) and blood urea nitrogen (BUN)
were measured using the picric acid and diacetyl monoxime methods
(17), respectively, in the
Department of Biochemistry, Liaoning Cancer Hospital and
Institute.
Serum levels of SOD and MDA were measured by
chemical absorbance spectroscopy (18) in the Department of Biochemistry,
Liaoning Cancer Hospital and Institute.
Histological examination
Kidneys embedded in paraffin were sectioned at 3
μm and stained with hematoxylin and eosin (H&E) by
standard methods. Depending on the percentage of tubules in the
corticomedullary junction that exhibited necrosis, loss of the
brush border, cast formation or tubular dilatation, markers of
tubular damage were scored from 0 to 5 (corresponding to none, ≤10,
11–25, 26–45, 46–75 and >76%, respectively) (19). Histological examination was
performed by two blinded observers. At least ten high-power fields
(HPFs; magnification, ×200) per section for each sample were
examined.
Electron microscopy
Following perfusion, kidneys were excised and
immersed in fresh fixative (2.5% glutaraldehyde in 0.1 M sodium
cacodylate buffer, pH 7.4) for 16 h at 4°C. For morphological
studies, the tissue blocks were post-fixed with 1% osmium tetroxide
and 0.8% potassium ferricyanide in 0.1 M cacodylate buffer, treated
with aqueous 1% uranyl acetate, dehydrated in a graded ethanol
series and embedded in PolyBed epoxy resin. Thin sections were cut,
collected on 200-μm mesh copper/rhodium grids, stained with
sodium acetate and lead citrate and then observed at 60 kV with a
transmission electron microscope (Hitachi, Tokyo, Japan).
RNA extraction and quantitative
polymerase chain reaction (PCR)
Total RNA was isolated from renal tissue using
TRIzol (Invitrogen Life Technologies, Carlsbad, CA, USA). Equal
amounts of RNA, measured by sfpectrophotometry and an RNA gel, were
used for first-strand cDNA synthesis with Superscript II
(Invitrogen Life Technologies) in a 20-μl reaction. The cDNA
product (1 μl) was then subjected to reverse
transcription-PCR (RT-PCR) with Taq polymerase (Boehringer Mannheim
GmbH, Mannheim, Germany). Quantitative RT-PCR was performed using a
Light Cycler system (Roche Diagnostics, Mannheim, Germany) and 2X
SYBR Premix Ex Taq (Takara Bio Inc., Shiga, Japan) was used to
detect PCR products. The comparative cycle threshold (Ct) method
(2−ΔΔCt) was used to analyze relative changes in gene
expression. β-actin (Actb) was used to normalize gene expression.
The primer sequences were as follows: BMP-2, sense:
5′-ACGATGCCGCCATTTGTG-3′ and antisense: 5′-CGC CTCGCCTTCTTCAGT-3′,
with the size of the PCR product being 349 bp; Actb, sense:
5′-GCCAACCGTGAAAAGATG-3′ and antisense: 5′-CCAGGATAGAGCCACCAAT-3′,
with the size of the PCR producting being 701 bp.
Kidney tissue protein extraction for
cytokine measurements
Pre-chilled CelLytic MT reagent (Sigma-Aldrich, St.
Louis, MO, USA) with a 1% protease inhibitor cocktail
(Sigma-Aldrich) for use with mammalian tissue extracts was added to
snap-frozen kidney tissue and then homogenized. The samples were
incubated for 30 min at 4°C and centrifuged at 16,000 × g at 4°C
for 15 min to pelletize the tissue debris. The supernatant was
stored at −70°C. Protein concentrations were determined by a
colorimetric protein assay (Bio-Rad, Hercules, CA, USA) using
protein standards from Sigma-Aldrich.
Enzyme-linked immunosorbent assay
(ELISA)
Cytokines were measured in kidney homogenates using
ELISA kits according to the manufacturer’s instructions. Kits for
interleukin (IL)-6, IL-8 and tumor necrosis factor (TNF)-α were
obtained from R&D Systems (Shanghai, China). Protein levels of
cytokines were corrected for the total amounts of protein and the
results were expressed in pg/ml.
Western blotting
Aliquots (50 μg) of kidney homogenates were
separated on 10% polyacrylamide gels (Sigma-Aldrich) and
transferred to a polyvinylidene fluoride membrane (PerkinElmer, San
Jose, CA, USA). The membrane was blocked overnight in Western
Blocker Solution (Sigma-Aldrich), incubated with anti-BMP2 antibody
(Nventa Biopharmaceuticals Corporation, San Diego, CA, USA) in
Western Blocker Solution for 1 h, washed, incubated with anti-mouse
IgG conjugated with horseradish peroxidase (Sigma-Aldrich) and then
washed. Positive bands were detected by chemiluminescence
technology (Sigma-Aldrich) using the G:BOX gel documentation and
analysis system (Syngene, Cambridge, UK). The membrane was also
probed with anti-Actb antibody (Sigma-Aldrich) for Actb expression.
The intensity of each band was quantified using Image J 1.32
software (National Institutes of Health, Bethesda, MD, USA).
Statistical analyses
Results are expressed as mean ± standard deviation
(SD). SPSS software (version 11.0; SPSS, Inc., Chicago, IL, USA)
was used for all statistical analyses. Multiple groups were
compared using one-way analysis of variance (ANOVA) with a post-hoc
Bonferroni correction (GraphPad Prism 5.0; GraphPad Software).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Rats are protected against kidney IRI by
propofol injection
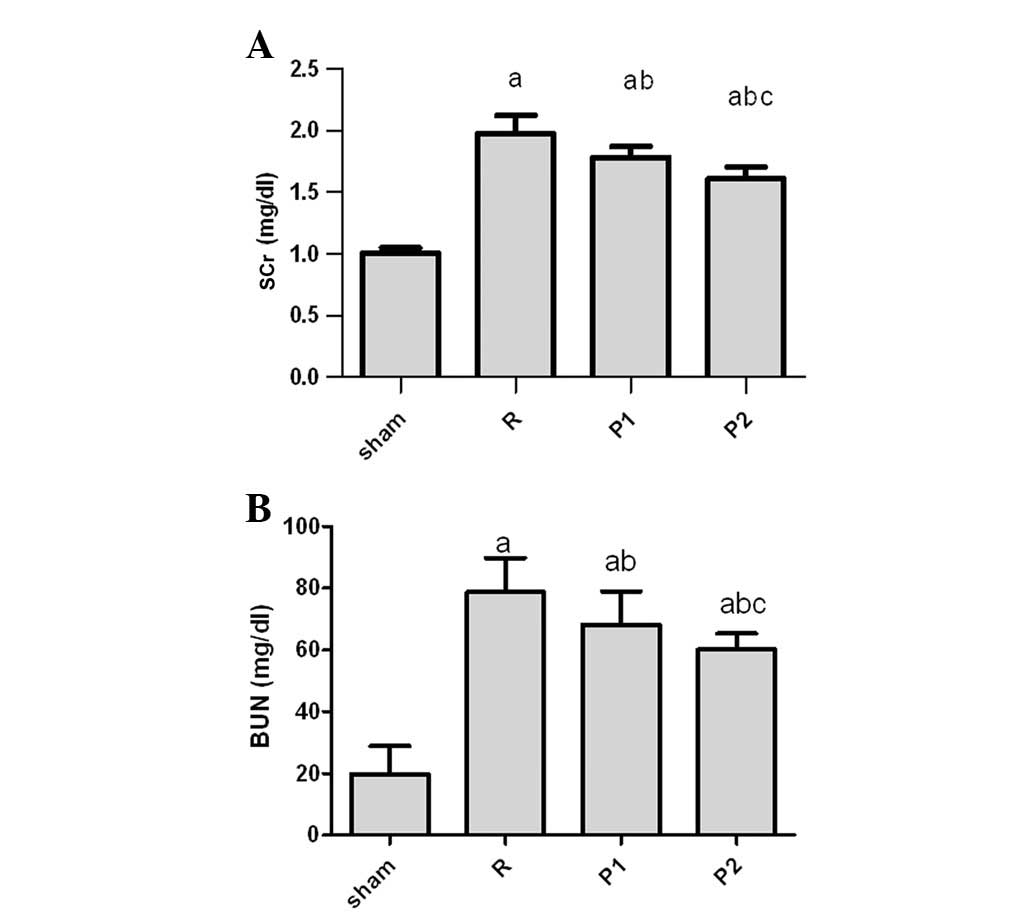
As shown in Fig.
1A, IRI caused kidney dysfunction in untreated rats with a peak
SCr of 1.92±0.44 mg/dl on day 3 after IRI as compared with
0.98±0.35 mg/dl in sham-operated rats. To determine the effects of
propofol on kidney IRI, rats were injected with 5 or 10 mg/kg
propofol (groups P1 or P2, respectively) in the IRI model. Rats
treated with propofol were protected against the effects of
ischemia, exhibiting significantly lower SCr and BUN levels than
untreated rats. By contrast, there was an increase in SCr and BUN
levels in rats treated with propofol on day 3, compared with sham
rats (Fig. 1). The SCr and BUN
levels in the P2 group were lower compared with those of the P1
group (Fig. 1).
The functional data correlated with histological
kidney tubular damage. Severe tubular damage was observed in
untreated IRI rats with no propofol treatment, as shown by
widespread tubular necrosis, loss of the brush border, cast
formation and tubular dilatation at the corticomedullary junction,
whereas IRI rats with propofol treatment demonstrated significantly
less tubular damage (Fig. 2A).
Sham-operated rats incurred no tubular injury. A blind review of
specimens from untreated IRI rats revealed greater tubular injury
in their kidneys. The mean histological score for the kidneys of
the propofol-treated (10 mg/kg) rats was 32.8±0.6% compared with
85.3±6.1% (P<0.01; Fig. 2B) for
the untreated IRI control. Tubular injury was attenuated as the
propofol dosage increased (Fig.
2B).
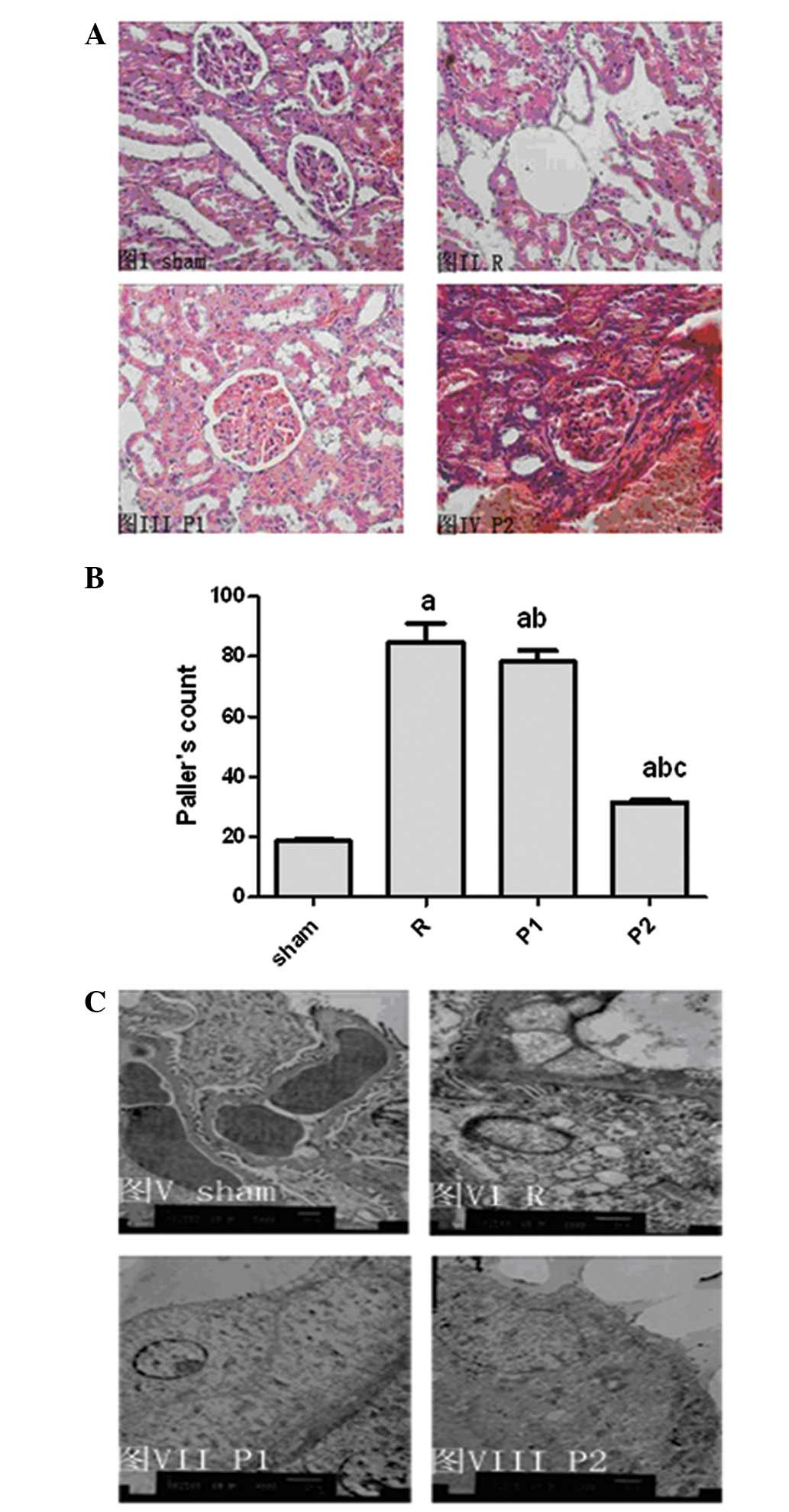 | Figure 2.Tubular injury in propofol-treated
ischemia/reperfusion injury (IRI) rats was significantly less
compared with that observed in kidneys from untreated IRI rats. (A)
Representative sections of the outer medulla from sham-operated,
untreated IRI rats, rats treated with 5 mg/kg propofol and rats
treated with 10 mg/kg propofol, three days after reperfusion
(H&E; magnification, ×400). (B) Semiquantitative analysis of
tubular damage in the kidneys of sham-operated rats, operated rats,
rats treated with 5 mg/kg propofol and rats treated with 10 mg/kg
propofol following reperfusion. Data are presented as mean ±
standard deviation (SD); n=8 per group. aP<0.05 vs.
S; bP<0.05 vs. R; cP<0.05 vs. P1. (C)
Representative electron micrograph sections from sham-operated
rats, operated rats, rats treated with 5 mg/kg propofol and rats
treated with 10 mg/kg propofol three days after reperfusion.
Original magnification, ×7,200. S, sham-operated rats; R, operated
rats; P1, rats treated with 5 mg/kg propofol; P2, rats treated with
10 mg/kg propofol. |
Morphological studies using electron microscopy
demonstrated a certain degree of heterogeneous loss of brush
border, bleb formation, cytoplasmic vacuolization, cellular
necrosis, mitochondrial loss or disappearance, chromatin
condensation and aggregation at the periphery of nuclei and nuclear
fragmentation, and tubular luminal debris and obstruction in
untreated kidneys. Damage was markedly reduced in propofol-treated
kidneys (Fig. 2C).
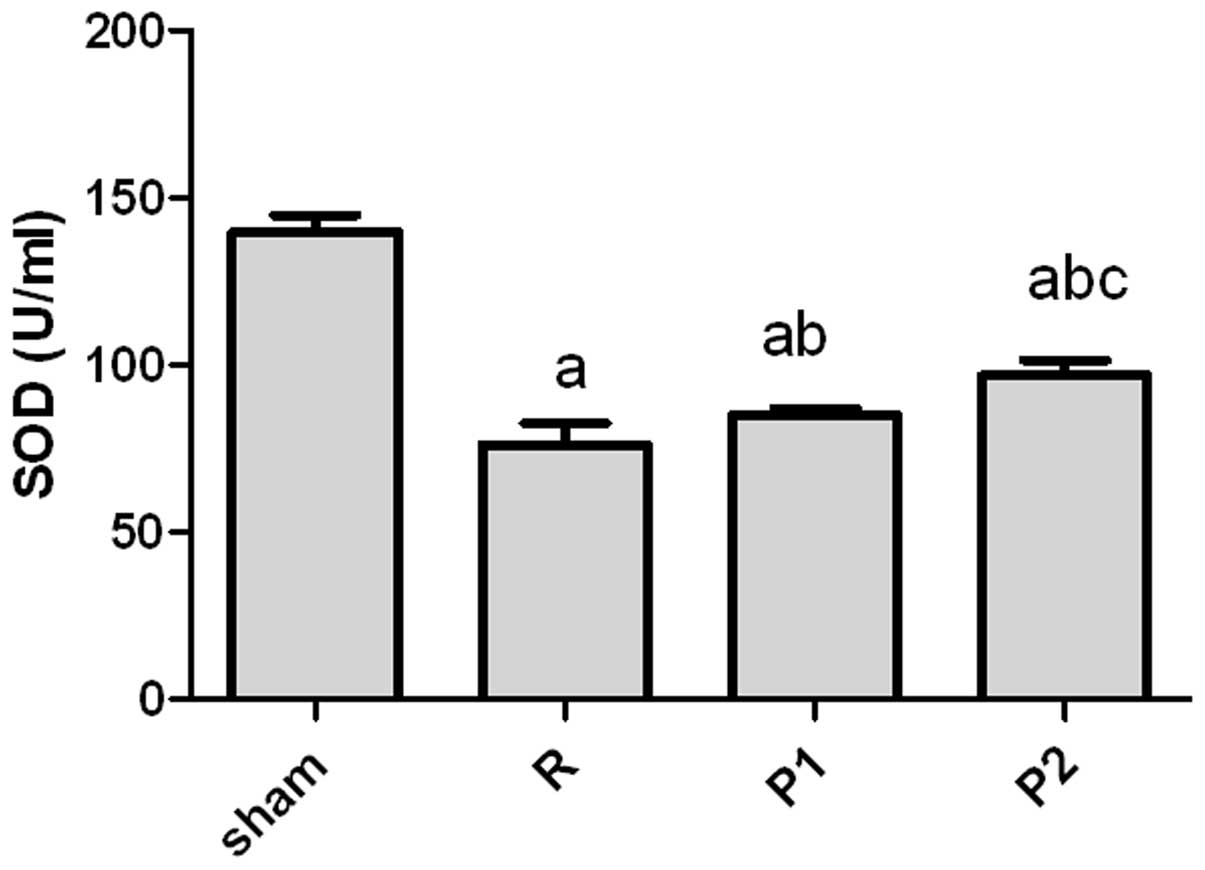
Oxygen free radical scavenger SOD levels
are increased in propofol-treated rats
Normally, tissues contain enough endogenous
scavengers to protect against damage induced by oxygen free
radicals (OFRs). The levels of SOD, one such scavenger, were
measured following IRI. The SOD levels were significantly reduced
following IRI compared with those in sham-operated controls
(Fig. 3). Propofol-treated rats
had significantly higher SOD levels compared with untreated rats
following IRI (Fig. 3). Moreover,
rats treated with 10 mg/kg propofol had significantly higher SOD
levels compared with those treated with 5 mg/kg propofol (Fig. 3).
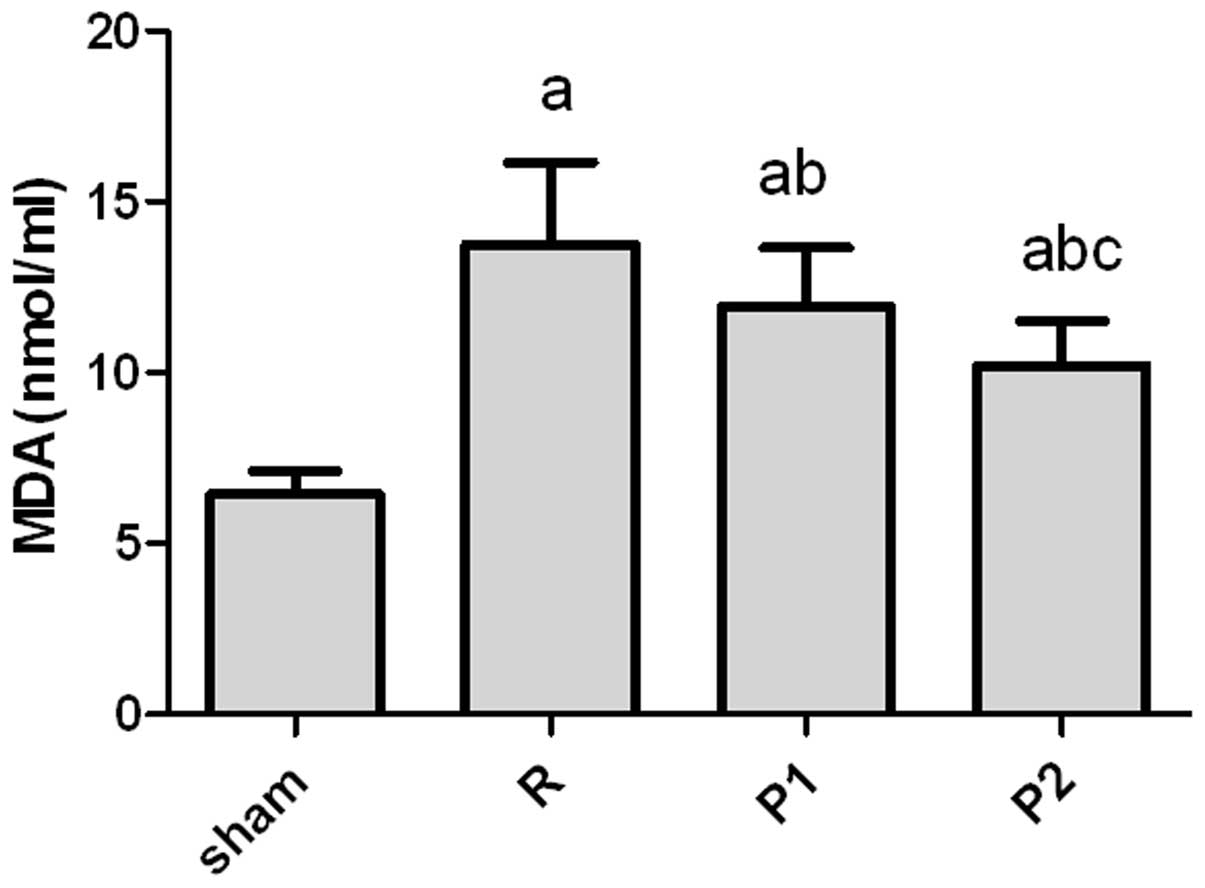
Lipid peroxidation by OFRs is reduced in
propofol-treated rats
The effect of IRI on lipid peroxidation was
evaluated in the rats. The lipid peroxidation by-product MDA was
measured as a marker for membrane lipid peroxidation. IRI resulted
in an increase in the whole kidney MDA levels of operated groups
compared with that of the sham-operated group (Fig. 4).
Propofol-treated rats demonstrated less lipid
peroxidation with significantly lower MDA levels compared with the
untreated IRI controls. Rats treated with 10 mg/kg propofol had
significantly lowere levels of MDA compared with those treated with
5 mg/kg propofol (Fig. 4).
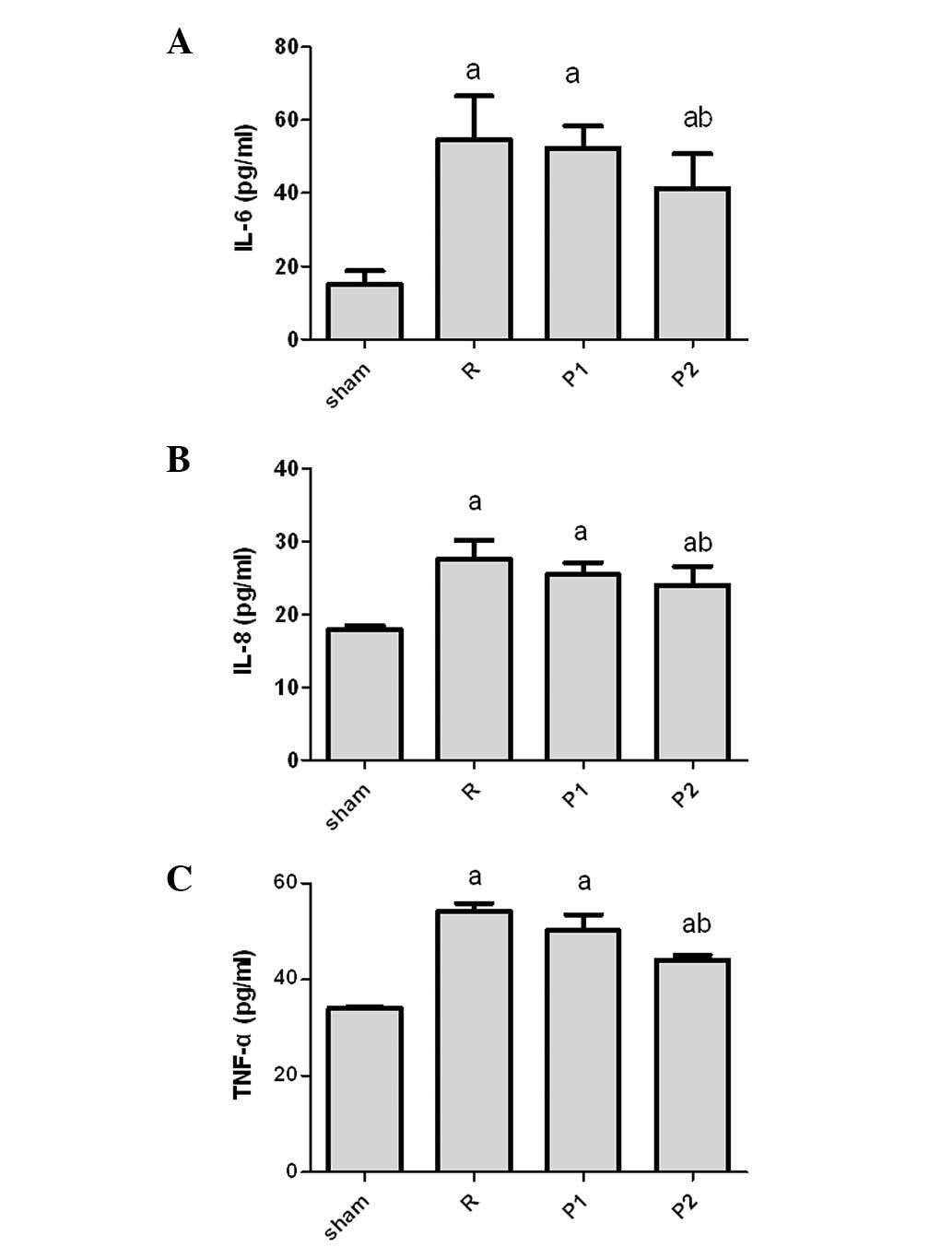
Propofol attenuates pro-inflammatory
cytokine expression in the kidney during IRI
To further determine the effects of propofol in the
IRI kidney model, we examined the expression of cytokines (Fig. 5). IL-6, IL-8 and TNF-α levels were
significantly increased in the IRI kidney compared with those in
sham-operated controls. Cytokine levels increased in rats treated
with 10 mg/kg propofol; however, the extent of the increase was
much less than that in untreated IRI rats. No significant
differences were identified between the two groups treated with
different doses of propofol (Fig.
5).
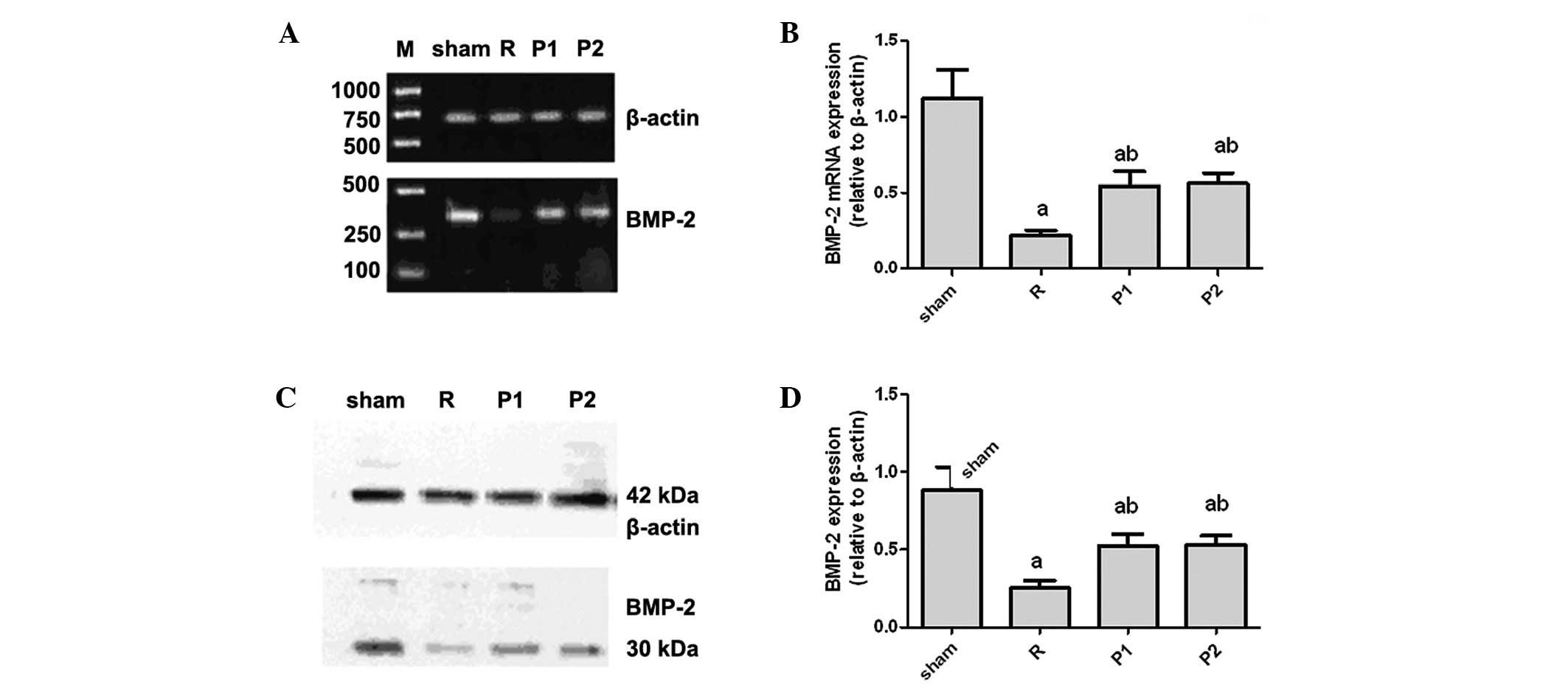
Role of BMP2 in the kidney during
IRI
To determine the role of BMP2 in our model, we
measured mRNA expression levels of BMP2 in the kidney by real-time
quantitative PCR. The level of BMP2 mRNA was significantly reduced
following IRI compared with that in sham-operated controls
(Fig. 6A and B). Sham-operated
kidneys expressed abundant BMP2, whereas BMP2 mRNA expression
decreased markedly in kidneys following IRI (Fig. 6A and B). By contrast, the reduction
in mRNA expression for BMP2 was less in propofol-treated kidneys
than in untreated kidneys (Fig. 6A and
B).
Consistent with the real-time PCR data, western
blots revealed that IRI induced a significant reduction in BMP2
expression in the kidneys of untreated IRI rats compared with that
in sham-operated controls (Fig. 6C and
D). Downregulation of BMP2 expression following IRI was greatly
attenuated in the propofol-treated rats.
Discussion
Previous experimental data suggests that IRI rapidly
induces the formation of OFRs, while SOD levels are reduced in the
kidneys following IRI (20,21).
In the present study we identified that IRI resulted in increased
production of the lipid peroxidation by-product MDA in homogenates
of whole kidneys. By contrast, propofol pretreatment reduced MDA
levels, increased SOD levels and improved renal dysfunction
following IRI. We identified that rats pretreated with propofol
were protected from kidney dysfunction and histological damage.
Protection was associated with a reduction in pro-inflammatory
cytokine generation and a concomitant increase in BMP2
expression.
The most common cause of acute renal failure is
renal ischemia, which causes functional impairment through a
combination of renal vasoconstriction, tubular obstruction, tubular
back-leakage of glomerular filtrate and reduced glomerular
permeability (22). IRI results in
the activation of multiple cell injury pathways that contribute to
organ dysfunction, including those resulting in the production of
OFRs (23).
OFRs are considered to cause cellular injury by
attacking membranes through the peroxidation of polyunsaturated
fatty acids (24,25) during IRI. This lipid peroxidation
results in increased membrane permeability in cells, mitochondria
and lysosomes. Peroxidative injury of erythrocyte membranes has
been reported to increase passive K+ permeability with a
loss of intracellular K+(26). Increased permeability of renal
tubular cell membranes may lead to a loss of transport functions,
whereas increased permeability of mitochondrial membranes impairs
oxidative phosphorylation. Increased lysosomal permeability may
result in the leakage of hydrolytic enzymes and acceleration of
cellular degradation. In the current study, we identified that IRI
increased the production of the lipid peroxidation by-product MDA
in the homogenates of whole rat kidneys.
Propofol has been reported to modulate IRI,
suggesting its potential for organ protection during surgery
involving abdominal aortic clamping and possibly as a means for
improving patient outcome (27).
Propofol is a lipophilic hypnotic drug with proven antioxidant
activity in in vitro and in vivo studies. This
results in part from its chemical structure, which is similar to
the natural antioxidant vitamin E (5,14).
Propofol has been demonstrated to act as a scavenger of OFRs,
reducing lipid peroxidation in the liver, kidney, heart and lung
(28). It was reported that in an
in vivo experimental model of reversible renal IRI, propofol
anesthesia was associated with diminished neutrophil infiltration,
and reductions in plasma pro-inflammatory cytokine levels,
production of OFRs, lipid peroxidation and inducible nitric oxide
synthase activity (29). Results
from the study by Yuzbasioglu et al (30) demonstrated that IRI was
significantly reduced in the presence of propofol and that the
protective effects of propofol may be due to its antioxidant
properties. Results from the study by Yuzer et al (31) demonstrated that IRI was
significantly reduced in the presence of propofol and thiopental.
The authors attributed the protective effects of these drugs to
their antioxidant properties. In the current study, propofol
pretreatment was observed to reduce lipid peroxidation, increase
SOD levels and suppress the production of inflammatory
cytokines.
BMPs are members of the TGF-β1 superfamily and play
important roles in diverse cell types. Vascular endothelial and
smooth muscle cells express BMP receptors and secrete BMPs
(30–32). Among them, BMP2 has been shown to
regulate a host of cellular functions (33), including cardiovascular
development, angiogenesis, neovascularization in tumors, vascular
calcification and smooth muscle cell chemotaxis, in response to
vascular injury (34,35). It is considered that BMP signaling
exerts important vasoprotective effects controlling the balance
between proliferation and activation of apoptosis in endothelial
and smooth muscle cells (36).
BMP2 is considered to signal primarily by activating
the mothers against decapentaplegic (SMAD) and mitogen-activated
protein kinase (MAPK) pathways (37), although evidence suggests that BMP2
may also activate nuclear factor κ-light-chain-enhancer of
activated B cells (NF-κB) (38).
The activation of BMP signaling, either by overexpression of BMP2
in vascular cells or administration of recombinant BMPs, results in
endothelial dysfunction, oxidative stress and enhanced monocyte
adhesiveness to the endothelium (39). BMP2 is selectively expressed by
late outgrowth endothelial progenitor cells and plays a role in
neoangiogenesis (40). Endothelial
colony-forming cells (ECFCs) express BMP2 morphogens. BMP2 may be
used as a marker of immaturity, the ECFC lineage and finally as an
angiogenic marker during ECFC commitment and expansion.
BMP-positive endothelial precursors correspond to ECFCs,
responsible for neovascularization, whereas BMP-negative
endothelial precursors correspond to proangiogenic hematopoietic
progenitor cells (41). A previous
study demonstrated that BMP2 is downregulated following IRI, which
may contribute to an imbalance between cell proliferation and
apoptosis, thereby causing renal injury (42). In the present study, propofol
pretreatment was observed to promote BMP2 expression following IRI
and may have contributed to neoangiogenesis, which may partly
explain its renal protective effect. Future studies are required to
elucidate the mechanism by which propofol regulates the expression
of BMP2.
In conclusion, regulation of BMP2 levels may be an
important mechanism for maintenance of cellular homeostasis.
Propofol pretreatment exerts a protective effect in rats in an IRI
model, which is partly correlated with upregulation of BMP2. This
study may open new avenues of investigation into the antioxidant
effects of propofol. An understanding of the mechanisms of action
of propofol in IRI may introduce new therapeutic approaches not
presently available.
Acknowledgements
This study was supported by the Doctor
Fund Project of the Ministry of Education of China (project grant
20070159020) and the National Natural Science Foundation of China
(81071503). The authors thank Medjaden Bioscience Ltd. for
assisting in the preparation of this manuscript.
References
|
1.
|
Bonventre JV and Zuk A: Ischemic acute
renal failure: an inflammatory disease? Kidney Int. 66:480–485.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Thurman JM: Triggers of inflammation after
renal ischemia/reperfusion. Clin Immunol. 123:7–13. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Ysebaert DK, De Greef KE, De Beuf A, et
al: T cells as mediators in renal ischemia/reperfusion injury.
Kidney Int. 66:491–496. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Erdogan H, Fadillioglu E, Yagmurca M, Ucar
M and Irmak MK: Protein oxidation and lipid peroxidation after
renal ischemia-reperfusion injury: protective effects of erdosteine
and N-acetylcysteine. Urol Res. 34:41–46. 2006. View Article : Google Scholar
|
|
5.
|
Li XL, Zou XM, Gao P, Li YL, Wang H and
Chen XW: Role of nitric oxide in ischemia-reperfusion injury and
acute rejection in rat intestinal transplantation. Transplant Proc.
40:3342–3345. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Xia Z, Godin DV, Chang TK and Ansley DM:
Dose-dependent protection of cardiac function by propofol during
ischemia and early reperfusion in rats: effects on
15-F2t-isoprostane formation. Can J Physiol Pharmacol. 81:14–21.
2003. View
Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Javadov SA, Lim KH, Kerr PM, Suleiman MS,
Angelini GD and Halestrap AP: Protection of hearts from reperfusion
injury by propofol is associated with inhibition of the
mitochondrial permeability transition. Cardiovasc Res. 45:360–369.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Kokita N, Hara A, Abiko Y, Arakawa J,
Hashizume H and Namiki A: Propofol improves functional and
metabolic recovery in ischemic reperfused isolated rat hearts.
Anesth Analg. 86:252–258. 1998.PubMed/NCBI
|
|
9.
|
Yang CY, Wong CS, Yu CC, Luk HN and Lin
CI: Propofol inhibits cardiac L-type calcium current in guinea pig
ventricular myocytes. Anesthesiology. 84:626–635. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Zhou W, Fontenot HJ, Liu S and Kennedy RH:
Modulation of cardiac calcium channels by propofol. Anesthesiology.
86:670–675. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Jordan JE, Zhao ZQ and Vinten-Johansen J:
The role of neutrophils in myocardial ischemia-reperfusion injury.
Cardiovasc Res. 43:860–878. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Simpson PJ, Fantone JC, Mickelson JK,
Gallagher KP and Lucchesi BR: Identification of a time window for
therapy to reduce experimental canine myocardial injury:
suppression of neutrophil activation during 72 hours of
reperfusion. Circ Res. 63:1070–1079. 1988. View Article : Google Scholar
|
|
13.
|
Shin IW, Lim BW, Chung YS, et al: The
effect of propofol on myocardial protection after regional
ischemia-reperfusion injury in an in vivo rat heart model. Korean J
Anesthesiol. 55:338–343. 2008. View Article : Google Scholar
|
|
14.
|
Nauth A, Ristiniemi J, McKee MD and
Schemitsch EH: Bone morphogenetic proteins in open fractures: past,
present, and future. Injury. 40(Suppl 3): S27–S31. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
El-Bizri N, Guignabert C, Wang L, et al:
SM22alpha-targeted deletion of bone morphogenetic protein receptor
1A in mice impairs cardiac and vascular development, and influences
organogenesis. Development. 135:2981–2991. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Hansmann G, de Jesus Perez VA, Alastalo
TP, et al: An antiproliferative BMP-2/PPARgamma/apoE axis in human
and murine SMCs and its role in pulmonary hypertension. J Clin
Invest. 118:1846–1857. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Wybenga DR, Di Giorgio J and Pileggi VJ:
Manual and automated methods for urea nitrogen measurement in whole
serum. Clin Chem. 17:891–895. 1971.PubMed/NCBI
|
|
18.
|
Kueltzo LA, Ersoy B, Ralston JP and
Middaugh CR: Derivative absorbance spectroscopy and protein phase
diagrams as tools for comprehensive protein characterization: a
bGCSF case study. J Pharm Sci. 92:1805–1820. 2003. View Article : Google Scholar
|
|
19.
|
Paller MS, Hoidal JR and Ferris TF: Oxygen
free radicals in ischemic acute renal failure in the rat. J Clin
Invest. 74:1156–1164. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Morsy MD, Mostafa OA and Hassan WN: A
potential protective effect of alpha-tocopherol on vascular
complication in spinal cord reperfusion injury in rats. J Biomed
Sci. 17:552010. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Sasaki M and Joh T: Oxidative stress and
ischemia-reperfusion injury in gastrointestinal tract and
antioxidant, protective agents. J Clin Biochem Nutr. 40:1–12. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
van der Heijden M, Versteilen AM, Sipkema
P, van Nieuw Amerongen GP, Musters RJ and Groeneveld AB:
Rho-kinase-dependent F-actin rearrangement is involved in the
inhibition of PI3-kinase/Akt during ischemia-reperfusion-induced
endothelial cell apoptosis. Apoptosis. 13:404–412. 2008.
|
|
23.
|
Bayrak O, Turgut F, Karatas OF, et al:
Oral beta-glucan protects kidney against ischemia/reperfusion
injury in rats. Am J Nephrol. 28:190–196. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Schramm L, Weierich T, Heldbreder E,
Zimmermann J, Netzer KO and Wanner C: Endotoxin-induced acute renal
failure in rats: effects of L-arginine and nitric oxide synthase
inhibition on renal function. J Nephrol. 18:374–381.
2005.PubMed/NCBI
|
|
25.
|
Spek CA, Brüggemann LW and Borensztajn KS:
Protease-activated receptor 2 blocking peptide counteracts
endotoxin-induced inflammation and coagulation and ameliorates
renal fibrin deposition in a rat model of acute renal failure.
Shock. 33:339author reply 339–340. 2010. View Article : Google Scholar
|
|
26.
|
Petronijević ND, Mićić DV, Duricić B,
Marinković D and Paunović VR: Substrate kinetics of erythrocyte
membrane Na, K-ATPase and lipid peroxides in schizophrenia. Prog
Neuropsychopharmacol Biol Psychiatry. 27:431–440. 2003.PubMed/NCBI
|
|
27.
|
Kato R and Foëx P: Myocardial protection
by anesthetic agents against ischemia-reperfusion injury: an update
for anesthesiologists. Can J Anaesth. 49:777–791. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Jin YC, Kim W, Ha YM, et al: Propofol
limits rat myocardial ischemia and reperfusion injury with an
associated reduction in apoptotic cell death in vivo. Vascul
Pharmacol. 50:71–77. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Sánchez-Conde P, Rodriguez-López JM,
Nicolás JL, et al: The comparative abilities of propofol and
sevoflurane to modulate inflammation and oxidative stress in the
kidney after aortic cross-clamping. Anesth Analg. 106:371–378.
2008.PubMed/NCBI
|
|
30.
|
Yuzbasioglu MF, Aykas A, Kurutas EB and
Sahinkanat T: Protective effects of propofol against
ischemia/reperfusion injury in rat kidneys. Ren Fail. 32:578–583.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Yuzer H, Yuzbasioglu MF, Ciralik H, et al:
Effects of intravenous anesthetics on renal ischemia/reperfusion
injury. Ren Fail. 31:290–296. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Dhore CR, Cleutjens JP, Lutgens E, et al:
Differential expression of bone matrix regulatory proteins in human
atherosclerotic plaques. Arterioscler Thromb Vasc Biol.
21:1998–2003. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Sorescu GP, Song H, Tressel SL, et al:
Bone morphogenic protein 4 produced in endothelial cells by
oscillatory shear stress induces monocyte adhesion by stimulating
reactive oxygen species production from a nox1-based NADPH oxidase.
Circ Res. 95:773–779. 2004. View Article : Google Scholar
|
|
34.
|
Shin V, Zebboudj AF and Boström K:
Endothelial cells modulate osteogenesis in calcifying vascular
cells. J Vasc Res. 41:193–201. 2004. View Article : Google Scholar
|
|
35.
|
Sorescu GP, Sykes M, Weiss D, et al: Bone
morphogenic protein 4 produced in endothelial cells by oscillatory
shear stress stimulates an inflammatory response. J Biol Chem.
278:31128–31135. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Abe J: Bone morphogenetic protein (BMP)
family, SMAD signaling and Id helix-loop-helix proteins in the
vasculature: the continuous mystery of BMPs pleiotropic effects. J
Mol Cell Cardiol. 41:4–7. 2006. View Article : Google Scholar
|
|
37.
|
Csiszar A, Lehoux S and Ungvari Z:
Hemodynamic forces, vascular oxidative stress, and regulation of
BMP-2/4 expression. Antioxid Redox Signal. 11:1683–1697. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Ten Dijke P, Goumans MJ, Itoh F and Itoh
S: Regulation of cell proliferation by Smad proteins. J Cell
Physiol. 191:1–16. 2002.PubMed/NCBI
|
|
39.
|
Funaki C, Hodges RR and Dartt DA: Role of
cAMP inhibition of p44/p42 mitogen-activated protein kinase in
potentiation of protein secretion in rat lacrimal gland. Am J
Physiol Cell Physiol. 293:C1551–C1560. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40.
|
Csiszar A, Ahmad M, Smith KE, et al: Bone
morphogenetic protein-2 induces proinflammatory endothelial
phenotype. Am J Pathol. 168:629–638. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41.
|
Csiszar A, Smith KE, Koller A, Kaley G,
Edwards JG and Ungvari Z: Regulation of bone morphogenetic
protein-2 expression in endothelial cells: role of nuclear
factor-kappaB activation by tumor necrosis factor-alpha,
H2O2, and high intravascular pressure.
Circulation. 111:2364–2372. 2005. View Article : Google Scholar
|
|
42.
|
Yang YL, Ju HZ, Liu SF, et al: BMP-2
suppresses renal interstitial fibrosis by regulating
epithelial-mesenchymal transition. J Cell Biochem. 112:2558–2565.
2011. View Article : Google Scholar : PubMed/NCBI
|