Introduction
Inflammatory bowel disease (IBD) is an inflammatory
condition of the gastrointestinal tract that comprises two forms:
Ulcerative colitis and Crohn's disease. IBD is characterized by
chronic inflammation, remitting and relapsing episodes, and a
progressive course at diagnosis (1).
Four aspects are known to be involved in the etiopathogenesis of
IBD, including the external environment, the genetic signature of
the patient, the microbiota and the immune system (2). Specific pathogenic agents or the
physiological microbial flora provide antigenic stimulation of
cell-mediated immune responses in genetically susceptible
individuals, while the presence of dysbiosis or impaired
gastrointestinal mucosal barriers, due to genetic factors or
environmentally-induced injury, represent risk factors for the
development of an inappropriate immune response (3,4).
Innate and adaptive immunity are known to play a
crucial role in triggering and maintaining inflammatory events in
IBD (5,6). Furthermore, an important underlying
mechanism is the production and release of cytokines and
chemokines, which are able to drive inflammatory events at a local
and systemic level. In a recent study, the normal production of
cytokines and chemokines was shown to vary consistently with the
individual growth of healthy subjects and the physiological
sharpening of the immune system (7).
The diagnosis of IBD is particularly challenging in patients of a
pediatric age, since presenting symptoms can vary widely and may
only consist of subtle extraintestinal manifestations. Often
children present IBD-like phenotypes and symptoms; however, they do
not suffer from IBD. A long period of unmanaged symptoms can
significantly impact on growth; thus, early treatments are
essential to preserve the long-term quality of life of patients
(8,9).
Although non-invasive tests for IBD already exist,
including antibody tests, imaging-based screens and fecal
biomarkers (10), a comprehensive
comparison of the serum levels of cytokines and chemokines in
pediatric IBD patients compared with normal age-matched controls
has yet to be performed. Therefore, the aim of the present study
was to assess a large panel (n=48) of inflammatory cytokines and
chemokines in pediatric IBD patients, and compare the results with
the same panel in age-matched healthy controls.
Subjects and methods
Patients and normal controls
The independent Ethics Review Board of the Institute
for Maternal and Child Health - IRCCS ‘Burlo Garofolo’ (Trieste,
Italy) provided approval of the study (n.185/08, 19/08/2008). For a
child to be eligible, informed consent was required from the
parents or guardians. For ethical reasons, the study population of
pediatric patients was restricted to those who were undergoing a
medically indicated peripheral venous blood sampling prior to
elective surgical interventions or within the scope of elective
diagnostic procedures. Furthermore, for the control group, subjects
were excluded from the study if they had an acute or chronic
infectious disease, any clinically significant disorder, or if they
were on any medication with a known effect on immunological
factors, such as corticosteroids. Blood samples were collected from
the control subjects (CTRL, n=37) and IBD patients (IBD, n=26). The
clinical characteristics of the subjects included in the study are
shown in Table I. Patient history,
with regard to breast-feeding, vaccinations, previous infectious
diseases and allergy, was documented but not evaluated as
covariates in the study, since subgroup analysis required a larger
population size.
 | Table I.Profile of the controls and the
patients with IBD. |
Table I.
Profile of the controls and the
patients with IBD.
| Group | Cases (n) | Age
(years)a | Gender, M/F (n) | Pathology |
|---|
| Controls | 37 | 11 (1/17) | 15/22 | - |
| IBD | 26 | 9 (1/16) | 14/12 | Crohn's disease
(n=15) |
|
|
|
|
| Ulcerative colitis
(n=11) |
Determination of the cytokine and
chemokine levels
The investigated panel comprised 48 cytokines or
chemokines known to or hypothesized to be involved in inflammatory
processes. The panel included interleukin (IL)-receptor antagonist
(RA), IL-1β, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-10, IL-12
(p70), IL-13, IL-15, IL-17, eotaxin, fibroblast growth factor
(FGF)-basic, granulocyte-colony stimulating factor (G-CSF),
granulocyte-macrophage colony-stimulating factor (GM-CSF),
interferon (IFN)-γ, IFN-γ-inducible protein (IP)-10, monocyte
chemoattractant protein (MCP)-1, macrophage inflammatory protein
(MIP)-1α, platelet-derived growth factor (PDGF)-BB, MIP-1β,
regulated on activation, normal T cell expressed and secreted
(RANTES), tumor necrosis factor (TNF)-α, vascular endothelial
growth factor (VEGF), IL-1α, IL-2Rα, IL-3, IL-12 (p40), IL-16,
IL-18, cutaneous T-cell-attracting chemokine (CTACK), growth
regulated oncogene (GRO)-α, hepatocyte growth factor (HGF), IFN-α2,
leukemia inhibitory factor (LIF), MCP-3, macrophage
colony-stimulating factor (M-CSF), macrophage migration inhibitory
factor (MIF), monokine induced by γ-IFN (MIG), β-nerve growth
factor (NGF), stem cell factor (SCF), stem cell growth factor
(SCGF)-β, stromal cell-derived factor (SDF)-1α, TNF-β and
TNF-related apoptosis-inducing ligand (TRAIL). Analysis of the
panel was performed with the collected serum samples, using a
magnetic bead-based multiplex immunoassay (Bio-Plex®; Bio-Rad
Laboratories, Inc., Milan, Italy), as previously described
(11–13). Experiments were performed according
to the manufacturer's instructions. Data from the reactions were
acquired using a Bio-Plex® 200 reader (Bio-Rad Laboratories, Inc.),
while a digital processor (Toshiba Intel® Celeron® CPU B820;
Toshiba, Tokyo, Japan) was used to manage the data output.
Application of Bio-Plex Manager® software (Bio-Rad Laboratories,
Inc.) enabled presentation of the data as the median fluorescence
intensity and concentration (pg/ml).
Statistical analysis
For each set of experiments, the cytokine levels are
presented as the median values with the interquartile range. To
compare the differences between the values in the two distinct
groups, a non-parametric Mann-Whitney rank-sum test was applied,
where P≤0.05 was considered to indicate a statistically significant
difference. In the case of multiple comparisons, the Bonferroni
correction was applied, where 0.05 was divided by the number of
multiple comparisons. Analyses were performed using GraphPad Prism
5.0 software (GraphPad Software, Inc., La Jolla, CA, USA) and
Stata/IC 11.2 software (StataCorp LP, College Station, TX,
USA).
Results
Pediatric IBD patients exhibit a
distinct cytokine profile when compared with the normal controls,
with 10 cytokines/chemokines exhibiting significantly higher values
in IBD patients
All the serum concentrations of cytokines and
chemokines, acquired following the magnetic bead-based multiplex
immunoassay, in the pediatric IBD patients and controls are
reported in Table II. Among the 48
investigated cytokines and chemokines, eight cytokines/chemokines
were not detectable in the sera of the controls or IBD pediatric
patients, which included IL-15, GM-CSF, IL-1α, IL-3, IL-12 (p40),
MCP-3, β-NGF and TNF-β. The majority of cytokines and chemokines
(n=27) did not exhibit a statistically significant difference when
comparing the values in the IBD and CTRL groups. These cytokines
and chemokines were IL-1RA, IL-4, IL-6, IL-8, IL-9, IL-10, IL-12
(p70), IL-13, eotaxin, FGF-basic, G-CSF, MCP-1, MIP-1α, PDGF-BB,
RANTES, TNF-α, VEGF, IL-2Rα, IL-18, GRO-α, HGF, M-CSF, MIF, SCF,
SCGF-β, SDF-1α and TRAIL. However, 10 cytokines/chemokines
exhibited significantly higher concentrations (P<0.05) in the
IBD patients when compared with the CTRL group. These cytokines and
chemokines included IL-1β, IL-5, IL-7, IFN-α2, IFN-γ, IP-10, CTACK,
IL-16, LIF and MIG (Fig. 1).
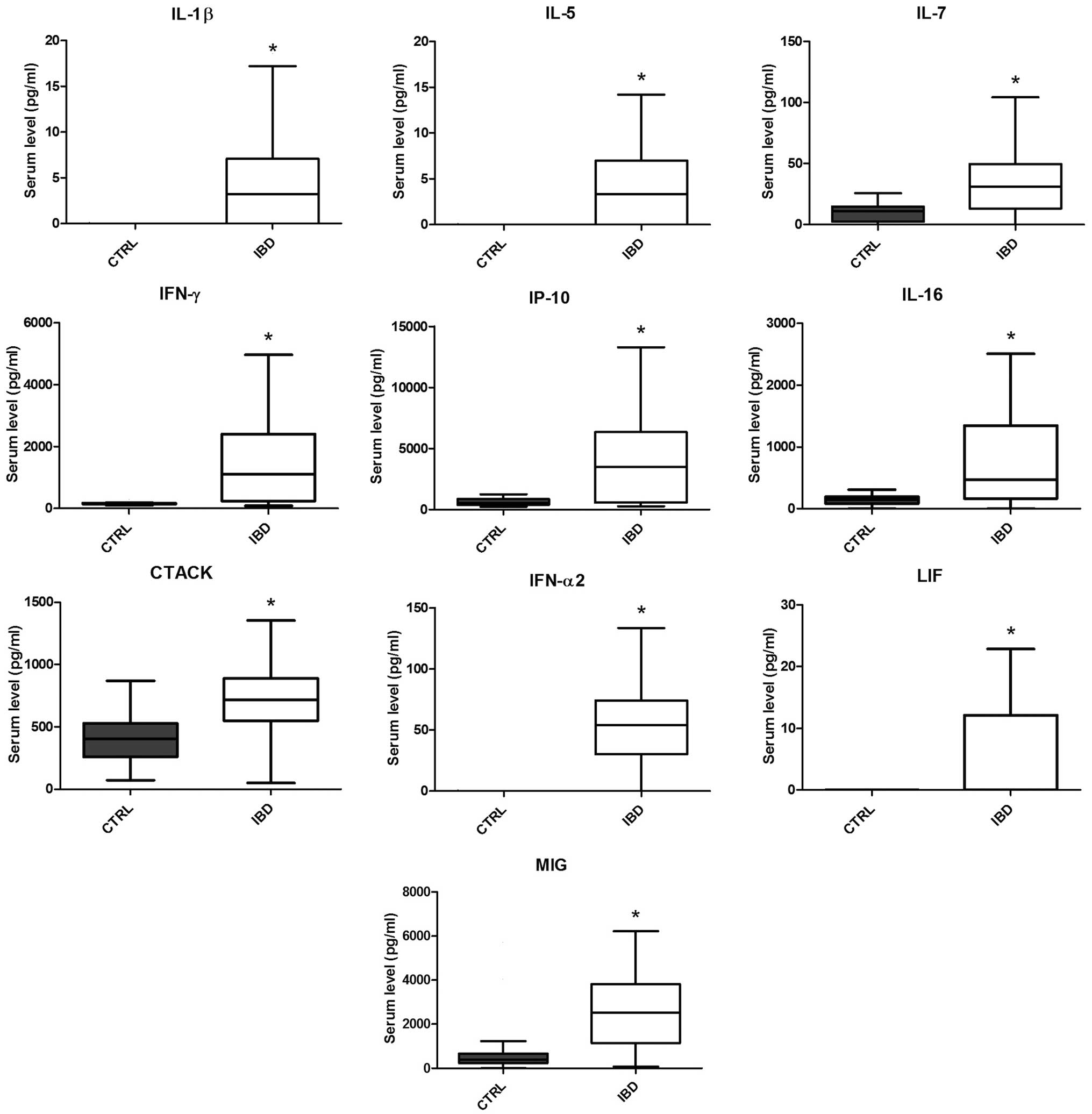 | Figure 1.Box plots showing the serum levels
(pg/ml) of IL-1β, IL-5, IL-7, IFN-γ, IP-10, IL-16, CTACK, IFN-α2,
LIF and MIG in the CTRL (gray) and IBD (white) groups. Whiskers
were calculated using the Tukey method and outliers are not shown.
*P<0.05, vs. CTRL group (Mann-Whitney test with Bonferroni
correction for multiple comparisons). IL, interleukin; IFN,
interferon; IP, IFN-γ-inducible protein; CTACK, cutaneous
T-cell-attracting chemokine; LIF, leukemia inhibitiory factor; MIG,
monokine induced by γ-IFN; CTRL, control; IBD, inflammatory bowel
disease. |
 | Table II.Concentrations of cytokines and
chemokines in the patients with IBD and the healthy controls. |
Table II.
Concentrations of cytokines and
chemokines in the patients with IBD and the healthy controls.
| Cyto/chemokine | Controls | IBD |
|---|
| IL-1β | nd | 3.21
(nd-7.09)a |
| IL-1RA | 160 (133.7–193) | 239.3
(139–370.9) |
| IL-2 | 12.86 (9.93–16) | 4.33
(1.31–12.62)a |
| IL-4 | 8.01 (7.75–8.7) | 10.29
(5.54–16.14) |
| IL-5 | nd | 3.31
(nd-6.97)a |
| IL-6 | 12.3
(10.41–15.22) | 18.16
(11.2–30.9) |
| IL-7 | 11.1
(2.29–14.97) | 31.17
(13.15–49.76)a |
| IL-8 | 32.28
(28.74–38.29) | 38.57
(24.67–64.12) |
| IL-9 | 23.5
(16.38–29.81) | 21.34
(12.93–33.6) |
| IL-10 | 9.35 (nd-11.43) | 5.29 (nd-16.54) |
| IL-12 (p70) | 40.5
(23.61–50.38) | 17.73
(10.38–43.35) |
| IL-13 | 9.36
(7.97–11.68) | 4.26 (1.52–8.13) |
| IL-15 | nd | nd |
| IL-17 | 111
(98.37–132.5) | 44.59
(15.46–99.85)a |
| Eotaxin | 11.73 (nd-46.26) | 42.52 (nd-171.8) |
| FGF-basic | 38.6
(33.23–49.16) | 35.07
(19.55–59.91) |
| G-CSF | 43.97
(34.28–53.47) | 52.88
(35.79–93.16) |
| GM-CSF | nd | nd |
| IFN-γ | 163.8
(147.1–177.4) | 1105
(238–2412)a |
| IP-10 | 542.5
(390.2–872.6) | 3486
(576.3–6348)a |
| MCP-1 | 50.78
(26.87–77.01) | 23.37
(13.37–45.71) |
| MIP-1α | 7.02 (6–8.02) | 5.84
(3.48–8.23) |
| PDGF-BB | 8479
(6714–10519) | 5182
(3387–11838) |
| MIP-1β | 99.38
(79.2–131.7) | 52.59
(35.96–72.8)a |
| RANTES | 4633 (nd-5457) | 1592
(nd-17660) |
| TNF-α | 30.44
(23.81–36.31) | 38.82
(23–55.57) |
| VEGF | 82.2
(46.72–124.9) | 91.71
(54.33–209.2) |
| IL-1α | nd | nd |
| IL-2Rα | 145.4
(76.49–199.7) | 173.6
(68–338.5) |
| IL-3 | nd | nd |
| IL-12 (p40) | nd | nd |
| IL-16 | 144
(83.92–195.8) | 473.4
(164.3–1343)a |
| IL-18 | 196
(102.1–284.4) | 120.6
(74.95–180.5) |
| CTACK | 406
(259.3–527.8) | 716.2
(548.6–889.3)a |
| GRO-α | 11.6
(78.63–195.9) | 158.3
(58.62–250.6) |
| HGF | 404.2
(290.3–555.5) | 382.1
(279.1–513.5) |
| IFN-α2 | nd | 54.05
(30.27–74.11)a |
| LIF | nd | nd
(nd-12.09)a |
| MCP-3 | nd | nd |
| M-CSF | 8.02
(nd-13.22) | nd (nd-15.05) |
| MIF | 393.5
(73.17–3127) | 3731
(572.1–7002) |
| MIG | 393
(228.3–676) | 2523
(1141–3821)a |
| β-NGF | nd | nd |
| SCF | 1048
(113–1514) | 135.5
(64.75–233.3) |
| SCGF-β | 36615
(22073–73267) | 39607
(22791–60520) |
| SDF-1α | 37.46
(17.34–75.02) | 47.59
(nd-352.3) |
| TNF-β | nd | nd |
| TRAIL | 85.28
(49.52–104.9) | 71.41
(49.84–118.4) |
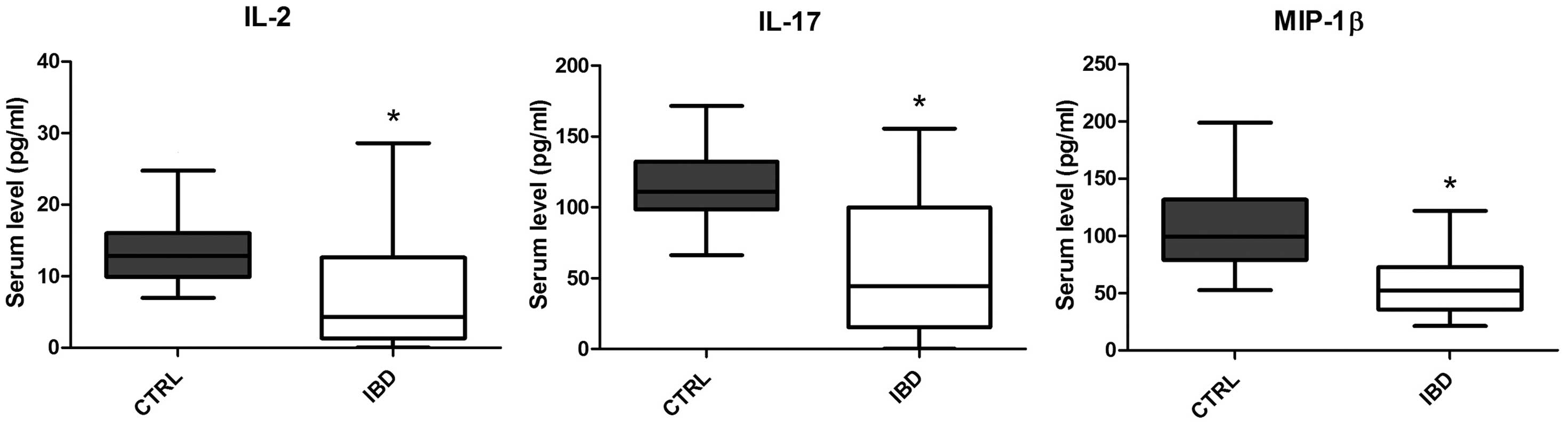
Levels of certain circulating
cytokines are significantly decreased in pediatric IBD patients
when compared with the normal controls
The majority of cytokines and chemokines were
unaffected or significantly (P<0.05) increased in the pediatric
IBD patients when compared with the age-matched control subjects.
However, it is particularly notable that two cytokines, IL-2 and
IL-17, and one chemokine, MIP-1β, exhibited significantly decreased
levels in the pediatric IBD patients when compared with the
age-matched controls (Fig. 2).
Discussion
The presence of deregulated cytokines and chemokines
has been previously reported in cases of IBD, primarily in studies
performed at the level of the inflamed mucosa, while studies on the
circulating levels of cytokines and chemokines in IBD are less
frequent (14–17). Among the panel of cytokines and
chemokines investigated in the present study, 10 proteins,
including IL-1β, IL-5, IL-7, IFN-α2, IFN-γ, IP-10, CTACK, IL-16,
MIG and LIF, were shown to be significantly upregulated in the
group of pediatric IBD patients, as compared with the age-matched
controls. While a number of these molecules, including IL-1β, IP-10
and IL-16 in particular, are known to have a strong proinflammatory
activity (18–24), certain other molecules, such as IFNs,
may account for compensatory mechanisms aimed at decreasing the
inflammatory status (25).
However, the most notable finding of the present
study was that a small number of cytokines and chemokines were
significantly downregulated in the serum of the IBD pediatric
patients when compared with the age-matched healthy controls. These
data are completely novel and unexpected, particularly considering
that in IBD patients, concentrations of MIP-1β, IL-2 and IL-17
(26–30) have been previously reported to be
upregulated at the mucosal level. The data concerning IL-17 are
particularly significant, since IL-17 has been proposed as a
potential therapeutic target for IBD (31). In addition, the results may aid the
interpretation of certain paradoxical effects resulting from
anti-TNF therapy that have been observed in IBD patients, such as
the occurrence of psoriasis in a subset of IBD patients (32). Therefore, the results demonstrate, in
accordance with the observations of a previous study (33), that the role of IL-17 in the
physiopathology of IBD is complex.
In conclusion, the results of the present study
indicate that the immunological characteristics of IBD are more
complex than originally hypothesized, and may comprise certain
aspects of immune-deficiency. To date, the precise balance between
proinflammatory and antiinflammatory cytokines/chemokines during
the progression of IBD remains unknown. The correct evaluation of
cytokines and chemokines involved in the progress of the disease is
crucial for identifying potential novel targets for drug
treatments.
Further studies are necessary to improve the
understanding of IBD etiology and to clarify whether there are
differences between Crohn's disease and ulcerative colitis. Other
parameters to be considered include genetic background, disease
onset, the age of the patients and pharmacological treatment
(34,35).
Acknowledgements
This study was supported by a grant from the
Institute for Maternal and Child Health - IRCCS ‘Burlo Garofolo’
(no. RC 40/2011).
References
|
1
|
Podolsky DK: Inflammatory bowel disease. N
Engl J Med. 347:417–429. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Scaldaferri F and Fiocchi C: Inflammatory
bowel disease: progress and current concepts of etiopathogenesis. J
Dig Dis. 8:171–178. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sartor RB: Mechanisms of disease:
pathogenesis of Crohn's disease and ulcerative colitis. Nat Clin
Pract Gastroenterol Hepatol. 3:390–407. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Raza A, Yousaf W, Giannella R and Shata
MT: Th17 cells: interactions with predisposing factors in the
immunopathogenesis of inflammatory bowel disease. Expert Rev Clin
Immunol. 8:161–168. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cassinotti A, Sarzi-Puttini P, Fichera M,
Shoenfeld Y, de Franchis R and Ardizzone S: Immunity, autoimmunity
and inflammatory bowel disease. Autoimmun Rev. 13:1–2. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Geremia A, Biancheri P, Allan P, Corazza
GR and Di Sabatino A: Innate and adaptive immunity in inflammatory
bowel disease. Autoimmun Rev. 13:3–10. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kleiner G, Marcuzzi A, Zanin V, Monasta L
and Zauli G: Cytokine levels in the serum of healthy subjects.
Mediators Inflamm. 2013:4340102013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Spray C, Debelle GD and Murphy MS: Current
diagnosis, management and morbidity in paediatric inflammatory
bowel disease. Acta Paediatr. 90:400–405. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Heikenen JB, Werlin SL, Brown CW and
Balint JP: Presenting symptoms and diagnostic lag in children with
inflammatory bowel disease. Inflamm Bowel Dis. 5:158–160. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lewis JD: The utility of biomarkers in the
diagnosis and therapy of inflammatory bowel disease.
Gastroenterology. 140:1817–1826. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Volpato S, Ferrucci L, Secchiero P, et al:
Association of tumor necrosis factor-related apoptosis-inducing
ligand with total and cardiovascular mortality in older adults.
Atherosclerosis. 215:452–458. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tisato V, Zauli G, Voltan R, et al:
Endothelial cells obtained from patients affected by chronic venous
disease exhibit a pro-inflammatory phenotype. PLoS One.
7:e395432012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tisato V, Zamboni P, Menegatti E, et al:
Endothelial PDGF-BB produced ex vivo correlates with relevant
hemodynamic parameters in patients affected by chronic venous
disease. Cytokine. 63:92–96. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Neurath MF: Cytokines in inflammatory
bowel disease. Nat Rev Immunol. 14:329–342. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Francescone R, Hou V and Grivennikov SI:
Cytokines, IBD, and Colitis-associated Cancer. Inflamm Bowel Dis.
21:409–418. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wallace KL, Zheng LB, Kanazawa Y and Shih
DQ: Immunopathology of inflammatory bowel disease. World J
Gastroenterol. 20:6–21. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jiang W, Su J, Zhang X, Cheng X, Zhou J,
Shi R and Zhang H: Elevated levels of Th17 cells and Th17-related
cytokines are associated with disease activity in patients with
inflammatory bowel disease. Inflamm Res. 63:943–950. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dinarello CA: Interleukin-1beta and the
autoinflammatory diseases. N Engl J Med. 360:2467–2470. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Seegert D, Rosenstiel P, Pfahler H,
Pfefferkorn P, Nikolaus S and Schreiber S: Increased expression of
IL-16 in inflammatory bowel disease. Gut. 48:326–332. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Marion R, Coëffier MM, Gargala G, Ducrotté
P and Déchelotte PP: Glutamine and CXC chemokines IL-8, Mig, IP-10
and I-TAC in human intestinal epithelial cells. Clin Nutr.
23:579–585. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Damen GM, Hol J, de Ruiter L, et al:
Chemokine production by buccal epithelium as a distinctive feature
of pediatric Crohn disease. J Pediatr Gastroenterol Nutr.
42:142–149. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mayer L, Sandborn WJ, Stepanov Y, et al:
Anti-IP-10 antibody (BMS-936557) for ulcerative colitis: a phase II
randomised study. Gut. 63:442–450. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Del Zotto B, Mumolo G, Pronio AM,
Montesani C, Tersigni R and Boirivant M: TGF-beta1 production in
inflammatory bowel disease: differing production patterns in
Crohn's disease and ulcerative colitis. Clin Exp Immunol.
134:120–126. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Watanabe M, Ueno Y, Yajima T, et al:
Interleukin 7 is produced by human intestinal epithelial cells and
regulates the proliferation of intestinal mucosal lymphocytes. J
Clin Invest. 95:2945–2953. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Seow CH, Benchimol EI, Griffiths AM and
Steinhart AH: Type I interferons for induction of remission in
ulcerative colitis. Cochrane Database Syst Rev.
3:CD0067902008.PubMed/NCBI
|
|
26
|
Mazzucchelli L, Hauser C, Zgraggen K, et
al: Differential in situ expression of the genes encoding the
chemokines MCP-1 and RANTES in human inflammatory bowel disease. J
Pathol. 178:201–206. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Grimm MC and Doe WF: Chemokines in
inflammatory bowel disease mucosa: Expression of RANTES, macrophage
inflammatory protein (MIP)-1α, MIP-1β and γ-interferon-inducible
protein-10 by macrophages, lymphocytes, endothelial cells and
granulomas. Inflamm Bowel Dis. 2:88–96. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Banks C, Bateman A, Payne R, Johnson P and
Sheron N: Chemokine expression in IBD. Mucosal chemokine expression
is unselectively increased in both ulcerative colitis and Crohn's
disease. J Pathol. 199:28–35. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Katz LH, Kopylov U, Fudim E, et al:
Expression of IL-2, IL-17 and TNF-alpha in patients with Crohn's
disease treated with anti-TNF antibodies. Clin Res Hepatol
Gastroenterol. 38:491–498. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ueno A, Jijon H, Chan R, et al: Increased
prevalence of circulating novel IL-17 secreting Foxp3 expressing
CD4+ T cells and defective suppressive function of
circulating Foxp3+ regulatory cells support plasticity
between Th17 and regulatory T cells in inflammatory bowel disease
patients. Inflamm Bowel Dis. 19:2522–2534. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fitzpatrick LR: Inhibition of IL-17 as a
pharmacological approach for IBD. Int Rev Immunol. 32:544–555.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tillack C, Ehmann LM, Friedrich M, et al:
Anti-TNF antibody-induced psoriasiform skin lesions in patients
with inflammatory bowel disease are characterised by
interferon-γ-expressing Th1 cells and IL-17A/IL-22-expressing Th17
cells and respond to anti-IL-12/IL-23 antibody treatment. Gut.
63:567–577. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Monteleone I, Sarra M, Pallone F and
Monteleone G: Th17-related cytokines in inflammatory bowel
diseases: friends or foes? Curr Mol Med. 12:592–597. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bianco AM, Girardelli M, Vozzi D, Crovella
S, Kleiner G and Marcuzzi A: Mevalonate kinase deficiency and IBD:
shared genetic background. Gut. 63:1367–1368. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Marcuzzi A, Girardelli M, Bianco AM,
Martelossi S, Magnolato A, Tommasini A and Crovella S: Inflammation
profile of four early onset Crohn patients. Gene. 493:282–285.
2012. View Article : Google Scholar : PubMed/NCBI
|