Introduction
Development of hypoxia in cancer poses a problem,
because it renders solid tumors resistant to chemotherapy and
radiation therapy (1–3). Tumor hypoxia is mainly caused by
insufficient vascularization and increased oxygen consumption as a
result of rapid cancer-cell proliferation. It has been reported to
have an adverse prognostic impact on various types of cancer,
including those of the cervix (4), the head and neck (5), and soft tissue sarcomas (6).
Hypoxia-inducible factor 1 (HIF-1) is a master
regulator of hypoxic adaptation (7,8).
This transcription factor is a heterodimer of HIF-1α and HIF-1β.
The HIF-1β protein is constitutively expressed regardless of the
oxygen concentration, whereas the HIF-1α protein is rapidly broken
down by ubiquitination and proteosomal degradation under normoxic
conditions (9). Under hypoxic
conditions, HIF-1α is stabilized and translocates from the
cytoplasm to the nucleus where it dimerizes with HIF-1β, and the
HIF-1 complex becomes transcriptionally activated. HIF-1 induces
the transcription of a wide variety of genes, including those
involved in glycolysis, angiogenesis, hematopoiesis, survival
pathways, and invasion.
The overexpression of HIF-1α has been reported in
many tumor types, including colon, breast, gastric, lung, skin,
ovarian, pancreatic, prostate and renal carcinomas (10). A correlation between HIF-1α
expression and tumor oxygenation was found in cervical carcinomas
(11,12). Significant associations between
HIF-1α overexpression and patient mortality have been shown in many
different cancers, including those of the brain, breast and cervix
(13–15).
HIF-1 is necessary for tumor growth. The disruption
of HIF-1α by the intratumoral injection of small-interfering RNA
(siRNA) was reported to result in the regression of human glioma
xenografts (16), and in the
tumor stasis of human cervical cancer and colon cancer xenografts
(17). Furthermore,
downregulation of HIF-1α by siRNA increased the sensitivity of
cancer cells to chemotherapies (18,19) and irradiation (20). These studies indicate that HIF-1
also plays important roles in resistance to therapies.
In the present study, we show that the chemical
compound ER-400583-00 is a novel HIF-1 inhibitor. We investigated
the mode of action, pharmacodynamics, and antitumor activity of
ER-400583-00. Our findings revealed that ER-400583-00 suppressed
the proliferation of cancer cells most prominently in areas distal
to the region of blood perfusion, where HIF-1α-expressing hypoxic
cancer cells were located.
Materials and methods
Cells and compounds
Human U251 glioma cells were purchased from the
Riken Cell Bank (Ibaraki, Japan). U251/vascular endothelial growth
factor (VEGF)-placental alkaline phosphatase (PLAP) cells harbor a
plasmid in which the PLAP reporter gene is under the control of a
VEGF promoter, as described previously (21). The cells were cultured in
Dulbecco's modified Eagle's medium supplemented with 10% fetal
bovine serum, 100 U/ml penicillin, and 100 μg/ml streptomycin. The
cells were incubated at 37°C in a humidified atmosphere containing
5% CO2 and either 21% O2 (normoxic
conditions) or 2% O2 (hypoxic conditions).
ER-400583-00 was synthesized at Eisai Co., Ltd
(Tokyo, Japan). For the cellular assays, ER-400583-00 was prepared
in dimethyl sulfoxide (DMSO) and then diluted in the culture
medium. The final concentration of DMSO in any incubation mixture
did not exceed 0.2% (v/v). For the animal studies, ER-400583-00 was
formulated in DMSO/Tween-80 (35:65), which was diluted 5-fold with
saline immediately before administration.
Cell-based HIF-1 reporter assay
HIF-1 reporter activity was determined as previously
described (21). Briefly,
U251/VEGF-PLAP cells were seeded onto 96-well plates at
4.0×104 cells/well. After overnight incubation, the test
compounds were added. The plates were incubated under hypoxic
conditions for an additional 18 h. PLAP activity in the culture
supernatant was then determined.
Quantitative polymerase chain reaction
(qPCR)
Total RNA was obtained using the RNeasy Mini kit
(Qiagen Inc., Valencia, CA) and reverse transcription was performed
using the High Capacity cDNA Reverse Transcription kit (Life
Technologies, Carlsbad, CA). qPCR was carried out using the
ABI7900HT Detection System and the TaqMan Universal PCR Master Mix
(Life Technologies). The following TaqMan Gene Expression assays
(Life Technologies) were used: Hs00153153_m1 (HIF-1α),
Hs00154208_m1 [carbonic anhydrase IX (CA9)], Hs00173626_m1 (VEGFA),
Hs00197884_m1 [solute carrier family 2, member 1 (SLC2A1)],
Hs99999904_m1 [peptidylprolyl isomerase A (PPIA)], Hs99999907_m1
[β-2-microglobulin (B2M)], and Hs99999901_s1 (18S rRNA).
HIF-1α enzyme-linked immunosorbent assay
(ELISA)
The HIF-1α protein concentration in the samples was
determined by a sandwich ELISA using the Human/Mouse Total HIF-1
alpha DuoSet IC (R&D Systems, Minneapolis, MN) according to the
manufacturer's recommended protocol. Briefly, cells in 96-well
plates were solubilized in 100 μl lysis buffer [50 mM Tris (pH
7.5), 300 mM NaCl, 10% glycerol, 3 mM EDTA, 1 mM MgCl2,
20 mM β-glycerophosphate, 25 mM NaF, 1% Triton X-100, and 1 mM
Na3VO4). The lysates were diluted 5-fold with
PBS containing 1% bovine serum albumin, and a 100 μl sample was
used for the sandwich ELISA.
Human tumor xenograft model
U251 cells were injected subcutaneously into the
flank or hind leg of female BALB/cA nu/nu athymic mice, and allowed
to grow to ~300 mm3. Tumor volumes were determined as
length × (diameter)2/2, where length was the longest
dimension and diameter was the shortest dimension. Local
irradiation (10 Gy) was delivered to the tumor xenografts using the
MBR-1520R-3 system (Hitachi, Tokyo, Japan) and a lead cage that
shielded the whole body except for the tumor-bearing hind limb. All
experiments were approved by the Animal Care and Use Committee at
the Eisai Tsukuba Research Laboratories (Ibaraki, Japan).
Pharmacodynamic studies
Mice bearing U251 tumor xenografts received a 10
ml/kg single oral administration of ER-400583-00. At various time
points, animals were sacrificed and the tumors were removed, frozen
in liquid nitrogen, and fragmented using a Multi-beads Shocker
(Yasui Kikai, Osaka, Japan). To determine the amount of HIF-1α
present, the fragmented tumors were lysed in lysis buffer [50 mM
Tris (pH 7.5), 300 mM NaCl, 10% glycerol, 3 mM EDTA, 1 mM
MgCl2, 20 mM β-glycerophosphate, 25 mM NaF, 1% Triton
X-100, and 1 mM Na3VO4) and centrifuged at
1500 × g for 5 min. The HIF-1α concentration in the supernatant was
determined by HIF-1α ELISA, as described above. The total protein
concentration of the sample was determined by the BCA Protein assay
kit (Thermo Fisher Scientific, Waltham, MA), and the HIF-1α protein
concentration in each sample was normalized to the total protein
concentration. For gene-expression analysis, the fragmented tumors
were lysed in TRIzol LS Reagent (Life Technologies), and total-RNA
was purified according to the manufacturer's instructions. qPCR was
conducted as described above.
Antitumor studies
Mice bearing U251 xenografts were stratified into
groups comprising five animals with approximately equal mean tumor
volumes. The animals received a 10 ml/kg oral administration of
ER-400583-00 or vehicle once daily for 11 days. The tumor volumes
were measured twice weekly, and the relative tumor volumes (the
ratio of tumor volume to initial size before treatment) were
calculated.
For combination studies of radiation therapy and
ER400583-00, U251 tumor xenografts were allowed to grow on both
hind legs of the mice. The mice were stratified into two groups of
five animals. The tumor xenografts on the left hind leg of each
mouse were locally irradiated on Day 0. The animals received an
oral administration of ER-400583-00 or vehicle once daily for the
following 4 days.
Immunohistochemistry and image
acquisition
For immunohistochemical analysis, mice bearing U251
tumor xenografts received an intraperitoneal injection of
5-bromo-2-deoxyuridine (BrdU, Sigma-Aldrich) as a 5 mg/ml solution
in PBS containing 0.007 N HCl at 50 mg/kg. After 6 h, the mice
received an intraperitoneal injection of pimonidazole
(Hypoxyprobe-1 Plus kit; Millipore Corporation, Billerica, MA) as a
10 mg/ml solution in saline at 60 mg/kg. After 40 min, the mice
were injected intravenously with 1 mg Hoechst 33342 fluorochrome as
a 10 mg/ml solution in water (Invitrogen). After 20 min, the mice
were sacrificed and the tumors were excised. They were embedded in
Tissue-Tek O.C.T. Compound (Sakura Finetechnical, Nagano, Japan) in
cryomolds, and stored at −80°C until sectioning.
The tumor cryosections were cut and air-dried for 5
min. Hoechst 33342 fluorescence images of entire tumor sections
were captured using an Axiovert 200M fluorescence microscope (Carl
Zeiss, Oberkochen, Germany) with a digital camera (Axiocam MRm,
Carl Zeiss) at a resolution of 9.7 μm/pixel for HIF-1α and BrdU
staining, and 2.4 μm/pixel for pimonidazole staining. The
AxioVision digital image processing software (Carl Zeiss) was used
for capturing the images. After image acquisition, the slides were
rinsed in water, fixed in 1% paraformaldehyde (PFA) in PBS for 10
min, rinsed twice in PBS, and then stained for HIF-1α, BrdU, or
pimonidazole.
HIF-1α was detected using a rabbit polyclonal
antibody NB100-449B (Novus Biologicals, Littleton, CO) at a 1:500
dilution with a horseradish peroxidase-conjugated avidin-biotin
detection system (Vectastain Elite ABC kit, Vector Laboratories,
Burlingame, CA) and diaminobenzidine (DAB, Muto Pure Chemicals,
Tokyo, Japan). Slides were counterstained with hematoxylin, and
mounted using Aquatek (Merck & Co., Inc.; Whitehouse Station,
NJ). Whole-slide red, green and blue (RGB) images were obtained
using an AP-200 slide imager (Kurabo Industries Ltd., Osaka, Japan)
at a resolution of 1.7 μm/pixel. Pimonidazole was detected using a
fluorescein isothiocyanate (FITC)-labeled hypoxyprobe-1 Mab1
monoclonal antibody (Hypoxyprobe-1 Plus kit) according to the
manufacturer's instructions.
Slides were mounted using Vectashield (Vector
Laboratories). FITC fluorescence images of entire tumor sections
were obtained using an Axiovert 200M fluorescence microscope at a
resolution of 2.5 μm/pixel. For BrdU detection, slides were treated
with 50% formamide in 2X SSC at 60°C for 3 h. The slides were then
rinsed twice in 2X SSC, treated with 2 N HCl at 37°C for 30 min,
neutralized twice in 0.1 M sodium borate, and rinsed twice in PBS.
BrdU was detected using the rat monoclonal antibody OBT0030
(Accurate Chemical & Scientific Corporation, Westbury, NY) at a
1:500 dilution with the horseradish peroxidase-conjugated
avidin-biotin detection system (Vectastain Elite ABC kit, Vector
Laboratories) and DAB (Muto Pure Chemicals). Slides were
counterstained with hematoxylin and mounted using Aquatek (Merck,
Inc.). Whole-slide RGB images were obtained using the AP-200 slide
imager (Kurabo Industries, Ltd.) at a resolution of 1.7
μm/pixel.
Image analysis
The fractions of tissue that were positive for
HIF-1, pimonidazole, and BrdU at each measured distance from the
region of blood perfusion were determined as follows. Initially,
Photoshop Elements software (Adobe Systems Inc., San Jose, CA) was
used to enlarge the Hoechst 33342 fluorescence images and to
overlay them with the corresponding images of HIF-1α, pimonidazole,
or BrdU staining. After the removal of necrotic tissue, skin, and
staining artifacts, three binary images (denoted Perfusion, Signal
and Tissue) were produced for each layered image.
The Perfusion images were produced by binarizing the
Hoechst 33342 fluorescence images with the threshold value fixed in
each experiment. The signal images for HIF-1α and BrdU were
produced from the RGB images by selecting all pixels within the
color range for DAB, which was determined separately for each
experiment. The Signal images for pimonidazole were produced by
binarizing the FITC fluorescence images with a threshold that was
determined separately for each image, which produced a
pimonidazole-positive fraction of 1% in the Hoechst 33342-positive
regions. The Tissue images for HIF-1α and BrdU were produced from
the RGB images by selecting all pixels with RGB values below or
equal to 200. The Tissue images for pimonidazole were produced by
manual delineation of the tumor boundaries. Photoshop Elements
software was used for binarizing the fluorescence images, and
Lumina Vision software (Mitani Corporation, Fukui, Japan) was used
for binarizing the RGB images.
All binary images for the HIF-1 and BrdU analysis
were reduced to 25% of the original size, and those for the
pimonidazole analysis were reduced to 50% of the original size. The
distance from each positive pixel in the Signal and Tissue images
to the nearest positive pixel in the corresponding Perfusion image
was then measured using JAVA software programmed by the authors.
The data were tabulated and the fraction of Signal-positive pixels
at each distance from the area of perfusion was determined by
dividing the number of pixels in the Signal image at each distance
by the number of pixels in the Tissue image at the same
distance.
Results
Discovery of ER-400583-00 as a
small-molecule HIF-1α inhibitor
In order to search for novel small molecules that
suppressed the HIF-1 pathway, we generated a human glioma cell line
expressing a PLAP reporter gene under the control of a VEGF
promoter, which contained the active HIF-1 binding site
(U251/VEGF-PLAP). The reporter activity was increased more than
10-fold when the cells were cultured under hypoxic conditions.
U251-based HIF-1 reporter assay has also been demonstrated by
another group (22). We performed
high-throughput screening of a small-molecule library of 43,000
compounds using U251/VEGF-PLAP. Through derivatization of a hit
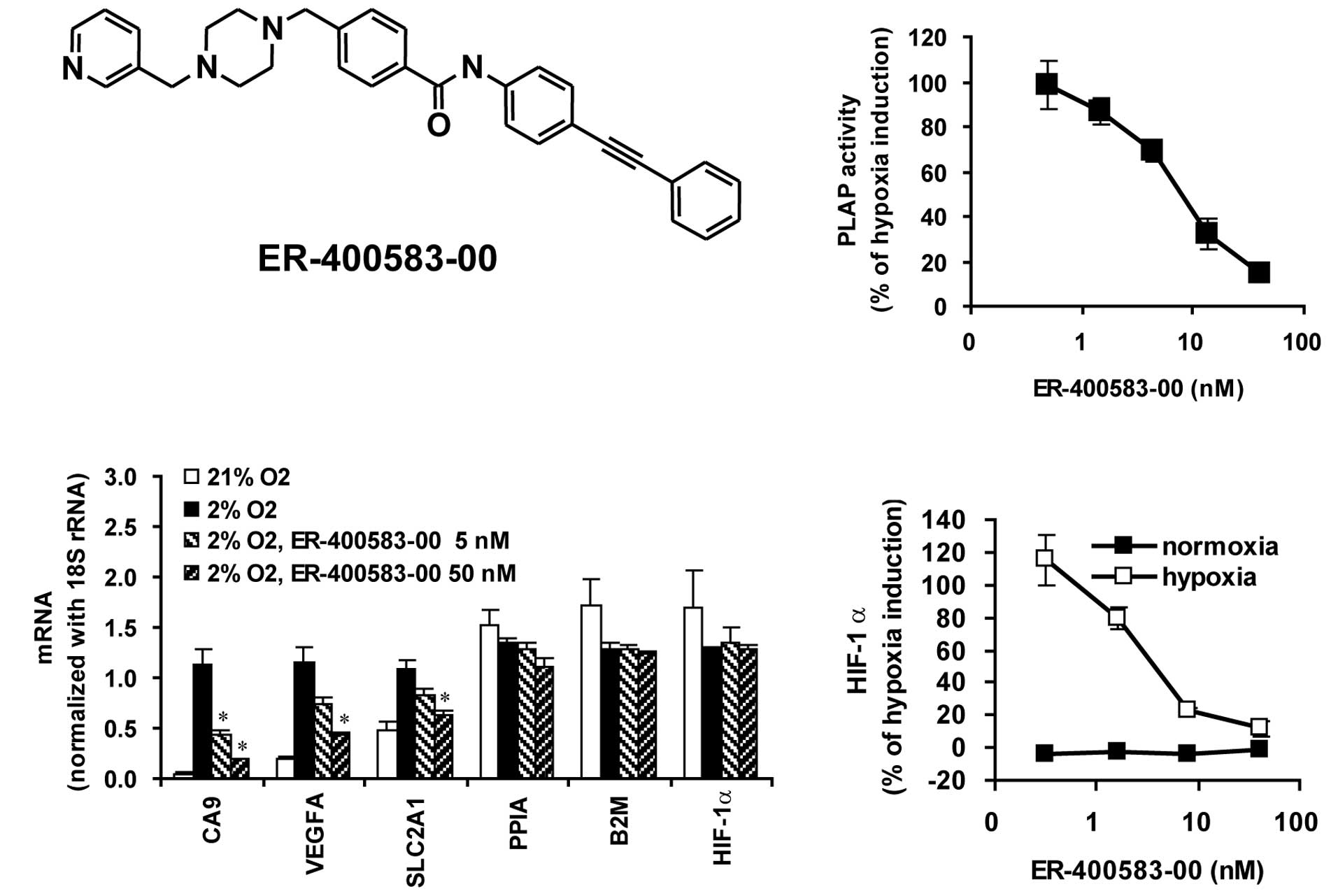
compound, we discovered ER-400583-00 (Fig. 1A), which inhibited the induction
of HIF-1 reporter activity in response to hypoxia, with a
half-maximal inhibitory concentration (IC50) value of
7.9 nM (Fig. 1B).
To examine whether ER-400583-00 suppressed the
induction of endogenous HIF-1 target genes in response to hypoxia,
U251 cells were cultured under normoxic or hypoxic conditions, with
or without ER-400583-00, and gene expression was examined. The
HIF-1 target genes CA9, VEGFA, and SLC2A1 were induced by hypoxia
(Fig. 1C). ER-400583-00
suppressed the hypoxia-induced accumulation of the messenger RNAs
(mRNAs) of these HIF-1 target genes. By contrast, ER-400583-00 did
not affect the mRNA levels of the housekeeping genes PPIA and B2M,
or those of HIF-1α. The amount of HIF-1α protein present was
measured. The mean ± SD HIF-1α levels in U251 cells incubated at
4×104 cells/well in 96-well plates under normoxic and
hypoxic conditions for 6 h were 37.8±13.4 pg/ml and 367.2±33.0
pg/ml, respectively. Incubation under hypoxic conditions for 6 h
thus resulted in a 9.7-fold increase of the amount of HIF-1α
protein in U251 cells. ER-400583-00 did not reduce the amount of
HIF-1α protein present in U251 cells under normoxic conditions.
However, it suppressed the induction of HIF-1α protein in response
to hypoxia, with an IC50 value of 3.7 nM (Fig. 1D).
Pharmacodynamics and antitumor activity
of ER-400583-00 in a human tumor xenograft model
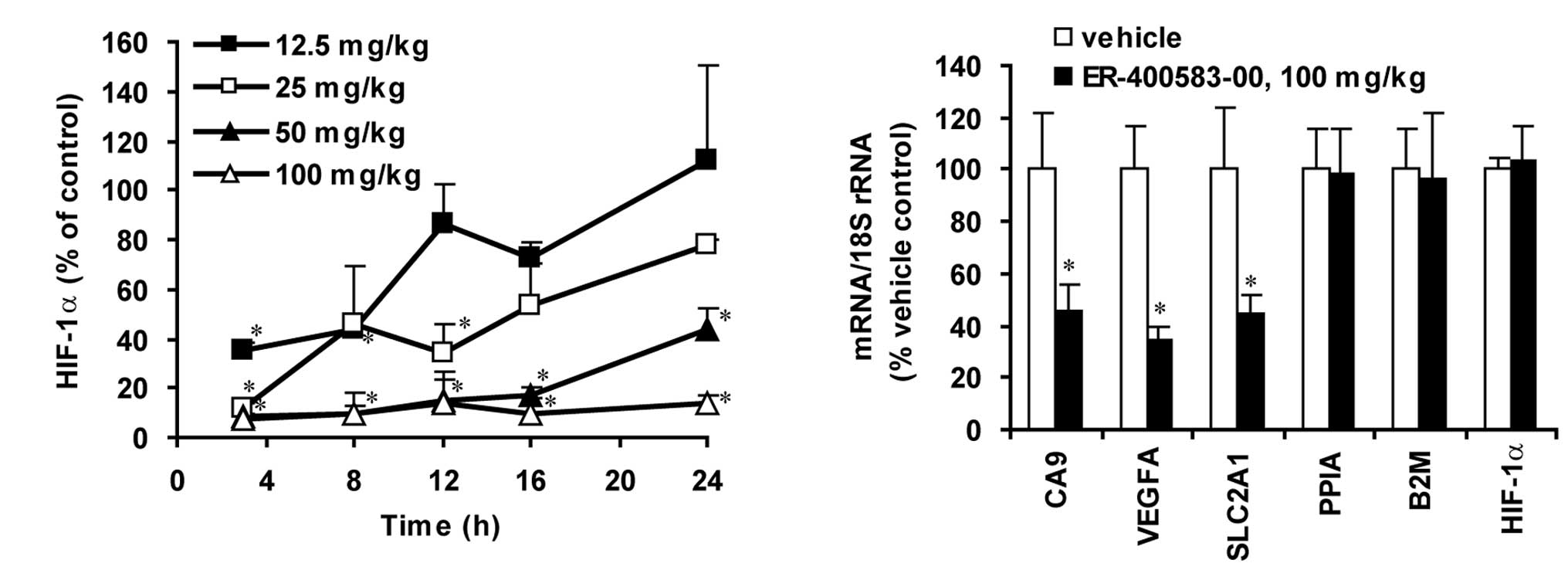
Next, we investigated the pharmacodynamics of
ER-400583-00 using a human U251 tumor xenograft model in athymic
mice. ER-400583-00 was administered orally as a single dose, and
the amount of HIF-1α present in tumors was determined over time. A
significant decrease in the amount of HIF-1α was observed in tumors
as early as 3 h after the administration of ER-400583-00 (Fig. 2A). The amount of HIF-1α returned
to the control level 12 h after the administration of ER-400583-00
at a dose of 12.5 mg/kg, but the effect was sustained for longer at
a higher dosage. ER-400583-00 at a dose of 100 mg/kg decreased the
amount of HIF-1α to less than 10% of that in control tumors 3 h
after administration, and the suppressive effect was sustained for
24 h. qPCR analysis revealed that the administration of
ER-400583-00 at 100 mg/kg significantly decreased mRNA levels of
the HIF-1 target genes CA9, VEGFA, and SLC2A1 24 h after treatment
(Fig. 2B). By contrast, the mRNA
expression levels of HIF-1α, and the house-keeping genes PPIA and
B2M, were not altered.
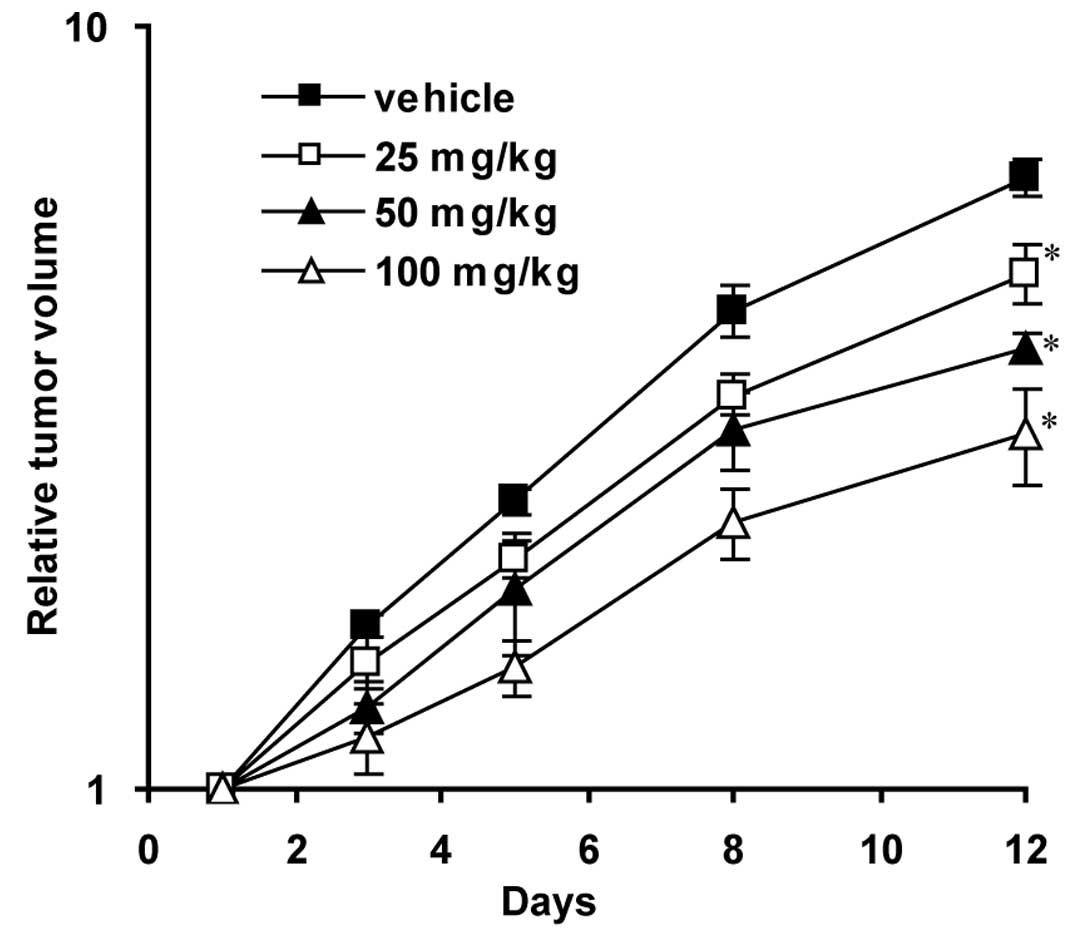
We then evaluated the antitumor activity of
ER-400583-00 in human U251 tumor xenografts. The daily oral
administration of ER-400583-00 significantly delayed tumor growth,
as assessed by changes in tumor volume (Fig. 3).
HIF-1α expression and hypoxia in
tumors
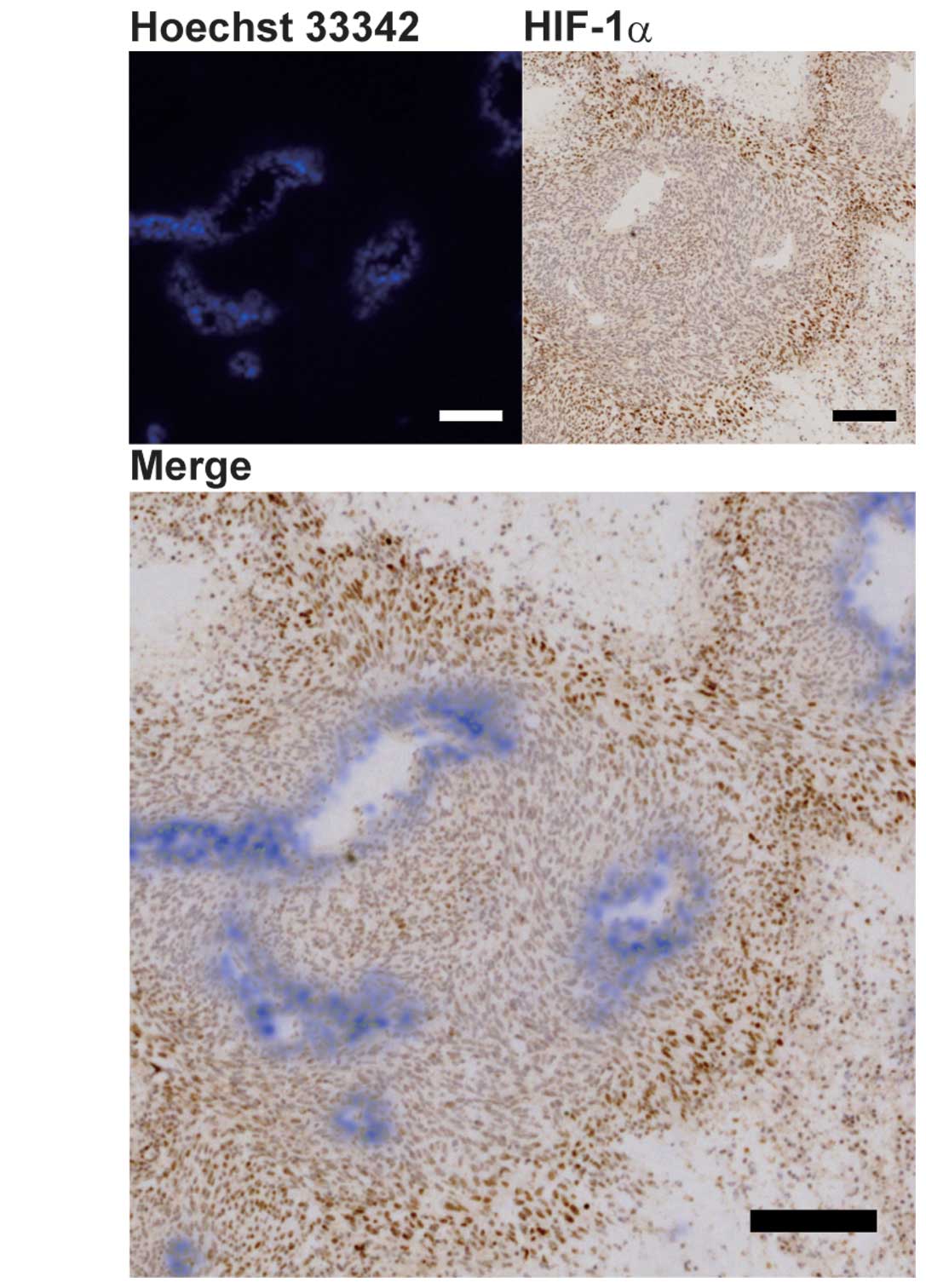
We examined the distributions of HIF-1α expression
and hypoxia in conjunction with blood perfusion in U251 tumor
xenografts, in order to determine whether ER-400583-00 exerted
antitumor activity against HIF-1α-expressing hypoxic cancer cells.
Blood perfusion was assessed using the distribution of Hoechst
33342 fluorochrome, which has limited tissue penetration (23). Tumor sections were then stained
for HIF-1α immunohistochemistry and the images for both signals
were merged. The results showed that cancer cells with higher
HIF-1α expression were located distal to the region of blood
perfusion (Fig. 4A).
Quantitative analysis of the merged images confirmed
that the size of the HIF-1α-positive area increased with distance
from the region of blood perfusion, and reached a maximum at a
distance between 100 and 200 μm (Fig.
4B). The distribution of hypoxia was also analyzed by a similar
method, using pimonidazole (23,24) as a marker, and appeared comparable
to that of HIF-1α (Fig. 4C).
Quantitative analysis revealed that the pimonidazole-positive area
also increased with distance from the region of blood perfusion,
and reached a maximum at a distance between 100 and 200 μm
(Fig. 4D). These results
indicated that, in U251 tumor xenografts, hypoxic tissues were
located distal to the region of blood perfusion and that HIF-1α was
induced in cancer cells in this region.
Antitumor activity of ER-400583-00
against a tumor hypoxic region
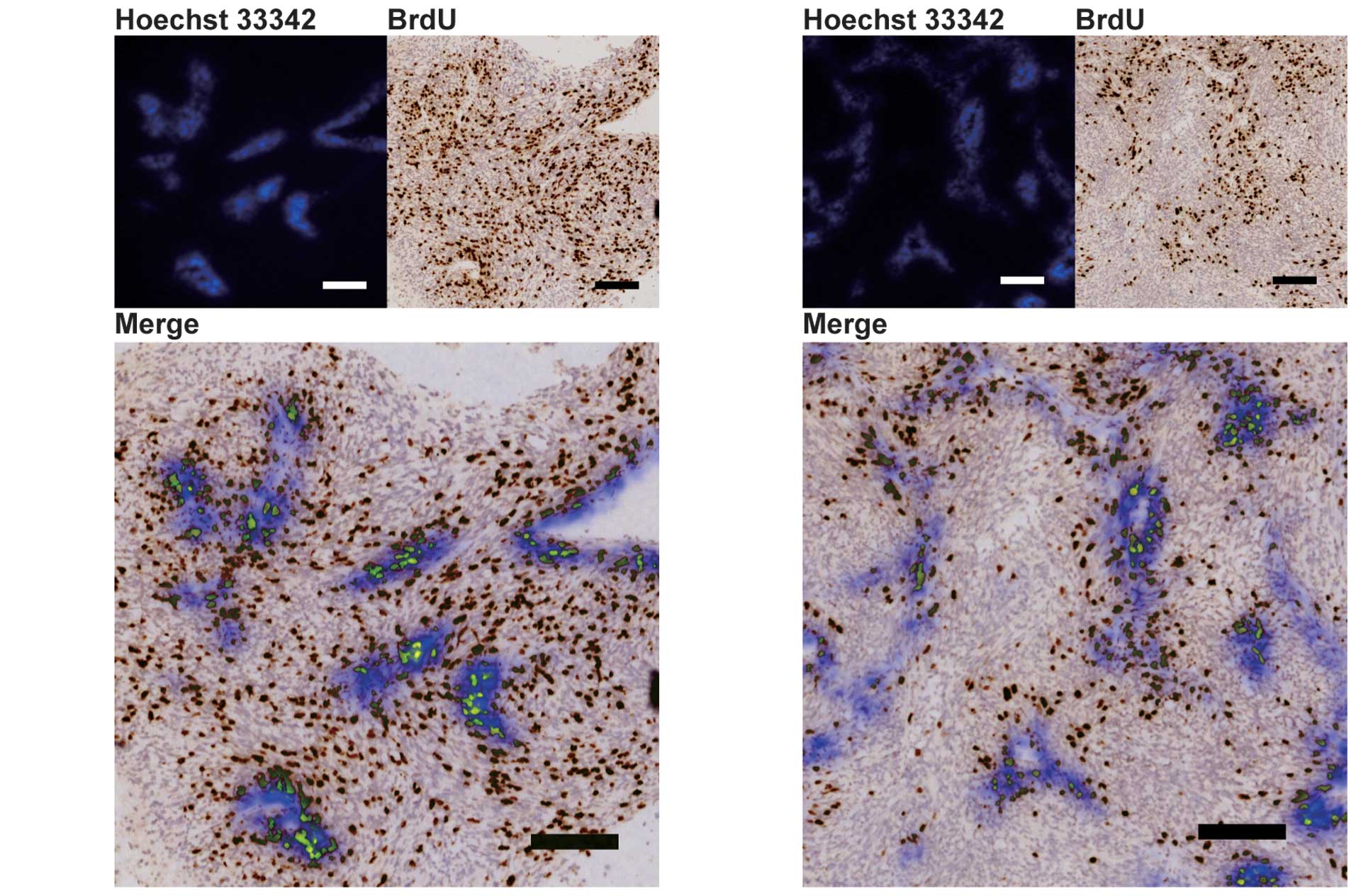
The distributions of proliferating cells were
analyzed using BrdU staining, in order to examine whether
ER-400583-00 acted against HIF-1α-expressing cancer cells in the
hypoxic region of U251 tumor xenografts. In ER-400583-00-treated
tumors, cancer cells in areas distal to the region of blood
perfusion appeared to be less proliferative than those in control
tumors (Fig. 5A and 5B).
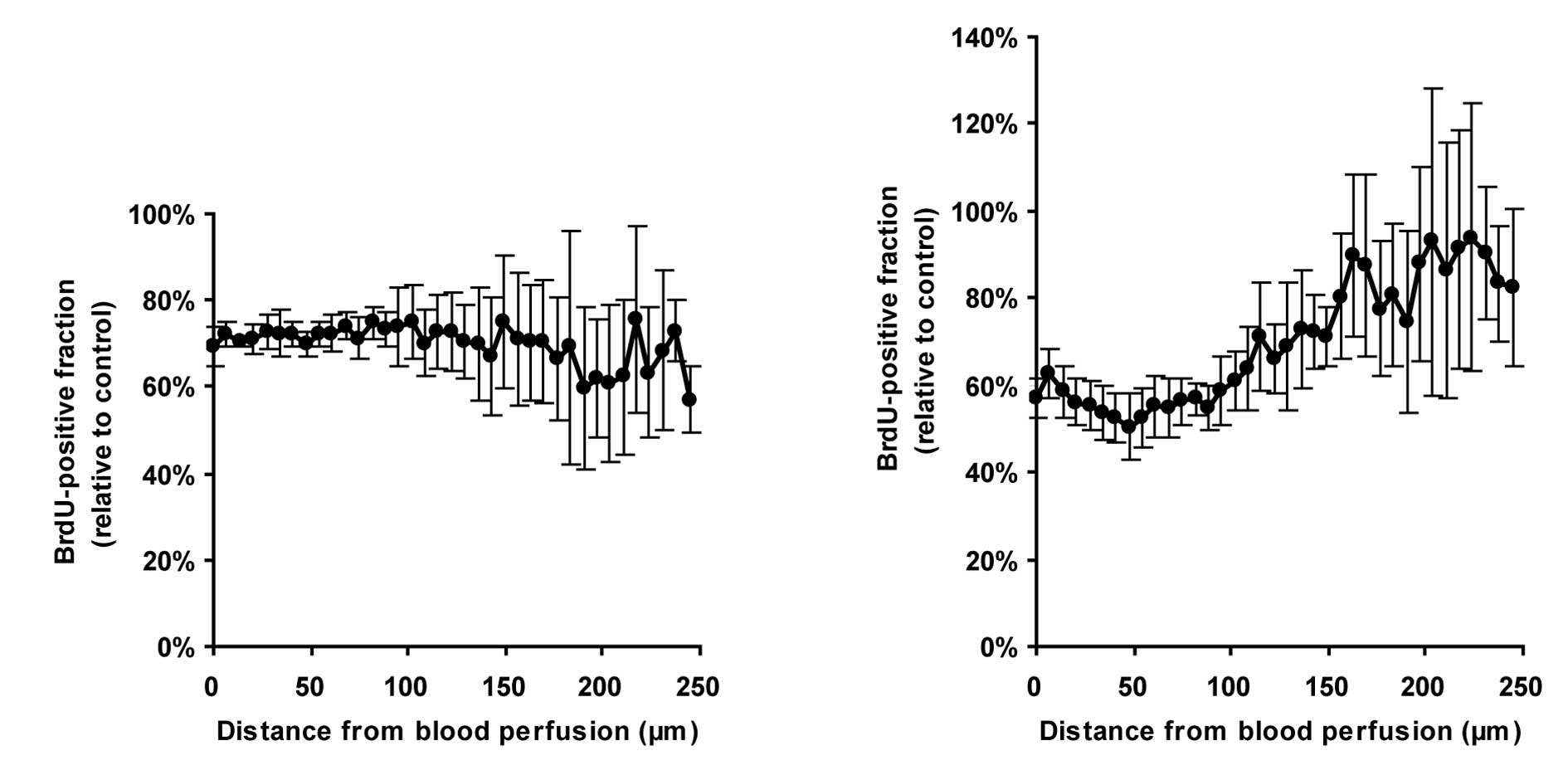
Quantitative analysis revealed that, in control
tumors, the values of proliferation indices decreased as the
distance from the region of blood perfusion increased (Fig. 5C). In ER-400583-00-treated tumors,
cancer cells near the region of blood perfusion showed similar
proliferative activity to those in control tumors. However, the
values of proliferation indices decreased more notably as the
distance increased (Fig. 5C), and
fell below 50% of those in control tumors in tissues at a distance
of 100 μm from the region of blood perfusion (Fig. 5D). These results indicated that
the growth-suppressive effect of ER-400583-00 was more prominent in
HIF-1α-expressing hypoxic cancer cells than in non-hypoxic cancer
cells.
Hypoxic cancer cells have been reported to be
radiation-resistant (25). To
determine whether hypoxic cancer cells in U251 tumor xenografts
were also resistant to radiation therapy, we analyzed the pattern
of BrdU incorporation in tumor cells after irradiation. The values
of proliferation indices were slightly decreased, regardless of the
distance from the region of blood perfusion, at 2 days
post-irradiation (Fig. 6A). At 7
days post-irradiation, the values of proliferation indices in
tissues near the region of blood perfusion remained low (Fig. 6B), whereas those in tissues distal
to the region of blood perfusion had recovered to control levels.
These results indicated that the hypoxic cancer cells in U251 tumor
xenografts were resistant to radiation therapy.
Combined antitumor activity of
ER-400583-00 and radiation therapy
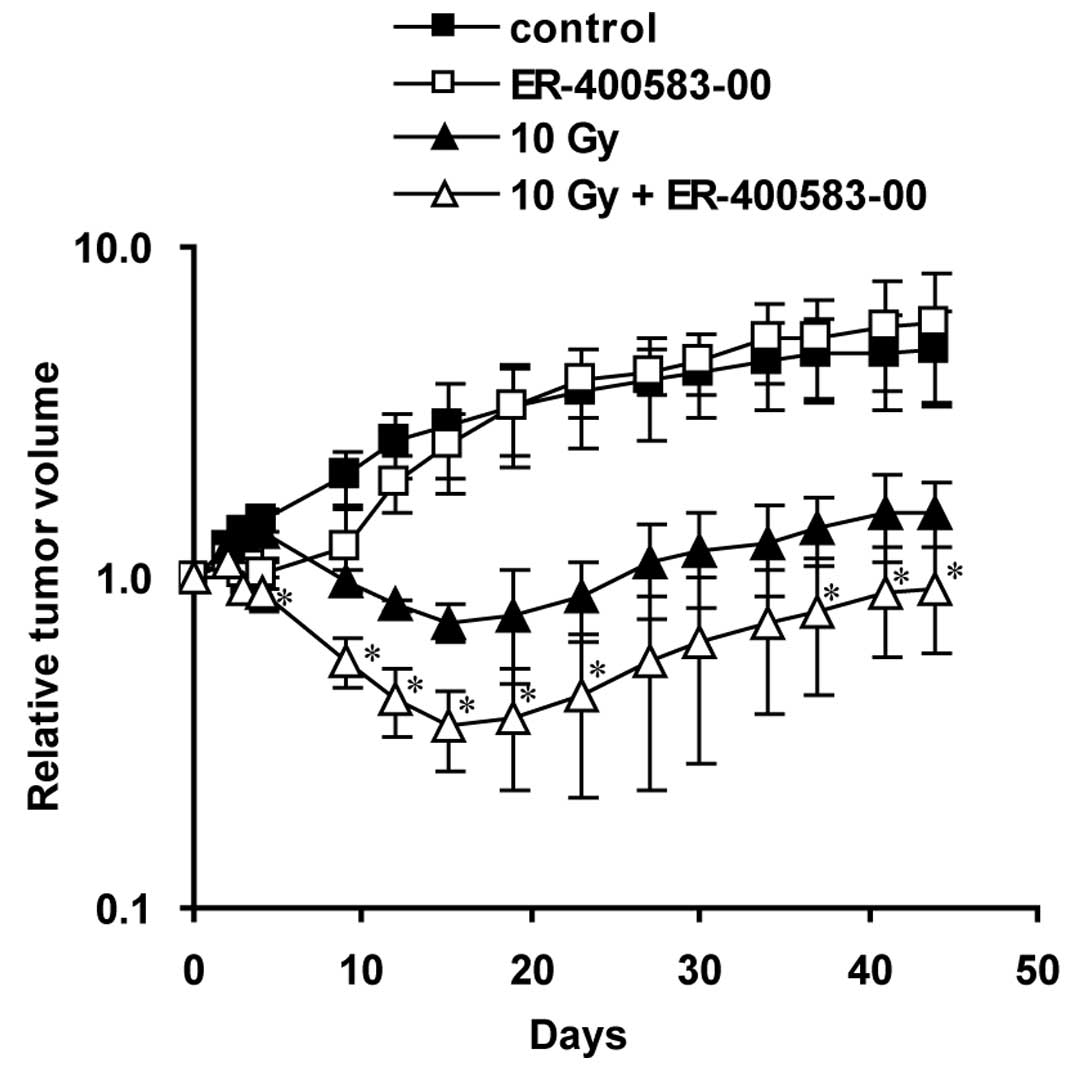
Finally, we evaluated the combined antitumor
activity of ER-400583-00 and radiation therapy in the U251 tumor
xenograft model (Fig. 7). In the
radiation-treated group, the tumors had shrunk by 4 days after
irradiation, and reached a minimum size at 15 days
post-irradiation. In the combined-treatment group, the tumors
shrank more rapidly and the minimum tumor size was significantly
lower than that of the radiation-treated group. A synergistic
interaction was observed at 15 days post-irradiation.
Discussion
This study identified ER-400583-00 as a novel
small-molecule HIF-1 inhibitor. ER-400583-00 suppressed the
accumulation of HIF-1α protein under hypoxic conditions but did not
affect HIF-1α mRNA levels, indicating that it acted on either the
translation or degradation of the HIF-1α protein. HIF-1α is
degraded by a proteasome-dependent pathway in normoxic cells. Under
normoxic conditions, HIF-1α is proline hydroxylated by specific
proline hydroxylases (26). This
hydroxylation promotes the binding of HIF-1α to the von Hippel
Lindau E3 ligase complex, which is followed by ubiquitination and
proteasomal degradation. Iron chelators such as deferoxamine and
cobalt inhibit the proline hydroxylases and induce HIF-1α
accumulation under normoxic conditions (27). Although ER-400583-00 suppressed
the HIF-1α protein accumulation induced by hypoxia, it did not
suppress the HIF-1α accumulation induced by deferoxamine (data not
shown); this indicated that ER-400583-00 enhanced the degradation
of HIF-1α under hypoxic conditions through a proline
hydroxylation-dependent ubiquitination pathway. Further studies are
needed to clarify the precise mechanism of ER-400583-00 action.
Our histological analysis revealed that cells
located at a distance between 100 and 200 μm away from blood
perfusion were pimonidazole-positive in U251 xenograft tumors.
Tissues far away (>200 μm) were necrotic and
pimonidazole-negative. This distance is well consistent with the
reported limitation of oxygen diffusion (28). The main pathogenetic mechanisms
involved in the development of hypoxia are related to diffusion,
anemia, or perfusion (2). Anemia
was not observed in this model (data not shown). Our histological
analysis showed that most platelet/endothelial cell-adhesion
molecule 1 (CD31)-positive vessels stained positive for Hoechst
33342 fluorochrome, and that pimonidazole staining was not observed
near the vessels. These data indicated that most vessels in this
model were functionally normal and well-perfused. In contrast to
perfusion-related hypoxia, which occurs abruptly and
intermittently, diffusion-related hypoxia is chronic. The chronic
nature of the hypoxia in this model facilitated the analysis of
differences in cellular responses among local
microenvironments.
Although hypoxia is a strong inducer of HIF-1α,
other factors such as growth signals and lactate accumulation have
been reported to induce this protein even under normoxic
conditions. We compared the amounts of HIF-1α in several cancer
cell lines, and found that U251 cells had relatively low expression
levels under normoxic conditions (data not shown); this indicated
that factors other than hypoxia were less effective at inducing
HIF-1α in U251 cells. The distribution of HIF-1α in U251 xenograft
tumors was well correlated with the distribution of hypoxia. These
data indicated that hypoxia was a dominant inducer of HIF-1α in
U251 xenograft tumors.
We found that ER-400583-00 suppressed the
proliferation of cancer cells distal to the region of blood
perfusion without affecting those near the vasculature in U251
xenograft tumors. This indicated that the cellular response to
HIF-1 blockade differed under different microenvironments. Hypoxic
conditions did not enhance the growth-inhibitory activity of
ER-400583-00 in vitro (data not shown). These data are
consistent with the results of previous studies showing that a loss
of HIF-1α had little effect on in vitro cell growth under
hypoxic conditions (29,30). Dominant-negative HIF-1α was shown
to suppress pancreatic cancer cell growth under hypoxic and
glucose-deprived conditions (31). Nutrients such as glucose and amino
acids are also transported by the blood, and are likely to be
scarce in areas distal to a region of blood perfusion. These
factors might enhance the growth-inhibitory activity of
ER-400583-00 in such areas.
ER-400583-00 augmented the antitumor effect of
radiation therapy in the U251 xenograft model. Our
immunohistochemical analysis revealed that hypoxic cancer cells
were resistant to radiation therapy in this model, and that
ER-400583-00 showed antitumor activity against them. Selective cell
killing by poly (ADP-ribose) polymerase 1 (PARP1) inhibitors was
reported recently (32). In this
study, the authors showed that PARP inhibitor-treated xenografts
displayed decreased clonogenic survival following experimental
radiotherapy. We hypothesized that the enhancement of antitumor
activity by ER-400583-00 was also achieved by targeting hypoxic
cancer cells. In our preliminary experiments, hypoxic cancer cells
in the U251 xenograft model were also resistant to cytotoxic agents
such as camptothecin-11 (CPT-11) and paclitaxel. Combining
ER-400583-00 with these drugs could therefore be a promising
approach, and we plan to evaluate this combined antitumor activity
in future studies.
In summary, this study discovered the orally active,
novel HIF-1 inhibitor ER-400583-00. Our results showed that
treatment with ER-400583-00 achieved sustained HIF-1α suppression
in xenograft tumors in animal studies. ER-400583-00 exhibited
enhanced cytotoxicity against hypoxic cancer cells in tumors and
enhanced antitumor activity in combination with radiation
therapy.
Acknowledgements
We thank Mr Masayuki Ikawa for developing a JAVA
software for the image analysis.
References
|
1
|
M HockelP VaupelTumor hypoxia: definitions
and current clinical, biologic, and molecular aspectsJ Natl Cancer
Inst93266276200110.1093/jnci/93.4.26611181773
|
|
2
|
P VaupelA MayerHypoxia in cancer:
significance and impact on clinical outcomeCancer Metastasis
Rev26225239200710.1007/s10555-007-9055-117440684
|
|
3
|
A WoutersB PauwelsF LardonJB
VermorkenReview: implications of in vitro research on the effect of
radiotherapy and chemotherapy under hypoxic
conditionsOncologist12690712200710.1634/theoncologist.12-6-69017602059
|
|
4
|
M HockelK SchlengerB AralM MitzeU
SchafferP VaupelAssociation between tumor hypoxia and malignant
progression in advanced cancer of the uterine cervixCancer
Res564509451519968813149
|
|
5
|
M NordsmarkSM BentzenV RudatPrognostic
value of tumor oxygenation in 397 head and neck tumors after
primary radiation therapy. An international multi-center
studyRadiother
Oncol771824200510.1016/j.radonc.2005.06.03816098619
|
|
6
|
M NordsmarkJ AlsnerJ KellerHypoxia in
human soft tissue sarcomas: adverse impact on survival and no
association with p53 mutationsBr J
Cancer8410701075200110.1054/bjoc.2001.172811308256
|
|
7
|
GL SemenzaTargeting HIF-1 for cancer
therapyNat Rev Cancer3721732200310.1038/nrc1187
|
|
8
|
GL SemenzaEvaluation of HIF-1 inhibitors
as anticancer agentsDrug Discov
Today12853859200710.1016/j.drudis.2007.08.00617933687
|
|
9
|
PH MaxwellMS WiesenerGW ChangThe tumour
suppressor protein VHL targets hypoxia-inducible factors for
oxygen-dependent
proteolysisNature399271275199910.1038/2045910353251
|
|
10
|
H ZhongAM De MarzoE LaughnerOverexpression
of hypoxia-inducible factor 1alpha in common human cancers and
their metastasesCancer Res5958305835199910582706
|
|
11
|
HK HauglandV VukovicM PintilieExpression
of hypoxia-inducible factor-1alpha in cervical carcinomas:
correlation with tumor oxygenationInt J Radiat Oncol Biol
Phys53854861200210.1016/S0360-3016(02)02815-812095550
|
|
12
|
GJ HutchisonHR ValentineJA
LoncasterHypoxia-inducible factor 1alpha expression as an intrinsic
marker of hypoxia: correlation with tumor oxygen, pimonidazole
measurements, and outcome in locally advanced carcinoma of the
cervixClin Cancer
Res1084058412200410.1158/1078-0432.CCR-03-0135
|
|
13
|
P BirnerB GatterbauerG OberhuberExpression
of hypoxia-inducible factor-1 alpha in oligodendrogliomas: its
impact on prognosis and on
neoangiogenesisCancer92165171200110.1002/1097-0142(20010701)92:1%3C165::AID-CNCR1305%3E3.0.CO;2-F11443623
|
|
14
|
M SchindlSF SchoppmannH
SamoniggOverexpression of hypoxia-inducible factor 1alpha is
associated with an unfavorable prognosis in lymph node-positive
breast cancerClin Cancer Res818311837200212060624
|
|
15
|
P BirnerM SchindlA ObermairC PlankG
BreiteneckerG OberhuberOverexpression of hypoxia-inducible factor
1alpha is a marker for an unfavorable prognosis in early-stage
invasive cervical cancerCancer Res6046934696200010987269
|
|
16
|
DL GillespieK WhangBT RagelJR FlynnDA
KellyRL JensenSilencing of hypoxia inducible factor-1alpha by RNA
interference attenuates human glioma cell growth in vivoClin Cancer
Res1324412448200710.1158/1078-0432.CCR-06-269217438103
|
|
17
|
X ZhangT KonH WangEnhancement of
hypoxia-induced tumor cell death in vitro and radiation therapy in
vivo by use of small interfering RNA targeted to hypoxia-inducible
factor-1alphaCancer
Res6481398142200410.1158/0008-5472.CAN-03-230115548675
|
|
18
|
L ChenP FengS LiEffect of
hypoxia-inducible factor-1alpha silencing on the sensitivity of
human brain glioma cells to doxorubicin and etoposideNeurochem
Res34984990200910.1007/s11064-008-9864-918937067
|
|
19
|
X SongX LiuW ChiHypoxia-induced resistance
to cisplatin and doxorubicin in non-small cell lung cancer is
inhibited by silencing of HIF-1alpha geneCancer Chemother
Pharmacol58776784200610.1007/s00280-006-0224-716532342
|
|
20
|
J KesslerA HahnelH WichmannHIF-1alpha
inhibition by siRNA or chetomin in human malignant glioma cells:
effects on hypoxic radioresistance and monitoring via CA9
expressionBMC Cancer10605201010.1186/1471-2407-10-60521050481
|
|
21
|
T SakaiT SameshimaM MatsufujiN KawamuraK
DobashiY MizuiPladienolides, new substances from culture of
Streptomyces platensis Mer-11107. I Taxonomy, fermentation,
isolation and screeningJ Antibiot (Tokyo)571731792004
|
|
22
|
A RapisardaB UranchimegDA
ScudieroIdentification of small molecule inhibitors of
hypoxia-inducible factor 1 transcriptional activation pathwayCancer
Res6243164324200212154035
|
|
23
|
LA HuxhamAH KyleJH BakerLK NykilchukAI
MinchintonMicroregional effects of gemcitabine in HCT-116
xenograftsCancer
Res6465376541200410.1158/0008-5472.CAN-04-098615374965
|
|
24
|
H OkuyamaH EndoT AkashikaK KatoM
InoueDownregulation of c-MYC protein levels contributes to cancer
cell survival under dual deficiency of oxygen and glucoseCancer
Res701021310223201010.1158/0008-5472.CAN-10-272020980433
|
|
25
|
D VordermarkA KatzerK BaierP KraftM
FlentjeCell type-specific association of hypoxia-inducible factor-1
alpha (HIF-1 alpha) protein accumulation and radiobiologic tumor
hypoxiaInt J Radiat Oncol Biol
Phys5812421250200410.1016/j.ijrobp.2003.11.03015001269
|
|
26
|
RK BruickSL McKnightA conserved family of
prolyl- 4-hydroxylases that modify
HIFScience29413371340200110.1126/science.106637311598268
|
|
27
|
P JaakkolaDR MoleYM TianTargeting of
HIF-alpha to the von Hippel-Lindau ubiquitylation complex by
O2-regulated prolyl
hydroxylationScience292468472200110.1126/science.105979611292861
|
|
28
|
DE SwinsonJL JonesD RichardsonCarbonic
anhydrase IX expression, a novel surrogate marker of tumor hypoxia,
is associated with a poor prognosis in non-small-cell lung cancerJ
Clin Oncol21473482200310.1200/JCO.2003.11.13212560438
|
|
29
|
B BlouwH SongT TihanThe hypoxic response
of tumors is dependent on their microenvironmentCancer
Cell4133146200310.1016/S1535-6108(03)00194-612957288
|
|
30
|
L LiX LinM StaverEvaluating
hypoxia-inducible factor-1 alpha as a cancer therapeutic target via
inducible RNA interference in vivoCancer
Res6572497258200510.1158/0008-5472.CAN-04-442616103076
|
|
31
|
J ChenS ZhaoK NakadaDominant-negative
hypoxia-inducible factor-1 alpha reduces tumorigenicity of
pancreatic cancer cells through the suppression of glucose
metabolismAm J
Pathol16212831291200310.1016/S0002-9440(10)63924-712651620
|
|
32
|
N ChanIM PiresZ BencokovaContextual
synthetic lethality of cancer cell kill based on the tumor
microenvironmentCancer
Res7080458054201010.1158/0008-5472.CAN-10-235220924112
|