Introduction
Cervical cancer is the third most commonly diagnosed
cancer and the fourth leading cause of female mortality worldwide,
with >85% of the associated deaths occurring in developing
countries (1). The American Cancer
Society estimates ~12,360 new cases of invasive cervical cancer and
~4,020 mortalities in the United States for 2014 (2). The one-year survival rate for females
with cervical cancer is 87% and the five-year survival rate is 68%.
When detected early, the five-year survival rate for patients with
invasive cervical cancer is 91% (3). Cervical cancer develops slowly,
taking 10–15 years to develop into cancer from a pre-cancerous
condition called dysplasia. Although fully treatable in the early
stages, once the cancer has metastasized, patient outcome is
poor.
Critical to tumor cell invasion are the processes of
cell attachment, proteolytic degradation of the extracellular
matrix (ECM) and migration through the disrupted matrix (4). Rath and Pauling (5) proposed that the use of nutrients,
such as lysine and ascorbic acid, to target plasmin-mediated
connective tissue degradation should be considered as a universal
approach to control tumor growth and expansion. Binding to
plasminogen active sites, lysine blocks plasminogen activation into
plasmin by tissue plasminogen activator; thus, it modulates the
plasmin-induced matrix metalloproteinase (MMP) activation cascade
(6). We have previously developed
strategies to inhibit cancer growth and its spread using complex
micronutrient supplementation with select natural compounds, such
as lysine, proline, ascorbic acid and green tea extract (7). This nutrient mixture (NM)
demonstrated pleiotropic synergistic anticancer activity in
vivo and in vitro in several cancer cell lines through
the inhibition of cancer cell growth, MMP secretion, invasion,
metastasis and angiogenesis (7).
In previous studies we found that NM significantly
inhibited the proliferation of cervical cancer HeLa cells in
vitro, the secretion of MMP-2 and -9, urokinase plasminogen
activator activity and Matrigel™ invasion, and enhanced tissue
inhibitor of matrix metalloproteinases 2 activity (8,9). In
the present study the in vivo effects of NM supplementation
on tumor growth and cancer markers in cervical cancer HeLa cell
tumor xenografts in female nude mice were investigated. The cancer
cell markers studied by tumor immunohistochemistry (IHC) were as
follows: Ki67 (proliferation marker); MMP-2 and -9 (metastasis
markers); vascular endothelial growth factor (VEGF) (angiogenesis
marker); terminal deoxynucleotidyl transferase dUTP nick end
labeling (TUNEL) and B-cell lymphoma 2 (Bcl-2) (apoptosis markers);
cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS)
(inflammatory markers) and glutathione S-transferase π (GSTπ)
(specific cancer marker).
Materials and methods
Animals
Female athymic nude mice, approximately five weeks
of age on arrival, were purchased from Simonsen Laboratories
(Gilroy, CA, USA) and maintained in microisolator cages under
pathogen-free conditions on a 12-h light/dark schedule for a week.
All procedures were performed according to a protocol approved by
an internal institutional Animal Safety Review Committee (Dr Rath
Research Institute, Santa Clara, CA, USA) and followed guidelines
for the humane and customary care and use of experimental
animals.
Experimental design
Following housing for a week, 5/6-week-old female
athymic nude mice (n=12) were inoculated subcutaneously with
3×106 HeLa cells in 0.2 ml phosphate-buffered saline and
0.1 ml Matrigel (BD Biosciences, Bedford, MA, USA). Following
injection, the mice were randomly divided into two groups: the
control group mice were fed regular Purina mouse chow (Laboratory
Rodent Diet 5001; Purina Mills, Richmond, IN, USA) and the NM mice
were fed the regular diet supplemented with 0.5% NM (w/w). During
the study, the mice consumed, on average, 4 g/day of their
respective diets; thus, the supplemented mice received ~20 mg
NM/day. After four weeks, the mice were sacrificed and their tumors
were excised, weighed and processed for histology. The mean weight
of the mice at the beginning and end of the study did not differ
significantly between the groups.
Composition of the NM
The NM was composed of 700 mg vitamin C (as ascorbic
acid and as Mg, Ca and palmitate ascorbate); 1,000 mg L-lysine; 750
mg L-proline; 500 mg L-arginine; 200 mg N-acetyl cysteine; 1,000 mg
standardized green tea extract; 30 μg selenium; 2 mg copper; and 1
mg manganese. All nutrients were obtained from Vita-Tech
International Inc. (Tustin, CA, USA). The certificate of analysis
for the green tea extract obtained from US Pharma Lab. (North
Brunswick, NJ, USA) indicated the following characteristics: Total
polyphenol, 80%; catechins, 60%; epigallocatechin gallate, 35%; and
caffeine 1.0%.
IHC
Tumors were placed in a formalin cassette and sent
to IDEXX (Sacramento, CA, USA) and HistoTox (Boulder, CO, USA) for
analyses. Formalin-fixed samples of tumors were trimmed, processed,
blocked, sectioned and stained with hematoxylin and eosin, and
evaluated microscopically by IDEXX Pathology. The IHC of the tumor
sections conducted by HistoTox Labs assessed Ki67, MMP-2 and MMP-9,
VEGF, TUNEL, Bcl-2 and iNOS.
Results
Tumor growth
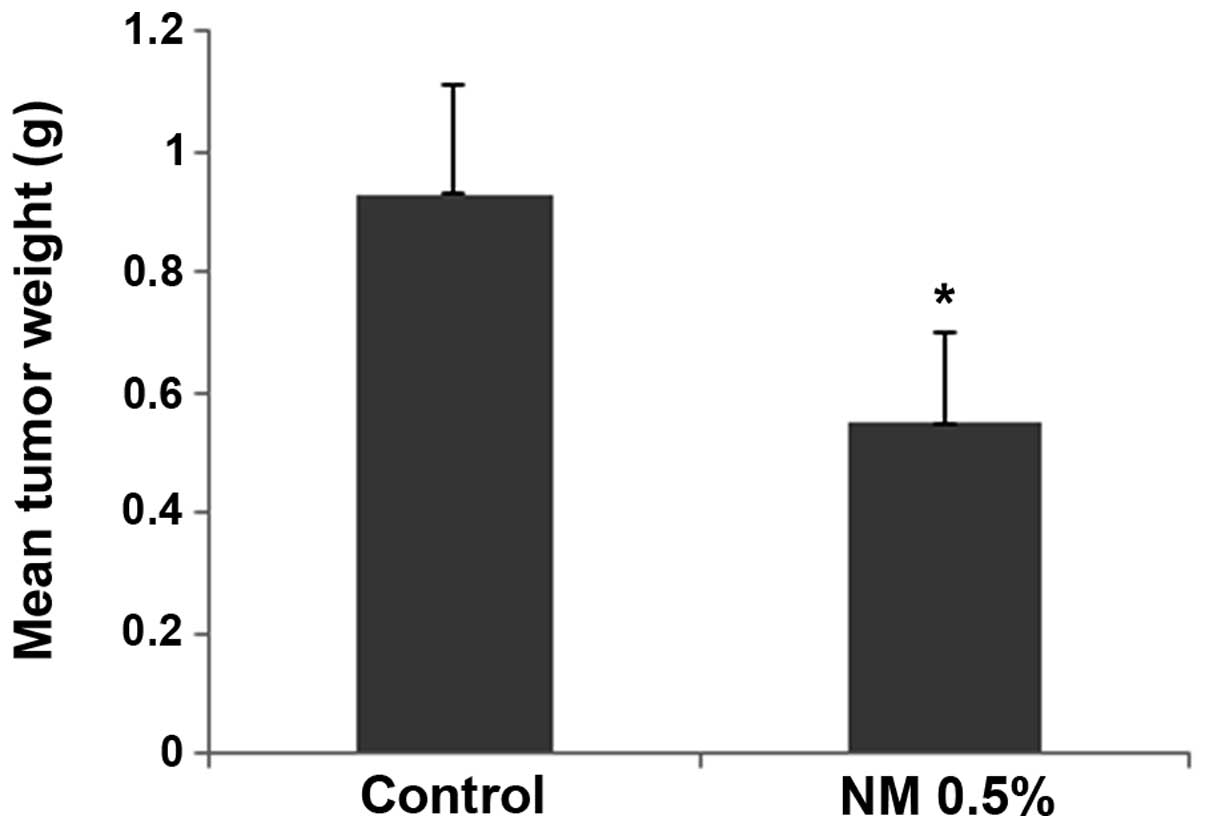
The NM strongly inhibited the growth of HeLa
xenografts in female nude mice. The mean tumor weight was reduced
to 59% (P=0.001) in mice with NM 0.5% dietary supplementation
compared with the tumor weight in the controlled-diet mice, as
shown in Fig. 1.
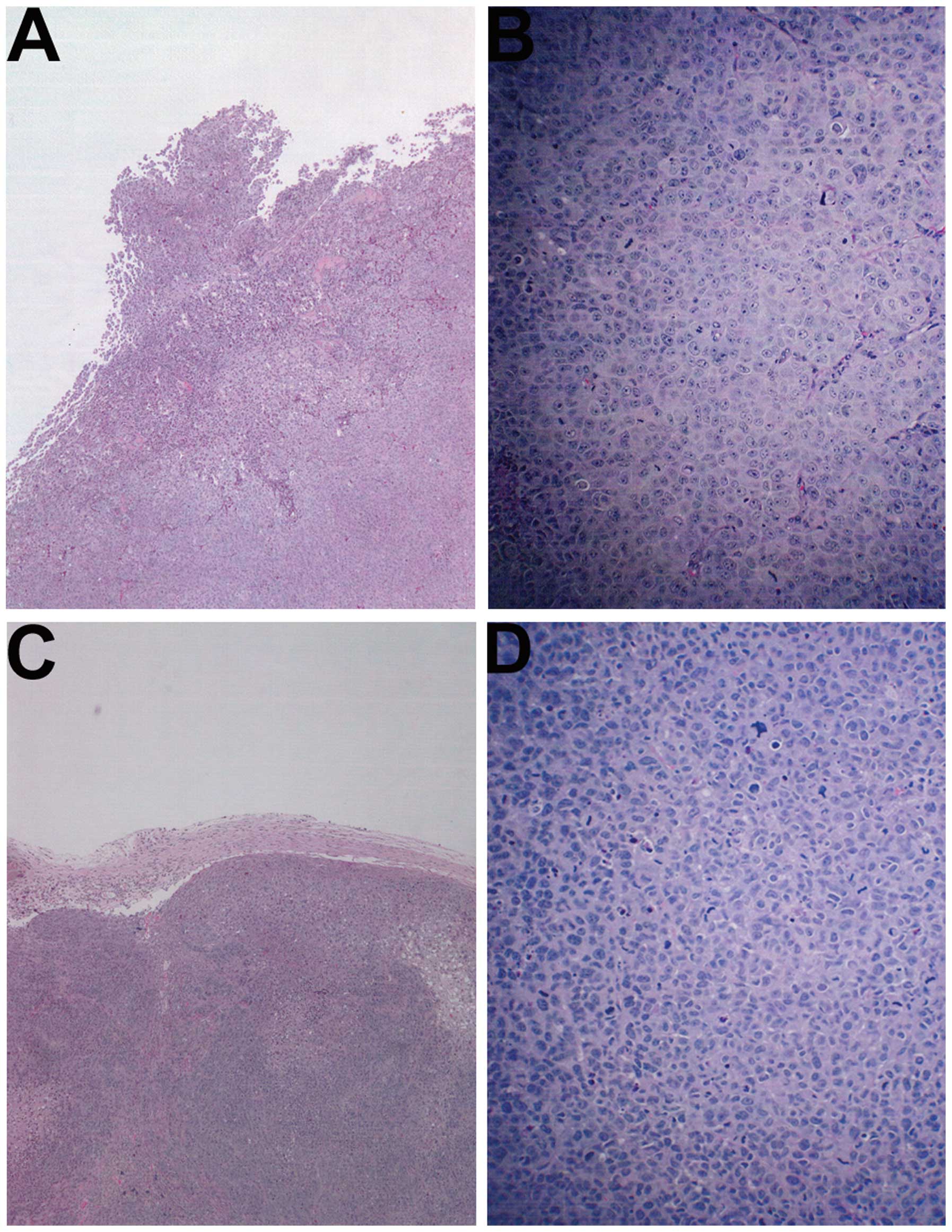
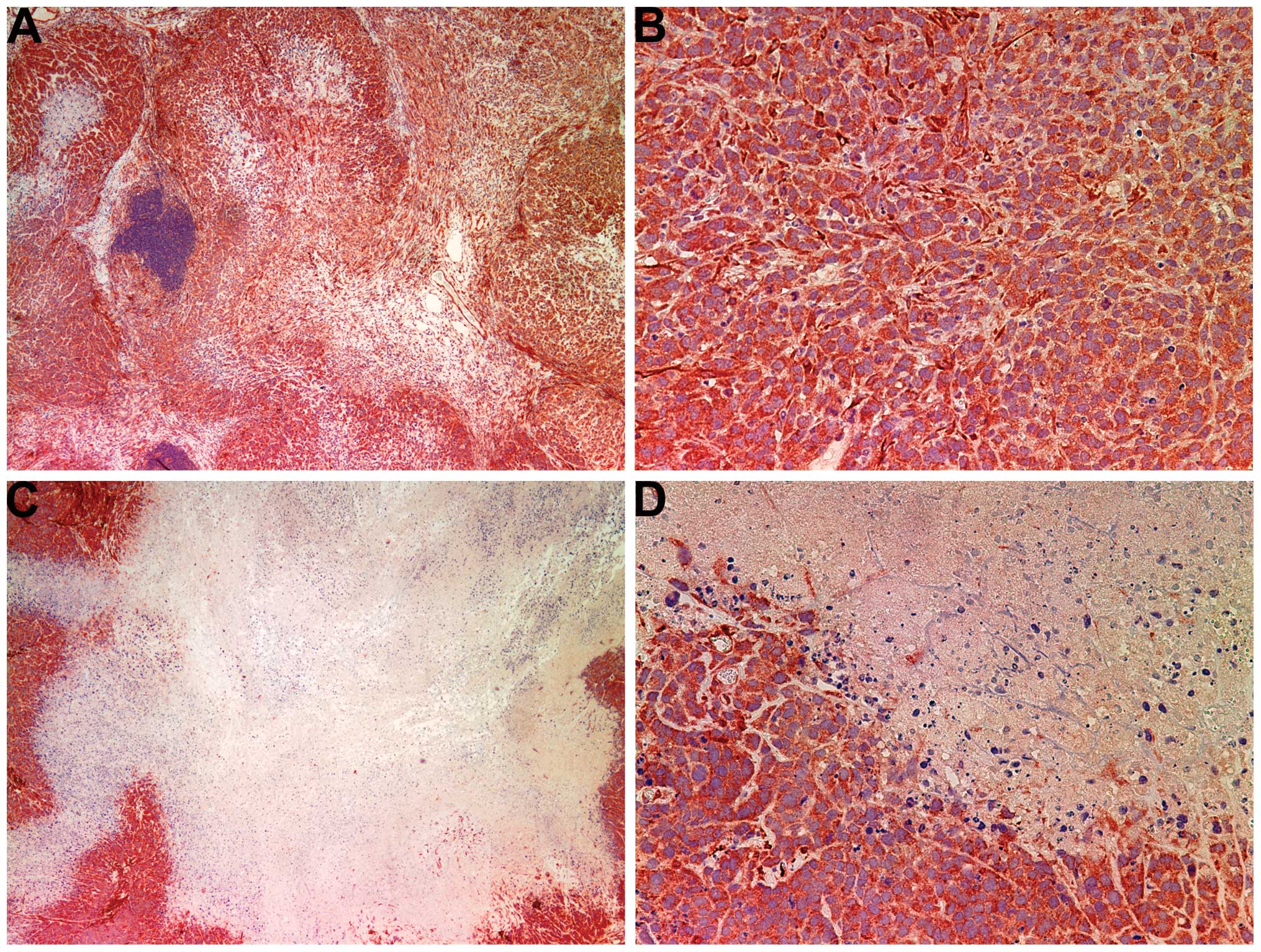
Histology of tumors
The histology of the tumors in both groups was
comparable, excepting a smaller tumor size in the NM-treated group.
A salient feature of the results was that the tumor border in the
untreated groups was ill defined, whereas in the NM group the
fibrous capsule was prominent (Fig.
2).
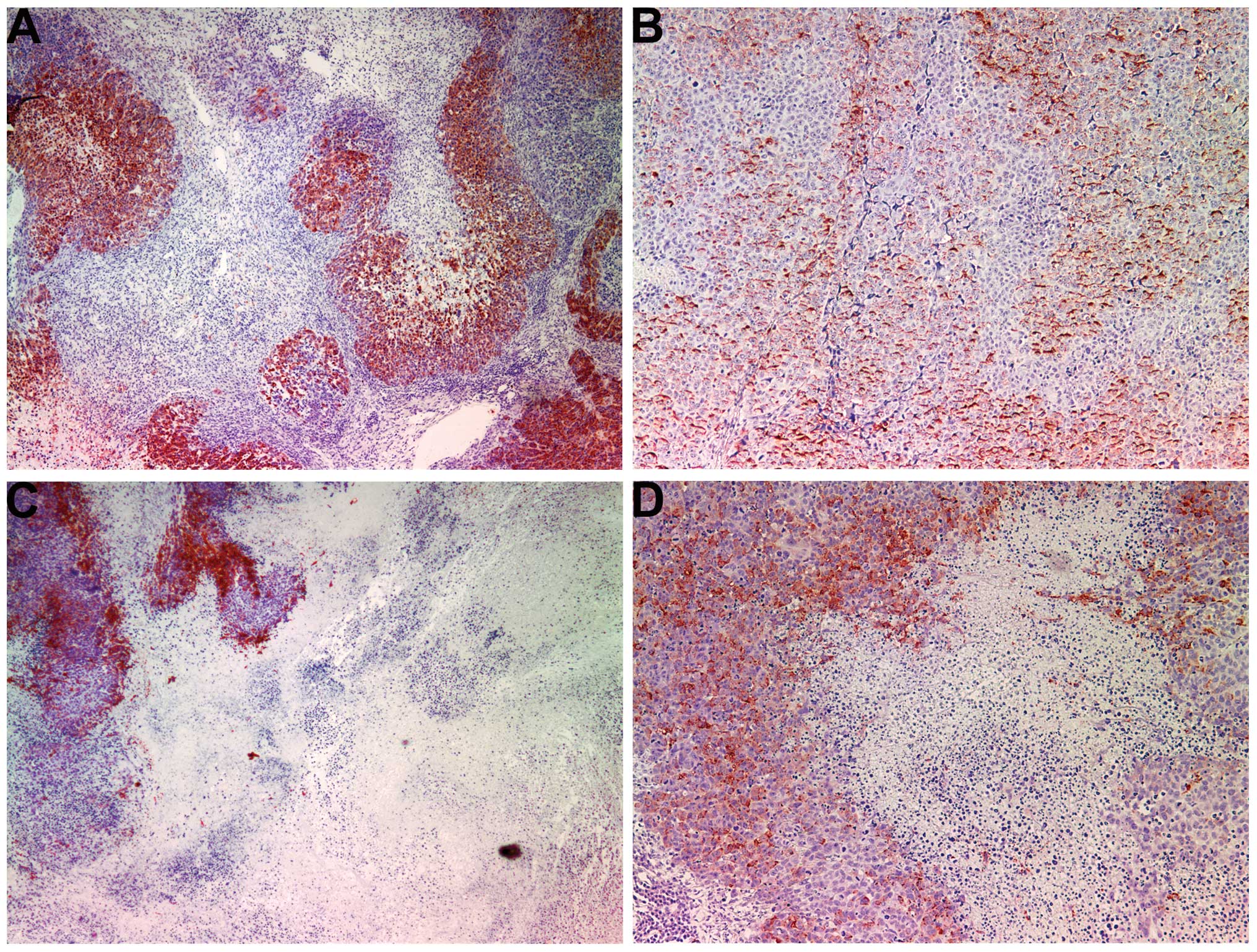
Proliferation: Ki67
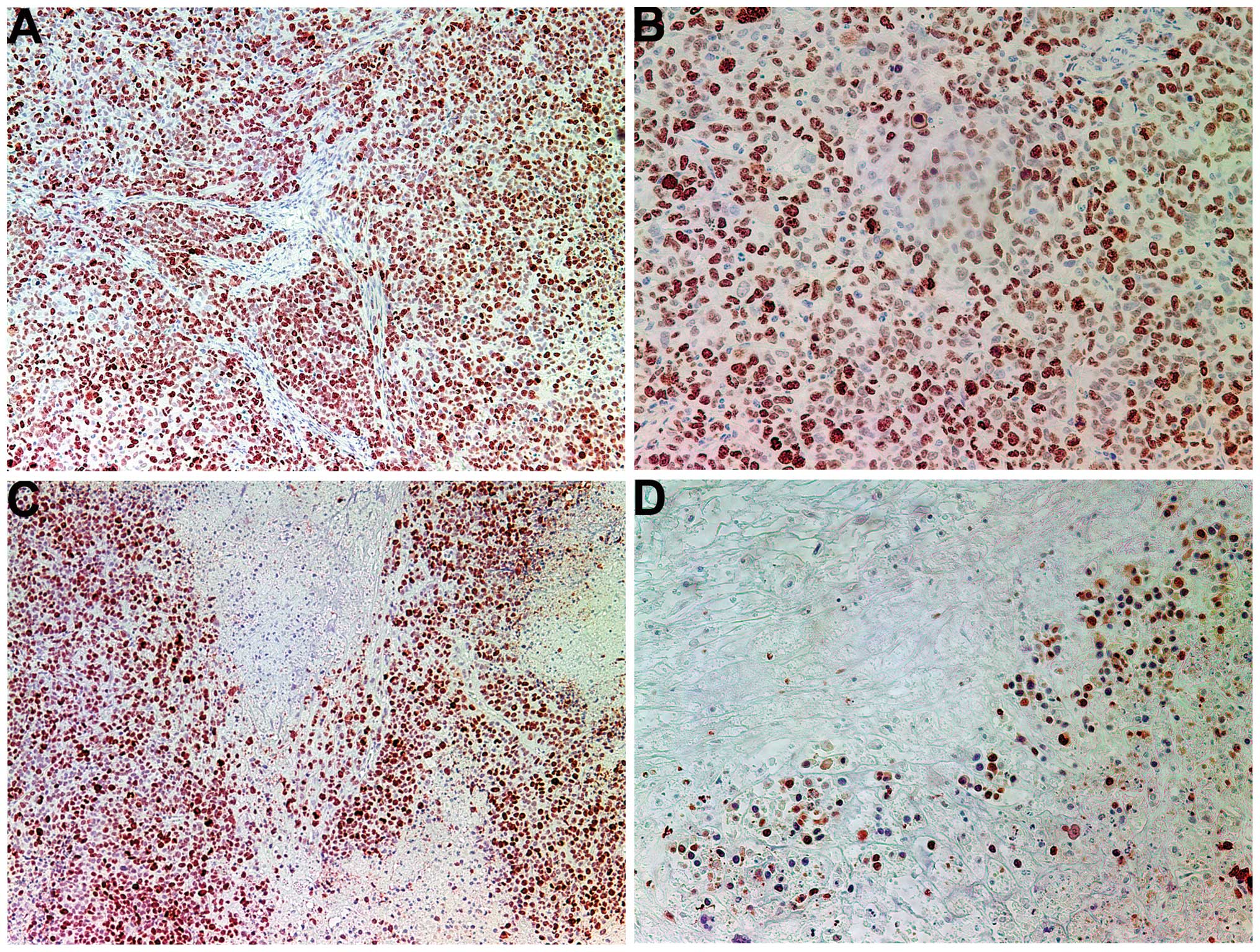
The proliferation marker Ki67 showed a lower
intensity and frequency of staining in the NM group compared with
that in the control group. Tumors from the control group showed
≥60% of cells positive for Ki67, with the positive cells dispersed
uniformly throughout the tumor amid the Ki67-negative cells
(Fig. 3A and B). The NM-treated
tumor sections exhibited large areas of Ki67-negative nucleated
cells amid areas of positive cells (Fig. 3C and D). The Ki67-positive cells
were observed to aggregate circumferentially within the tumor
capsule, away from the core, in the NM-treated tumors, whereas in
the untreated tumors the Ki67-positive cells were found to be
distributed uniformly throughout.
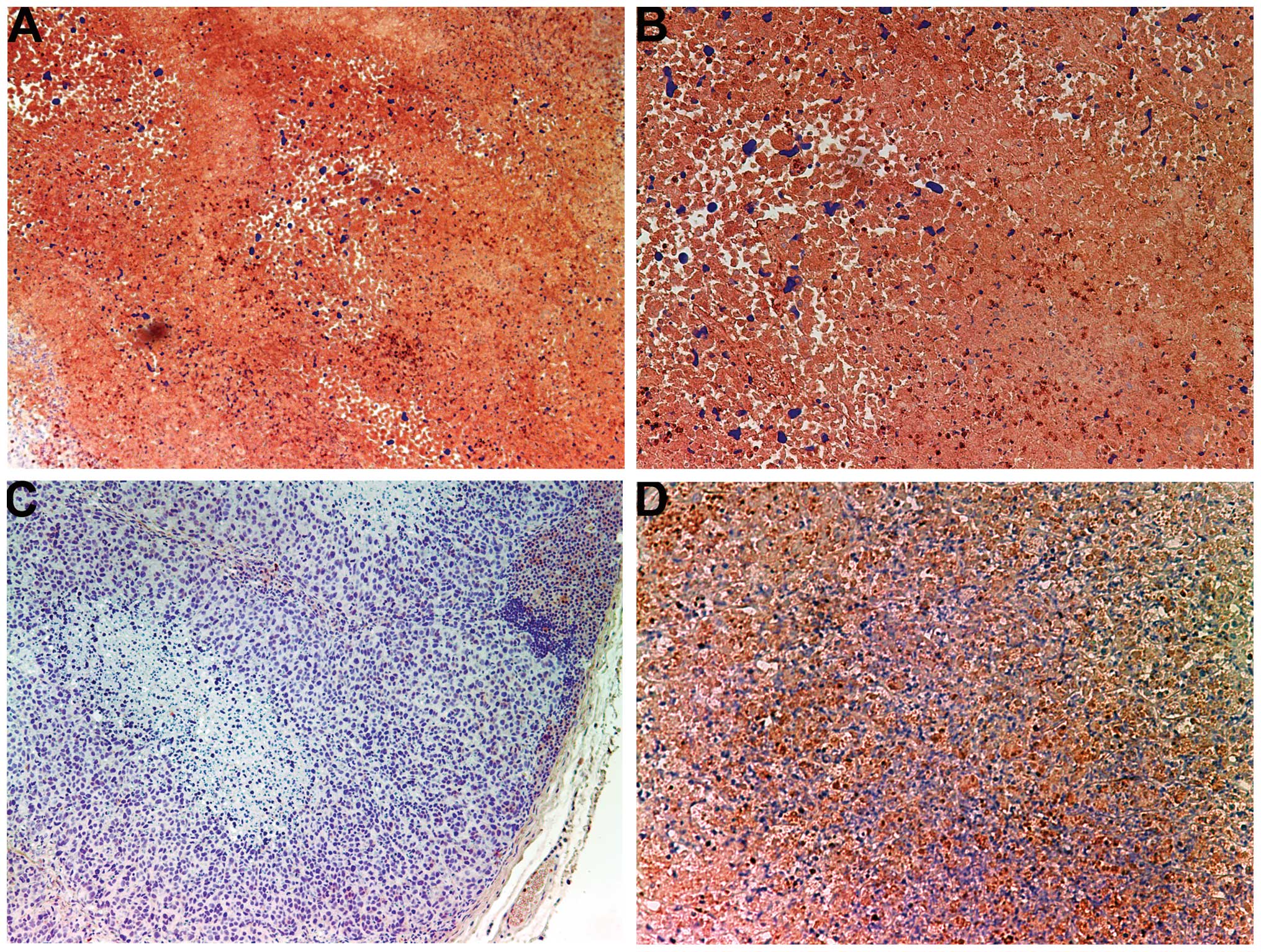
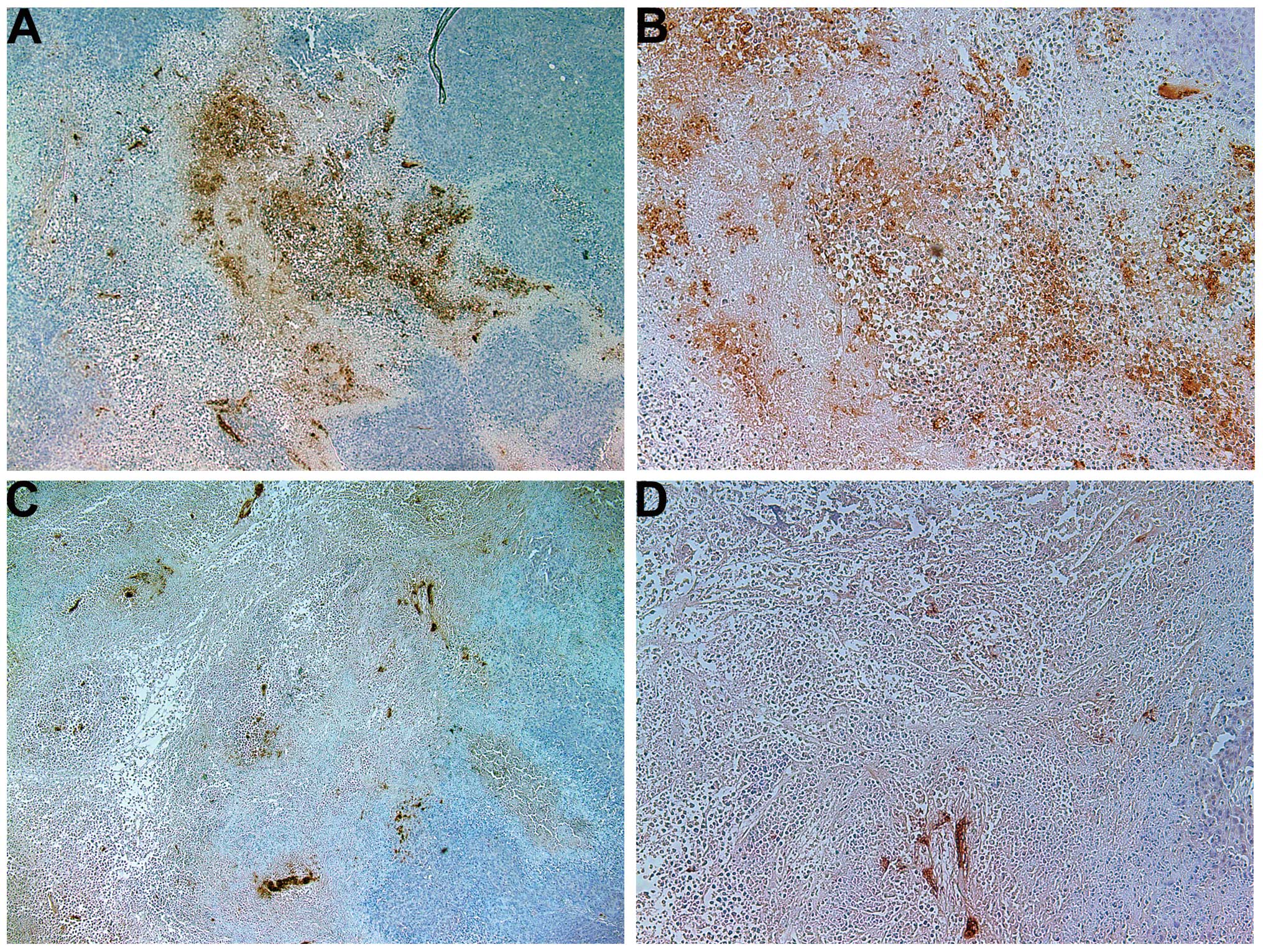
Invasion/metastasis: MMP-2 and -9
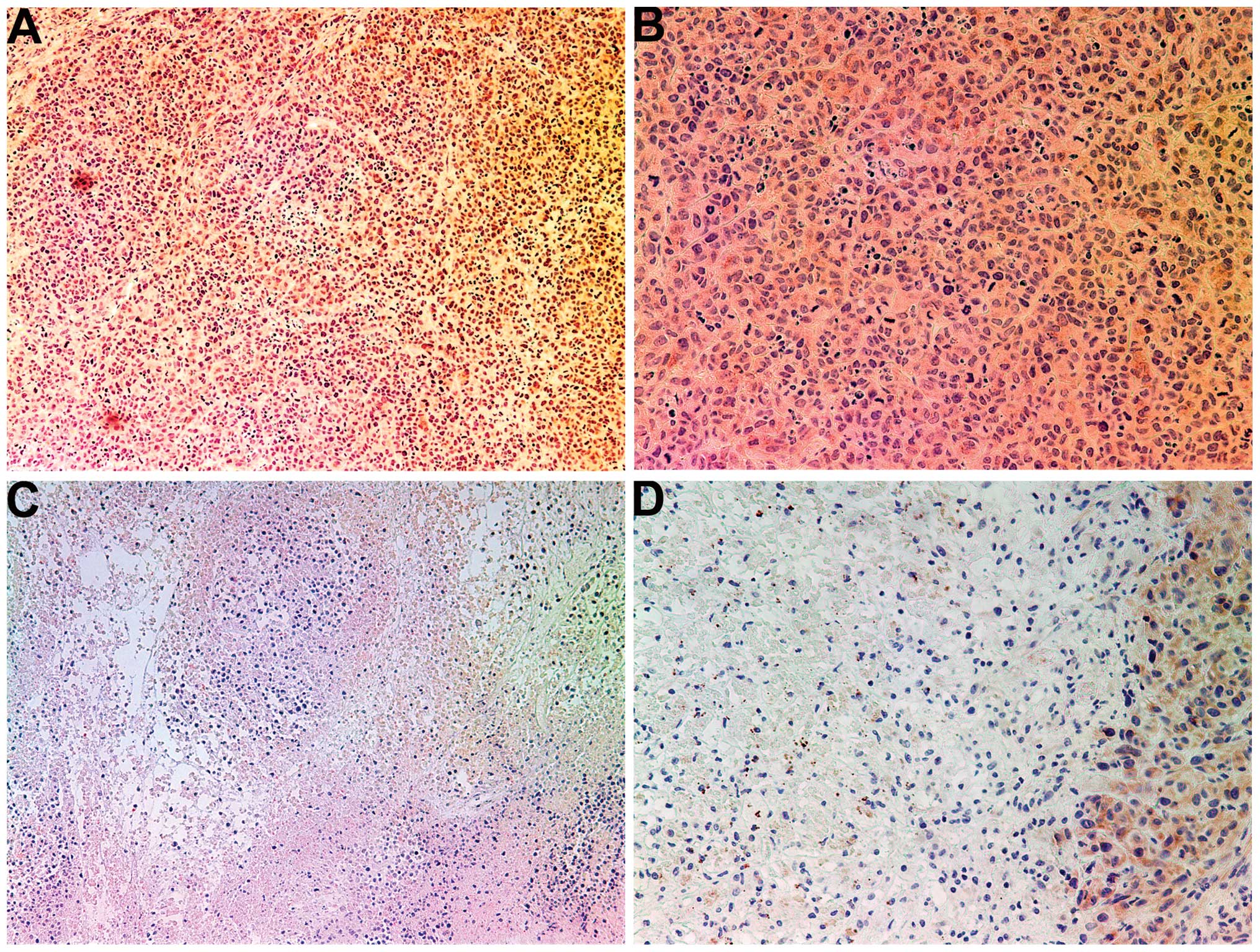
MMP-2
Tumors from the control group showed intense uniform
staining of MMP-2 in and around each cell (Fig. 4A and B), while the NM-treated
tumors exhibited greatly reduced central expression and secretion
of MMP-2 with a ring of peripheral cells positive for MMP-2
(Fig. 4C and D).
MMP-9
The NM-treated tumors showed less MMP-9 staining
than did the control-group tumors. Untreated tumors exhibited large
areas of MMP-9 (Fig. 5A and B),
while NM-treated tumors had large areas of cells with no MMP-9
surrounding smaller populations of cells with abundant MMP-9
(Fig. 5C and D).
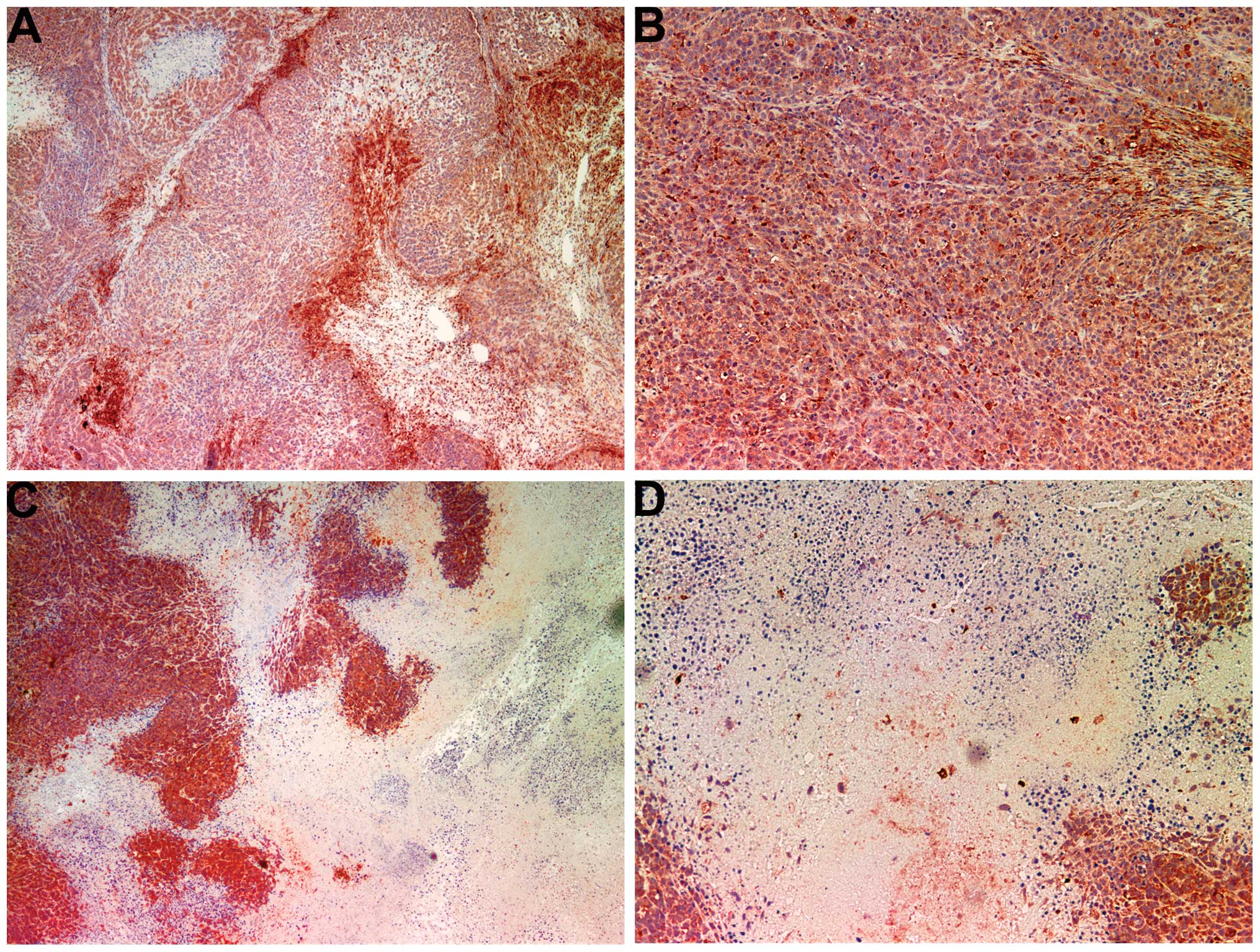
Angiogenesis: VEGF
The majority of the cross-sectional areas of
untreated tumors exhibited VEGF expression in a uniform
distribution pattern (Fig. 6A and
B). The NM-treated tumors showed markedly reduced VEGF
staining, and large central areas of cells with no VEGF expression
(Fig. 6C and D) were observed.
Apoptosis: TUNEL and Bcl-2
TUNEL
Abundant apoptosis occurred in both treated and
untreated tumors with more apoptosis occurring in the NM-treated
group. The control group tumors showed regions of cells that were
not undergoing apoptosis within larger regions of cells undergoing
apoptosis for a heterogeneous pattern of normal interspersed with
apoptotic cells (Fig. 7A and B).
The NM-treated tumors had uniform areas of homogeneous apoptotic
cells with peripheral areas of non-apoptotic cells (Fig. 7C and D). Some apoptosis was
detected in the cells of the fibrous capsule of NM-treated
tumors.
Bcl-2
High staining for Bcl-2, a pro-survival,
anti-apoptotic protein, was observed in untreated tumors, whereas a
reduced level of Bcl-2 was detected in the NM-treated tumors. The
untreated tumors had extensive regions of Bcl-2-positive cells
inside the tumors adjacent to and infiltrating regions of cells
without Bcl-2 (Fig. 8A and B). The
NM-treated tumors had vast regions of Bcl-2-free cells, which were
clearly nucleated with considerably less Bcl-2 expression (Fig. 8C and D).
Inflammation: COX-2 and iNOS
COX-2
Tumors from the control group exhibited large
dispersed regions of central COX-2 enzymes (Fig. 9A and B). The NM-treated tumors
showed decreased levels of COX-2 (Fig.
9C and D).
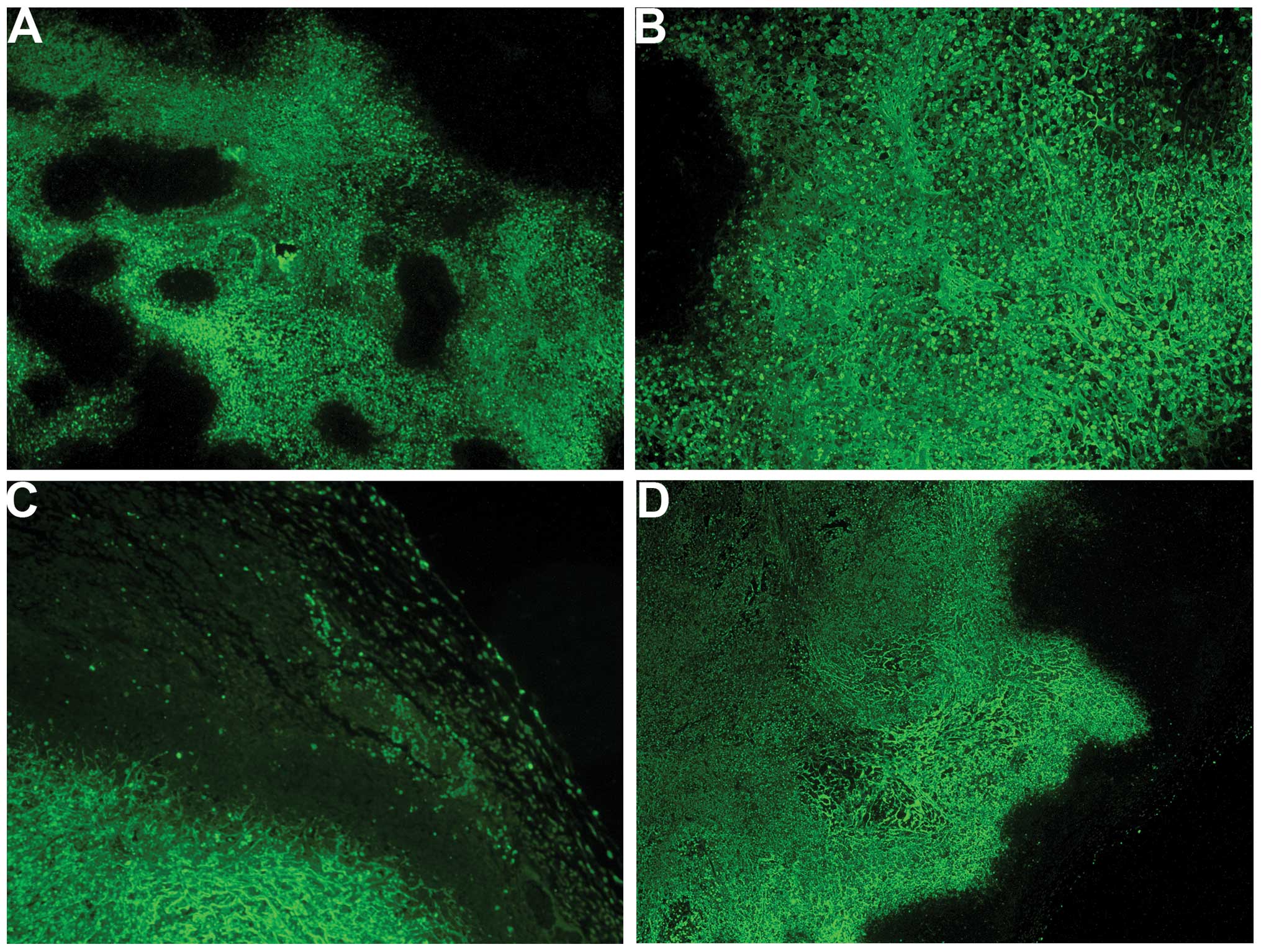
iNOS
Tumors from the control group had extensive diffuse
cytoplasmic as well as intense punctate iNOS staining within the
tumor, with the majority of the tumor positive for iNOS (Fig. 10A and B). By contrast, in the
NM-treated group, tumors exhibited large areas devoid of iNOS
(Fig. 10C and D).
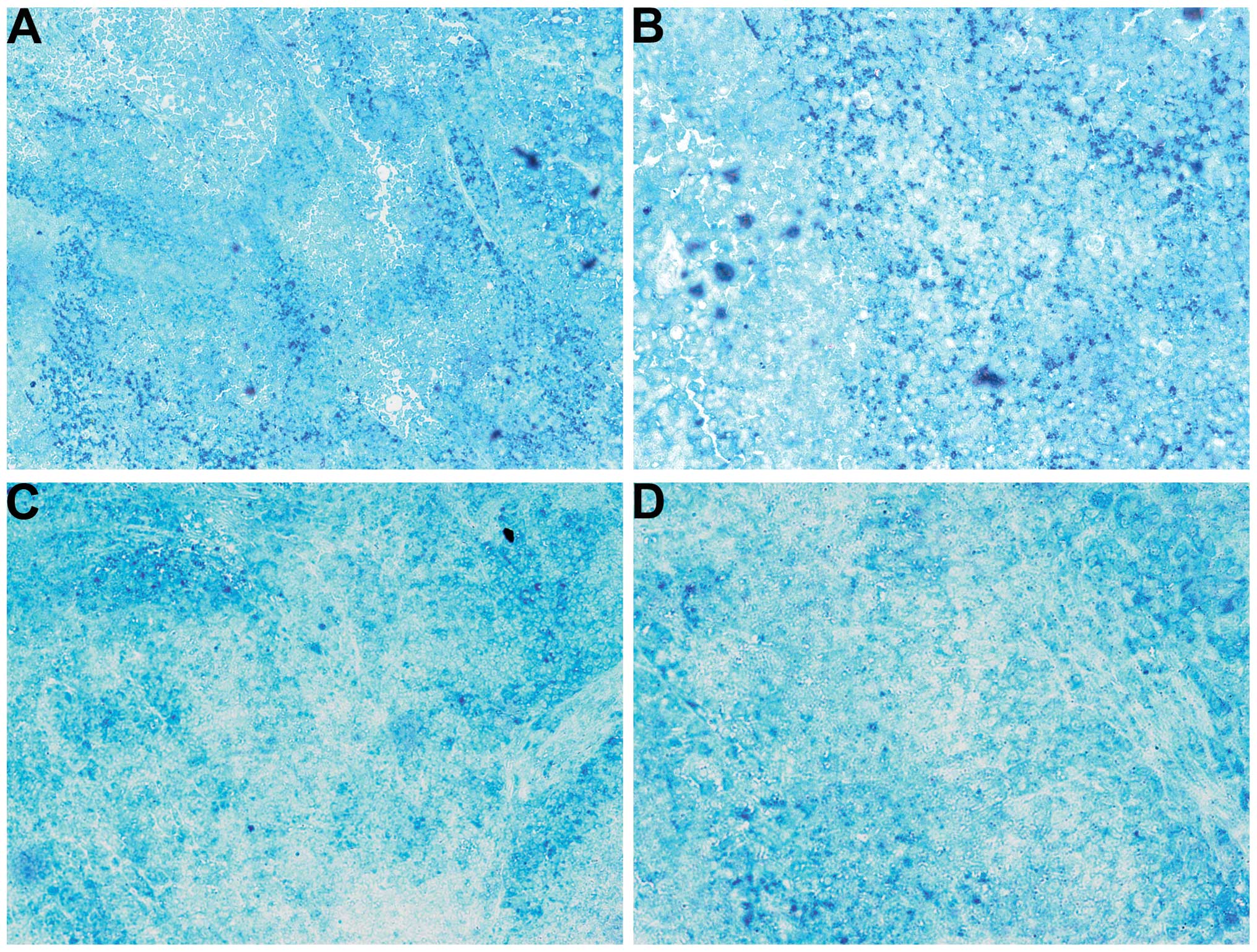
Cancer marker GSTπ
The untreated tumors had homogeneous diffuse GSTπ
expression as well as intense punctate staining (Fig. 11A and B). The NM-treated tumors
exhibited a similar pattern but also with regions free of GSTπ
(Fig. 11C and D).
Discussion
In the present study, NM dietary supplementation of
female nude mice challenged with HeLa xenografts resulted in a
profound reduction in mean tumor weight compared with the control
group. IHC staining of tumor markers confirmed this observation.
The proliferation marker Ki67 was markedly reduced in the tumors
from NM-supplemented mice. TUNEL staining showed abundant apoptosis
in both tumor groups, with more apoptosis occurring in the
NM-treated group. The control-group tumors showed regions of cells
not undergoing apoptosis within larger regions of cells undergoing
apoptosis. The NM-treated tumors showed uniform areas of apoptotic
cells with peripheral areas of non-apoptotic cells.
The activity of MMPs, particularly MMP-2 and-9, on
the degradation of the ECM plays a critical role in the formation
of tumors and metastasis, and high MMP-9 levels have been found to
correlate with the aggressiveness of cancers (10). Clinical studies have noted the
association of MMP expression with the progression of cervical
cancers (11,12). Bonfil et al (13,14)
reported that tumor necrosis was an important source of
gelatinase/type IV collagenase, mainly in its 92-kDa form, and thus
played a major role in tumor invasion. In the present study, tumors
from the control group showed intense uniform staining of MMP-2 in
and around each cell, while tumors from the NM-supplemented mice
exhibited greatly reduced central MMP-2 expression surrounded by a
ring of peripheral cells positive for MMP-2. In addition, tumors
from NM-supplemented mice showed less MMP-9 staining than did
control-group tumors. Tumors from the control group exhibited large
areas of MMP-9, whereas NM-treated tumors showed large areas of
cells with no MMP-9 surrounding smaller areas with abundant MMP-9.
In a previous in vitro study, cervical HeLa cells showed an
intense band corresponding to MMP-2 and a faint band corresponding
to MMP-9, which was enhanced with phorbol 12-myristate 13-acetate
treatment. The NM completely blocked MMP-9 expression at 500 μg/ml
and MMP-2 expression at 1,000 μg/ml (8).
The expression of the pro-angiogenic factor VEGF,
which is critical for primary tumor growth and metastasis, was also
found to be significantly lower in tumors from NM-supplemented mice
than that in tumors from the control group. The majority of the
cross-sectional areas of the control-group tumors exhibited VEGF
expression in a uniform distribution pattern. The NM-treated tumors
showed markedly reduced VEGF staining with large central areas of
cells having no VEGF expression. In examining specimens
immunohistochemically from normal cervical epithelium, carcinoma
in situ, microinvasive carcinoma and invasive cervical
squamous carcinoma (SCC), Fujiwaki et al (15) reported that VEGF expression
progressively increased along a continuum from normal epithelium to
invasive SCC (P<0.0001). Mathur et al (16) found serum VEGF-C upregulation to be
a unique marker for the early diagnosis of cervical cancer
metastasis.
In addition to promoting the progression of cancer,
elevated pro-inflammatory cytokine levels have been associated with
a variety of pathologies, such as fatigue, depression and cachexia
(17,18). In studying the inflammatory markers
COX-2 and iNOS in the present study, NM supplementation of the
mouse diet was found to decrease COX-2 and iNOS expression. It was
found that tumors from the NM-supplemented mice exhibited reduced
COX-2 staining in comparison with tumors from the control group,
which exhibited large dispersed regions of central COX-2 enzymes.
Furthermore, tumors from the control group had extensive diffuse
cytoplasmic as well as intense punctate iNOS staining within the
tumor, with the majority of the tumor positive for iNOS. By
contrast, in the NM-treated group, tumors exhibited large areas
devoid of iNOS. In previous studies, the NM has been shown to have
an inhibitory effect on inflammatory mediators, such as COX-2, in
various cancer cell lines (7,19,20).
Nuclear GSTπ is important in the diagnosis of cancer
as it is expressed abundantly in tumor cells (21). This enzyme plays a role in the
detoxification of both endogenous and exogenous electrophiles that
can react with cellular components such as DNA. In the present
study, tumors from the control group exhibited diffuse, uniform
GSTπ expression, as well as intense punctate staining. Tumors from
the NM-treated mice exhibited a similar pattern, but with further
regions free of GSTπ.
Rath and Pauling (5) proposed that nutrients such as lysine
and ascorbic acid could potentially modulate tumor growth and
expansion by acting as natural inhibitors of ECM degradation,
inhibiting MMP activity and strengthening the integrity of the
connective tissue surrounding cancer cells. Based on this approach,
a complex of nutrients was developed to affect multiple key cancer
mechanisms at once through their synergistic effects. The roles of
the individual components of this mixture in relation to the
critical aspects of cancer are described below. Optimal ECM
structure depends upon adequate supplies of ascorbic acid, lysine
and proline to maintain the synthesis and hydroxylation of collagen
fibers. Furthermore, lysine contributes to the stability of the ECM
as a natural inhibitor of plasmin-induced proteolysis (5,22).
Manganese and copper are essential cofactors for collagen
formation, and the potency of green tea extract in modulating
certain processes associated with cancer progression, including
cancer cell growth, metastasis and, angiogenesis, is well
documented (23–27). N-acetyl cysteine and selenium have
been demonstrated to inhibit tumor cell MMP-9 and invasive
activities, as well as the migration of endothelial cells through
the ECM (28–30). Ascorbic acid demonstrates cytotoxic
and antimetastatic actions on malignant cell lines (31–36)
and patients with cancer have been found to have low levels of
ascorbic acid (37,38). Low levels of arginine, a precursor
of nitric oxide (NO), can limit the production of NO, which has
been demonstrated to mainly act as an inducer of apoptosis
(39).
The results presented in this study showed the
potent NM-induced inhibition of tumor growth and cancer markers in
female nude mice injected with HeLa xenografts, suggesting the
therapeutic value of this specific nutrient complex in the
treatment of cervical cancer. Supplementation with the NM has
beneficial effects in modulating cancer markers of proliferation
(Ki67), invasion/metastasis (MMP-2 and -9), angiogenesis (VEGF),
apoptosis (TUNEL and Bcl-2) and inflammation (COX-2 and iNOS), as
well as the general cancer marker GSTπ. Furthermore, in contrast to
the toxic side effects of current treatments, the micronutrient
mixture has been shown to be a safe therapeutic agent. In a
previous in vivo study addressing safety issues, we found
that gavaging adult female Osteogenic Disorder Shionogi rats
(weighing 250–300 g) with NM (at 30, 90 or 150 mg/day for seven
days) resulted in neither adverse effects on vital organs (heart,
liver and kidney) nor on the associated functional serum enzymes,
indicating the safety of this mixture even at these high doses,
which far exceed the normal equivalent dosage of the nutrient
(40).
Acknowledgements
This study was funded by the Dr Rath Health
Foundation, a non-profit organization. The authors would like to
thank Professor Rajesh Agarwal, Department of Pharmaceutical
Science, University of Colorado (Denver, CO, USA) for providing
COX-2 IHC staining and Professor Ezio Laconi, Department of
Biomedical Sciences, University of Cagliari School of Medicine
(Cagliari, Italy) for the GSTπ IHC staining. Special thanks to Earl
Rainey for maintenance of the animal colony.
References
|
1
|
Jemal A, Bray F, Center MM, Ferley J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
American Cancer Society. Cervical cancer:
What are the key statistics about cervical cancer? http://www.cancer.org/cancer/cervicalcancer/detailedguide/cervical-cancer-key-statisticsuri.
Last revised January 31, 2014. accessed June 9, 2014
|
|
3
|
Cancer.net. Cervical cancer: Statistics.
http://www.cancer.net/cancer-types/cervical-cancer/statisticsuri.
Last revised April 2014. accessed June 2014
|
|
4
|
Fidler IJ: Molecular biology of cancer:
Invasion and metastasis. Cancer: Principles and Practice of
Oncology. DeVita VT Jr, Hellman S and Rosenberg SA: 5th edition.
Lippincott-Raven; Philadelphia, PA: pp. 135–152. 1997
|
|
5
|
Rath M and Pauling L: Plasmin-induced
proteolysis and the role of apoprotein(a), lysine and synthetic
lysine analogs. J Orthomol Med. 7:17–23. 1992.
|
|
6
|
Andreasen PA, Kjøller L, Christensen L and
Duffy MJ: The urokinase-type plasminogen activator system in cancer
metastasis: a review. Int J Cancer. 72:1–22. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Niedzwiecki A, Roomi MW, Kalinovsky T and
Rath M: Micronutrient synergy - a new tool in effective control of
metastasis and other key mechanisms of cancer. Cancer Metastasis
Rev. 29:529–542. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Roomi MW, Kalinovsky T, Rath M and
Niedzwiecki A: Modulation of u-PA, MMPs and their inhibitors by a
novel nutrient mixture in human female cancer cell lines. Oncol
Rep. 28:768–776. 2012.PubMed/NCBI
|
|
9
|
Roomi MW, Ivanov V, Kalinovsky T,
Niedzwiecki A and Rath M: Suppression of human cervical cancer cell
lines HeLa and DoTc2 4510 by a mixture of lysine, proline, ascorbic
acid and green tea extract. Int J Gynecol Cancer. 16:1241–1247.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Stetler-Stevenson WG: The role of matrix
metalloproteinases in tumor invasion, metastasis and angiogenesis.
Surg Oncol Clin N Am. 10:383–392. 2001.
|
|
11
|
Asha Nair S, Karunagaran D, Nair MB and
Sudhakaran PR: Changes in matrix metalloproteinases and their
endogenous inhibitors during tumor progression in the uterine
cervix. J Cancer Res Clin Oncol. 129:123–131. 2003.PubMed/NCBI
|
|
12
|
Zhou CY, Yao JF and Chen XD: Expression of
matrix metalloproteinase-2,9 and their inhibitor-TIMP 1,2 in human
squamous cell carcinoma of uterine cervix. Ai Zheng. 21:735–739.
2002.(In Chinese). PubMed/NCBI
|
|
13
|
Bonfil RD, Bustuoabad OD, Ruggiero RA,
Meiss RP and Pasqualini CD: Tumor necrosis can facilitate the
appearance of metastases. Clin Exp Metastasis. 6:121–129. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bonfil RD, Medina PA, Gómez DE, Farías E,
Lazarowski A, Lucero Gritti MF, Meiss RF and Bustuoabad OD:
Expression of gelatinase/type IV collagenase in tumor necrosis
correlates with cell detachment and tumor invasion. Clin Exp
Metastasis. 10:211–220. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fujiwaki R, Hata K, Lida K, Maede Y and
Miyazaki K: Vascular endothelial growth factor expression in
progression of cervical cancer: correlation with thymidine
phosphorylase expression, angiogenesis, tumor cell proliferation
and apoptosis. Anticancer Res. 20:1317–1322. 2000.PubMed/NCBI
|
|
16
|
Mathur SP, Mathur RS, Gray EA, Lane D,
Underwood PG, Kohler M and Creasman WT: Serum vascular endothelial
growth factor C (VEGF-C) as a specific biomarker for advanced
cervical cancer: Relationship to insulin-like growth factor II
(IGF-II), IGF binding protein 3 (IGF-BP3) and VEGF-A [corrected].
Gynecol Oncol. 98:467–483. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Argilés JM, Busquets S, Toledo M and
López-Soriano FJ: The role of cytokines in cancer cachexia. Curr
Opin Support Palliat Care. 3:263–268. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Deans C and Wigmore SJ: Systemic
inflammation, cachexia and prognosis in patients with cancer. Curr
Opin Clin Nutr Metab Care. 8:265–269. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Roomi MW, Kalinovsky T, Roomi NW, Rath M
and Niedzwiecki A: Inhibition of growth and expression of
inflammation mediators in human leukemic cell line U-937 by a
nutrient mixture. Exp Oncol. 35:180–186. 2013.PubMed/NCBI
|
|
20
|
Roomi MW, Kalinovsky T, Niedzwiecki A and
Rath M: Pleiotropic effects of a micronutrient mixture on critical
parameters of bladder cancer. Bladder Cancer: Etymology, Diagnosis
and Treatments. Nilsson WE: Nova Science Publishers Inc; Hauppage,
NY: pp. 229–244. 2009
|
|
21
|
Aliya S, Reddanna P and Thyagaraju K: Does
glutathione S-transferase-Pi (GST-Pi) a marker protein for cancer?
Mol Cell Biochem. 253:319–327. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sun Z, Chen YH, Wang P, et al: The
blockage of high-affinity lysine binding sites of plasminogen by
EACA significantly inhibits prourokinase-induced plasminogen
activation. Biochem Biophys Acta. 1596:182–192. 2002.
|
|
23
|
Valcic S, Timmermann BN, et al: Inhibitory
effect of six green tea catechins and caffeine on the growth of
four selected human tumor cell lines. Anticancer Drugs. 7:461–468.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mukhtar H and Ahmed N: Tea polyphenols:
prevention of cancer and optimizing health. Am J Clin Nutr. (6
Suppl)71:1698S–1702S. 2000.PubMed/NCBI
|
|
25
|
Yang GY, Liao J, Kim K, Yurtow EJ and Yang
CS: Inhibition of growth and induction of apoptosis in human cancer
cell lines by tea polyphenols. Carcinogenesis. 19:611–616. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Taniguchi S, Fujiki H, Kobayashi H, Go H,
Miyado K, Sadano H and Shimikawa R: Effect of (-)epigallocatechin
gallate, the main constituent of green tea, on lung metastasis with
mouse B16 melanoma cell lines. Cancer Lett. 65:51–54. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hara Y: Green tea: Health Benefits and
Applications. Marcel Dekker Inc; New York, NY: 2001, View Article : Google Scholar
|
|
28
|
Kawakami S, Kageyama Y, Fujii Y, Kihara K
and Oshima H: Inhibitory effects of N-acetyl cysteine on invasion
and MMP-9 production of T24 human bladder cancer cells. Anticancer
Res. 21:213–219. 2001.PubMed/NCBI
|
|
29
|
Morini M, Cai T, Aluigi MG, et al: The
role of the thiol N-acetyl cysteine in the prevention of tumor
invasion and angiogenesis. Int J Biol Markers. 14:268–271.
1999.
|
|
30
|
Yoon SO, Kim MM and Chung AS: Inhibitory
effects of selenite on invasion of HT1080 tumor cells. J Biol Chem.
276:20085–20092. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cha J, Roomi MW, Ivanov V, Kalinovsky T,
Niedzwiecki A and Rath M: Ascorbate supplementation inhibits growth
and metastasis of B16FO melanoma and 4T1 breast cancer cells in
vitamin C-deficient mice. Int J Oncol. 42:55–64. 2013.
|
|
32
|
Naidu KA, Karl RC and Coppola D:
Antiproliferative and proapoptotic effect of ascorbyl stearate in
human pancreatic cancer cells: association with decreased
expression of insulin-like growth factor 1 receptor. Dig Dis Sci.
48:230–237. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Anthony HM and Schorah CJ: Severe
hypovitaminosis C in lung-cancer patients: the utilization of
vitamin C in surgical repair and lymphocyte-related host
resistance. Br J Cancer. 46:354–367. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Maramag C, Menon M, Balaji KC, Reddy PG
and Laxmanan S: Effect of vitamin C on prostate cancer cells in
vitro: effect on cell number, viability and DNA synthesis.
Prostate. 32:188–195. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Koh WS, Lee SJ, Lee H, Park C, Park MH,
Kim WS, Yoon SS, Park K, Hong SI, Chung MH and Park CH:
Differential effects and transport kinetics of ascorbate
derivatives in leukemic cell lines. Anticancer Res. 18:2487–2493.
1998.PubMed/NCBI
|
|
36
|
Chen Q, Espey MG, Krishna MC, Mitchell JB,
Corpe CP, Buettner GR, Shacter E and Levine M: Pharmacologic
ascorbic acid concentrations selectively kill cancer cells: action
as a pro-drug to deliver hydrogen peroxide to tissues. Proc Natl
Acad Sci USA. 102:13604–13609. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Nuñez Martin C and Ortiz de Apodaca y Ruiz
A: Ascorbic acid in the plasma and blood cells of women with breast
cancer. The effect of consumption of food with an elevated content
of this vitamin. Nutr Hosp. 10:368–372. 1995.(In Spanish).
|
|
38
|
Kurbacher CM, Wagner U, Kolster B,
Andreotti PE, Krebs D and Bruckner HW: Ascorbic acid (vitamin C)
improves the antineoplastic activity of doxorubicin, cisplatin and
paclitaxel in human breast carcinoma cells in vitro. Cancer Lett.
103:183–189. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Cooke JP and Dzau VJ: Nitric oxide
synthase: role in the genesis of vascular disease. Annu Rev Med.
48:489–509. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Roomi MW, Ivanov V, Netke SP, Niedzwiecki
A and Rath M: Serum markers of the liver, heart, and kidney and
lipid profile and histopathology in female ODS rats treated with
nutrient synergy. In: Presented at: American College of Nutrition;
Nashville, TN. (abstract 86). 22. pp. 4772003
|