Introduction
Chronic prostatitis (CP) has been described as one
of the most common illnesses in males aged <50 years (1), and exhibits different clinical
presentations (2). According to
the classification of the National Institutes of Health (NIH)
(3), class III CP/chronic pelvic
pain syndrome (CP/CPPS) is the most frequent category (4), in which either genitourinary symptoms
or pain are usually found and the impact on quality of life is
considerable (5). The efficacies
of current therapies for CP/CPPS are unsatisfactory (6). Phytotherapeutics are a noteworthy
option due to their generally minimal side-effects; however, few
have been subjected to scientific scrutiny and prospective
controlled clinical trials (7,8). In
previous years, a number of studies have shown that pollen extract
preparations are able to yield a durable and marked reduction of
symptoms in young males with CP/CPPS, with an improvement in semen
quality and a significant reduction in the NIH-Chronic Prostatitis
Symptom Index (CPSI) score (9–11).
Previously, Wagenlehner et al demonstrated that a
standardized pollen extract significantly improved the total
symptoms, pain and Quality of Well-Being (QoL) scores in patients
with inflammatory CP/CPPS without severe side-effects, highlighting
the role of the anti-inflammatory activity of pollen extract
(12). In the last year, Cai et
al demonstrated that pollen extract in association with
vitamins significantly improved the total symptoms, pain and QoL
scores in patients with non-inflammatory CP/CPPS without severe
side-effects in a phase II study (10). Furthermore, the association with
vitamins is likely to improve the antioxidant activity of the
pollen extract as well as the protective effect on nerves and also
reduce the pain in patients with inflammatory or non-inflammatory
CP/CPPS (10). The aim of the
present study was to assess the safety and efficacy of pollen
extract in association with vitamins in comparison with ibuprofen
in order to improve the quality of life of patients affected by
CP/CPPS by the relief of pain.
Materials and methods
Study design
In order to assess the safety and efficacy of pollen
extract in association with vitamins (DEPROX 500®) in
males with CP/CPPS, all consecutive patients with a clinical and
instrumental diagnosis of CP/CPPS (class IIIa or b), attending the
same urologic centre (Santa Chiara Regional Hospital, Trento,
Italy) between March and October 2012 were screened for this
prospective randomised controlled phase III study. The design of
the study was in accordance with the guidelines for clinical trials
in CP/CPPS described by the NIH Chronic Prostatitis Collaborative
Research Network (13). No placebo
arm was included. The possible biases caused by the lack of placebo
arm were considered in the results analysis. No placebo run-in
period was considered necessary due to the fact that all enrolled
patients were not blinded. The main outcome measure was the
improvement of quality of life at the end of the whole study
period, defined as the symptomatic improvement in the pain domain
of the NIH-CPSI. Clinical failure was defined as the persistence of
low quality of life following the treatment (failure to obtain a
reduction of the NIH-CPSI total score by ≥25%), or the suspension
of therapy for significant reported adverse effects (12). In addition, spontaneously reported
adverse events, or those noted by the investigator, were recorded
during the whole study period. The study was conducted in line with
Good Clinical Practice guidelines, with the ethical principles laid
down in the latest version of the Declaration of Helsinki. Written
informed consent was obtained from all patients prior to treatment.
Furthermore, this study was conducted in line with the Consolidated
Standards of Reporting Trials statement (The Ottawa Hospital
Research Institute, Ottawa, ON, Canada).
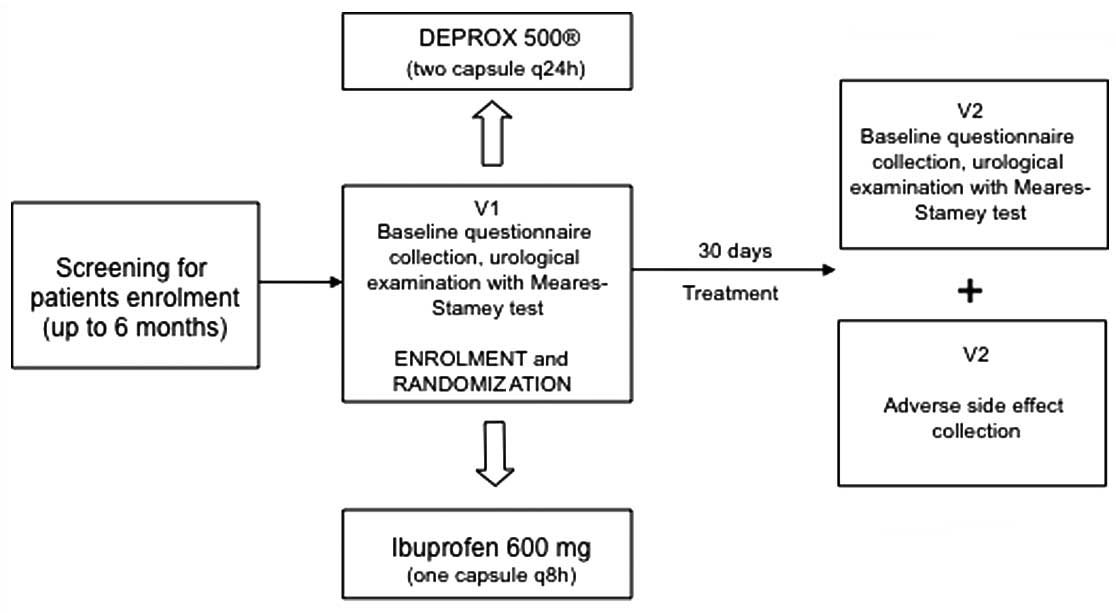
Study schedule
On arrival at the center, all eligible individuals
provided their written informed consent and underwent baseline
questionnaires, urological examination and the Meares-Stamey test
that was performed by the same urologist in accordance with the
procedure described in the European Association of Urology (EAU)
guidelines (14). All patients who
met the inclusion criteria undertook oral administration of DEPROX
500® (two capsules every 24 h) or ibuprofen (600 mg, one
tablet three times a day) for four weeks. Ibuprofen was selected in
accordance with the results obtained by Lee et al (15). Proton-pump inhibitors (PPIs) were
not routinely used due to the fact that all patients with
gastrointestinal bleeding or a history of duodenal or gastric
ulcers were excluded. Enrolled patients were not blinded to the
preventative treatment. All patients were assigned to the two
groups (DEPROX 500® and ibuprofen) according to a 1:1
randomization (Fig. 1). All
patients were contacted by telephone on day 14 of the therapy to
ensure correct timing and dose of treatment. Each subject was
scheduled for a follow-up examination at 30 days from starting
therapy, with a urological and microbiological examination and
questionnaire collection.
Inclusion and exclusion criteria
Inclusion criteria were the presence of symptoms of
pelvic pain for at least three months during the six months before
study entry, according to the EAU guidelines, a score in the pain
domain of the NIH-CPSI (14) of
>7 and a negative four-glass result in the Meares-Stamey test
(12). Subjects <18 and >65
years of age, affected by major concomitant diseases, with known
anatomical abnormalities of the urinary tract or with evidence of
other urological diseases, and with residual urine volume >50 ml
resulting from bladder outlet obstruction were excluded. Males with
a reported allergy to pollen extract, who had recently (<4
weeks) undergone oral or parental treatment or who were currently
using prophylactic antibiotic drugs were also excluded.
Additionally, all patients with a history of gastrointestinal
bleeding or duodenal or gastric ulcers were excluded. All patients
positive to tests for Chlamydia trachomatis (Ct),
Ureaplasma urealyticum, Neisseria gonorrhoeae, herpes
viruses (HSV 1/2) and human papillomavirus (HPV) were also
excluded.
Composition and characterization of the
extracts used
All patients who were randomized to the DEPROX
500® group underwent oral administration of two tablets
of DEPROX 500® in a single dose daily in the evening, in
line with our previous study (10)
and with the manufacturer’s instructions (IDI Integratori Dietetici
Italiani S.r.l, Sicily, Italy). Each administration contained 1 g
pollen extract (500 mg per tablet), and vitamins B1, B2, B6, B9,
B12 and PP. All compound analyses were carried out in accordance
with the procedures described by Fiamegos et al (16). All patients randomized to the
ibuprofen group received ibuprofen (600 mg) three times per
day.
Questionnaires and urological
examinations
The validated Italian versions of the NIH-CPSI
(17) and the International
Prostate Symptom Score (IPSS) (18) questionnaires were administered to
each patient. The questionnaires were self-administered when the
patient arrived at the urologic centre. Furthermore, patient
quality of life was measured by using an Italian version of the QoL
scale, a validated, multi-attribute health scale (19). This scale was selected because it
has been successfully applied to acute illnesses, whereas other
quality of life scales, including the Short Form-36 (SF-36) Health
Survey, are more suitable in chronic cases (20). Higher scores on the QoL scale
reflect a higher quality of life. In accordance with the study by
Nickel et al (21),
prostatitis-like symptoms were considered significant at a pain
score of ≥4. The NIH-CPSI was also used in determining clinical
therapy efficacy (21).
Sample collection and laboratory
procedures
All samples were collected during the urological
examination and immediately taken to the laboratory, under
refrigerated conditions, analysed for cultures and aliquoted for
DNA extraction and polymerase chain reaction for Ct, Neisseria
gonorrhoeae, HSV 1/2 and HPV detection. All subjects included
in the study underwent urinary culture for common bacteria, yeasts
and urogenital mycoplasma. Microbiological culture was carried out
in accordance with the methods described by Mazzoli et al
(22). DNA extraction and
purification from urine were performed using the EZ1 DNA Tissue kit
(Qiagen SpA, Milan, Italy), as described in our previous study
(22).
Statistical analysis
The primary target of the study was the symptomatic
improvement in the pain domain of the NIH-CPSI. In order to analyse
the homogeneity of the two groups, the baseline characteristics
were compared using the Student’s t-test and Mann-Whitney U
test for continuous variables and by the χ2 test for
categorical variables. The normal distribution of the variables was
assessed using the Kolmogorov-Smirnov test. Data were analysed
based on the intention-to-treat (ITT) approach. General
characteristics of the study participants were expressed using
descriptive statistics (means, standard deviations and ranges). The
required sample size for the present study was calculated under the
following conditions: Difference between the groups, 2±1 score
points in the NIH-CPSI pain domain; α error level, 0.05 two-sided;
statistical power, 80%; and anticipated effect size, Cohen’s d=0.5.
The calculation yielded 2×39 individuals per group. Randomization
based on a single sequence of random assignments (simple
randomization) was performed using a pseudo-random number generator
software (Research Randomizer Version 4.0, Social Psychology
Network, Wesleyan University, Middletown, CT, USA). Analysis of
variance (ANOVA) was used for comparing the means. The Bonferroni
adjustment test was also used at the second stage of the ANOVA. The
effect size between the means (Cohen’s d) was also calculated. The
differences between the groups regarding the NIH-CPSI results were
obtained using an ANOVA test. Statistical significance was achieved
when P<0.05. All reported P-values were two-sided. Statistical
analyses were performed using SPSS software, version 11.0 (SPSS,
Inc., Chicago, IL, USA) for Apple-Macintosh.
Results
Patients
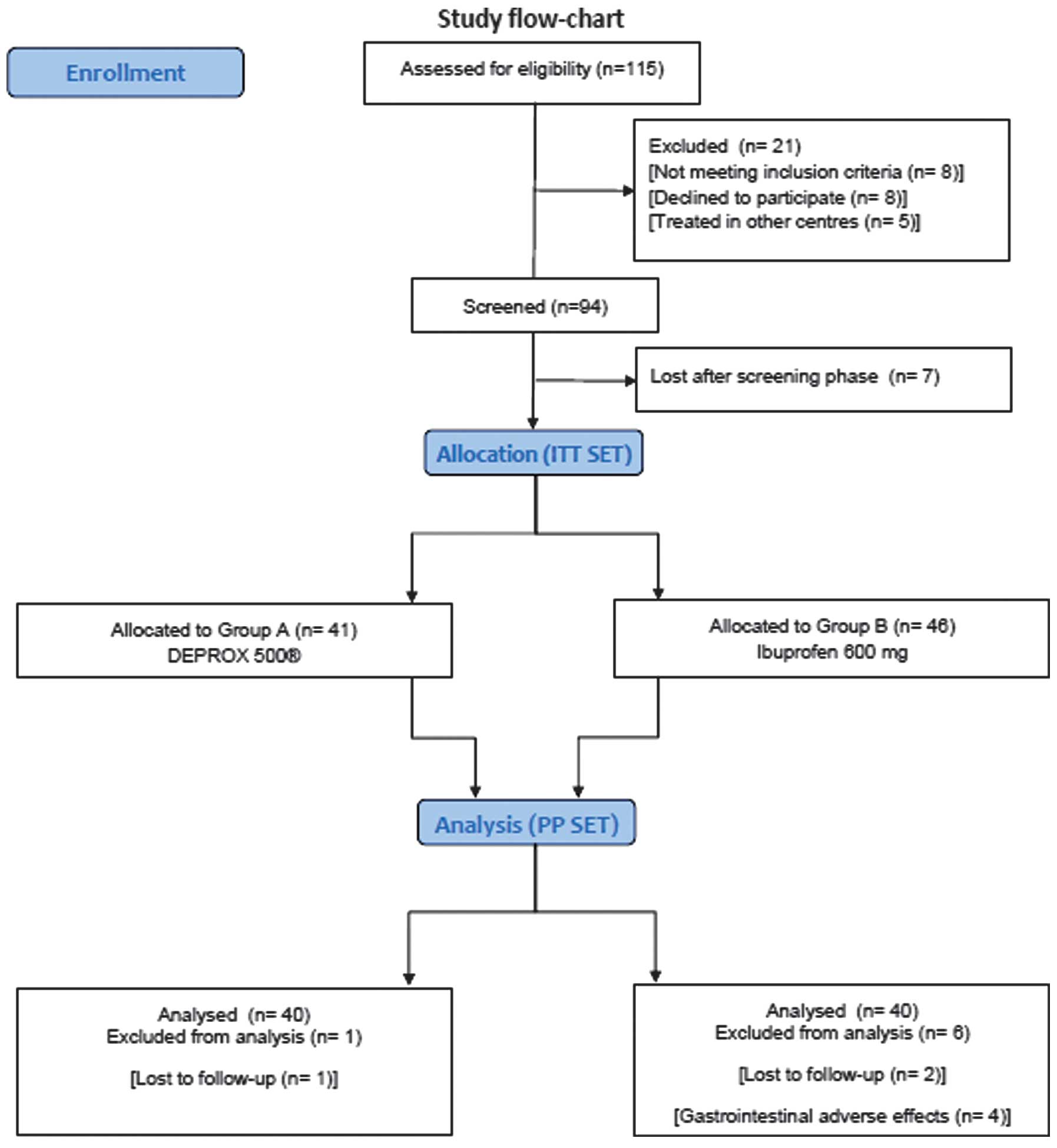
From the 115 patients attending the center for
prostatitis-like symptoms during the study period, 94 were
eventually enrolled and randomised. Out of the 21 patients excluded
from the study, eight refused to be enrolled, six reported adverse
effects to nonsteroidal anti-inflammatory drugs, two reported a
clinical history of gastrointestinal ulcers and five elected to be
treated in other centres. Additionally, seven patients were lost
subsequent to randomisation and 87 males were finally enrolled
(Fig. 2). The baseline
questionnaire mean scores were 25.9±2.1, 8.0±3.6 and 0.55±0.15 for
NIH-CPSI, IPSS and QoL, respectively. Historical medical
information and clinical data at enrolment are described in
Table I. No statistically
significant differences between the groups were identified.
 | Table IBaseline characteristics and clinical
parameters at enrolment. |
Table I
Baseline characteristics and clinical
parameters at enrolment.
| Parameter | DEPROX
500® group | Ibuprofen group |
|---|
| Patients, n | 41 | 46 |
| Age, yearsa | 33.8±6.78 | 33.7±5.44 |
| Marital status, n
(%) |
| Married | 19 (46.3) | 18 (39.1) |
| Unmarried | 22 (53.7) | 28 (60.8) |
| Educational
qualification, n (%) |
| Primary school | 5 (12.2) | 7 (15.2) |
| High school | 29 (70.7) | 27 (58.6) |
| University | 7 (17.1) | 12 (26.2) |
| Smoker status, n
(%) |
| Yes | 11 (26.8) | 13 (28.2) |
| No | 30 (73.2) | 33 (71.8) |
| Sexually active in
the past month, n (%) | 39 (95.1) | 41 (89.1) |
| Sexual behaviour, n
(%) |
| 1 partner | 33 (80.4) | 37 (80.4) |
| >1 partners | 8 (19.6) | 9 (19.6) |
| Contraceptive use, n
(%) |
| Condom | 29 (70.3) | 34 (73.9) |
| Coitus
interruptus | 12 (29.7) | 12 (26.1) |
| Start of CP history
(months)a | 18.7±4.28 | 19.1±3.99 |
| Symptoms score at
baselinea |
| NIH-CPSI | 24.9±2.1 | 25.5±3.0 |
| IPSS | 8.3±3.6 | 8.0±2.5 |
| QoL | 0.57±0.17 | 0.55±0.15 |
| Clinical
presentation, n (%) |
| Dysuria | 12 (29.2) | 14 (30.4) |
| Urgency | 4 (9.7) | 5 (10.8) |
| Dysuria +
frequency | 6 (14.6) | 5 (10.8) |
| Burning | 7 (17.0) | 9 (19.5) |
| Pain, n (%) |
| Perineal | 19 (46.4) | 21 (45.6) |
| Scrotal | 4 (9.7) | 4 (8.7) |
| Suprapubic | 8 (19.6) | 9 (19.6) |
| Lower
abdominal | 10 (24.3) | 12 (26.1) |
| Pain frequency, n
(%) |
| Daily | 33 (80.4) | 36 (78.2) |
| Weekly | 8 (19.6) | 10 (21.8) |
| Sexual Symptoms, n
(%) |
| ED | 12 (29.2) | 13 (28.2) |
| PE | 7 (17.0) | 6 (13.0) |
| ED + EP | 4 (9.7) | 4 (8.6) |
| CP/CPPS type, n
(%) |
| Type a | 12 (29.2) | 13 (28.2) |
| Type b | 29 (70.8) | 33 (71.8) |
Randomisation
Of the 87 enrolled patients (mean age 33.6±5.9
years), 41 received DEPROX 500® (group A), and 46
received 600 mg ibuprofen (group B). The treatment arms were
comparable for all variables at the enrolment and randomisation
visits.
Compliance with treatment schedule and
adverse effects
In group A, 1/41 patients (2.4%) had mild adverse
effects that did not require additional treatment (nausea), while
in group B, 7/46 patients (15.2%) reported nausea and epigastric
pain. In group A, 40 patients (97.5%) were analysed subsequent to
one being lost in follow-up. In group B, 38 patients (82.6%) were
analysed subsequent to two being lost to follow-up and four
discontinuing therapy due to gastrointestinal adverse effects. The
DEPROX 500® treatment was well tolerated in all the
patients analysed, and no significant drug-related side-effects
were identified. The analyses were carried out in the ITT (pollen
extract, n=41; ibuprofen, n=46) and per protocol (PP) populations
(pollen extract, n=40; ibuprofen, n=40) (Fig. 2).
Clinical and laboratory results at
follow-up (after one month of treatment)
At the follow-up examination, in the ITT set and
DEPROX 500® group, 31/41 patients (75.6%) reported an
improvement of quality of life, defined as a reduction of the
NIH-CPSI total score by ≥25%, compared with 19/46 (41.3%) in the
control group (P=0.002). In the PP set and DEPROX 500®
group, 31/40 patients (77.5%) reported an improvement of quality of
life, compared with 20/40 (50.0%) in the control group (P=0.019).
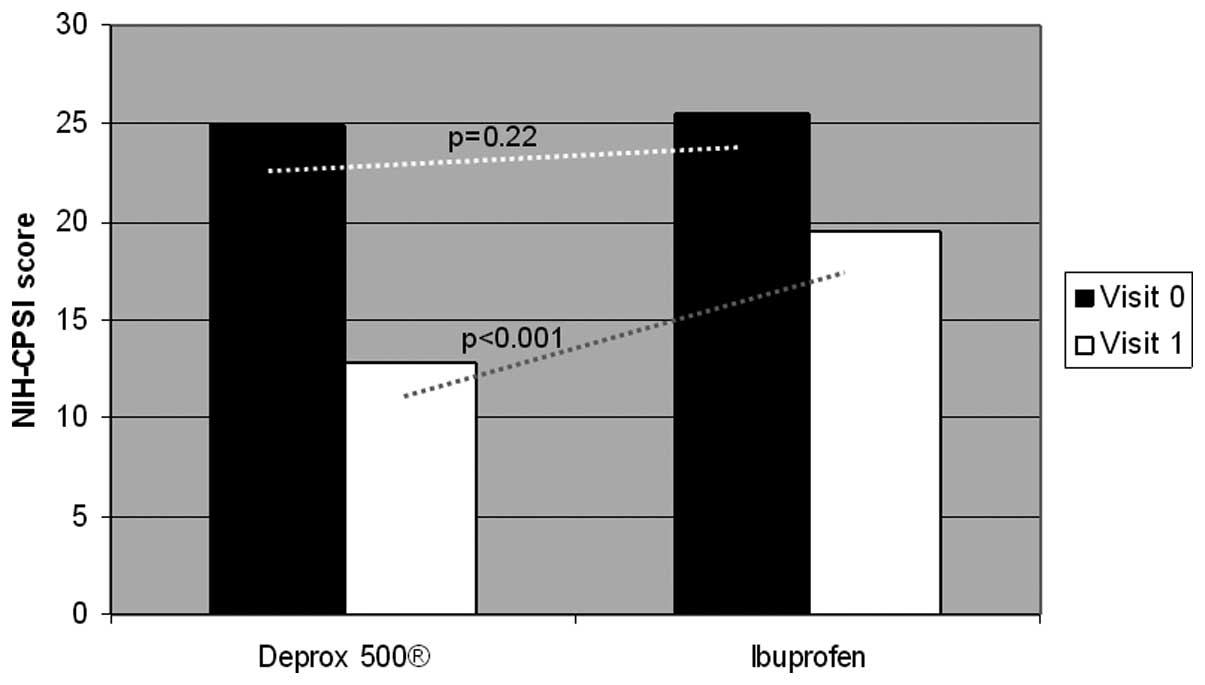
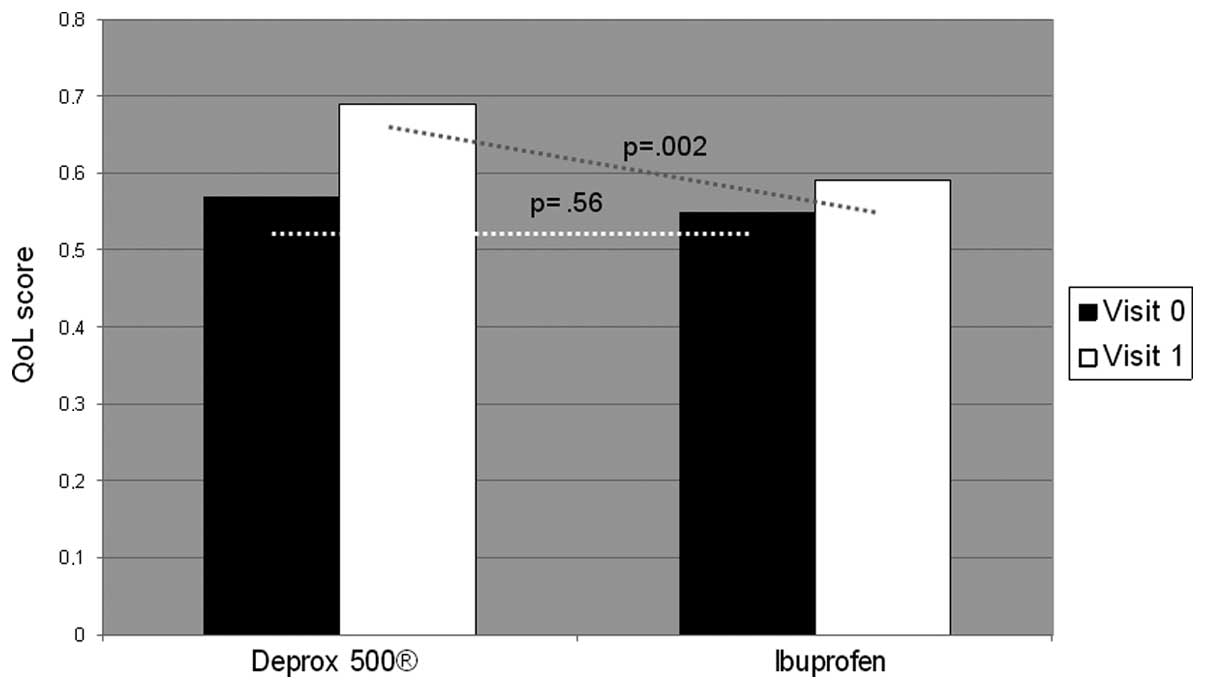
The questionnaire results at one month after treatment were as
follows: NIH-CPSI, 12.8±2.20; IPSS, 7.6±1.58; and QoL, 0.69±0.10 in
the DEPROX 500® group. By contrast the results in the
control group were: NIH-CPSI, 19.5±2.10; IPSS, 8.00±2.81; and QoL,
0.59±0.18. The higher improvement in the DEPROX 500®
group compared with the ibuprofen group was statistically
significant (treatment difference in NIH-CPSI pain domain: ITT,
−2.14±0.51, P<0.001; PP, −1.76±0.22, P<0.001). Statistically
significant differences were also reported in the NIH-CPSI
(P<0.001), and QoL (P=0.002) scores between the two visits in
the DEPROX 500® group and between the two groups
(Figs. 3 and 4). No statistically significant
differences were identified in the IPSS scores (P=0.43). All
patients were negative at the Meares-Stamey test evaluation. All
questionnaire results at the follow-up visit are presented in
Table II. The results of the
physical examinations, including vital signs, and laboratory
examinations showed no relevant changes from the baseline.
 | Table IIQuestionnaire results at the
follow-up visit. |
Table II
Questionnaire results at the
follow-up visit.
| Variable | DEPROX
500® group | Ibuprofen
group |
|---|
| NIH-CPSI
(P<0.001)a | 12.8±2.20 | 19.5±2.10 |
| IPSS
(P=0.87)a | 7.6±1.58 | 8.00±2.81 |
| QoL
(P=0.002)a | 0.69±0.10 | 0.59±0.18 |
| Reduction in
NIH-CPSI pain domainb, n
(%) | 31 (77.5) | 19 (47.5) |
| Efficacy outcomes
(NIH-CPSI pain domain)a,c | −4.36±0.51 | −2.22±0.53 |
| Efficacy outcomes
(NIH-CPSI pain domain)a,d | −3.86±0.21 | −2.10±0.20 |
Sub-analysis on the basis of CP/CPPS
type
Of the 87 patients, 25 (28.7%) showed inflammatory
CP/CPPS (type IIIa), while 62 (71.3%) exhibited type IIIb. A
statistically significant difference was identified between the two
groups in terms of pain relief and QoL improvement when stratified
by CP/CPPS type. In fact, in the DEPROX 500® group,
patients affected by type IIIb CP/CPPS showed higher QoL results
and a lower pain level following treatment (the NIH-CPSI score was
24.8±1.8 at the enrolment versus 11.7±1.7 at the follow-up visit;
P<0.001) when compared with type IIIa CP/CPPS patients (Table III). No differences were reported
between the ITT or PP sets.
 | Table IIIResults of the sub-analysis on the
basis of CP/CPPS type a or b. |
Table III
Results of the sub-analysis on the
basis of CP/CPPS type a or b.
| Variable | DEPROX
500® group | Ibuprofen
group |
|---|
| Patients, n | 40 | 40 |
| Type a, n (%) | 14 (35) | 11 (27.5) |
| Type b, n (%) | 29 (65) | 32 (72.5) |
| NIH-CPSIa |
| Type a | 13.1±1.8 | 20.2±1.9 |
| Type b | 11.7±1.7 | 19.1±2.7 |
| IPSSa |
| Type a | 7.9±0.9 | 7.9±3.1 |
| Type b | 7.4±1.5 | 8.0±2.2 |
| QoLa |
| Type a | 0.61±0.3 | 0.57±0.2 |
| Type b | 0.70±0.1 | 0.60±0.1 |
Discussion
The major finding of the present study was that
DEPROX 500® is able to provide early pain relief and
improve the quality of life in patients with CP/CPPS without severe
side-effects, when compared with ibuprofen. Furthermore, it was
revealed that patients affected by type IIIb CP/CPPS may obtain
greater advantages from this therapy. These findings lead to
several points of discussion. Firstly, the early pain relief. In
2006, Elist (23), using a
double-blind study with random distribution versus placebo,
demonstrated the superiority of pollen extract versus placebo in
terms of improvement in pain score and the filling and emptying
symptoms from the start to the end of the treatment after six
months of therapy. Additionally, in 2009, Wagenlehner et al
(12) showed that pollen extract
improved symptoms, pain and quality of life after 12 weeks of
treatment in patients with this condition, with differences in
favour of pollen extract at six weeks of treatment compared with
the placebo, and the treatment being well tolerated. These two
studies treated the patients for at least six weeks (12,23).
Consistent with our previous study (10), 30 days of treatment with DEPROX
500® in the present study was able to provide
significant results in terms of pain reduction when compared with
ibuprofen. This effect is possibly due to the association between
the pollen extract and vitamins B6 and B12 that improve the
antioxidant activity of pollen extract with the protective effect
on nerves. B vitamins including thiamine (B1), pyridoxine (B6) and
cyanocobalamin (B12) are capable of antinociception in experimental
animals with acute and chronic pain evoked by electrical, chemical
and thermal stimulation, primary neuronal injury and diabetes
(24,25). Notably, several studies have
demonstrated that certain B vitamins, particularly B6 and B12, are
able to protect neurons from certain injuries (26,27).
The B vitamins, B1, B6 and B12, are clinically useful in the
treatment of certain painful conditions including lumbago,
sciatica, trigeminal neuralgia and chronic pain associated with
diabetic polyneuropathy (28).
Finally, we hypothesized that the early improvement on pain relief
is due to the protective effect on nerves, and the following
improvement in quality of life could be due to the antioxidant
activity of pollen extract. Indeed, previous studies in which only
pollen extract was administered demonstrated an improvement in
quality of life and pain relief after ≥6 weeks of treatment.
Furthermore, on the basis of the sub-analysis, the patients that
best obtained the important advantages from this therapy were those
with non-inflammatory CP/CPPS. Contrasting with previous studies,
the present study revealed that patients with non-inflammatory
CP/CPPS showed improved results compared with those with
inflammatory CP/CPPS. This is possibly due to the fact that the
protective effect on nerves of B vitamins occurs earlier than the
anti-inflammatory effect of pollen extract. In this sense, DEPROX
500® is able to provide improved results in terms of
early pain reduction in patients with non-inflammatory CP/CPPS.
DEPROX 500® was generally well tolerated over the full
study period.
The present study had a few limitations that should
be taken into account: The small number of enrolled patients, a
short follow-up period, a selected patient population, the lack of
control group and that this was not a blinded study. Given the lack
of prove efficacy of conventional therapies, alternative treatment
options are urgently required and pollen extract in association
with vitamins should be an noteworthy option due to its generally
low side-effects and promising results in terms of quality of life
improvement.
In conclusion, given the aforementioned limitations,
DEPROX 500® significantly improved the total symptoms,
pain, and quality of life compared with ibuprofen in patients with
CP/CPPS, without severe side-effects.
Acknowledgements
The authors would like to thank Professor John
Denton (Department of Modern Philology, University of Florence,
Florence, Italy) for language revision of the study.
References
|
1
|
Collins MM, Stafford RS, O’Leary MP and
Barry MJ: How common is prostatitis? A national survey of physician
visits. J Urol. 159:1224–1228. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Barbalias GA: Clinical and therapeutical
guidelines for chronic prostatitis. from bacteriological importance
to neuromuscular considerations. Eur Urol. 37:116–117. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Workshop Committee of the National
Institute of Diabetes and Digestive and Kidney Disease (NIDDK).
Chronic Prostatitis Workshop; Bethesda, MD. 7–8 December, 1995;
|
|
4
|
Schaeffer AJ: Classification (traditional
and National Institutes of Health) and demographics of prostatitis.
Urology. 60(6 Suppl): 5–7. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nickel JC: Role of alpha1-blockers in
chronic prostatitis syndromes. BJU Int. 101(Suppl 3): 11–16. 2008.
View Article : Google Scholar
|
|
6
|
Tuğcu V, Taşçi AI, Fazlioğlu A, et al: A
placebo-controlled comparison of the efficiency of triple- and
monotherapy in category III B chronic pelvic pain syndrome (CPPS).
Eur Urol. 51:1113–1118. 2007.PubMed/NCBI
|
|
7
|
Herati AS and Moldwin RM: Alternative
therapies in the management of chronic prostatitis/chronic pelvic
pain syndrome. World J Urol. 31:761–766. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shoskes DA, Zeitlin SI, Shahed A and
Rajfer J: Quercetin in men with category III chronic prostatitis: a
preliminary prospective, double-blind, placebo-controlled trial.
Urology. 54:960–963. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rugendorff EW, Weidner W, Ebeling L and
Buck AC: Results of treatment with pollen extract (Cernilton N) in
chronic prostatitis and prostatodynia. Br J Urol. 71:433–438. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cai T, Luciani LG, Caola I, et al: Effects
of pollen extract in association with vitamins (DEPROX
500®) for pain relief in patients affected by chronic
prostatitis/chronic pelvic pain syndrome: results from a pilot
study. Urologia. 80(Suppl 22): 5–10. 2013.PubMed/NCBI
|
|
11
|
Kamijo T, Sato S and Kitamura T: Effect of
cernitin pollen-extract on experimental nonbacterial prostatitis in
rats. Prostate. 49:122–131. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wagenlehner FM, Schneider H, Ludwig M,
Schnitker J, Brähler E and Weidner W: A pollen extract (Cernilton)
in patients with inflammatory chronic prostatitis-chronic pelvic
pain syndrome: a multicentre, randomised, prospective,
double-blind, placebo-controlled phase 3 study. Eur Urol.
56:544–551. 2009. View Article : Google Scholar
|
|
13
|
Propert KJ, Alexander RB, Nickel JC, et
al; Chronic Prostatitis Collaborative Research Network. Design of a
multicenter randomized clinical trial for chronic
prostatitis/chronic pelvic pain syndrome. Urology. 59:870–876.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Grabe M, Bjerklund-Johansen TE, Botto H,
et al: Guidelines on Urological Infections. European Association of
Urology; Arnhem, The Netherlands: pp. 662012
|
|
15
|
Lee CB, Ha US, Lee SJ, Kim SW and Cho YH:
Preliminary experience with a terpene mixture versus ibuprofen for
treatment of category III chronic prostatitis/chronic pelvic pain
syndrome. World J Urol. 24:55–60. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fiamegos YC, Nanos CG, Vervoort J and
Stalikas CD: Analytical procedure for the in-vial
derivatization-extraction of phenolic acids and flavonoids in
methanolic and aqueous plant extracts followed by gas
chromatography with mass-selective detection. J Chromatogr A.
1041:11–18. 2004. View Article : Google Scholar
|
|
17
|
Giubilei G, Mondaini N, Crisci A, et al:
The Italian version of the National Institutes of Health Chronic
Prostatitis Symptom Index. Eur Urol. 47:805–811. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Badía X, García-Losa M and Dal-Ré R:
Ten-language translation and harmonization of the International
Prostate Symptom Score: developing a methodology for multinational
clinical trials. Eur Urol. 31:129–140. 1997.PubMed/NCBI
|
|
19
|
Kaplan RM, Bush JW and Berry CC: Health
status: types of validity and the index of well-being. Health Serv
Res. 11:478–507. 1976.PubMed/NCBI
|
|
20
|
Ernst EJ, Ernst ME, Hoehns JD and Bergus
GR: Women’s quality of life is decreased by acute cystitis and
antibiotic adverse effects associated with treatment. Health Qual
Life Outcomes. 3:452005.
|
|
21
|
Nickel JC, Downey J, Hunter D and Clark J:
Prevalence of prostatitis-like symptoms in a population based study
using the National Institutes of Health chronic prostatitis symptom
index. J Urol. 165:842–845. 2001. View Article : Google Scholar
|
|
22
|
Mazzoli S, Cai T, Rupealta V, et al:
Interleukin 8 and anti-chlamydia trachomatis mucosal IgA as
urogenital immunologic markers in patients with C.
trachomatis prostatic infection. Eur Urol. 51:1385–1393. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Elist J: Effects of pollen extract
preparation Prostat/Poltit on lower urinary tract symptoms in
patients with chronic nonbacterial prostatitis/chronic pelvic pain
syndrome: a randomized, double-blind, placebo-controlled study.
Urology. 67:60–63. 2006. View Article : Google Scholar
|
|
24
|
Jolivalt CG, Mizisin LM, Nelson A, et al:
B vitamins alleviate indices of neuropathic pain in diabetic rats.
Eur J Pharmacol. 612:41–47. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yu CZ, Liu YP, Liu S, Yan M, Hu SJ and
Song XJ: Systematic administration of B vitamins attenuates
neuropathic hyperalgesia and reduces spinal neuron injury following
temporary spinal cord ischaemia in rats. Eur J Pain. 18:76–85.
2014. View Article : Google Scholar
|
|
26
|
Wang ZB, Gan Q, Rupert RL, Zeng YM and
Song XJ: Thiamine, pyridoxine, cyanocobalamin and their combination
inhibit thermal, but not mechanical hyperalgesia in rats with
primary sensory neuron injury. Pain. 114:266–277. 2005. View Article : Google Scholar
|
|
27
|
Hung KL, Wang CC, Huang CY and Wang SJ:
Cyanocobalamin, vitamin B12, depresses glutamate release through
inhibition of voltage-dependent Ca2+ influx in rat
cerebrocortical nerve terminals (synaptosomes). Eur J Pharmacol.
602:230–237. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mäder R, Deutsch H, Siebert GK, et al:
Vitamin status of inpatients with chronic cephalgia and dysfunction
pain syndrome and effects of a vitamin supplementation. Int J Vitam
Nutr Res. 58:436–441. 1988.PubMed/NCBI
|