Introduction
Calcium (Ca2+) is a ubiquitous and
essential second messenger in cells, which mediates a broad of
cellular functions including secretion, excitation, contraction,
metabolism, transcription, growth, proliferation, division and
apoptosis (1). In general,
Ca2+ signals are controlled by Ca2+ flux in
(influx) and out (efflux) through plasma membrane channels in order
to achieve Ca2+ homeostasis within the cell (2). Store-operated Ca2+ entry
(SOCE) is a major mechanism of Ca2+ import from
extracellular to intracellular space, which has been demonstrated
to consistently increase intracellular Ca2+ levels
(3).
Recently, two key factors involved in SOCE have been
identified. These are the endoplasmic reticulum (ER)
Ca2+ sensor protein stromal interaction molecule 1
(STIM1) and the plasma membrane Ca2+ channel protein
Orai1 (4). STIM1 is a transmembrane
protein located in the ER where it has a Ca2+ sensor
function, and it has a Ca2+-binding EF-hand motif
located within the ER lumen (5).
Orai1 is an essential component of Ca2+
release-activated Ca2+ (CRAC) channels in the plasma
membrane (6). STIM1 is predominantly
located in the ER lumina, the main intracellular Ca2+
store. When the store of Ca2+ is depleted and inositol
1,4,5-trisphosphate (IP3) receptors are activated, STIM1
translocates into ER/plasma membrane junctions where it couples to
activate Orai1 and, possibly, transient receptor potential channels
(TRPCs). At these junctions, STIM proteins bind to and activate the
highly Ca2+-selective Orai channels, thereby mediating
finely controlled Ca2+ signals and homeostatically
balancing cellular Ca2+ levels (7,8).
The traditional Chinese herbal medicine Fructus
Corni (Cornus officinalis Sieb. et Zucc.), known as
Shan-zhu-yu in Chinese, has a long history of use in the treatment
of diabetes, cancer, inflammation and shock, and for
hepatoprotection (9). Fructus Corni
contains gallic acid, malic acid, tartaric acid, ursolic acid,
morroniside, loganin and sweroside (10,11).
Recent studies have shown Fructus Corni extract (FCE) to be
effective against cerebral ischemia-reperfusion injury, and to have
anti-oxidant and anti-aging functions (12–14).
However, its role and effect in the nervous system remain
unknown.
In the present study, the aim was to investigate the
effects of FCE on neuronal differentiation. The effects of FCE
treatment on neurite outgrowth, intracellular Ca2+ and
the expression of STIM1 provide useful information to the
therapeutic potential of FCE in neurodegenerative diseases.
Materials and methods
Preparation of FCE
Fresh Fructus Corni (C. officinalis Sieb. et
Zucc.) was purchased from Tong Ren Tang (Beijing, China). The
Fructus Corni (100 g) was extracted by heating in 1,000 ml
distilled water at 80°C for 2 h, and then concentrated using a
rotary evaporator (Rotary evaporator R-480; Büchi Labortechnik AG,
Flawil, Switzerland). The concentrated extracts were lyophilized
using a freeze dryer (FDU-540; Elaya, Tokyo, Japan). Following the
lyophilization, a powder was obtained (yield, 25.82 g) and was
dissolved in dimethyl sulfoxide (DMSO; Sigma-Aldrich, St. Louis,
MO, USA) to provide a stock solution of concentration 100 mg/ml and
stored at 4°C. The stock solution of FCE was then diluted with
medium to the desired concentration prior to use.
Materials
Rat β-nerve growth factor (NGF) was purchased from
R&D Systems (Minneapolis, MN, USA).
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium (MTT) was
obtained from Sigma-Aldrich. Rabbit polyclonal anti-STIM1 (S6072)
and mouse monoclonal anti-β-actin (A5316) antibodies were purchased
from Sigma-Aldrich.
Cell culture
PC12 (adrenal gland pheochromocytoma) cells (that
were a gift from Professor Yanhong Liao of Huazhong University of
Science and Technology, Wuhan, China) were cultured in Dulbecco's
modified Eagle's medium (DMEM; Hyclone™, GE Healthcare, Logan, UT,
USA) supplemented with 10% heat-inactivated horse serum (Gibco,
Grand Island, NY, USA), 5% heat-inactivated fetal bovine serum
(Gibco), and antibiotics (100 µg/ml streptomycin and 100 U/ml
penicillin; Gibco) at 37°C in a 5% CO2 incubator. Cells
were supplied with fresh medium three times per week, and were
split at a 1:3 ratio twice per week.
MTT assay
PC12 cells were seeded into 96-well plates (200
µl/well) at a density of 5×104 cells/ml. They were
treated with various concentrations (0, 10, 20, 40, 60, 80 and 100
µg/ml) of FCE for 48 h. At the end of the drug incubation period,
MTT (Sigma-Aldrich) working solution (0.5 mg/ml) was added to each
well, and the plates incubated for an additional 4 h at 37°C.
Following centrifugation at 350 × g for 5 min, the medium was
replaced with DMSO. The absorbance of reduced MTT at 570 nm was
measured with a plate reader (Bio-Rad Laboratories, Hercules, CA,
USA). The rate of inhibition of cell proliferation was calculated
from the optical density (OD) values as follows: Inhibitory rate of
PC12 cell proliferation (%) = (OD value control group - OD value
test group) × 100/OD value control group. Based on the
IC50 of FCE in PC12 cells, a single concentration of 60
µg/ml was determined according to the results and it was selected
for evaluation in the following tests. The MTT assay was also
conducted using 60 µg/ml FCE with various incubation times (0, 1,
6, 12, 18, 24, 48 and 72h) to evaluate whether the effect of FCE
was time-dependent. Based on the results a 48-h culture time was
used for the PC12 cells treated in different groups.
Lentivirus-delivered RNA
interference
Lentiviral plasmids carrying STIM1 short hairpin RNA
(shRNA) were purchased from Sigma-Aldrich. The lentivirus was
produced by the Hope Center Viral Vectors Core of Washington
University (St. Louis, MO, USA). For control infection,
non-targeted shRNA was used.
Transduction of shRNA
STIM1 shRNA and non-targeted shRNA were transduced
into PC12 cells using lentivirus (Hope Center Viral Vectors Core of
Washington University) according to the instructions provided by
the manufacturer. Briefly, PC12 cells were infected with lentivirus
at day 8 in vitro, and selected with puromycin
dihydrochloride (Santa Cruz Biotechnology, Inc.) for 1 week. At 3
days after infection, western blotting or immunofluorescence
staining was performed.
Evaluation of neurite outgrowth
PC12 cells (1×105 cells/ml) were seeded
onto 24-well plates and cultured for 1 day, after which time 60
µg/ml FCE or 50 ng/ml NGF was added and the cells were cultured for
an additional 2 days. The cells were then fixed with 4%
paraformaldehyde (Sigma-Aldrich) in phosphate-buffered saline
(PBS), and cell morphology was assessed under a phase-contrast
microscope (IBE2003; Chongqing COIC Industrial Co., Ltd, Chongqing,
China). STIM1 shRNA-treated cells were also cultured and assessed,
without 60 µg/ml FCE or 50 ng/ml NGF addition. Neurite extension
from the PC12 cells was regarded as an index of neuronal
differentiation. Processes with a length equivalent to ≥1 diameters
of the cell body were regarded as neurites. The differentiation of
PC12 cells was evaluated by examining the proportion of
neurite-bearing cells to total cells in randomly selected fields.
The mean differentiation score was obtained for >100 PC12 cells
in each well. In certain experiments, images of the cells were
captured, and the total length of the neurite extension per
positive cell and average length of neurites in positive cells were
determined in randomly chosen fields using Motic Images Plus
software (version 2.0S; Motic Instruments Inc., Richmond,
Canada).
Immunofluorescence staining
Cells were blocked with 0.1% Tween-20 and 5% goat
serum in PBS for 30 min and then stained for 2 h at room
temperature with monoclonal mouse anti-Myc-Cy3 (1:200, cat.
SAB4700448, Sigma-Aldrich) and polyclonal rabbit anti-GAP-43 (1:50,
cat. BA0878, Boster, Pleasanton, CA, USA) antibodies. Primary
antibody was then visualized using ECL™ donkey monoclonal
anti-rabbit secondary antibody (1:1,000, cat. A-21206, Thermo
Fisher Scientific Inc., Waltham, MA, USA). Fluorescent images were
captured with a confocal microscope (Olympus Fluoview 500; Olympus,
Tokyo, Japan).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted using TRIzol reagent
(Invitrogen Life Technologies, Carlsbad, CA, USA) and reverse
transcribed using M-MLV-Reverse Transcriptase (Promega Corporation,
Madison, WI, USA), according to the manufacturer's instructions.
qPCR was performed using the SYBR-Green Master PCR mix (Applied
Biosystems, Foster City, CA, USA) on the TP800 Real Time PCR System
(Takara Bio, Otsu, Japan). qPCR of cDNA was performed using the
following forward (F) and reverse (R) primer sequences: STIM1: F,
5′-GGACGATGATGCCAATGGTGATGT-3′; R, 5′-TTCCACAGGTCCTCCACGCTGAT-3′;
β-actin: F, 5′-TGGACATCCGCAAAGAC-3′; R, 5′-GAAAGGGTGTAACGCAACTA-3′.
The amplification conditions were: 5 min at 94°C; 35 cycles of 45
sec at 94°C, 1 min at 56°C and 1 min at 72°C; followed by 10 min at
72°C. All quantification was normalized to an endogenous gene,
β-actin. For relative quantification, 2−(Ct-Cc) where Ct
and Cc are the mean threshold cycle differences after normalizing
to β-actin, was calculated and used as an indication of the
relative expression levels (15).
Western blotting
PC12 cells were collected, washed once with ice-cold
PBS, and lysed with a lysis buffer (50 mM Tris HCl pH 7.5, 150 mM
NaCl, 1 mM EDTA, 0.1% SDS, 0.2% deoxycholic acid and 1:100 protease
inhibitor cocktail). Lysates were centrifuged for 10 min at 12,000
× g and supernatants were analyzed for protein concentration using
a BCA Protein Assay kit (Pierce Biotechnology, Inc., Rockford, IL,
USA). Equal amounts of proteins were resolved by SDS-PAGE,
transferred to polyvinylidene difluoride membranes, and probed with
antibodies against STIM1 (1:1,000) and β-actin (1:1,000),
respectively. Immunoreactive bands were visualized by enhanced
chemiluminescence. Images of the bands were captured using a
scanner (HP Scanjet 7400C; Hewlett-Packard, Palo Alto, CA, USA),
and the intensities of the were quantified using Image J software
(National Institutes of Health, Bethesda, MD, USA).
Intracellular Ca2+
measurements
Ratiometric imaging of intracellular Ca2+
using cells loaded with fura-2 was measured as previously described
(16). All cells for these
experiments were grown in round coverslips (30 mm) under normal
tissue culture conditions, except where specified. Coverslips with
cells were placed in a cation-safe solution composed of (in mM):
107 NaCl, 7.2 KCl, 1.2 MgCl2, 11.5 glucose and 20
HEPES-NaOH pH 7.3, and loaded with fura-2-acetoxymethyl ester (2 µM
final concentration) for 30 min at 37°C. Cells were washed, and
de-esterification was allowed for a minimum of 15 min.
Ca2+ measurements were made using a Leica DMI6000 B
fluorescence microscope (Leica Microsystems, Wetzlar, Germany)
controlled by SlideBook software (Intelligent Imaging Innovations,
Denver, CO, USA). Fluorescence emission at 505 nm was monitored
while excitation wavelengths were alternated between 340 and 380 nm
at a frequency of 0.5 Hz; intracellular Ca2+
measurements were determined as a 340/380 nm ratio obtained from
groups (n=15-25) of single cells. External solutions were (in mM):
135 NaCl, 5.4 KCl, 10 HEPES, 0.02 NaH2PO4, 2
Mg2+ and 10 glucose pH 7.4. Measurements shown are
representative of a minimum of three independent experiments.
Statistical analysis
All results are expressed as the means ± standard
error of the mean of data obtained from triplicate experiments.
Data in two groups were analyzed by Student's t-test. Multiple
comparisons of the data were performed by analysis of variance
followed by Tukey's test. All the analyses were performed using the
SPSS software package, version 17.0 (SPSS, Inc., Chicago, IL, USA).
Differences with P<0.05 were considered statistically
significant.
Results
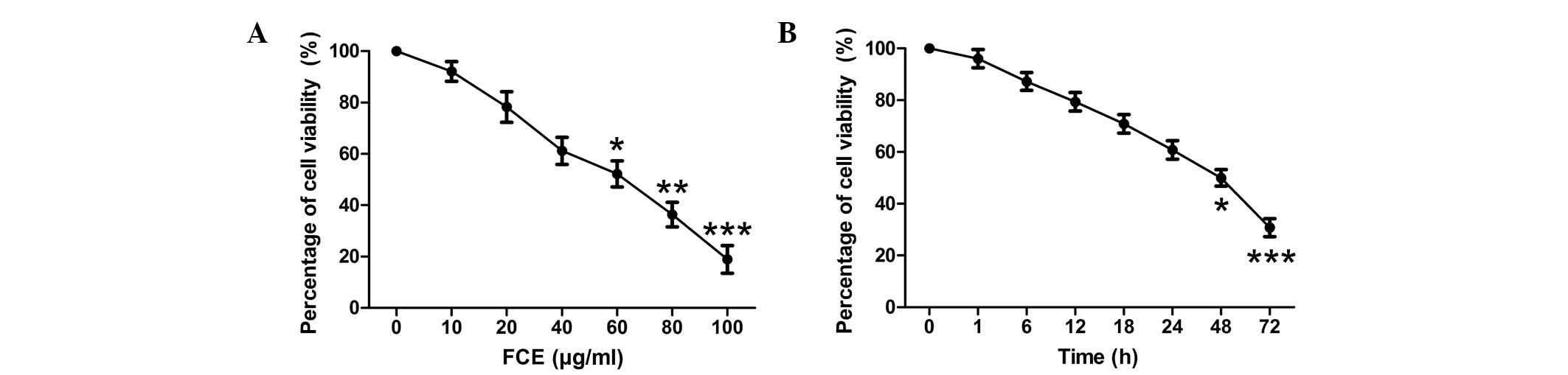
FCE treatment inhibits the
proliferation of PC12 cells
To examine the effects of FCE treatment on cell
proliferation, MTT assays were used to detect the viability of PC12
cells. When the PC12 cells were treated with various concentrations
of FCE for 48 h, the proliferation of PC12 cells was inhibited in a
concentration-dependent manner (Fig.
1A). According to the inhibitory rates of FCE at different
concentrations on PC12 cells (data not shown), the IC50
was 58.248 µg/ml. A concentration of 60 µg/ml FCE was selected for
use in this study. Furthermore, a time-dependent reduction in cell
viability was observed (Fig. 1B).
The PC12 cells were incubated for 48 h with different treatments
prior to the following experiments.
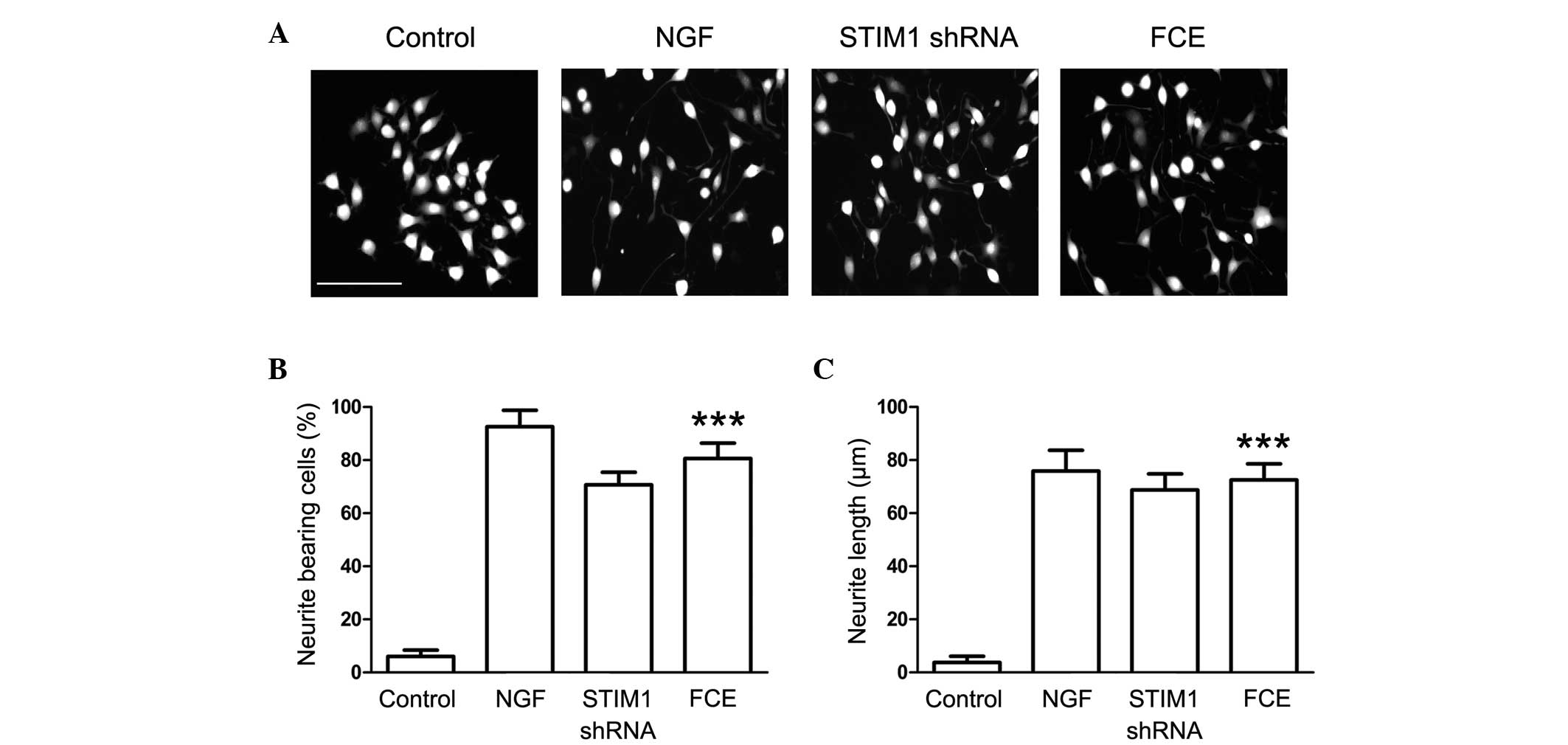
FCE treatment induces neurite
outgrowth in PC12 cells
Following treatment with 60 µg/ml FCE, morphological
changes indicating neurite outgrowth were observed in the PC12
cells (Fig. 2A). NGF, a prominent
neurotrophic factor, was used to induce neurite outgrowth in PC12
cells as a positive control. In addition, the morphological changes
induced by the knockdown of STIM1 expression with specifically
targeted shRNA were observed to elucidate the effect of STIM1 on
neurite outgrowth from PC12 cells. The results demonstrated that
FCE treatment stimulated neurite extension in PC12 cells in a
similar manner to NGF. In addition, neurite outgrowth from the PC12
cells also was observed in the STIM1 shRNA-treated group,
suggesting that STIM1 may be involved in the process of neural
differentiation (Fig. 2A).
Furthermore, FCE significantly increased the percentage of
neurite-bearing cells and the length of the branches (Fig. 2B and C).
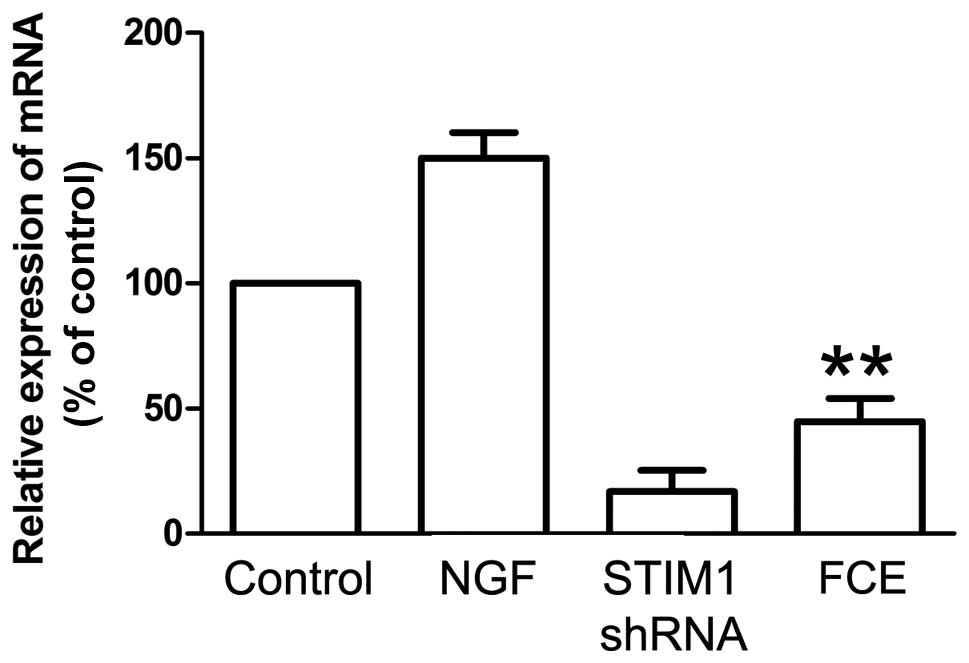
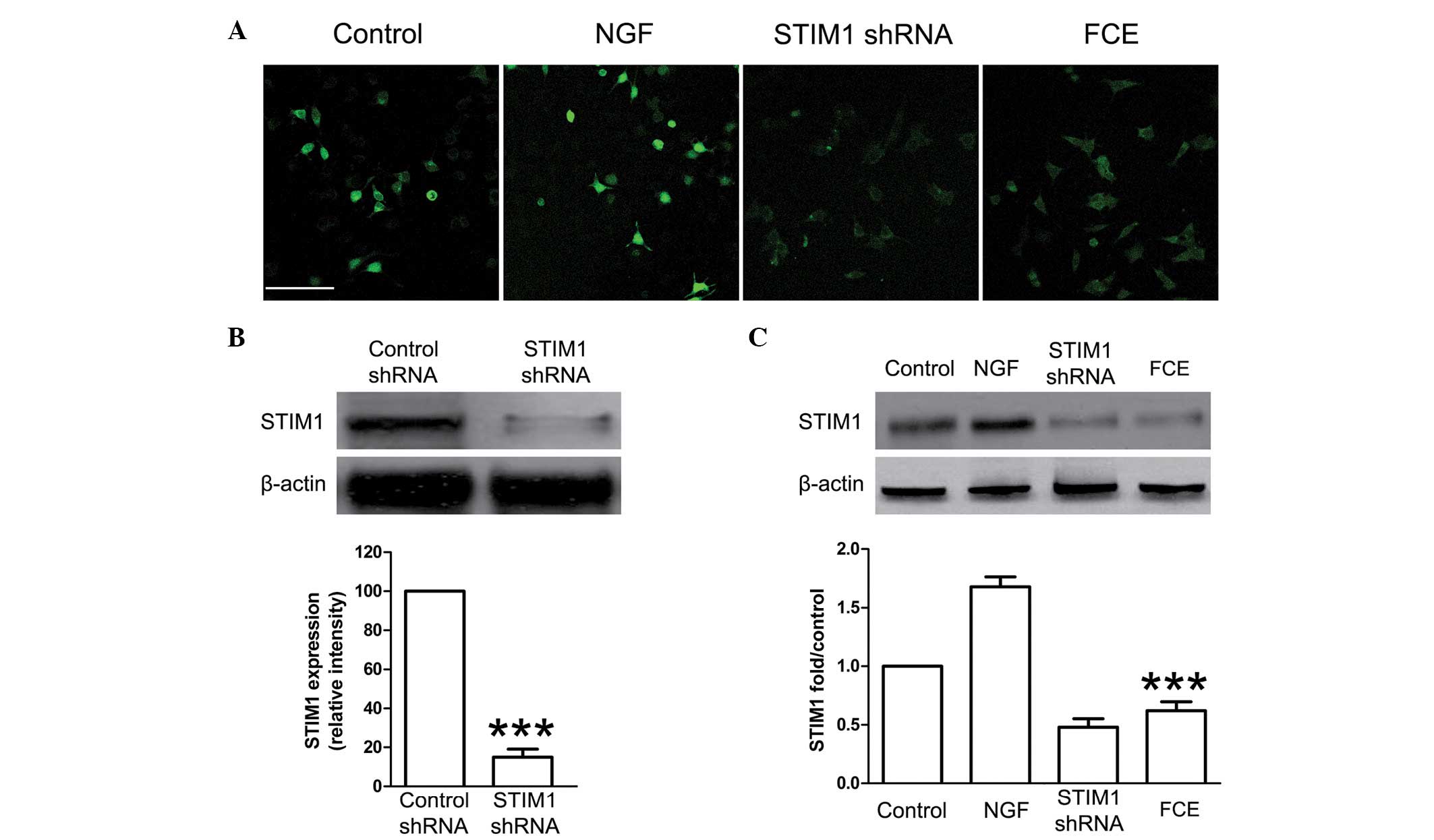
FCE treatment suppresses the
expression of STIM1 mRNA and protein in PC12 cells
SOCCs, including STIM1, play a important role in
cellular proliferation and differentiation. In this study, the
knockdown of STIM1 expression with specifically targeted shRNA was
carried out, and it effectively reduced the expression of STIM1
mRNA (Fig. 3) and protein (Fig. 4B). Treatment with FCE also reduced
the mRNA expression of STIM1 in the PC12 cells (Fig. 3), concurrently with its inducing
effect on neurite outgrowth. Immunofluorescence staining confirmed
that FCE treatment suppressed the expression of STIM1 protein in
PC12 cells (Fig. 4A). Consistent
with these results, the expression of STIM1 protein was also found
to be significantly decreased in the FCE treatment group compared
with the control group by western blot assay (Fig. 4C).
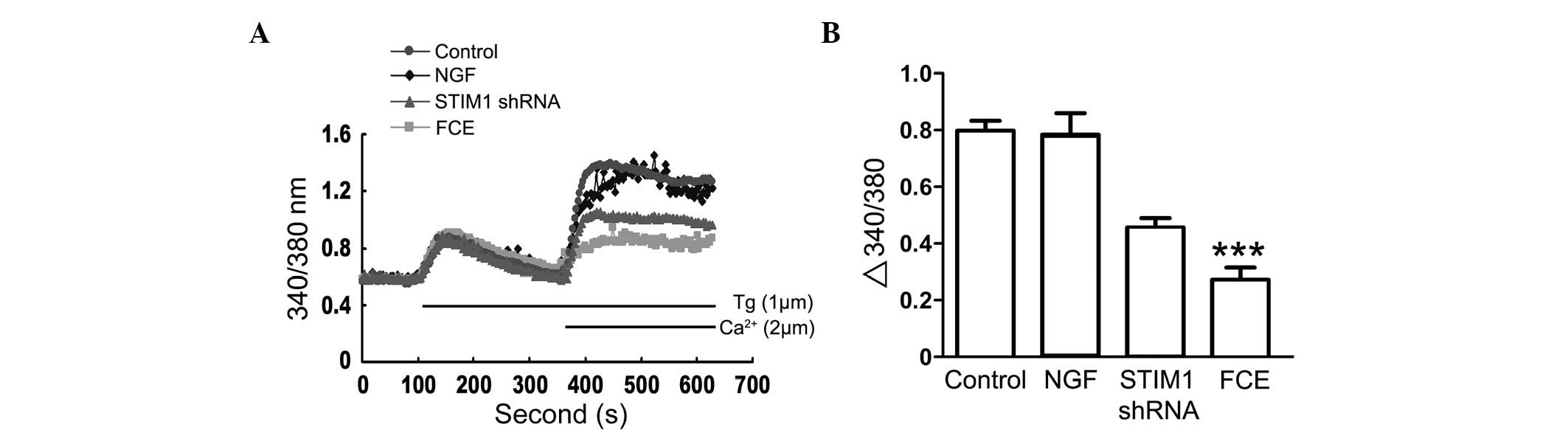
Inhibition of extracellular
Ca2+ influx is involved in FCE-induced neurite
growth
To examine whether sustained Ca2+ influx
is critical for neurite growth in PC12 cells with FCE treatment,
cytosolic Ca2+ was measured by the fura 2 assay
(Fig. 5). FCE treatment inhibited
Ca2+ influx in a time-dependent manner in PC12 cells,
which differed from the NGF and STIM1 shRNA groups. These results
suggest that FCE-induced neurite growth may involve a signaling
pathway and mechanism different from that of NGF.
Discussion
In the present study, it was shown that FCE promotes
neurite outgrowth and differentiation of PC12 cells. Notably, the
FCE-induced neuritogenesis was demonstrated to be associated with
the downregulation of STIM1 expression at the mRNA and protein
levels, as well as the inhibition of extracellular Ca2+
influx.
The second messenger Ca2+ plays a crucial
role in regulating a number of different cellular processes by
modulating Ca2+-regulated proteins and the corresponding
signaling pathways. There are two main sources of intracellular
free Ca2+: Ca2+ released from intracellular
Ca2+ storage organelles, most notably the ER, and
Ca2+ influx from sources external to the cell (17). It has been recognized that
Ca2+ influx into neuronal subcellular compartments (for
example, dendrites, somata, spines and axons) is mediated by two
principal means of Ca2+ entry. These routes are
voltage-gated Ca2+ channels (VGCCs) and ionotropic
neurotransmitter receptors; both routes elicit crucial rises in
cytosolic Ca2+ levels in response to different stimuli
(18). VGCCs are widely expressed in
excitable cells and they trigger Ca2+ influx over
specific ranges of membrane potentials (19). Neurons along with other cell types
display an alternative Ca2+ entry mode that is coupled
to intracellular Ca2+stores. This alternative type of
entry, known as capacitative Ca2+ entry, is triggered
upon the depletion of Ca2+ stores to facilitate SOCE
(20). SOCE is a major mechanism by
which Ca2+ is imported from extracellular to
intracellular space. In general, the stimulation of cell surface
receptors leads to the activation of IP3, which evokes a rapid and
transient release of Ca2+ from the ER store through IP3
receptor channels. The resulting reduction of Ca2+
concentration in the ER is detected by the EF-hand motif of STIMs.
This causes the STIMs to translocate to the plasma membrane, where
they interact with Orai (also known as CRACM1)
Ca2+channel subunits, resulting in an influx of
Ca2+ from the extracellular space in order to restore
the Ca2+ concentration in the ER (21).
The essential roles of STIM1 and Orai1 in SOCE have
been confirmed in a number of studies. STIM1 is a single-pass
transmembrane protein in the ER membrane that functions as a
Ca2+ sensor in the ER, while Orai1 is the pore-forming
subunit of SOC channels in cell membranes (22). STIM1 predominantly exists in the
sarcoplasmic reticulum (SR) or ER and has its N-terminal sensing
Ca2+ domain in the SR/ER lumen and its C-terminal Orai1
coupling site in the cytosol (23).
STIM1 acts as both an ER Ca2+ sensor and activator of
SOCE. When Ca2+ in the ER is depleted and STIM1 migrates
to the plasma membrane, STIM1 forms aggregates at plasma membrane
sites of Ca2+ entry, where it interacts with and
activates Orai1 channels to induce SOCE (24). Several studies have investigated the
potential involvement of SOCE-mediated Ca2+ metabolism
with a focus on STIM1. For example, STIM proteins and Orai channels
have been indicated to be associated with neuronal development and
memory, and it has been shown that abnormal SOCE may participate in
the Ca2+ overload of neurons in the early stages
following diffuse axonal injury via an increase in STIM1 expression
(25). In addition, it has been
observed that STIM1- and Orai1-regulated SOCE is associated with
changes in the proliferation of endothelial cells (26). In vascular smooth muscle cells, the
knockdown of either STIM1 or Orai1 has been demonstrated to inhibit
the proliferation and migration of the cells (27). Furthermore, STIM1-knockdown inhibited
serum-induced epidermoid carcinoma cell migration (28). The data from the present study
demonstrate that STIM1 plays an important role in FCE-induced
neuritogenesis, suggesting that inhibiting STIM1-mediated
SOCE-associated Ca2+ metabolism dysfunction might be a
strategy for the treatment of neuronal injury.
The upstream pathway leading to SOCE in PC12 cells
has not been elucidated. Ca2+ levels in the ER are
mainly controlled by the phospholipase C (PLC)/IP3/IP3 receptor
pathway with activation by tyrosine kinase-type or Gq-related G
protein-coupled receptors (29). In
colorectal cancer cells, SOCE has been shown to be controlled by
tyrosine kinase-type receptors rather than by Gq-coupled receptors
(30). In pulmonary arterial smooth
muscle cells, platelet-derived growth factor was found to activate
SOCE via Akt signaling (31). In
addition, the phosphorylation of STIM1 at extracellular
signal-regulated kinase 1/2 (ERK1/2) target sites has been
demonstrated to regulate the association of STIM1 with EB1
(end-binding protein 1), a regulator of growing microtubule ends
(32). Another study revealed that
ERK1/2 phosphorylates STIM1 in vitro at Ser575, Ser608 and
Ser621, and that STIM1 regulates SOCE in HEK293 cells via
phosphorylation at ERK1/2 target sites (33). An investigation of the functional
association among tyrosine kinase-type receptors, SOCE and ERK
signaling in PC12 cells would be of interest.
In conclusion, the findings of the present study
provide the first evidence of induction of neurite outgrowth in
PC12 cells by the traditional Chinese herbal medicine FCE.
Additionally, the results demonstrate that FCE-induced
neuritogenesis is associated with the suppression of STIM1
expression and the inhibition of Ca2+ influx. These
results provide a useful information regarding the therapeutic
potential of FCE for the treatment of neurodegenerative
disease.
Glossary
Abbreviations
Abbreviations:
|
FCE
|
Fructus Corni extract
|
|
NGF
|
nerve growth factor
|
|
MTT
|
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium
|
|
DMSO
|
dimethyl sulfoxide
|
|
SOCE
|
store-operated Ca2+
entry
|
|
ER
|
endoplasmic reticulum
|
|
STIM1
|
stromal interaction molecule 1
|
|
CRAC
|
Ca2+ release-activated
Ca2+
|
|
VGCC
|
voltage-gated Ca2+
channel
|
|
IP3
|
inositol 1,4,5-trisphosphate
|
|
TRPC
|
transient receptor potential
channel
|
References
|
1
|
Soboloff J, Rothberg BS, Madesh M and Gill
DL: STIM proteins: dynamic calcium signal transducers. Nat Rev Mol
Cell Biol. 13:549–565. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stathopulos PB, Schindl R, Fahrner M, et
al: STIM1/Orai1 coiled-coil interplay in the regulation of
store-operated calcium entry. Nat Commun. 4:29632013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jozsef L, Tashiro K, Kuo A, et al:
Reticulon 4 is necessary for endoplasmic reticulum tubulation,
STIM1-Orai1 coupling and store-operated calcium entry. J Biol Chem.
289:9380–9395. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pozo-Guisado E and Martin-Romero FJ: The
regulation of STIM1 by phosphorylation. Commun Integr Biol.
6:e262832013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ross DG, Smart CE, Azimi I,
Roberts-Thomson SJ and Monteith GR: Assessment of ORAI1-mediated
basal calcium influx in mammary epithelial cells. BMC Cell Biol.
14:572013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang H, Clemens RA, Liu F, et al: STIM1
calcium sensor is required for activation of the phagocyte oxidase
during inflammation and host defense. Blood. 123:2238–2249. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Harraz OF and Altier C: STIM1-mediated
bidirectional regulation of Ca2+ entry through
voltage-gated calcium channels (VGCC) and calcium-release activated
channels (CRAC). Front Cell Neurosci. 8:432014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lee KP, Choi S, Hong JH, et al: Molecular
determinants mediating gating of transient receptor potential
canonical (TRPC) channels by stromal interaction molecule 1
(STIM1). J Biol Chem. 289:6372–6382. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Poon TY, Ong KL and Cheung BM: Review of
the effects of the traditional chinese medicine rehmannia six
formula on diabetes mellitus and its complications. J Diabetes.
3:184–200. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cai H, Cao G and Cai B: Rapid simultaneous
identification and determination of the multiple compounds in crude
Fructus Corni and its processed products by HPLC-MS/MS with
multiple reaction monitoring mode. Pharm Biol. 51:273–278. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chu Q, Amano O, Kanda Y, Kunii S, Wang Q
and Sakagami H: Tumor-specific cytotoxicity and type of cell death
induced by gefitinib in oral squamous cell carcinoma cell lines.
Anticancer Res. 29:5023–5031. 2009.PubMed/NCBI
|
|
12
|
Wu Y, Wang X, Shen B, Kang L and Fan E:
Extraction, structure and bioactivities of the polysaccharides from
Fructus Corni. Recent Pat Food Nutr Agric. 5:57–61. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang W, Xu J, Li L, et al: Neuroprotective
effect of morroniside on focal cerebral ischemia in rats. Brain Res
Bull. 83:196–201. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hong SY, Jeong WS and Jun M: Protective
effects of the key compounds isolated from Corni fructus against
β-amyloid-induced neurotoxicity in PC12 cells. Molecules.
17:10831–10845. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-∆∆CT) method. Methods. 25:402–428. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liao Y, Plummer NW, George MD, et al: A
role for Orai in TRPC-mediated Ca2+ entry suggests that a TRPC:
Orai complex may mediate store and receptor operated Ca2+ entry.
Proc Natl Acad Sci USA. 106:3202–3206. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Smyth JT and Putney JW: Regulation of
store-operated calcium entry during cell division. Biochem Soc
Trans. 40:119–123. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cahalan MD: STIMulating store-operated
Ca2+ entry. Nat Cell Biol. 11:669–677. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kilch T, Alansary D, Peglow M, et al:
Mutations of the Ca2+-sensing stromal interaction
molecule STIM1 regulate Ca2+ influx by altered
oligomerization of STIM1 and by destabilization of the
Ca2+ channel Orai1. J Biol Chem. 288:1653–1664. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Umemura M, Baljinnyam E, Feske S, et al:
Store-operated Ca2+ entry (SOCE) regulates melanoma
proliferation and cell migration. PLoS One. 9:e892922014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Smyth JT and Putney JW: Regulation of
store-operated calcium entry during cell division. Biochem Soc
Trans. 40:119–123. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Johnstone LS, Graham SJ and Dziadek MA:
STIM proteins: integrators of signalling pathways in development,
differentiation and disease. J Cell Mol Med. 14:1890–1903. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu H, Hughes JD, Rollins S, Chen B and
Perkins E: Calcium entry via ORAI1 regulates glioblastoma cell
proliferation and apoptosis. Exp Mol Pathol. 9:753–760. 2011.
View Article : Google Scholar
|
|
24
|
Chiu TY, Teng HC, Huang PC, Kao FJ and
Yang DM: Dominant role of Orai1 with STIM1 on the cytosolic entry
and cytotoxicity of lead ions. Toxicol Sci. 110:353–362. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li Y, Song J, Liu X, Zhang M, et al: High
expression of STIM1 in the early stages of diffuse axonal injury.
Brain Res. 1495:95–102. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Abdullaev IF, Bisaillon JM, Potier M,
Gonzalez JC, Motiani RK and Trebak M: Stim1 and Orai1 mediate CRAC
currents and store-operated calcium entry important for endothelial
cell proliferation. Circ Res. 103:1289–1299. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Potier M, Gonzalez JC, Motiani RK, et al:
Evidence for STIM1- and Orai1-dependent store-operated calcium
influx through ICRAC in vascular smooth muscle cells: role in
proliferation and migration. FASEB J. 23:2425–2437. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yoshida J, Iwabuchi K, Matsui T, Ishibashi
T, Masuoka T and Nishio M: Knockdown of stromal interaction
molecule 1 (STIM1) suppresses store-operated calcium entry, cell
proliferation and tumorigenicity in human epidermoid carcinoma A431
cells. Biochem Pharmacol. 84:1592–1603. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pozo-Guisado E, Campbell DG, Deak M, et
al: Phosphorylation of STIM1 at ERK1/2 target sites modulates
store-operated calcium entry. J Cell Sci. 123:3084–3093. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang JY, Chen BK, Wang YS, et al:
Involvement of store-operated calcium signaling in EGF-mediated
COX-2 gene activation in cancer cells. Cell Signal. 24:162–169.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ogawa A, Firth AL, Smith KA, Maliakal MV
and Yuan JX: PDGF enhances store-operated Ca2+ entry by
upregulating STIM1/Orai1 via activation of Akt/mTOR in human
pulmonary arterial smooth muscle cells. Am J Physiol Cell Physiol.
302:C405–C411. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Pozo-Guisado E, Casas-Rua V, Tomas-Martin
P, Lopez-Guerrero AM, Alvarez-Barrientos A and Martin-Romero FJ:
Phosphorylation of STIM1 at ERK1/2 target sites regulates
interaction with the microtubule plus-end binding protein EB1. J
Cell Sci. 126:3170–3180. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fedida-Metula S, Feldman B, Koshelev V,
Levin-Gromiko U, Voronov E and Fishman D: Lipid rafts couple
store-operated Ca2+ entry to constitutive activation of
PKB/Akt in a Ca2+/calmodulin-, Src- and PP2A-mediated
pathway and promote melanoma tumor growth. Carcinogenesis.
33:740–750. 2012. View Article : Google Scholar : PubMed/NCBI
|