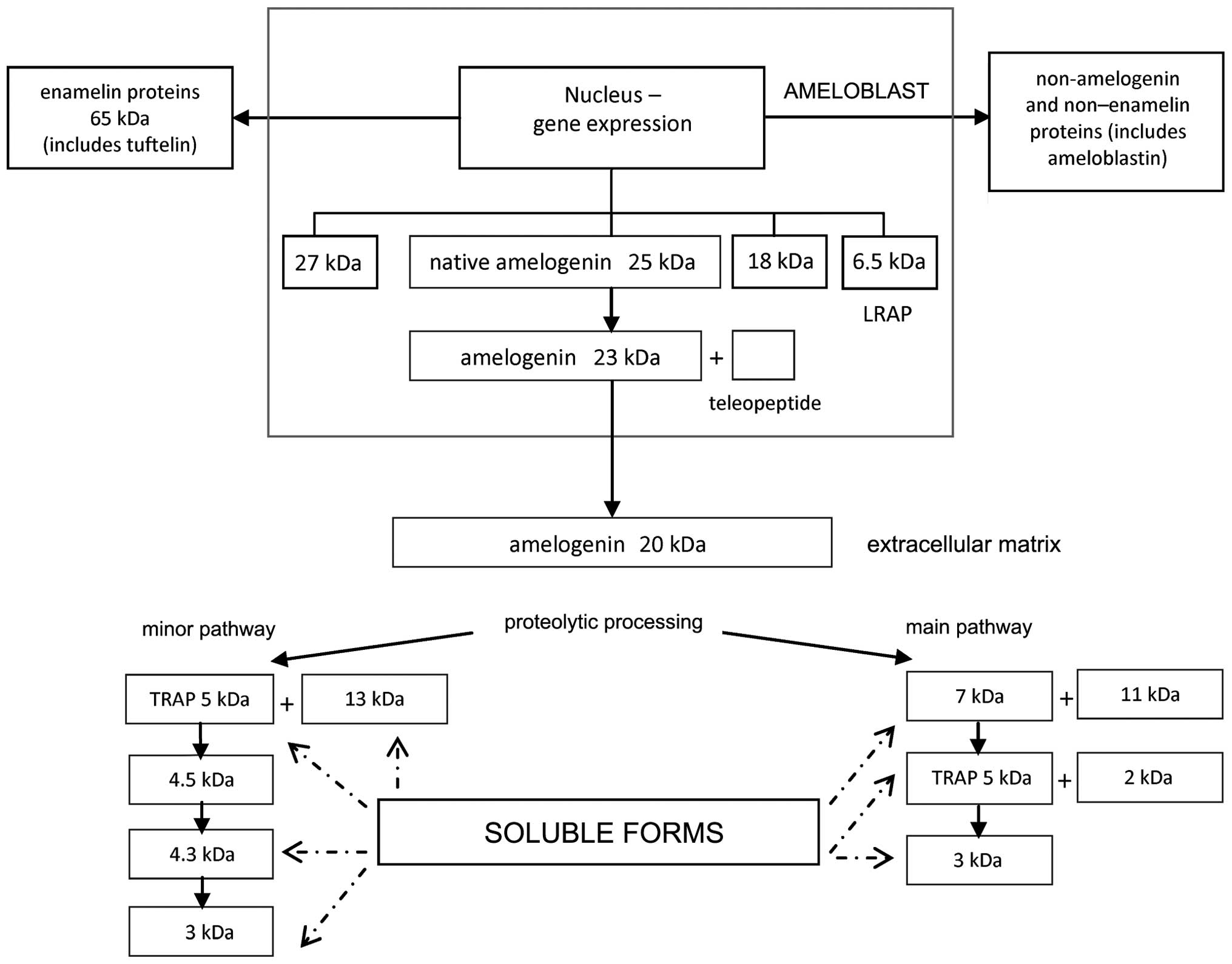
In this review, the growth factor and growth
factor-like activities of enamel matrix proteins (EMPs) were
examined. Enamel matrix derivative (EMD), a mixture of EMPs,
emerged almost two decades ago as an agent capable of periodontal
regeneration. Although numerous studies and review papers have been
published on this topic, the understanding of the cellular and
molecular mechanisms of action of EMD is far from exhaustive
(1–7); thus this subject is revisited in the
present comprehensive review.
Growth factors regulate important cellular events
involved in numerous physiological and pathological processes by
binding to specific cell surface receptors (8). A number of polypeptide growth factors
have been identified that regulate cell proliferation, chemotaxis
or differentiation. Certain growth and differentiation factors,
such as insulin-like growth factor 1 (IGF-1), platelet-derived
growth factor (PDGF), basic fibroblast growth factor (bFGF),
transforming growth factor-β (TGF-β) and bone morphogenetic protein
(BMP)-2, are able to stimulate the cellular activities associated
with periodontal regeneration in periodontal ligament (PDL) cells
(9–19).
Ameloblasts synthesize and secrete a number of EMPs,
including amelogenins, ameloblastin, amelotin, tuftelin and
enamelin (20,21). EMPs are associated with the process
of amelogenesis and they play a crucial role in the formation of
enamel and periodontal attachment during tooth development;
however, in wound healing and tissue regeneration EMPs show several
novel functions.
Clinically, EMPs are applied as an extract of
porcine fetal tooth enamel matrix (known as EMD) for the
periodontal regeneration of teeth affected by periodontitis, root
coverage procedures and tooth implantation. EMD has also been used
in in vitro research, for dentin repair, tooth movement,
anti-cancer treatment evaluation and skin wound healing (22). The regeneration of several types of
periodontal tissue, including alveolar bone, cellular cementum and
collagenous ligaments, and the formation of an extracellular matrix
(ECM) layer in adjacent tissues has been observed following EMD
application (22). The predominant
(>90%) component of EMD is amelogenin, which in its native form
is slowly degraded by the proteinases enamelysin and kallikrein-4
(20). Cleavage produces different,
shorter forms of amelogenin. These amelogenin-derived peptides are
classified into two groups: Leucine-rich amelogenin peptide (LRAP)
and tyrosine-rich amelogenin peptide (TRAP) (Fig. 1). While EMD presents a number of
growth factor-like effects, it is the TGF-β activity that is
predominately known (23–26). EMD has also been reported to contain
other cytokines, such as a BMP-like growth factor and bone
sialoprotein (BSP)-like molecules (27,28).
To date, which of the EMD fractions are crucial for
BMP- or TGF-β-like activity has not been definitively clarified. In
one study, the chromatographic separation of EMD resulted in 22
protein fractions. Fractions 4–6 had BMP-like activity while
fractions 8–13 had TGF-β-like activity, and fractions 4–13 were
found to contain 10–25-kDa peptides (26). It was observed that BMP-2 signal
transduction activity was inhibited by authentic TGF-β1 and the
TGF-β1 or TGF-β-like activity in an EMD gel, but that signal
transduction by TGF-β was not suppressed by BMP-2. It was
hypothesized that TGF-β could not completely inhibit the activity
of BMP, since BMP and TGF-β activate SMAD intracellular
transcription factors (26). In oral
epithelial cells and fibroblasts isolated from gingiva, EMD
stimulated the rapid translocation of SMAD2 protein from the
cytoplasm to the cell nucleus, which suggests the involvement of
TGF-β-like factors (24).
TGF-β is a member of the TGF-β superfamily, which
consists of five isoforms of TGF-β and associated homologous
proteins including activins, inhibins, BMPs, growth differentiation
factors and the glial cell line-derived neurotrophic factor family
(29–31). These structurally related
polypeptides are characterized by the presence of a common sequence
and specifically positioned structures, namely a ̔cystine knot̓
composed of six cysteine residues (32). The structural differences between the
TGF-β proteins and the BMPs have been found to lie within four
regions of the polypeptide chain. These are the N-terminal segment,
the loops at the end of fingers 1 and 2 and the C-terminus of helix
α3 (33). In addition, the receptor
activation differs, despite the fact that in each case it involves
the recruitment of pairs of type I and type II receptor molecules
by dimeric ligands to form signaling complexes. Sequential binding
is typical of TGF-β and activin ligand receptors, while a fully
cooperative interaction is characteristic of BMP ligand receptors
(33). Mature TGF-β is a homodimer,
composed of two 12.5-kDa polypeptides joined by a disulfide bond
between two cysteine 77 residues and by hydrophobic interactions
(34).
TGF-β regulates various cellular processes including
cell growth, apoptosis, homeostasis, differentiation, migration,
wound healing, fibrosis, angiogenesis and carcinogenesis (35–37).
TGF-β1 has been indicated to play an important role in the
modulation of tissue formation and development of the periodontium
(38). It is also a
transcription-regulating factor (29,30,39,40).
Notably, the response can differ considerably according to the type
of cell and the stimulation context, even though the activation is
induced by the same receptor. It is, therefore, critical,
particularly in carcinogenesis, to know where TGF-β may act as a
suppressor and where as a stimulator. The role of TGF-β in
carcinogenesis appears to involve a signaling pathway involving
SMAD proteins, which is induced by TGF-β (41). Non-SMAD signaling pathways in TGF-β
signaling include extracellular signal-regulated kinases (ERK),
c-Jun N-terminal kinases (JNK), Rho-associated protein kinases and
P21-activated kinase-2, depending on the cell line (42). It has been suggested that
TGF-β-mediated apoptosis is regulated by the modulation of SMAD
activation (43). Furthermore, TGF-β
has been indicated to participate in carcinogenesis by immune
suppression (44). Several studies
have indicated that TGF-β arrests growth in the majority of cell
types (29,45). This effect has been observed in
primary embryonic fibroblasts; however, in fibroblasts from
SMAD3-null mice, the growth inhibitory effect of TGF-β was
suppressed (45).
Several studies of EMD have demonstrated that it
contains TGF-β1 or a TGF-β-like substance, and that EMD rapidly
translocates SMAD2, an effector of the TGF-β signaling pathway,
into the nucleus and modulates the proliferation of human gingival
fibroblasts and oral epithelial cells in a cell type-specific
manner (24,26,46,47).
Furthermore, experiments in vitro on epithelial and
fibroblastic cells with anti-TGF-β antibodies, in which the
TGF-β1-induced SMAD2 translocation was blocked, showed that the
EMD-induced translocation of SMAD2 was strongly reduced. This may
indicate that they act via the same mechanism (48). In human PDL fibroblasts, EMD
stimulated the release of TGF-β1 (45). PDL cell metabolism was significantly
increased when EMD was present in cultures, and an increased
autocrine production of TGF-β1, interleukin 6 (IL-6) and PDGF-AB
was detected when compared with that in control cultures (49).
It has been postulated that EMD may contain an
additional mitogenic factor, which acts in combination with TGF-β1
to fully stimulate fibroblastic proliferation. Kawase et al
(46) investigated the effects of
EMD, TGF-β1 and neutralizing TGF-β antibody on epithelial and
fibroblastic cells. It was found that porcine EMD translocated
SMAD2 into the nucleus of cells, as does TGF-β1 or a TGF-β-like
substance. SMAD2 is an effector of the TGF-β signaling pathway that
modulates the proliferation of gingival fibroblastic and oral
epithelial cells. In the study, cells were treated with porcine
TGF-β1 in order to compare its actions with those of EMD. In the
epithelial and fibroblastic cells, TGF-β1 replicated the action of
EMD in the nuclear accumulation of SMAD2, the phosphorylation of
mitogen activated protein (MAP) kinase family members and,
consequently, cell growth induction. Neutralizing TGF-β antibody
blocked certain actions of EMD. The anti-TGF-β antibody prevented
TGF-β1-induced SMAD2 translocation and blocked other actions of
EMD, such as p38-MAP kinase phosphorylation and p21WAF1/cip1
expression in epithelial cells. It has been suggested that TGF-β1
or a TGF-β-like substance is a principal bioactive factor in EMD,
but the TGF-β1-neutralizing antibody did not block EMD-induced
fibroblast proliferation, strongly implying that EMD contains
unidentified mitogenic factor(s).
The effects of EMD vary according to the origin of
the cell line; EMD has been found to increase the proliferation of
gingival fibroblasts but decrease the proliferation of epithelial
cells (50,51). Notably, no apoptotic effect was
observed when epithelial cells were treated with EMD (51), which led to the conclusion that EMD
acted as a cytostatic rather than a cytotoxic agent for epithelial
cells. EMD has also been demonstrated to have a growth-inhibitory
effect on epithelial (HeLa) cells and human squamous cell
carcinoma-derived-25 cells (51).
Kawase et al (46) postulated
that EMD reduced DNA synthesis, suggesting that a reduction in
epithelial cell growth could be mediated by TGF-β-like activity.
EMD and TGF-β are also able to stimulate the production of matrix
metalloproteinases (MMPs), which are crucial in tumorigenesis and
in benign keratinocytes (27).
The findings concerning the effect of EMD on the
other special epithelial cells, endothelial cells, which are
required for the healing and regeneration of periodontal tissue,
are contradictory and include either a stimulatory effect (52) or no effect at all (53) on proliferation. A low concentration
of EMD stimulated the proliferation and migration of endothelial
cells, whereas a higher concentration inhibited them (54). It was hypothesized that TGF-β present
in the EMD-conditioned media may be responsible for the effects of
EMD on the proliferation and viability of human umbilical vein
endothelial cells (54).
The effect of EMD is also dependent upon its
specific fraction. Full-length amelogenin molecules have been shown
to stimulate the autocrine production of BMP, while smaller
fractions like LRAP and TRAP stimulate the autocrine production of
TGF-β in the human PDL (4). The
TGF-β protein, however, has not been found in the composition of
EMD (50). The aforementioned
studies suggest that specific amelogenin molecules may stimulate
the autocrine release of growth factors that coordinate the
regenerative effects of EMD.
BMPs bind to BMP receptors of types I and II. Type I
receptors include activin receptor-like kinase (ALK)-2, ALK-3 (BMP
receptor IA; expressed in most types of cells) and ALK-6 (BMP
receptor IB; chondrocytes and osteoclasts express only this type of
BMP receptor), and mainly determine the specificity of the
intracellular signals. Type II receptors include BMP type II
receptor, activin type II receptor and activin type IIB receptor.
BMPs activate intracellular transcription factors SMAD-1, −5 and
−8, which dimerize with SMAD-4 prior to translocation into the
nucleus (67,69–71).
Osteopontin, osteoprotegerin (OPG), BMP-7 and SMAD-1 are activated
by BMP through the SMAD activation mechanism (72–74).
BMPs also stimulate MAP kinase, phosphoinositide-3 kinase and JNK
by SMAD-independent signals (75,76).
In the periodontium the presence of BMP-2 and BMP-4
between sections of human periodontal structures is distinct.
Immunohistochemistry has shown intense staining in the PDL with
almost no detection in the cementum, alveolar bone and gingival
connective tissue (77). This
finding did not correlate with the expression of mRNA for these
proteins. In vitro the gingival and PDL fibroblasts
expressed mRNA for BMP-2 and −4, and while the BMP-4 mRNA level was
similar in the gingival and periodontal fibroblasts, the BMP-2
expression was higher in the gingival fibroblasts (77).
EMD has been shown to contain or stimulate growth
factors such as TGF-β, BMP-2, −4 and −7 (24,26,78–80). It
has also been noted that amelogenin stimulates BSP gene
transcription in osteoblasts by inducing the expression of nuclear
proteins that bind to FGF-2 response elements and TGF-β1 activation
elements in BSP gene promoters (4).
Amelogenin has comparable osteogenic activities to recombinant
human BMP-2 and induces the formation of a reparative dentin
bridge, in a manner comparable with that of BMP-7 and calcium
hydroxide (81). In response to EMD
treatment, human dental follicle cells have exhibited increased
expression levels of BMP-2, BMP-7, BSP and two cementum markers,
namely cementum attachment protein and cementum protein-23
(78).
The investigation of osteoprogenitor cells (C2C12)
and human microvascular endothelial cells showed that noggin, a
molecule that prevents BMPs from binding to their receptors
(82,83), abolishes alkaline phosphatase
activity in C2C12 cells. This suggests that the effect on
osteoprogenitor cell differentiation results from the action of
BMP-like proteins, whereas the effects on proliferation and
angiogenesis are associated with lower molecular weight proteins
from EMD (84). By contrast, the
osteoinductive activity of LRAP has been found to be comparable
with the effect of BMP-2 on the osteogenesis of mouse embryonic
stem cells (85).
VEGF induces endothelial proliferation, migration
and specialization in new and developing vascular beds (86) during embryogenesis and later
development, wound healing and menstruation. It is also a potent
promoter of angiogenesis in numerous types of tumors (87), diabetes, rheumatoid fever and
psoriasis (88).
In the periodontium, VEGF has been shown to be
involved in the regulation of bone remodeling by attracting
endothelial cells and osteoclasts and by stimulating osteoblast
differentiation (89). VEGF has been
found in a higher concentration in crevicular fluid during
gingivitis (90). Angiogenesis is
central to tissue healing. EMD, directly or indirectly, positively
influences this process. EMD has been shown to have a chemotactic
effect on endothelial cells in vitro (53) and to stimulate human microvascular
endothelial cells (HMVECs) as well as their production of VEGF
(52,84). Additionally, EMD enhances the
communication between HMVECs and PDL fibroblasts (52). Human periodontal and dermal
fibroblasts cultured with EMD also exhibit increased VEGF
production (52,91). One of the possible mechanisms by
which EMD stimulates angiogenesis is by increasing the expression
of MMP-2 in human microvascular pericytes (91,92).
Another route could be through the EMD-induced stimulation of VEGF
production, which occurs partially via TGF-β1 and FGF-2 in human
gingival fibroblasts (37). It is
notable that EMD and its major component, amelogenin, stimulate
angiogenesis but the small tyrosine-rich and leucine-rich
polypeptides present in a 5-kD protein fraction derived from EMD do
not (93). This stimulation is
dose-dependent (94). At low
concentrations EMD stimulates PDL fibroblast proliferation by
HMVECs but in higher concentrations it does not (52).
PDGF stimulates the activation of proliferation,
migration and matrix synthesis in gingival and PDL fibroblasts,
cementoblasts, pre-osteoblasts and osteoblasts in a dose- and
time-dependent manner (15,95–100).
It is suggested that during wound healing, PDGF cooperates with
other growth factors, such as IGF-1 (101), TGF-β (99) or VEGF (102). PDGF has VEGF-like effects on
angiogenesis. The three main VEGF receptors are structurally
similar to the family of PDGF receptor III class of tyrosine kinase
receptors (RTK class III) (103).
The RTK-ERK 1/2 signaling pathway induced by EMD is similar to that
activated by epidermal growth factor (EGF) (103). PDGF upregulates the expression of
integrin collagen receptors in rat fibroblasts (104) and also stimulates actin filament
reorganization in cytoskeletal proteins (105). EMD induces PDL fibroblasts to
secrete TGF-β1, IL-6 and PDGF-AB by intracellular cyclic adenosine
monophosphate signaling; epithelial cell growth is inhibited by the
same signal (49). A
combination of EMD and PDGF-BB produced greater proliferative and
wound-fill effects on PDL cells than either protein by itself
(106).
EGF enhances the cellular proliferation and
differentiation of epidermal and epithelial cells, fibroblasts,
cartilage and bone-derived cells during growth, maturation and
healing processes (124–129), and is also a potent mitogenic
factor (130–132). The treatment of human gingival
fibroblasts with EMD results in an autocrine/paracrine EGF receptor
(EGFR) transactivation. There are two suggested independent
mechanisms of EGFR transactivation: i) An intracellular pathway
mediated by the src family of non-receptor tyrosine kinases
(133,134); and ii) an extracellular pathway
mediated by the shedding of a transmembrane pro-form of EGFR
ligands by metalloproteinases (135–137).
The capacity of PDL cells to bind to EGF and EMD has been assessed
in a 125I-EGF radioligand binding assay. The assay
showed that there was no significant competitive binding between
EGF and EMD, indicating that the EGFR is not the binding site for
EMD (103). These results indicate
that EMD does not contain biologically effective amounts of EGF and
supports a study in which no EGF was detected in EMD by
radioimmunoassay (50). Other
studies have demonstrated cross-talk between TGF-β and
EGF-stimulated pathways (138) and
suggested that the molecular mechanisms by which TGF-β1 and EGF
interact to elicit these phenotypic changes may involve MAP
kinases, SMADs, activator protein and upstream stimulatory factor
transcription factors (139,140).
IGF-1 is a multifunctional peptide that regulates
growth, differentiation and the expression of ECM proteins
(9). It is also thought to be a key
mediator of wound healing, inducing epithelial and mesenchymal cell
proliferation (8). IGF is reported
to stimulate cell migration (126,130,132)
and has been successfully used for dentine-pulp complex
regeneration (141). EMD stimulates
IGF-1, TGF-β1, PDGF and IL-6 production in PDL fibroblasts
(49,142); however, it has no effect on IGF-1,
BMP-2 or IL-6 in HeLa and MG-63 cell lines (49,143).
PDGF and IGF together synergistically enhance gingival fibroblast
contractility, and may have had a synergistic effect on wound
healing (101,144). Furthermore, cementum-derived growth
factor (CGF) has been characterized as an IGF-1-like molecule
(145). CGF has been shown to be
mitogenic for both PDL and gingival fibroblasts, to promote the
migration and growth of progenitor cells adjacent to the dentin
matrix, and to participate in their differentiation into
cementoblasts (146).
The effects of EMD on periodontal tissue
regeneration have been well documented, however, the mechanism of
action remains unknown. To date, no receptors specific for
amelogenin have been identified, to the best of our knowledge.
However, there are putative receptors, such as lysosomal-associated
membrane proteins (LAMP); LAMP-1 interacts with LRAP, and LAMP-3
with longer amelogenin protein isoforms. Notably, neither of these
receptors interacts with both of the amelogenin molecules (147). The role of amelogenin derivatives
in the periodontium is also unclear. Studies carried out on LRAP
have shown its induction effect on the expression of bone acidic
glycoprotein-75, BSP (148) and OPG
in mineralized tissues, including cementoblasts (149). When evaluating whether the action
of EMD on cells is dependent on direct cell-matrix contact or
mediated by growth factors released from EMD or stimulated by it,
the close interaction between growth factors presents a challenge.
It has been suggested that the soluble growth factors contained in
EMD may be responsible for the stimulating effects. TGF-β and small
amelogenin peptides are potential candidates for the factors
mediating the action of EMD (150),
however further studies are required to investigate this
further.
This study was supported in part by grants from the
Polish Ministry of Science (no. 403283040), the Frank Stranahan
Endowed Chair and the Children Miracle Network.
|
1
|
Bosshardt DD: Biological mediators and
periodontal regeneration: a review of enamel matrix proteins at the
cellular and molecular levels. J Clin Periodontol. 35:(Suppl).
87–105. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gibson CW: The amelogenin ̔enamel
proteins̓ and cells in the periodontium. Crit Rev Eukaryot Gene
Expr. 18:345–360. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Grandin HM, Gemperli AC and Dard M: Enamel
matrix derivative: a review of cellular effects in vitro and a
model of molecular arrangement and functioning. Tissue Eng Part B
Rev. 18:181–202. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lyngstadaas SP, Wohlfahrt JC, Brookes SJ,
Paine ML, Snead ML and Reseland JE: Enamel matrix proteins; old
molecules for new applications. Orthod Craniofac Res. 12:243–253.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Miron RJ, Guillemette V, Zhang Y, Chandad
F and Sculean A: Enamel matrix derivative in combination with bone
grafts: A review of the literature. Quintessence Int. 45:475–487.
2014.PubMed/NCBI
|
|
6
|
Rathe F, Junker R, Chesnutt BM and Jansen
JA: The effect of enamel matrix derivative (Emdogain) on bone
formation: a systematic review. Tissue Eng Part B Rev. 15:215–224.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zeichner-David M: Is there more to enamel
matrix proteins than biomineralization? Matrix Biol. 20:307–316.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Giannobile WV: Periodontal tissue
engineering by growth factors. Bone. 19:(Suppl). 23S–37S. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Blom S, Holmstrup P and Dabelsteen E: The
effect of insulin-like growth factor-I and human growth hormone on
periodontal ligament fibroblast morphology, growth pattern, DNA
synthesis and receptor binding. J Periodontol. 63:960–968. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Brady TA, Piesco NP, Buckley MJ, Langkamp
HH, Bowen LL and Agarwal S: Autoregulation of periodontal ligament
cell phenotype and functions by transforming growth factor-beta1. J
Dent Res. 77:1779–1790. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dennison DK, Vallone DR, Pinero GJ,
Rittman B and Caffesse RG: Differential effect of TGF-beta 1 and
PDGF on proliferation of periodontal ligament cells and gingival
fibroblasts. J Periodontol. 65:641–648. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kobayashi M, Takiguchi T, Suzuki R, et al:
Recombinant human bone morphogenetic protein-2 stimulates
osteoblastic differentiation in cells isolated from human
periodontal ligament. J Dent Res. 78:1624–1633. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lynch SE, Williams RC, Polson AM, et al: A
combination of platelet-derived and insulin-like growth factors
enhances periodontal regeneration. J Clin Periodontol. 16:545–548.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Matsuda N, Lin WL, Kumar NM, Cho MI and
Genco RJ: Mitogenic, chemotactic and synthetic responses of rat
periodontal ligament fibroblastic cells to polypeptide growth
factors in vitro. J Periodontol. 63:515–525. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nishimura F and Terranova VP: Comparative
study of the chemotactic responses of periodontal ligament cells
and gingival fibroblasts to polypeptide growth factors. J Dent Res.
75:986–992. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Oates TW, Rouse CA and Cochran DL:
Mitogenic effects of growth factors on human periodontal ligament
cells in vitro. J Periodontol. 64:142–148. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Takayama S, Murakami S, Miki Y, et al:
Effects of basic fibroblast growth factor on human periodontal
ligament cells. J Periodontal Res. 32:667–675. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Terranova VP, Odziemiec C, Tweden KS and
Spadone DP: Repopulation of dentin surfaces by periodontal ligament
cells and endothelial cells. Effect of basic fibroblast growth
factor. J Periodontol. 60:293–301. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Terranova VP and Wikesjö UM: Extracellular
matrices and polypeptide growth factors as mediators of functions
of cells of the periodontium. A review. J Periodontol. 58:371–380.
1987. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bartlett JD and Simmer JP: Proteinases in
developing dental enamel. Crit Rev Oral Biol Med. 10:425–441. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Margolis HC, Beniash E and Fowler CE: Role
of macromolecular assembly of enamel matrix proteins in enamel
formation. J Dent Res. 85:775–793. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sculean A, Schwarz F, Becker J and Brecx
M: The application of an enamel matrix protein derivative
(Emdogain) in regenerative periodontal therapy: a review. Med Princ
Pract. 16:167–180. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Heijl L, Heden G, Svärdström G and Ostgren
A: Enamel matrix derivative (EMDOGAIN) in the treatment of
intrabony periodontal defects. J Clin Periodontol. 24:705–714.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kawase T, Okuda K, Momose M, Kato Y,
Yoshie H and Burns DM: Enamel matrix derivative (EMDOGAIN) rapidly
stimulates phosphorylation of the MAP kinase family and nuclear
accumulation of smad2 in both oral epithelial and fibroblastic
human cells. J Periodontal Res. 36:367–376. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Petinaki E, Nikolopoulos S and Castanas E:
Low stimulation of peripheral lymphocytes, following in vitro
application of Emdogain. J Clin Periodontol. 25:715–720. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Suzuki S, Nagano T, Yamakoshi Y, et al:
Enamel matrix derivative gel stimulates signal transduction of BMP
and TGF-β. J Dent Res. 84:510–514. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Laaksonen M, Sorsa T and Salo T: Emdogain
in carcinogenesis: a systematic review of in vitro studies. J Oral
Sci. 52:1–11. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nikolopoulos S, Peteinaki E and Castanas
E: Immunologic effects of emdogain in humans: one-year results. Int
J Periodontics Restorative Dent. 22:269–277. 2002.PubMed/NCBI
|
|
29
|
Massagué J, Blain SW and Lo RS: TGFbeta
signaling in growth control, cancer, and heritable disorders. Cell.
103:295–309. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Patterson GI and Padgett RW: TGF
beta-related pathways. Roles in Caenorhabditis elegans development.
Trends Genet. 16:27–33. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Roberts AB, Sporn MB, Assoian RK, et al:
Transforming growth factor type beta: rapid induction of fibrosis
and angiogenesis in vivo and stimulation of collagen formation in
vitro. Proc Natl Acad Sci USA. 83:4167–4171. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sun PD and Davies DR: ccccccccccccc. Annu
Rev Biophys Biomol Struct. 24:269–291. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Innis CA, Shi J and Blundell TL:
Evolutionary trace analysis of TGF-beta and related growth factors:
implications for site-directed mutagenesis. Protein Eng.
13:839–847. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Daopin S, Piez KA, Ogawa Y and Davies DR:
Crystal structure of transforming growth factor-beta 2: an unusual
fold for the superfamily. Science. 257:369–373. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gruber R, Roos G, Caballé-Serrano J, Miron
R, Bosshardt DD and Sculean A: TGF-βRI kinase activity mediates
Emdogain-stimulated in vitro osteoclastogenesis. Clin Oral
Investig. 18:1639–1646. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gruber R, Bosshardt DD, Miron RJ, Gemperli
AC, Buser D and Sculean A: Enamel matrix derivative inhibits
adipocyte differentiation of 3T3-L1 cells via activation of TGF-βRI
kinase activity. PloS One. 8:e710462013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sakoda K, Nakajima Y and Noguchi K: Enamel
matrix derivative induces production of vascular endothelial cell
growth factor in human gingival fibroblasts. Eur J Oral Sci.
120:513–519. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gao J, Symons AL and Bartold PM:
Expression of transforming growth factor-beta 1 (TGF-beta1) in the
developing periodontium of rats. J Dent Res. 77:1708–1716. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Akhurst RJ and Derynck R: TGF-beta
signaling in cancer-a double-edged sword. Trends Cell Biol.
11:(Suppl). S44–S51. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ten Dijke P, Goumans MJ, Itoh F and Itoh
S: Regulation of cell proliferation by Smad proteins. J Cell
Physiol. 191:1–16. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lampropoulos P, Zizi-Sermpetzoglou A,
Rizos S, Kostakis A, Nikiteas N and Papavassiliou AG: TGF-beta
signalling in colon carcinogenesis. Cancer Lett. 314:1–7. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Blanchette F, Rivard N, Rudd P, Grondin F,
Attisano L and Dubois CM: Cross-talk between the p42/p44 MAP kinase
and Smad pathways in transforming growth factor beta 1-induced
furin gene transactivation. J Biol Chem. 276:33986–33994. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Jang CW, Chen CH, Chen CC, Chen JY, Su YH
and Chen RH: TGF-beta induces apoptosis through Smad-mediated
expression of DAP-kinase. Nat Cell Biol. 4:51–58. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Rahimi RA and Leof EB: TGF-β signaling: A
tale of two receptors. J Cell Biochem. 102:593–608. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Datto MB, Frederick JP, Pan L, Borton AJ,
Zhuang Y and Wang XF: Targeted disruption of Smad3 reveals an
essential role in transforming growth factor beta-mediated signal
transduction. Mol Cell Biol. 19:2495–2504. 1999.PubMed/NCBI
|
|
46
|
Kawase T, Okuda K, Yoshie H and Burns DM:
Anti-TGF-beta antibody blocks enamel matrix derivative-induced
upregulation of p21WAF1/cip1 and prevents its inhibition of human
oral epithelial cell proliferation. J Periodontal Res. 37:255–262.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wada Y, Yamamoto H, Nanbu S, Mizuno M and
Tamura M: The suppressive effect of enamel matrix derivative on
osteocalcin gene expression of osteoblasts is neutralized by an
antibody against TGF-beta. J Periodontol. 79:341–347. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Vayalil PK, Iles KE, Choi J, Yi AK,
Postlethwait EM and Liu RM: Glutathione suppresses TGF-beta-induced
PAI-1 expression by inhibiting p38 and JNK MAPK and the binding of
AP-1, SP-1 and Smad to the PAI-1 promoter. Am J Physiol Lung Cell
Mol Physiol. 293:L1281–L1292. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Lyngstadaas SP, Lundberg E, Ekdahl H,
Andersson C and Gestrelius S: Autocrine growth factors in human
periodontal ligament cells cultured on enamel matrix derivative. J
Clin Periodontol. 28:181–188. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Gestrelius S, Andersson C, Lidström D,
Hammarström L and Somerman M: In vitro studies on periodontal
ligament cells and enamel matrix derivative. J Clin Periodontol.
24:685–692. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Kawase T, Okuda K, Yoshie H and Burns DM:
Cytostatic action of enamel matrix derivative (EMDOGAIN) on human
oral squamous cell carcinoma-derived SCC25 epithelial cells. J
Periodontal Res. 35:291–300. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Schlueter SR, Carnes DL Jr and Cochran DL:
In vitro effects of enamel matrix derivative on microvascular
cells. J Periodontol. 78:141–151. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Yuan K, Chen CL and Lin MT: Enamel matrix
derivative exhibits angiogenic effect in vitro and in a murine
model. J Clin Periodontol. 30:732–738. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Bertl K, An N, Bruckmann C, et al: Effects
of enamel matrix derivative on proliferation/viability, migration
and expression of angiogenic factor and adhesion molecules in
endothelial cells in vitro. J Periodontol. 80:1622–1630. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Wozney JM, Rosen V, Celeste AJ, et al:
Novel regulators of bone formation: molecular clones and
activities. Science. 242:1528–1534. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Bragdon B, Moseychuk O, Saldanha S, King
D, Julian J and Nohe A: Bone morphogenetic proteins: a critical
review. Cell Signal. 23:609–620. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Chen D, Zhao M, Harris SE and Mi Z: Signal
transduction and biological functions of bone morphogenetic
proteins. Front Biosci. 9:349–358. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Hogan BL: Bone morphogenetic proteins:
multifunctional regulators of vertebrate development. Genes Dev.
10:1580–1594. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Wordinger RJ and Clark AF: Bone
morphogenetic proteins and their receptors in the eye. Exp Biol Med
(Maywood). 232:979–992. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Ogata T, Wozney JM, Benezra R and Noda M:
Bone morphogenetic protein 2 transiently enhances expression of a
gene, Id (inhibitor of differentiation), encoding a
helix-loop-helix molecule in osteoblast-like cells. Proc Natl Acad
Sci USA. 90:9219–9222. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Myllylä RM, Haapasaari KM, Palatsi R, et
al: Multiple miliary osteoma cutis is a distinct disease entity:
four case reports and review of the literature. Br J Dermatol.
164:544–552. 2011.PubMed/NCBI
|
|
62
|
Plikus MV, Mayer JA, de la Cruz D, et al:
Cyclic dermal BMP signalling regulates stem cell activation during
hair regeneration. Nature. 451:340–344. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Kramer J, Hegert C, Guan K, Wobus AM,
Müller PK and Rohwedel J: Embryonic stem cell-derived chondrogenic
differentiation in vitro: activation by BMP-2 and BMP-4. Mech Dev.
92:193–205. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Rui YF, Du L, Wang Y, et al: Bone
morphogenetic protein 2 promotes transforming growth factor
β3-induced chondrogenesis of human osteoarthritic synovium-derived
stem cells. Chin Med J (Engl). 123:3040–3048. 2010.PubMed/NCBI
|
|
65
|
Hu J, Cui D, Yang X, et al: Bone
morphogenetic protein-2: a potential regulator in scleral
remodeling. Mol Vis. 14:2373–2380. 2008.PubMed/NCBI
|
|
66
|
Blanco Calvo M, Bolós Fernández V, Medina
Villaamil V, Aparicio Gallego G, Díaz Prado S and Grande Pulido E:
Biology of BMP signalling and cancer. Clin Transl Oncol.
11:126–137. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Miyazono K, Maeda S and Imamura T: BMP
receptor signaling: transcriptional targets, regulation of signals
and signaling cross-talk. Cytokine Growth Factor Rev. 16:251–263.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Ryoo HM, Lee MH and Kim YJ: Critical
molecular switches involved in BMP-2-induced osteogenic
differentiation of mesenchymal cells. Gene. 366:51–57. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Holtzhausen A, Golzio C, How T, et al:
Novel bone morphogenetic protein signaling through Smad2 and Smad3
to regulate cancer progression and development. FASEB J.
28:1248–1267. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Matsumoto Y, Otsuka F, Hino J, et al: Bone
morphogenetic protein-3b (BMP-3b) inhibits osteoblast
differentiation via Smad2/3 pathway by counteracting Smad1/5/8
signaling. Mol Cell Endocrinol. 350:78–86. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Nohe A, Keating E, Knaus P and Petersen
NO: Signal transduction of bone morphogenetic protein receptors.
Cell Signal. 16:291–299. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Hullinger TG, Pan Q, Viswanathan HL and
Somerman MJ: TGFbeta and BMP-2 activation of the OPN promoter:
roles of smad- and hox-binding elements. Exp Cell Res. 262:69–74.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Stopa M, Anhuf D, Terstegen L, Gatsios P,
Gressner AM and Dooley S: Participation of Smad2, Smad3 and Smad4
in transforming growth factor beta (TGF-beta)-induced activation of
Smad7. THE TGF-beta response element of the promoter requires
functional Smad binding element and E-box sequences for
transcriptional regulation. J Biol Chem. 275:29308–29317. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Wan M, Shi X, Feng X and Cao X:
Transcriptional mechanisms of bone morphogenetic protein-induced
osteoprotegrin gene expression. J Biol Chem. 276:10119–10125. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Guicheux J, Lemonnier J, Ghayor C, Suzuki
A, Palmer G and Caverzasio J: Activation of p38 mitogen-activated
protein kinase and c-Jun-NH2-terminal kinase by BMP-2 and their
implication in the stimulation of osteoblastic cell
differentiation. J Bone Miner Res. 18:2060–2068. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Osyczka AM and Leboy PS: Bone
morphogenetic protein regulation of early osteoblast genes in human
marrow stromal cells is mediated by extracellular signal-regulated
kinase and phosphatidylinositol 3-kinase signaling. Endocrinology.
146:3428–3437. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Ivanovski S, Li H, Haase HR and Bartold
PM: Expression of bone associated macromolecules by gingival and
periodontal ligament fibroblasts. J Periodontal Res. 36:131–141.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Kémoun P, Laurencin-Dalicieux S, Rue J, et
al: Human dental follicle cells acquire cementoblast features under
stimulation by BMP-2/-7 and enamel matrix derivatives (EMD) in
vitro. Cell Tissue Res. 329:283–294. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Saito K, Konishi I, Nishiguchi M, Hoshino
T and Fujiwara T: Amelogenin binds to both heparan sulfate and bone
morphogenetic protein 2 and pharmacologically suppresses the effect
of noggin. Bone. 43:371–376. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Takayama T, Suzuki N, Narukawa M, Tokunaga
T, Otsuka K and Ito K: Enamel matrix derivative stimulates core
binding factor alpha1/Runt-related transcription factor-2
expression via activation of Smad1 in C2C12 cells. J Periodontol.
76:244–249. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Goldberg M, Six N, Decup F, et al:
Bioactive molecules and the future of pulp therapy. Am J Dent.
16:66–76. 2003.PubMed/NCBI
|
|
82
|
Larrain J, Bachiller D, Lu B, Agius E,
Piccolo S and De Robertis EM: BMP-binding modules in chordin: a
model for signalling regulation in the extracellular space.
Development. 127:821–830. 2000.PubMed/NCBI
|
|
83
|
Zimmerman LB, De Jesús-Escobar JM and
Harland RM: The Spemann organizer signal noggin binds and
inactivates bone morphogenetic protein 4. Cell. 86:599–606. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Johnson DL, Carnes D, Steffensen B and
Cochran DL: Cellular effects of enamel matrix derivative are
associated with different molecular weight fractions following
separation by size-exclusion chromatography. J Periodontol.
80:648–656. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Warotayanont R, Zhu D, Snead ML and Zhou
Y: Leucine-rich amelogenin peptide induces osteogenesis in mouse
embryonic stem cells. Biochem Biophys Res Commun. 367:1–6. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Li C, Shintani S, Terakado N, et al:
Microvessel density and expression of vascular endothelial growth
factor, basic fibroblast growth factor and platelet-derived
endothelial growth factor in oral squamous cell carcinomas. Int J
Oral Maxillofac Surg. 34:559–565. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Johnstone S and Logan RM: The role of
vascular endothelial growth factor (VEGF) in oral dysplasia and
oral squamous cell carcinoma. Oral Oncol. 42:337–342. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Kerbel R and Folkman J: Clinical
translation of angiogenesis inhibitors. Nat Rev Cancer. 2:727–739.
2002. View
Article : Google Scholar : PubMed/NCBI
|
|
89
|
Deckers MM, Karperien M, van der Bent C,
Yamashita T, Papapoulos SE and Löwik CW: Expression of vascular
endothelial growth factors and their receptors during osteoblast
differentiation. Endocrinology. 141:1667–1674. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Johnson RB, Serio FG and Dai X: Vascular
endothelial growth factors and progression of periodontal diseases.
J Periodontol. 70:848–852. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Mirastschijski U, Konrad D, Lundberg E,
Lyngstadaas SP, Jorgensen LN and Agren MS: Effects of a topical
enamel matrix derivative on skin wound healing. Wound Repair Regen.
12:100–108. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Neeley WW II, Carnes DL and Cochran DL:
Osteogenesis in an in vitro coculture of human periodontal ligament
fibroblasts and human microvascular endothelial cells. J
Periodontol. 81:139–149. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Kauvar AS, Thoma DS, Carnes DL and Cochran
DL: In vivo angiogenic activity of enamel matrix derivative. J
Periodontol. 81:1196–1201. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Thoma DS, Villar CC, Carnes DL, Dard M,
Chun YH and Cochran DL: Angiogenic activity of an enamel matrix
derivative (EMD) and EMD-derived proteins: an experimental study in
mice. J Clin Periodontol. 38:253–260. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Bartold PM and Raben A: Growth factor
modulation of fibroblasts in simulated wound healing. J Periodontal
Res. 31:205–216. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Chang PC, Dovban AS, Lim LP, Chong LY, Kuo
MY and Wang CH: Dual delivery of PDGF and simvastatin to accelerate
periodontal regeneration in vivo. Biomaterials. 34:9990–9997. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Coimbra LS, Steffens JP, Rossa C Jr,
Graves DT and Spolidorio LC: Clopidogrel enhances periodontal
repair in rats through decreased inflammation. J Clin Periodontol.
41:295–302. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Ojima Y, Mizuno M, Kuboki Y and Komori T:
In vitro effect of platelet-derived growth factor-BB on collagen
synthesis and proliferation of human periodontal ligament cells.
Oral Dis. 9:144–151. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Saygin NE, Tokiyasu Y, Giannobile WV and
Somerman MJ: Growth factors regulate expression of mineral
associated genes in cementoblasts. J Periodontol. 71:1591–1600.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Strayhorn CL, Garrett JS, Dunn RL,
Benedict JJ and Somerman MJ: Growth factors regulate expression of
osteoblast-associated genes. J Periodontol. 70:1345–1354. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Lynch SE, de Castilla GR, Williams RC, et
al: The effects of short-term application of a combination of
platelet-derived and insulin-like growth factors on periodontal
wound healing. J Periodontol. 62:458–467. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
van der Geer P, Hunter T and Lindberg RA:
Receptor protein tyrosine kinases and their signal transduction
pathways. Annu Rev Cell Biol. 10:251–337. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Matsuda N, Horikawa M, Watanabe M,
Kitagawa S, Kudo Y and Takata T: Possible involvement of
extracellular signal-regulated kinases 1/2 in mitogenic response of
periodontal ligament cells to enamel matrix derivative. Eur J Oral
Sci. 110:439–444. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Gullberg D, Gehlsen KR, Turner DC, et al:
Analysis of alpha 1 beta 1, alpha 2 beta 1 and alpha 3 beta 1
integrins in cell-collagen interactions: identification of
conformation dependent alpha 1 beta 1 binding sites in collagen
type I. EMBO J. 11:3865–3873. 1992.PubMed/NCBI
|
|
105
|
Hammacher A, Mellström K, Heldin CH and
Westermark B: Isoform-specific induction of actin reorganization by
platelet-derived growth factor suggests that the functionally
active receptor is a dimer. EMBO J. 8:2489–2495. 1989.PubMed/NCBI
|
|
106
|
Chong CH, Carnes DL, Moritz AJ, et al:
Human periodontal fibroblast response to enamel matrix derivative,
amelogenin and platelet-derived growth factor-BB. J Periodontol.
77:1242–1252. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Bouma-ter Steege JC, Mayo KH and Griffioen
AW: Angiostatic proteins and peptides. Crit Rev Eukaryot Gene Expr.
11:319–334. 2001.PubMed/NCBI
|
|
108
|
Traver D and Zon LI: Walking the walk:
migration and other common themes in blood and vascular
development. Cell. 108:731–734. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Batouli S, Miura M, Brahim J, et al:
Comparison of stem-cell-mediated osteogenesis and dentinogenesis. J
Dent Res. 82:976–981. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Javed F, Al-Askar M, Al-Rasheed A and
Al-Hezaimi K: Significance of the platelet-derived growth factor in
periodontal tissue regeneration. Arch Oral Biol. 56:1476–1484.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Asahara T, Bauters C, Zheng LP, et al:
Synergistic effect of vascular endothelial growth factor and basic
fibroblast growth factor on angiogenesis in vivo. Circulation.
92:(Suppl). II365–II371. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Goto F, Goto K, Weindel K and Folkman J:
Synergistic effects of vascular endothelial growth factor and basic
fibroblast growth factor on the proliferation and cord formation of
bovine capillary endothelial cells within collagen gels. Lab
Invest. 69:508–517. 1993.PubMed/NCBI
|
|
113
|
Pepper MS, Ferrara N, Orci L and Montesano
R: Potent synergism between vascular endothelial growth factor and
basic fibroblast growth factor in the induction of angiogenesis in
vitro. Biochem Biophys Res Commun. 189:824–831. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Mason JC, Lidington EA, Ahmad SR and
Haskard DO: bFGF and VEGF synergistically enhance endothelial
cytoprotection via decay-accelerating factor induction. Am J
Physiol Cell Physiol. 282:C578–C587. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Han L and Gotlieb AI: Fibroblast growth
factor-2 promotes in vitro mitral valve interstitial cell repair
through transforming growth factor-β/Smad signaling. Am J Pathol.
178:119–127. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Schwartz Z, Carnes DL Jr, Pulliam R, et
al: Porcine fetal enamel matrix derivative stimulates proliferation
but not differentiation of pre-osteoblastic 2T9 cells, inhibits
proliferation and stimulates differentiation of osteoblast-like
MG63 cells and increases proliferation and differentiation of
normal human osteoblast NHOst cells. J Periodontol. 71:1287–1296.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Canalis E, Centrella M and McCarthy T:
Effects of basic fibroblast growth factor on bone formation in
vitro. J Clin Invest. 81:1572–1577. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Hurley MM, Abreu C, Harrison JR, Lichtler
AC, Raisz LG and Kream BE: Basic fibroblast growth factor inhibits
type I collagen gene expression in osteoblastic MC3T3-E1 cells. J
Biol Chem. 268:5588–5593. 1993.PubMed/NCBI
|
|
119
|
Mizutani S, Tsuboi T, Tazoe M, Koshihara
Y, Goto S and Togari A: Involvement of FGF-2 in the action of
Emdogain on normal human osteoblastic activity. Oral Dis.
9:210–217. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Cheng T, Cao W, Wen R, Steinberg RH and
LaVail MM: Prostaglandin E2 induces vascular endothelial growth
factor and basic fibroblast growth factor mRNA expression in
cultured rat Müller cells. Invest Ophthalmol Vis Sci. 39:581–591.
1998.PubMed/NCBI
|
|
121
|
Sabbieti MG, Marchetti L, Abreu C, et al:
Prostaglandins regulate the expression of fibroblast growth
factor-2 in bone. Endocrinology. 140:434–444. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Pickering JG, Ford CM, Tang B and Chow LH:
Coordinated effects of fibroblast growth factor-2 on expression of
fibrillar collagens, matrix metalloproteinases and tissue
inhibitors of matrix metalloproteinases by human vascular smooth
muscle cells. Evidence for repressed collagen production and
activated degradative capacity. Arterioscler Thromb Vasc Biol.
17:475–482. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Yanagita M, Kojima Y, Kubota M, et al:
Cooperative effects of FGF-2 and VEGF-A in periodontal ligament
cells. J Dent Res. 93:89–95. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Carpenter G and Cohen S: Epidermal growth
factor. J Biol Chem. 265:7709–7712. 1990.PubMed/NCBI
|
|
125
|
Cohen S: Nobel lecture. Epidermal growth
factor. Biosci Rep. 6:1017–1028. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Furfaro F, Ang ES, Lareu RR, Murray K and
Goonewardene M: A histological and micro-CT investigation in to the
effect of NGF and EGF on the periodontal, alveolar bone, root and
pulpal healing of replanted molars in a rat model-a pilot study.
Prog Orthod. 15:22014. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Guajardo G, Okamoto Y, Gogen H, et al:
Immunohistochemical localization of epidermal growth factor in cat
paradental tissues during tooth movement. Am J Orthod Dentofacial
Orthop. 118:210–219. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Keeve PL, Dittmar T, Gassmann G, Grimm WD,
Niggemann B and Friedmann A: Characterization and analysis of
migration patterns of dentospheres derived from periodontal tissue
and the palate. J Periodontal Res. 48:276–285. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Pyrc K, Milewska A, Kantyka T, et al:
Inactivation of epidermal growth factor by Porphyromonas gingivalis
as a potential mechanism for periodontal tissue damage. Infect
Immun. 81:55–64. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Dereka XE, Markopoulou CE and Vrotsos IA:
Role of growth factors on periodontal repair. Growth Factors.
24:260–267. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Lee J, Stavropoulos A, Susin C and Wikesjö
UM: Periodontal regeneration: focus on growth and differentiation
factors. Dent Clin North Am. 54:93–111. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Okuda K, Kawase T, Momose M, et al:
Platelet-rich plasma contains high levels of platelet-derived
growth factor and transforming growth factor-beta and modulates the
proliferation of periodontally related cells in vitro. J
Periodontol. 74:849–857. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Biscardi JS, Maa MC, Tice DA, Cox ME, Leu
TH and Parsons SJ: c-Src-mediated phosphorylation of the epidermal
growth factor receptor on Tyr845 and Tyr1101 is associated with
modulation of receptor function. J Biol Chem. 274:8335–8343. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Zeldich E, Koren R, Dard M, Nemcovsky C
and Weinreb M: EGFR in Enamel Matrix Derivative-induced gingival
fibroblast mitogenesis. J Dent Res. 87:850–855. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Edwin F, Wiepz GJ, Singh R, et al: A
historical perspective of the EGF receptor and related systems.
Methods Mol Biol. 327:1–24. 2006.PubMed/NCBI
|
|
136
|
Prenzel N, Zwick E, Daub H, et al: EGF
receptor transactivation by G-protein-coupled receptors requires
metalloproteinase cleavage of proHB-EGF. Nature. 402:884–888.
1999.PubMed/NCBI
|
|
137
|
Xu KP, Yin J and Yu FS: SRC-family
tyrosine kinases in wound- and ligand-induced epidermal growth
factor receptor activation in human corneal epithelial cells.
Invest Ophthalmol Vis Sci. 47:2832–2839. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Kutz SM, Higgins CE, Samarakoon R, et al:
TGF-beta 1-induced PAI-1 expression is E box/USF-dependent and
requires EGFR signaling. Exp Cell Res. 312:1093–1105. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Allen RR and Higgins PJ: Plasminogen
activator inhibitor type-1 expression and the pathophysiology of
TGF-β1-induced epithelial-to-mesenchymal transition. Recent Res Dev
Physiol. 95:918–931. 2004.
|
|
140
|
Davies M, Robinson M, Smith E, Huntley S,
Prime S and Paterson I: Induction of an epithelial to mesenchymal
transition in human immortal and malignant keratinocytes by
TGF-beta1 involves MAPK, Smad and AP-1 signalling pathways. J Cell
Biochem. 95:918–931. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Lovschall H, Fejerskov O and Flyvbjerg A:
Pulp-capping with recombinant human insulin-like growth factor I
(rhIGF-I) in rat molars. Adv Dent Res. 15:108–112. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Okubo K, Kobayashi M, Takiguchi T, et al:
Participation of endogenous IGF-I and TGF-beta 1 with enamel matrix
derivative-stimulated cell growth in human periodontal ligament
cells. J Periodontal Res. 38:1–9. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Lee AZ, Jiang J, He J, Safavi KE,
Spangberg LS and Zhu Q: Stimulation of cytokines in osteoblasts
cultured on enamel matrix derivative. Oral Surg Oral Med Oral
Pathol Oral Radiol Endod. 106:133–138. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
MacNeil RL, D'Errico J, Strayhorn C,
Pickrum H and Somerman MJ: Agents with periodontal regenerative
potential regulate cell-mediated collagen lattice contraction in
vitro. J Dent Res. 75:903–911. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Ikezawa K, Hart CE, Williams DC and
Narayanan AS: Characterization of cementum derived growth factor as
an insulin-like growth factor-I like molecule. Connect Tissue Res.
36:309–319. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Yonemura K, Raines EW, Ahn NG and
Narayanan AS: Mitogenic signaling mechanisms of human
cementum-derived growth factors. J Biol Chem. 268:26120–26126.
1993.PubMed/NCBI
|
|
147
|
Xu L, Harada H and Taniguchi A: The
effects of LAMP1 and LAMP3 on M180 amelogenin uptake, localization
and amelogenin mRNA induction by amelogenin protein. J Biochem.
144:531–537. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Veis A, Tompkins K, Alvares K, et al:
Specific amelogenin gene splice products have signaling effects on
cells in culture and in implants in vivo. J Biol Chem.
275:41263–41272. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Boabaid F, Gibson CW, Kuehl MA, et al:
Leucine-rich amelogenin peptide: a candidate signaling molecule
during cementogenesis. J Periodontol. 75:1126–1136. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
150
|
He J, Jiang J, Safavi KE, Spangberg LS and
Zhu Q: Direct contact between enamel matrix derivative (EMD) and
osteoblasts is not required for EMD-induced cell proliferation.
Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 98:370–375. 2004.
View Article : Google Scholar : PubMed/NCBI
|