Introduction
Differentiation between stimulation-induced
thyrotoxicosis and destruction-induced thyrotoxicosis is important
(1). The former refers to Graves'
disease (GD), while the latter includes subacute thyroiditis,
painless thyroiditis (PT, also called silent thyroiditis) and
postpartum thyroiditis. Therapy for the two entities is completely
different. Antithyroid drugs (ATDs), radioactive iodine 131
(131I) and thyroidectomy are the treatments for GD,
whereas destruction-induced thyrotoxicosis can be managed
conservatively. Treating PT as if it were GD would be completely
inappropriate (1). The diagnosis of
subacute thyroiditis is not difficult because is caused by viral
infection and is characterized by fever and thyroid pain. However,
PT is easily overlooked or misdiagnosed and perhaps mistreated
since its symptoms resemble those of early onset or recurrence of
GD. It is often problematic to discriminate GD from PT unless
radioactive iodine uptake (RAIU) is determined, because
occasionally GD and PT are associated with each other. PT may
develop following the complete remission of GD, and PT can be
followed by GD (2–10).
Although RAIU is the most reliable method to
differentiate GD from PT (11–14), it
is contraindicated when patients are lactating, and not all clinics
are well equipped to perform RAIU. Therefore, a number of studies
have been undertaken to search for simple and practical parameters
that might allow differentiation between the two entities.
Thyrotropin receptor antibody (TRAb) assessment has been shown to
be a useful marker to make a distinction between the two diseases
(2–3,15–16).
TRAb is also able to differentiate relapse of GD from development
of PT in patients who appear to be in remission following ATD
treatment for GD (2). However, 5–10%
of GD patients are negative for TRAb, and some PT patients are
positive (17–19). Amino et al demonstrated that
the serum triiodothyronine (T3)/thyroxine (T4) ratio (20) or free triiodothyronine (FT3)/free
thyroxine(FT4) ratio (21) was
useful for differentiating PT from GD. However, these observations
have not been confirmed by other groups.
Several methods of thyroid imaging can be used for
differential diagnosis. Thyroid scintigraphy using
99mTc-pertechnetate has been well established for use in
the assessment of thyroid uptake ability. Although
99mTc-pertechnetate does not undergo organification in
the thyroid, the pertechnetate ion is transported into the thyroid
by the sodium/iodide symporter. Thus, thyroid scintigraphy embodies
and enables the visualization of thyroid RAIU (12). Thyroid volume and blood flow
quantitative measurement by ultrasonography has been shown to be
effective for differential diagnosis (22). Diffusion-weighted magnetic resonance
imaging (DWI) of the thyroid with the assessment of an apparent
diffusion coefficient (ADC) value is a relatively new topic in
thyroid imaging studies. There appears to be only one study in
which DWI has been used to differentiate between GD and
thyroiditis. Tezuka et al (23) demonstrated that the ADC values of
patients with GD were significantly higher than those of patients
with subacute thyroiditis and Hashimoto thyroiditis. However, to
the best of our knowledge, no prior study has investigated whether
DWI is useful for discriminating between GD and PT. Furthermore,
the total number of cases in the study by Tezuka et al was
only 34, and the results of the study require verification.
In this study, the aim was to systematically
evaluate the ADC value in DWI for the differentiation between GD
and PT, and to compare it with RAIU (the reference method), thyroid
scintigraphy, TRAb and other serum indices. Parameters were
compiled and statistically analyzed to determine sensitivity,
specificity, accuracy, positive predictive value (PPV) and negative
predictive value (NPV) for differentiation diagnosis. Tissue
histopathology of GD and PT was also investigated.
Materials and methods
Patients
From August 2010 until August 2013, a series of 102
patients with GD and 37 patients with PT were consecutively
enrolled in this prospective study. The Institutional Review Board
of Tianjin Medical University General Hospital (Tianjin, China)
approved the ethical and methodological aspects of this
investigation. All participants provided their written informed
consent to participate in this study. Diagnosis was made according
to the generally recognized guidelines (1), with consensus. In brief, GD was
diagnosed on the basis of clinical findings and laboratory tests
showing high values of free thyroid hormone, low levels of
thyroid-stimulating hormone (TSH), high RAIU and/or increased TRAb
activity. PT was diagnosed by increased free thyroid hormone levels
and low TSH levels for <3 months, low RAIU and/or later
development of transient hypothyroidism.
Evaluation of serum parameters
Assays to determine the levels of FT3 (reference,
3.50–6.50 pmol/l; maximum, 30.80 pmol/l), FT4 (reference,
11.50–23.50 pmol/l; maximum, 154.80 pmol/l) and TSH (reference,
0.30–5.00 µIU/ml) were performed on a fully automated ADVIA Centaur
analyzer (Siemens Healthcare Diagnostics, Tarrytown, NY, USA).
These assays were based on a chemiluminescent reaction principle.
Thyroid globulin antibody (TgAb; reference, 0–40.00 IU/ml; maximum,
3,000.00 IU/ml) and thyroid peroxidase antibody (TpoAb; reference,
0–35.00 IU/ml; maximum 1,000.00 IU/ml) were also assessed by
chemiluminescent reaction on a fully automated IMMULITE 2000
analyzer (Siemens Healthcare Diagnostics, Los Angeles, CA, USA).
TRAb (reference, 0–1.50 IU/l; maximum, 40.00 IU/l) was determined
by a competitive enzyme immunoassay (Medizym T.R.A., Medipan GmbH,
Berlin, Germany).
DWI and ADC
Magnetic resonance (MR) images were obtained with a
superconducting 3.0-T MR imaging unit (Signa HDx; GE Healthcare,
Milwaukee, WI, USA) using an anterior neck array coil. The neck
array coil was carefully placed in order to position the thyroid
gland in the center of the field of view. T1-weighted images were
acquired using the following parameters: repetition time (TR), 780
msec; echo time (TE), 11 msec; slice thickness, 5.0 mm;
intersection gap, 1.0 mm; matrix, 320×224; field of view (FOV),
240×240 mm2; and echo train length, 3. T2-weighted
images were obtained by using the following parameters: TR, 5,000
msec; TE, 102 msec; slice thickness, 5.0 mm; intersection gap, 1.0
mm; matrix, 288×256; FOV, 240×240 mm2; and echo train
length, 18. DWI was conducted with the following parameters: TR,
5,000 msec; TE, 75 msec; slice thickness, 5.0 mm; intersection gap,
1.0 mm; matrix, 128×128; FOV, 240×240 mm2; and echo
train length, 20. Imaging was performed with b values of 0 and
1,000 sec/mm2. Afterwards, an ADC map was constructed
and ADC values were automatically calculated in units of
x10−3 mm2/sec on an ADW 4.3 workstation (GE
Healthcare, Waukesha, WI, USA). The MR imaging signal intensities
of the thyroid gland were measured with an electronic cursor to
define the region of interest (ROI). On each patient, ROIs were
drawn around bilateral thyroid parenchyma at the level of the upper
pole, central portion and the lower pole, while avoiding artifacts
from focal lesions, vascular motion, chemical shift or magnetic
susceptibility. The final ADC per subject that used for statistical
analysis was the average of the above ADC values in one
patient.
RAIU and thyroid scintigraphy
As reference methods, RAIU and thyroid scintigraphy
were performed as previously described (24,25).
Briefly, the thyroid RAIU protocol was performed as follows:
Radioactivities of the thyroid as well as the decayed tracer source
were measured at 24 h after oral intake of the tracer dose (74 kBq
of 131I) using a multifunctional nuclear medicine
instrument (MN-6300XT; University of Science and Technology of
China, Hefei, China). The RAIU was calculated using the formula:
RAIU (%) = [(radioactivity of the thyroid -
background)/(radioactivity of the decayed tracer source -
background)] × 100. The thyroid scintigraphy protocol was
implemented as follows: 30 min after the injection of 185 MBq
99mTc-pertechnetate, acquisition was performed using a
high-resolution low-energy parallel-hole collimator equipped
dual-detector scanner (Discovery VH; GE Healthcare, Milwaukee, WI,
USA). Thyroid scintigraphy was performed subsequent to RAIU
measurement.
Treatments and tissue sampling
Treatments were determined according to the
generally accepted guidelines (1).
GD was managed by one of the following therapies: ATD,
131I or thyroidectomy. Decisions about the therapy were
made at a consultation meeting among endocrinologists, nuclear
medicine physicians and surgeons. PT was given symptomatic
treatments, for example, β-adrenergic blockers, nonsteroidal
anti-inflammatory agents, and sometimes corticosteroids to
ameliorate symptoms. Tissue samples were obtained from patients
with GD following thyroidectomy, and from patients with PT
following biopsy. Tissue samples were fixed in neutral buffered
formalin (pH 7.4), embedded in paraffin, and sliced into ~4-µm
sections by a routine procedure. Sections were stained with
hematoxylin and eosin in order to examine the general histology.
Images were acquired and observed under a microscope (BX51;
Olympus, Tokyo, Japan).
Statistical analysis
All data are presented as mean ± standard deviation.
Statistics were performed with SPSS software, version 17.0 (SPSS,
Inc., Chicago, IL, USA). Differences of indices between two groups
were analyzed by independent samples t-test. The Pearson
χ2 test was used to check whether gender had a
significant effect on inter-group differences. Pearson bivariate
correlation was performed among the variables. Receiver operating
characteristic (ROC) curves were drawn and diagnostic efficacies
were determined by comparing the areas under the curves. Then,
optimal cut-off values were selected, and the sensitivity,
specificity, diagnostic accuracy, PPV and NPV of various factors
for differential diagnosis were assessed, respectively. P-values
not exceeding 0.05 were considered statistically significant.
Results
Comparisons of clinical indices
between different groups of patients
For the GD group, there were 31 males (age range,
16–59 years) and 71 females (age range, 19–65 years). For the PT
group, there were 10 males (age range, 21–60 years) and 27 females
(age range, 21–61 years). Gender did not have any substantial
impact on the differential diagnosis of the two diseases, with a
Pearson χ2 value of 0.148 (P=0.701).
Clinical indices of the two groups of patients are
listed and compared in Table I. The
ADC, FT3 and TRAb levels and RAIU were significantly higher in the
GD group than in the PT group (P<0.05). However, no significant
differences were identified among the other parameters, namely age,
FT4, TSH, TgAb, TpoAb and FT3/FT4 (P>0.05).
 | Table I.Comparisons among the factors of the
two groups of patients in the study. |
Table I.
Comparisons among the factors of the
two groups of patients in the study.
| Factor | Graves' disease
(n=102) | Painless
thyroiditis (n=37) | F value
(P-value)a |
|---|
| Age |
37.971±13.856 |
35.054±12.326 | 2.083 (0.151) |
| ADC |
2.212±0.209 |
1.508±0.318 | 5.979 (0.016) |
| FT3 |
15.555±6.507 |
11.336±4.111 | 4.491 (0.036) |
| FT4 |
68.868±31.608 |
54.598±22.049 | 1.479 (0.226) |
| TSH |
0.007±0.008 |
0.008±0.016 | 0.884 (0.349) |
| TRAb |
9.378±6.877 |
1.123±1.472 | 42.951
(<0.001) |
| TgAb |
317.001±718.324 |
415.379±630.150 | 0.006 (0.940) |
| TpoAb |
444.047±389.482 |
464.341±337.990 | 3.625 (0.059) |
| RAIU |
66.358±11.799 |
3.678±4.075 | 25.466
(<0.001) |
| FT3/FT4 |
0.237±0.045 |
0.219±0.051 | 1.649 (0.201) |
Pearson bivariate correlations were carried out.
Table II demonstrates that for ADC,
the three highest correlation coefficients were with RAIU, TRAb and
FT3. For TRAb they were ADC, RAIU and FT3, and for RAIU they were
ADC, TRAb and FT3. Correlation coefficients among RAIU, ADC, TRAb
were >0.700. Therefore, RAIU, ADC and TRAb were closely and
positively correlated with each other.
 | Table II.Pearson bivariate correlation. |
Table II.
Pearson bivariate correlation.
| Factor | ADC | TRAb | RAIU |
|---|
| Age | 0.009 | −0.055 | 0.079 |
| ADC | – | 0.777a | 0.902a |
| FT3 | 0.524a | 0.730a | 0.479a |
| FT4 | 0.436a | 0.652a | 0.401a |
| TSH | −0.060 | −0.055 | −0.075 |
| TRAb | 0.777a | – | 0.731a |
| TgAb | −0.043 | −0.037 | −0.095 |
| TpoAb | 0.015 | −0.252a | −0.082 |
| RAIU | 0.902a | 0.731a | – |
| FT3/FT4 | 0.031 | −0.085 | 0.051 |
Imaging performance
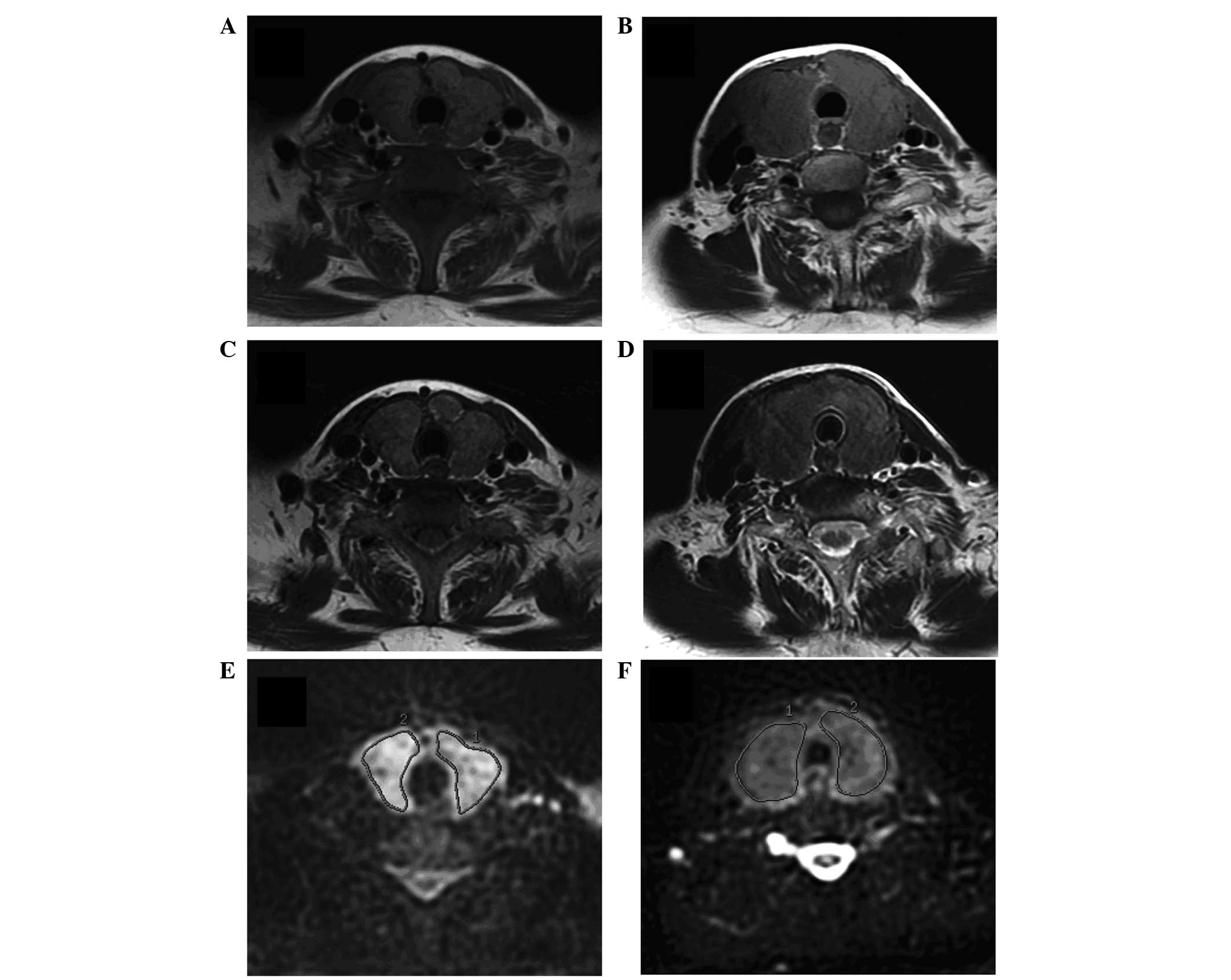
The MR images of an index case of GD and an index
case of PT are presented in Fig. 1.
Signal intensities of T1-weighted images (A,D) and T2-weighted
images (B,E) were not different, while ADC maps from DWI displayed
significantly higher signal intensity in GD than in PT (C,F).
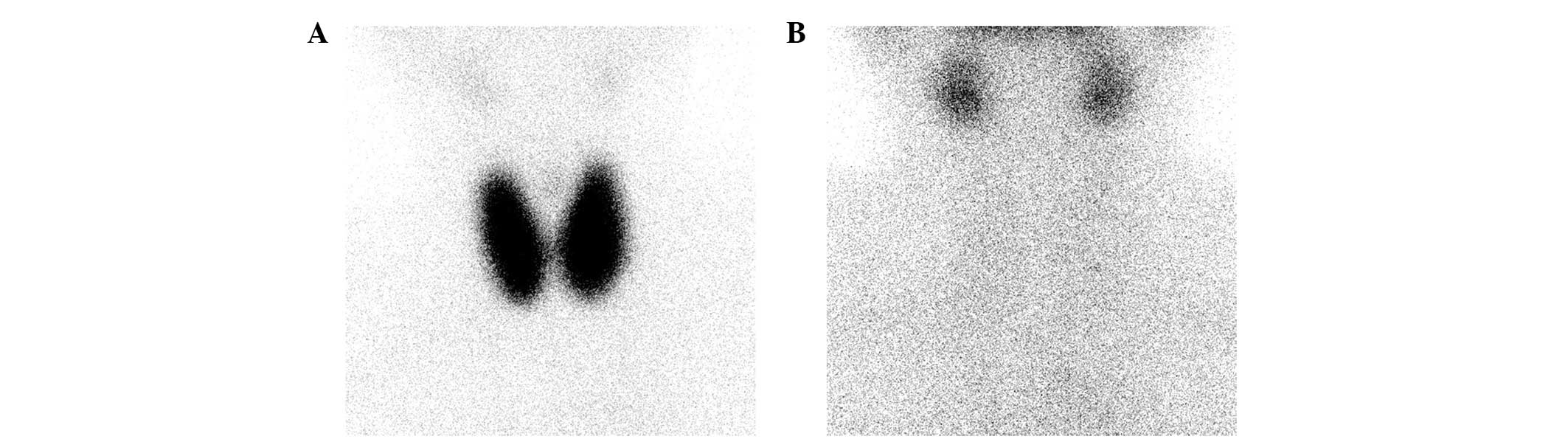
Thyroid scintigraphy results of an index case of GD and an index
case of PT are presented in Fig. 2.
The uptake of radionuclide 99mTc-pertechnetate was
significantly higher in the patient with GD (Fig. 2A) than in the patient with PT
(Fig. 2B), which visually reflected
the difference in RAIU between the two diseases.
Diagnostic efficacy of various
indices
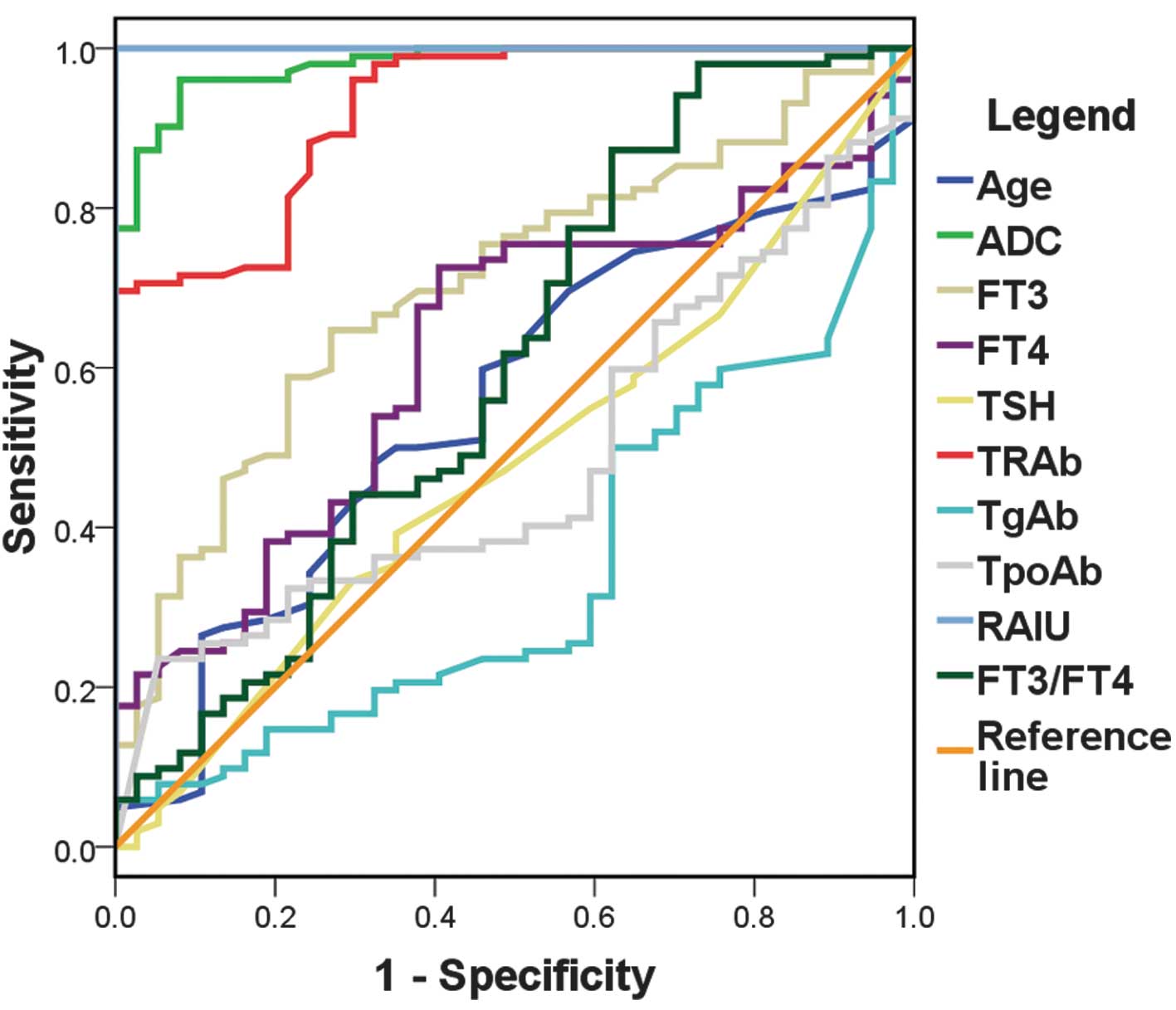
ROC curves were drawn and are shown in Fig. 3. Diagnostic capabilities, indicated
by area under the curve (Az) value, decreased in the following
order: RAIU > ADC > TRAb > FT3 > FT4 > FT3/FT4 >
age > TSH > TpoAb > TgAb (Table III). There were three factors
(RAIU, ADC and TRAb) that had an Az >0.900, which indicated that
they had excellent diagnostic efficacy. The cut-off value,
sensitivity, specificity, diagnostic accuracy, PPV and NPV of ADC,
TRAb and RAIU are listed in Table
IV. RAIU was used as the reference method. When the optimal
threshold of 24.500% was set, the sensitivity, specificity,
accuracy, PPV and NPV were all 100%. For ADC and TRAb, the optimal
thresholds of 1.837×10−3 mm2/sec and 1.350
IU/ml were determined. ADC had better diagnostic capability than
TRAb, as the sensitivity, specificity, accuracy, PPV and NPV were
all higher for the former index.
 | Table III.Az values in receiver operating
characteristic curve among factors. |
Table III.
Az values in receiver operating
characteristic curve among factors.
| Factor | Az | P-value |
|---|
| Age | 0.554 | 0.331 |
| ADC | 0.980 | <0.001 |
| FT3 | 0.706 | <0.001 |
| FT4 | 0.620 | 0.030 |
| TSH | 0.479 | 0.701 |
| TRAb | 0.925 | <0.001 |
| TgAb | 0.348 | 0.006 |
| TpoAb | 0.484 | 0.780 |
| RAIU | 1.000 | <0.001 |
| FT3/FT4 | 0.603 | 0.064 |
 | Table IV.Receiver operating characteristic
curve-related data and diagnostic indices. |
Table IV.
Receiver operating characteristic
curve-related data and diagnostic indices.
| Factor | ADC | TRAb | RAIU |
|---|
| Cut-off value |
1.837×10−3
mm2/sec | 1.350 IU/ml | 24.500 |
| Sensitivity
(%) | 96.078 | 88.235 | 100.000 |
| Specificity
(%) | 91.892 | 75.676 | 100.000 |
| Accuracy (%) | 95.000 | 84.892 | 100.000 |
| PPV (%) | 97.059 | 90.909 | 100.000 |
| NPV (%) | 89.474 | 70.000 | 100.000 |
Therapeutic management and
histopathological comparison
For the 102 patients with GD, treatment with
131I, ATD and thyroidectomy was given to 78, 18 and 6
patients respectively. All 37 patients with PT were managed with
β-adrenergic blockers. In addition, nonsteroidal anti-inflammatory
agents were prescribed to 21 patients and corticosteroids were
administrated to 8 patients. Tissue samples were obtained from the
6 patients with GD who received thyroidectomy and 2 patients with
recurrent PT who agreed to undergo biopsy, and were observed by
microscopy.
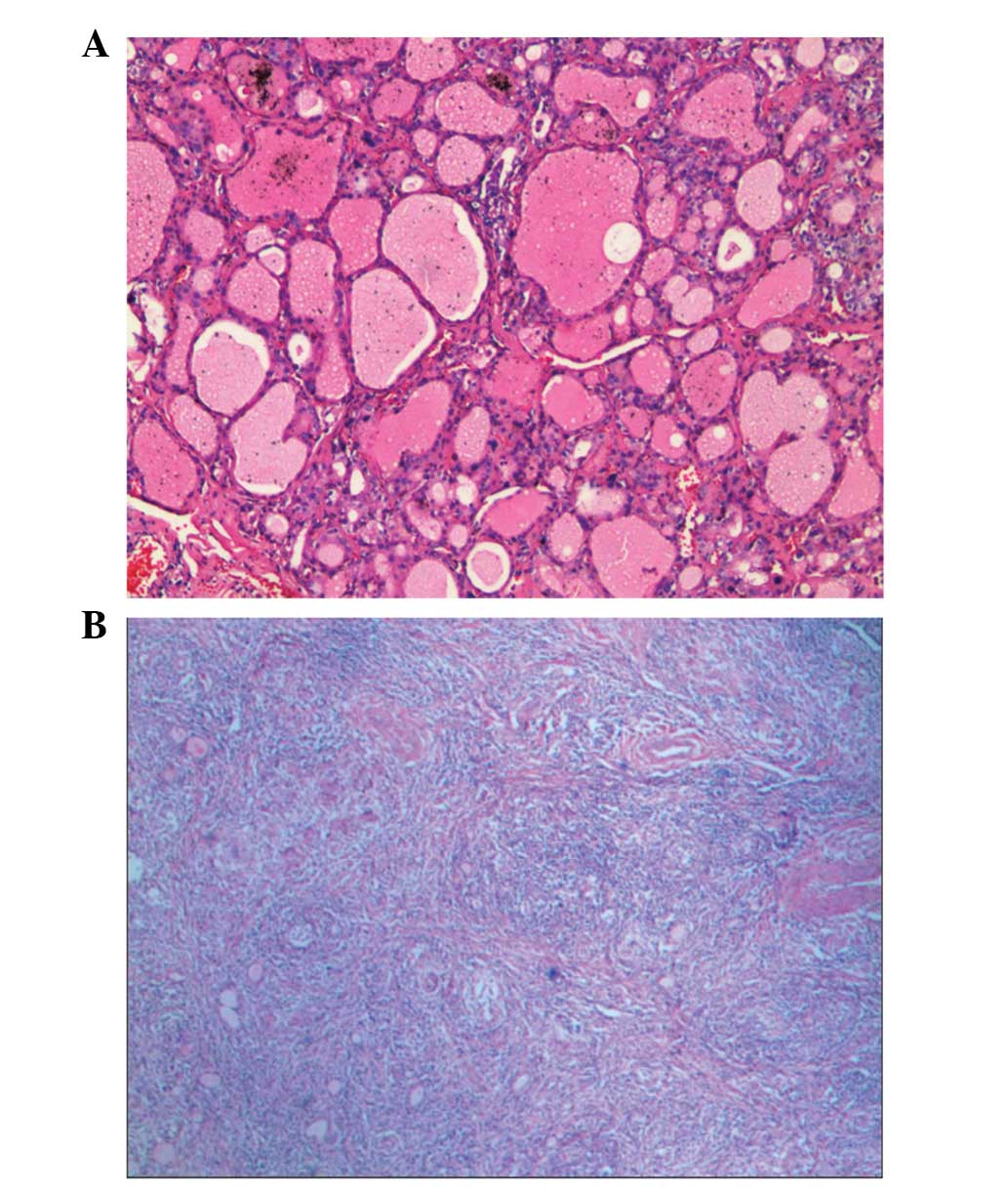
For GD, the follicular epithelial cells were tall
and more crowded than those of a normal thyroid gland. Some small
papillae were formed, which projected into the follicular lumen and
encroached on the colloid. The colloid within the follicular lumen
was pale, with scalloped margins. Lymphoid infiltrates were present
in the interstitium (Fig. 4A). For
PT, the most prominent and specific histopathological feature was
the massive lymphocytic infiltration with hyperplastic germinal
centers within the thyroid parenchyma. Thyroid follicles were
disrupted and collapsed (Fig. 4B).
It was evident that the tissue cellularity in PT was much higher
than that in GD.
Discussion
GD and PT are two distinct clinical entities within
the wide spectrum of autoimmune thyroid diseases. Thyrotoxicosis
induced by PT is usually self-limited, and therefore only requires
symptomatic treatment. Therapies intended for GD, in particular
ATD, 131I or thyroidectomy, are usually contraindicated
for PT. Differential diagnosis of these two types of thyrotoxicosis
has recently become an important clinical concern. However,
differentiation is sometimes challenging on the basis of clinical
findings only. First, PT is not rare. PT has a relatively high
prevalence, accounting for 9–23% of all thyrotoxicosis cases
(26). PT often present with milder
symptoms, so many patients with PT will often go to a general
practitioner, rather than a specialized thyroid clinic. Therefore,
the actual prevalence of PT is likely to be even higher than is
recognized. Secondly, hyperthyroidism, ophthalmopathy, diffuse
goiter and the peau d'orange appearance of pretibial dermopathy are
the major manifestations of GD (27), although not all GD patients exhibit
these signs. Without clear clinical manifestations, differentiation
will be more difficult. Thirdly, these two diseases are often
intertwined with each other. PT can often develop following the
remission of GD, PT can also be followed by GD, and in rare cases
simultaneous occurrence of the two can happen (10).
Generally, GD is characterized by persistent
thyrotoxicosis associated with a hyperfunctional thyroid gland that
gives rise to avid uptake of radioiodine. By contrast, PT is a
clinical syndrome manifested by spontaneously resolving
thyrotoxicosis associated with thyroidal destruction and marked
suppression of thyroid iodine uptake. Pathologically, GD is
characterized by diffuse hyperplasia and hypertrophy of follicular
cells with retention of lobular architecture and prominent vascular
congestion. Tall follicular cells with papillae are often observed.
PT is a syndrome of thyrotoxicosis due to the release of preformed
thyroid hormones from disrupted thyroid follicles. The diffuse
infiltration of the thyroid by lymphocytes implies that it is also
an autoimmune disorder (28,29). RAIU testing or thyroid scintigraphy
is of high diagnostic value for differentiating between GD and PT,
and has been demonstrated to be reliable (11–14);
however, RAIU and thyroid scintigraphy reflect only one type of
change in the pathogenesis of the two diseases. In brief, enhanced
thyrocyte uptake and processing of iodine in the former, and
disruption of thyroid follicles and suppressed thyrocyte uptake in
the latter. The more condense cellular structure of PT in
comparison with that in GD, due to massive lymphocyte infiltration
with hyperplastic germinal centers, is another hallmark
histopathological difference, which has not been studied in terms
of imaging so far.
DWI has a high sensitivity in the detection of
changes in the microscopic cellular environment. In particular, DWI
noninvasively probes the random microscopic motion of free water
molecules (known as Brownian motion) in a defined voxel by means of
the application of motion-probing gradients. The movement of water
is affected by cell organization, density, microstructure,
microcirculation and interaction with tissue compartments. DWI is
quantified by measuring the ADC value in units of
mm2/sec, which defines the average area covered by a
molecule per unit time. The ADC value can be calculated by
assessing the signal attenuation that occurs in DWI performed at
different b values. Usually, low ADC values indicate restricted
diffusion (high cellular density), while high ADC values indicate
more free diffusion (low tissue cellularity) (30–32).
DWI has a wide range of diagnostic applications. It
has primarily been used clinically for brain disorders,
particularly for the early detection of ischemic stroke or
infarction (33–36). Numerous studies have put forward DWI
as a new and valuable cancer imaging biomarker for detection,
diagnosis, staging, detecting metastasis or relapse, and assessing
treatment response (30–32,37–44).
Studies have also demonstrated that, for malignancy imaging, the
quantitative analysis of ADC and the standard uptake value (SUV) in
fluorodeoxyglucose positron emission tomography (FDG PET)/CT are
inversely correlated (37–42). ADC and SUV play complementary roles
in the provision of functional information concerning cancer, which
can now be successfully applied in head and neck cancers, after
overcoming the motion artifacts from swallowing or respiration and
susceptibility artifacts due to air/soft tissue/bone interfaces
(38–40). FDG PET/CT traces glucose metabolism,
a nonspecific process essential for tumor growth. DWI provides
information on the random motion of water molecules in tissues
indicating cellularity, as well as intra-cellular and
inter-cellular membranes. Tissue glucose metabolism and cellularity
represent two different facets of tumor biology and
pathophysiology. These successful applications of DWI prompted the
investigation in the present study of whether DWI can be used for
differential diagnosis of GD and PT.
To the best of our knowledge, the current
investigation is the first to apply DWI to discriminate GD from PT,
and make correlations and comparisons among ADC, RAIU and serum
indices. The findings of the present study demonstrate the definite
confirmative diagnostic ability of RAIU with 100% accuracy. It was
also found that the RAIU values are positively and closely
associated with those of ADC and TRAb. The performance of ADC was
better than that of TRAb (Fig. 3,
Tables III and IV). Sensitivity, specificity, accuracy,
PPV and NPV were between 89 and 97% when the optimal cut-off value
of 1.837×10−3 mm2/sec was determined for ADC.
TRAb has been demonstrated to be a useful serum marker to
differentiate GD from PT, although false positives and false
negatives remain (2–3,15–19). The
present study revealed that the sensitivity, specificity, accuracy,
PPV and NPV were between 70 and 90% when the optimal cut-off value
of 1.350 IU/ml was determined for TRAb. It is hypothesized that ADC
in DWI reflects the cellular structure being more condense in PT
than in GD due to massive lymphocyte infiltration with hyperplastic
germinal center formation. Hypercellularity in PT results in more
numerous structural components and membranes, resulting in greater
impedance and restriction of the motion/diffusion of water
molecules, which engenders low ADC values. Tezuka et al
(23) conducted an investigation of
24 patients with GD, 5 patients with subacute thyroiditis and 5
patients with Hashimoto thyroiditis. They found that ADC values
obtained from patients with GD were significantly higher than those
from patients with subacute thyroiditis and Hashimoto thyroiditis.
Tezuka et al (23) proposed
another hypotheses, which was that perfusion due to augmented
intrathyroidal blood flow and vascularity (characteristic of GD)
might account for the significantly higher ADC values of GD, as
compared with those of subacute thyroiditis and Hashimoto
thyroiditis. It is also evident that, as in PT, lymphocytic
infiltration is a prominent feature in subacute thyroiditis and
Hashimoto thyroiditis (29), which
will result in hypercellularity. This may explain why the results
of the present study are consistent with those of Tezuka et
al.
MR imaging is non-invasive, since no injection of
contrast material is required. Another advantage is the lack of
ionizing radiation, which is of particular relevance if patients
are pregnant or nursing, or if a patient with recurrent disease
requires repeated follow-up examinations. In addition, if the
facility is not equipped to perform RAIU or thyroid scintigraphy,
ADC could be a good option for imaging due to the broader
availability of MR scanners. The MR examination time is longer than
that of RAIU and is more uncomfortable for the patient. However for
RAIU, the patient has to wait for 24 h prior to the measurement of
thyroid radioactivity under the protocol used at Tianjin Medical
University General Hospital. In addition, for thyroid scintigraphy,
an uptake time of ~30 min must also be added. MR imaging,
particularly DWI, has certain limitations. For instance, DWI is
highly sensitive to motion artifacts, such as swallowing or
breathing. Moreover, patients with pacemakers or incompatible metal
implants cannot be examined by MR, although such cases were not
encountered in the present study.
Several major limitations of the present study are
acknowledged. First, the study population was small, so the results
can only be considered to be preliminary. An extension of the study
to a larger patient population is planned. Secondly, the number of
cases of tissue sampling for histopathological evidence was
limited, particularly for PT, where only 2 patients with several
episodes of recurrence agreed to undergo a biopsy. It was not
possible to conduct a statistical analysis of the pathological
data, and no correlation was established between histopathology and
ADC. Thirdly, image resolution improvement was required for ADC
maps. Although no standard method for measuring ADC has been
established, different methods of measuring ADC were not compared.
Further evaluations that complement these limitations are
necessary.
In conclusion, the present study showed that RAIU,
ADC and TRAb are all of diagnostic value for reliably
differentiating between GD and PT. Rationale for evaluating DWI to
differentiate GD from PT were provided. ADC determined by DWI
reflected tissue cellularity and intra-cellular and inter-cellular
membranes, whereas changes in thyroid uptake were displayed by RAIU
and thyroid scintigraphy.
Acknowledgements
This study was supported by Tianjin Medical
University New Century Excellent Talent Program; Young and
Middle-aged Innovative Talent Training Program from Tianjin
Education Committee; and Talent Fostering Program (the 131 Project)
from Tianjin Human Resources and Social Security Bureau (all
awarded to Zhaowei Meng). This investigation was also supported by
the National Key Clinical Specialty Project (awarded to the
Departments of Nuclear Medicine and Radiology).
References
|
1
|
Bahn Chair RS, Burch HB, Cooper DS, et al:
American Thyroid Association; American Association of Clinical
Endocrinologists: Hyperthyroidism and other causes of
thyrotoxicosis: management guidelines of the American Thyroid
Association and American Association of Clinical Endocrinologists.
Thyroid. 21:593–646. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Izumi Y, Takeoka K and Amino N: Usefulness
of the 2nd generation assay for anti-TSH receptor antibodies to
differentiate relapse of Graves ‘thyrotoxicosis from development of
painless thyroiditis after antithyroid drug treatment for Graves’
disease. Endocr J. 52:493–497. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kamijo K, Murayama H, Uzu T, Togashi K and
Kahaly GJ: A novel bioreporter assay for thyrotropin receptor
antibodies using a chimeric thyrotropin receptor (Mc4) is more
useful in differentiation of Graves' disease from painless
thyroiditis than conventional thyrotropin-stimulating antibody
assay using porcine thyroid cells. Thyroid. 20:851–856. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kubota S, Tamai H, Ohye H, et al:
Transient hyperthyroidism after withdrawal of antithyroid drugs in
patients with Graves' disease. Endocr J. 51:213–217. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Iitaka M, Morgenthaler NG, Momotani N, et
al: Stimulation of thyroid-stimulating hormone (TSH) receptor
antibody production following painless thyroiditis. Clin Endocrinol
(Oxf). 60:49–53. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Misaki T, Miyamoto S, Kasagi K, Mori T and
Konishi J: Serial occurrence of two types of postpartum thyroid
disorders. Usefulness of Tc-99m pertechnetate uptake. Clin Nucl
Med. 21:460–462. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nagai Y, Toya T, Fukuoka K, et al:
Occurrence and spontaneous remission of Graves' hyperthyroidism
preceded by painless thyroiditis. Endocr J. 44:881–885. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sarlis NJ, Brucker-Davis F, Swift JP,
Tahara K and Kohn LD: Graves' disease following thyrotoxic painless
thyroiditis. Analysis of antibody activities against the
thyrotropin receptor in two cases. Thyroid. 7:829–836. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Umena S, Takano T, Iijima T, et al: A case
of repeated painless thyroiditis followed by Graves' disease.
Endocr J. 42:821–826. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ho SC, Eng PH, Fok AC, Ng DC and Khoo DH:
Thyrotoxicosis due to the simultaneous occurrence of silent
thyroiditis and Graves' disease. Thyroid. 9:1127–1132. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hiraiwa T, Ito M, Imagawa A, et al: High
diagnostic value of a radioiodine uptake test with and without
iodine restriction in Graves' disease and silent thyroiditis.
Thyroid. 14:531–535. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kidokoro-Kunii Y, Emoto N, Cho K and
Oikawa S: Analysis of the factors associated with Tc-99m
pertechnetate uptake in thyrotoxicosis and Graves' disease. J
Nippon Med Sch. 73:10–17. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Osaki Y, Sakurai K, Arihara Z, Hata M and
Fukazawa H: Prediction of late (24-h) radioactive iodine uptake
using early (3-hour) uptake values in Japanese patients with
Graves' disease. Endocr J. 59:173–177. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Morris LF, Waxman AD and Braunstein GD:
Accuracy considerations when using early (four-or six-h)
radioactive iodine uptake to predict twenty-four-hour values for
radioactive iodine dosage in the treatment of Graves' disease.
Thyroid. 10:779–787. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kamijo K: Study on cutoff value setting
for differential diagnosis between Graves' disease and painless
thyroiditis using the TRAb (Elecsys TRAb) measurement via the fully
automated electrochemiluminescence immunoassay system. Endocr J.
57:895–902. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yoshimura Noh J, Miyazaki N, Ito K, et al:
Evaluation of a new rapid and fully automated
electrochemiluminescence immunoassay for thyrotropin receptor
autoantibodies. Thyroid. 18:1157–1164. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ilicki A, Gamstedt A and Karlsson FA:
Hyperthyroid Graves' disease without detectable thyrotropin
receptor antibodies. J Clin Endocrinol Metab. 74:1090–1094. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Costagliola S, Morgenthaler NG, Hoermann
R, et al: Second generation assay for thyrotropin receptor
antibodies has superior diagnostic sensitivity for Graves' disease.
J Clin Endocrinol Metab. 84:90–97. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Morita T, Tamai H, Oshima A, et al: The
occurrence of thyrotropin binding-inhibiting immunoglobulins and
thyroid-stimulating antibodies in patients with silent thyroiditis.
J Clin Endocrinol Metab. 71:1051–1055. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Amino N, Yabu Y, Miyai K, et al:
Differentiation of thyrotoxicosis induced by thyroid destruction
from Graves' disease. Lancet. 2:344–346. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Izumi Y, Hidaka Y, Tada H, et al: Simple
and practical parameters for differentiation between
destruction-induced thyrotoxicosis and Graves' thyrotoxicosis. Clin
Endocrinol (Oxf). 57:51–58. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ota H, Amino N, Morita S, et al:
Quantitative measurement of thyroid blood flow for differentiation
of painless thyroiditis from Graves' disease. Clin Endocrinol
(Oxf). 67:41–45. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tezuka M, Murata Y, Ishida R, et al: MR
imaging of the thyroid: correlation between apparent diffusion
coefficient and thyroid gland scintigraphy. J Magn Reson Imaging.
17:163–169. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zheng W, Jian T, Guizhi Z, Zhaowei M and
Renfei W: Analysis of 13 I therapy and correlation
factors of Graves' disease patients: A 4-year retrospective study.
Nucl Med Commun. 33:97–101. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang RF, Tan J, Zhang GZ, Meng ZW and
Zheng W: A comparative study of influential factors correlating
with early and late hypothyroidism after 131I therapy
for Graves' disease. Chin Med J (Engl). 123:1528–1532.
2010.PubMed/NCBI
|
|
26
|
Vitug AC and Goldman JM: Silent (painless)
thyroiditis: Evidence of a geographic variation in frequency. Arch
Intern Med. 145:473–475. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Meng Z, Zhu M and Tan J: Elephantiasis
legs. Am J Med Sci. 347:2482014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mittra ES and McDougall IR: Recurrent
silent thyroiditis: A report of four patients and review of the
literature. Tfhyroid. 17:671–675. 2007. View Article : Google Scholar
|
|
29
|
LiVolsi VA: The pathology of autoimmune
thyroid disease: A review. Thyroid. 4:333–339. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Malayeri AA, El Khouli RH, Zaheer A, et
al: Principles and applications of diffusion-weighted imaging in
cancer detection, staging and treatment follow-up. Radiographics.
31:1773–1791. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Woodhams R, Ramadan S, Stanwell P, et al:
Diffusion-weighted imaging of the breast: Principles and clinical
applications. Radiographics. 31:1059–1084. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Padhani AR, Liu G, Koh DM, et al:
Diffusion-weighted magnetic resonance imaging as a cancer
biomarker: Consensus and recommendations. Neoplasia. 11:102–125.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu Z and Xiao X: The use of multi b
values diffusion-weighted imaging in patients with acute stroke.
Neuroradiology. 55:371–376. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Della Nave R, Foresti S, Tessa C, et al:
ADC mapping of neurodegeneration in the brainstem and cerebellum of
patients with progressive ataxias. Neuroimage. 22:698–705. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Pienaar R, Paldino MJ, Madan N, et al: A
quantitative method for correlating observations of decreased
apparent diffusion coefficient with elevated cerebral blood
perfusion in newborns presenting cerebral ischemic insults.
Neuroimage. 63:1510–1518. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Forbes KP, Pipe JG, Karis JP and Heiserman
JE: Improved image quality and detection of acute cerebral
infarction with PROPELLER diffusion-weighted MR imaging. Radiology.
225:551–555. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ahn SJ, Park MS, Kim KA, et al:
18F-FDG PET metabolic parameters and MRI perfusion and
diffusion parameters in hepatocellular carcinoma: A preliminary
study. PLoS One. 8:e715712013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Choi SH, Paeng JC, Sohn CH, et al:
Correlation of 18F-FDG uptake with apparent diffusion
coefficient ratio measured on standard and high b value diffusion
MRI in head and neck cancer. J Nucl Med. 52:1056–1062. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Fruehwald-Pallamar J, Czerny C,
Mayerhoefer ME, et al: Functional imaging in head and neck squamous
cell carcinoma: Correlation of PET/CT and diffusion-weighted
imaging at 3 Tesla. Eur J Nucl Med Mol Imaging. 38:1009–1019. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Nakajo M, Kajiya Y, Tani A, et al: FDG
PET/CT and diffusion-weighted imaging of head and neck squamous
cell carcinoma: Comparison of prognostic significance between
primary tumor standardized uptake value and apparent diffusion
coefficient. Clin Nucl Med. 37:475–480. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ohno Y, Koyama H, Yoshikawa T, et al: N
stage disease in patients with non-small cell lung cancer: Efficacy
of quantitative and qualitative assessment with STIR turbo
spin-echo imaging, diffusion-weighted MR imaging and
fluorodeoxyglucose PET/CT. Radiology. 261:605–615. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wu X, Pertovaara H, Korkola P, et al:
Correlations between functional imaging markers derived from PET/CT
and diffusion-weighted MRI in diffuse large B-cell lymphoma and
follicular lymphoma. PLoS One. 9:e849992014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Thoeny HC, De Keyzer F and King AD:
Diffusion-weighted MR imaging in the head and neck. Radiology.
263:19–32. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ho KC, Lin G, Wang JJ, et al: Correlation
of apparent diffusion coefficients measured by 3T
diffusion-weighted MRI and SUV from FDG PET/CT in primary cervical
cancer. Eur J Nucl Med Mol Imaging. 36:200–208. 2009. View Article : Google Scholar : PubMed/NCBI
|