Introduction
Host defense is achieved by two different immune
systems, innate and adaptive immunity. Innate immunity is the first
line of defense process to protect the host from microbial
pathogens and is primarily mediated by phagocytes such as
macrophages and dendritic cells (1–4).
The innate immunity recognizes microorganisms via a limited number
of pattern-recognition receptors (PRRs), which recognize microbial
components, known as pathogen-associated molecular patterns
(PAMPs). Toll-like receptors (TLRs) and Nod-like receptors (NLRs)
are the representative PRRs.
They recognize microbial molecules including
bacterial lipoprotein, lipopolysaccharide (LPS), flagellin, and
viral nucleic acids, at the cell surface or endosomal membrane and
subsequently activate NF-κB and MAPK to trigger the inflammatory
process (1). In addition to
microbial molecules, a variety of endogenous ligands, such as heat
shock proteins, high mobility group box 1 (HMGB1), hyaluronan
fragments, heparin sulphate, and fibronectin are recognized by TLR2
or TLR4 (5). In contrast, NLRs
are intracellular, cytoplasmic sensors for microbial components and
danger signals (6,7). There are 23 NLR family members in
humans and at least 34 NLR genes in mice (7). NLRs are expressed in non-immune
cells including epithelial and mesothelial cells as well as immune
cells. Nucleotide-binding oligomerization domain (NOD)1 and NOD2,
the first identified NLRs consist of an N-terminal caspase
recruitment domain (CARD), an intermediate NOD, and a C-terminal
leucine-rich repeats (LRRs) domain (8). NOD1 and NOD2 recognize the
peptidoglycan derivatives, meso-diaminopimelic acid
(meso-DAP) and muramyl dipeptide (MDP), respectively
(8). After stimulation by their
specific bacterial molecules, NOD1 and NOD2 associate with the
adaptor molecule, RICK/Rip2/CARDIAK, through CARD-CARD interaction,
which leads to activation of NF-κB and MAPK, followed by induction
of numerous genes involved in the inflammatory process (9–11).
Periodontitis is a chronic inflammatory disease
initiated on the periodontium by toxin and oxygen produced from
periodontopathic bacteria (12),
which results in tooth loss and periodontal bone resorption because
the supportive tissue surrounding the teeth was destructed.
Gram-negative bacteria such as Porphyromonas gingivalis,
Aggregatibacter actinomycetemcomitans and Fusobacterium
necleatum have been considered to be important pathogenic
microorganisms associated with periodontitis (12–14).
Periodontal ligament (PDL) cells not only function
as supporting cells for periodontal tissues but also produce
inflammatory mediators that recognize various molecules including
LPS (15). There is evidence that
TLRs mediate immune responses of PDL cells against periodontal
infections (16,17). However, little is known about the
role of NOD1 and NOD2 in innate immune responses of PDL cells.
Therefore, in the present study, we examined the function of NOD1
and NOD2 in the innate immune responses of human PDL cells.
Materials and methods
Cell culture and reagents
A human periodontal ligament cell line was a gift
from Dr Maeda (Kyushu University Hospital, Fukuoka, Japan). This
cell line was immortalized by SV40 T-antigen and hTERT gene
transfer (18). THP-1 cells, a
human monocytic leukemia cell line, were used as a positive
control. PDL cells were cultured in Minimum Essential Medium α
(Gibco, Grand Island, NY, USA) containing 1X
penicillin/streptomycin and 10% fetal bovine serum in 5%
CO2 incubator at 37°C. Tri-DAP,
Pam3CSK4, LPS, and recombinant human IFN-γ
were purchased from Invivogen, Inc. (San Diego, CA, USA) and
muramyl dipeptide [MDP;
Ac-(6-O-strearoyl)-muramyl-Ala-D-Glu-NH2] was from
Bachem, Inc. (Torrance, CA, USA).
RT-PCR
Total-RNA was extracted from the cell using
easy-BLUE (Intron Biotechnology, Daejeon, Korea) according to the
manufacturer’s instruction. One microgram of total-RNA was reverse
transcribed into cDNA, and PCR was performed using the Power cDNA
Synthesis kit (Intron Biotechnology) and One-step RT-PCR with
AccuPower® HotStart PCR PreMix (Bioneer, Daejeon,
Korea). The following primer sets were used. Human NOD1, forward,
5′-CCACTTCACAGCTGGAG ACA-3′ and reverse,
3′-TGAGTGGAAGCAGCATTTTG-5′; human NOD2, forward,
5′-GAATGTTGGGCACCTCAAGT-3′ and reverse, 3′-CAAGGAGCTTAGCCATGGAG-5′;
human GAPDH, forward, 5′-GTCGGAGRCAACGGATT-3′ and reverse,
3′-AAGCTTCCCGTTCTCAG-3′.
The PCR reaction condition included pre-denaturing
at 94°C for 30 sec, then 35–40 cycle of 56°C for 30 sec, 72°C for 1
min. PCR products were then electrophoresed on a 1.5% agarose gel
and visualized using a gel documentation system.
Measurement of IL-6 and IL-8
The cells in triplicate were treated with the
indicated doses of Tri-DAP and MDP or combination with TLR agonist
or IFN-γ for 24 h and the culture supernatant was collected. The
concentration of IL-6 and IL-8 in the culture supernatants were
determined using a commercial ELISA kit (R&D Systems,
Minneapolis, MN, USA)
Western blotting
The cells (1×106/well) were plated in
35-mm culture dishes. The cells were treated with 10 μg/ml of
Tri-DAP and MDP and were lysed in buffer containing 1% Nonidet-P40
supplemented with a complete protease inhibitor cocktail (Roche)
and 2 mM dithiothreitol. Lysates were resolved by 10% SDS-PAGE,
transferred to a polyvinylidene fluoride (PVDF) membrane, and
immunoblotted with primary antibodies against regular- and
phospho-IκB-α, p38, ERK and JNK (Cell Signaling Technology, Inc.,
Beverly, MA, USA). After immunoblotting with secondary antibodies,
proteins were detected with an enhanced chemiluminescence (ECL)
reagent (Intron Biotechnology).
Statistical analysis
The differences among the mean values of the
different groups were assessed, and the values are expressed as the
mean ± SD. All of the statistical calculations were performed by
one-way ANOVA using the GraphPad Prism version 5.01 (GraphPad
Software, San Diego, CA, USA). Values of P<0.05 were considered
significant.
Results
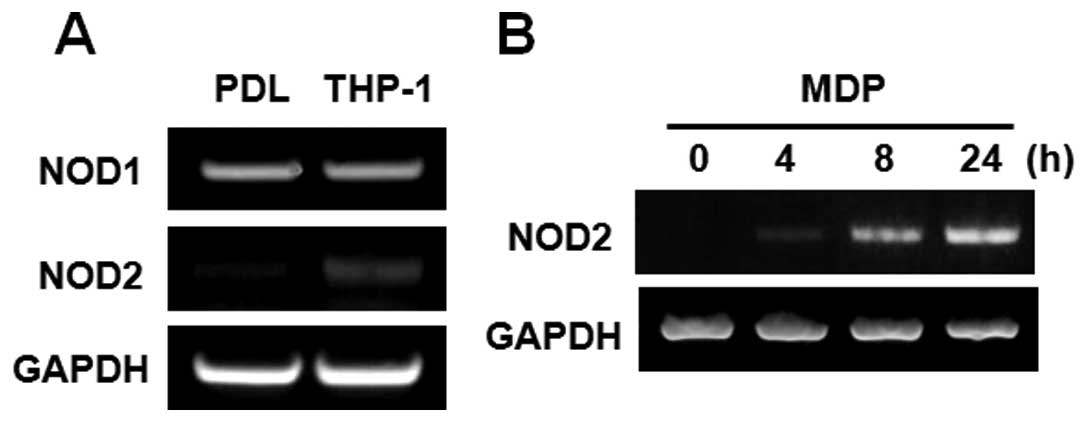
Expression of NOD1 and NOD2 in PDL
cells
The gene expression of NOD1 and NOD2 in PDL cells
were examined by RT-PCR. THP-1 cells, human monocyte leukemia
cells, were used as a positive control. The gene of NOD1 was
strongly expressed in PDL cells, and NOD1 levels were comparable to
that in THP-1 cells. In contrast, NOD2 expression was found to be
low level in PDL cells, as compared to THP-1 cells (Fig. 1A). However, the stimulation with
MDP, a specific NOD2 agonist, augmented the gene expression of NOD2
in a time-dependent manner in PDL cells (Fig. 1B).
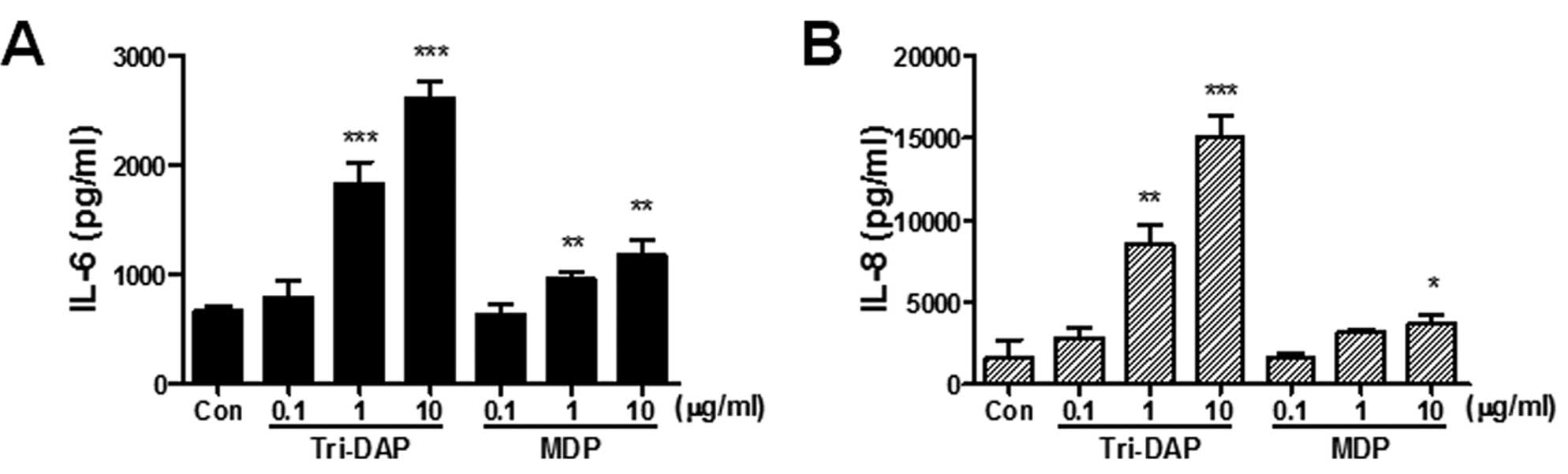
NOD1 and NOD2 stimulation leads to
increased production of IL-6 and IL-8 in PDL cells
To determine whether the stimulation of NOD1 and
NOD2 leads to the production of pro-inflammatory
cytokines/chemokines, the cells were treated with Tri-DAP (NOD1
agonist) and MDP (NOD2 agonist) and the production of IL-6 and IL-8
from culture supernatants was determined by ELISA. Both Tri-DAP and
MDP can lead to increased production of IL-6 and IL-8 production in
PDL cells in a dose-dependent manner (Fig. 2). Both IL-6 and IL-8 production
was more increased by Tri-DAP than MDP, suggesting that NOD1 may
play a more important role in the immune response of PDL cells than
NOD2.
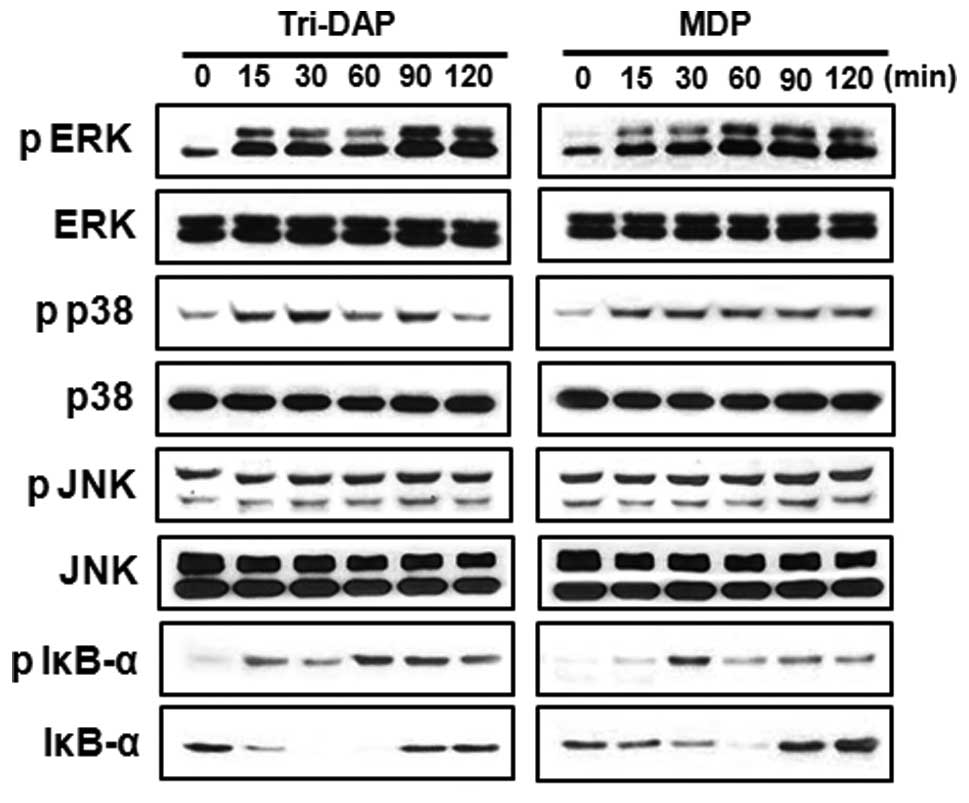
Tri-DAP and MDP induce NF-κB and MAPK
activation in PDL cells
Sensing of microbial molecules by NOD1 and NOD2
induce the activation of NF-κB and MAPK in various cell types
including macrophages (19–21). To determine whether NOD1 and NOD2
stimulation leads to NF-κB and MAPK activation in PDL cells, the
cells were treated with Tri-DAP or MDP and extracts were prepared
at different times after stimulation. Subsequently, immunoblotting
was performed using antibodies that recognize activated forms of
IκB-α, p38, JNK and ERK. Results showed that both Tri-DAP and MDP
induced phosphorylation of IκB-α, p38 and ERK, but not JNK
(Fig. 3). The kinetics of IκB-α
phosphorylation and degradation were different between Tri-DAP and
MDP. Fifteen minutes after stimulation, Tri-DAP induced
phosphorylation of IκB-α and maximal phosphorylation was detected
at 60 and 90 min after stimulation. However, MDP induced optimal
phosphorylation of I-κBα at 30 min after stimulation, which was
reduced after that time (Fig.
3).
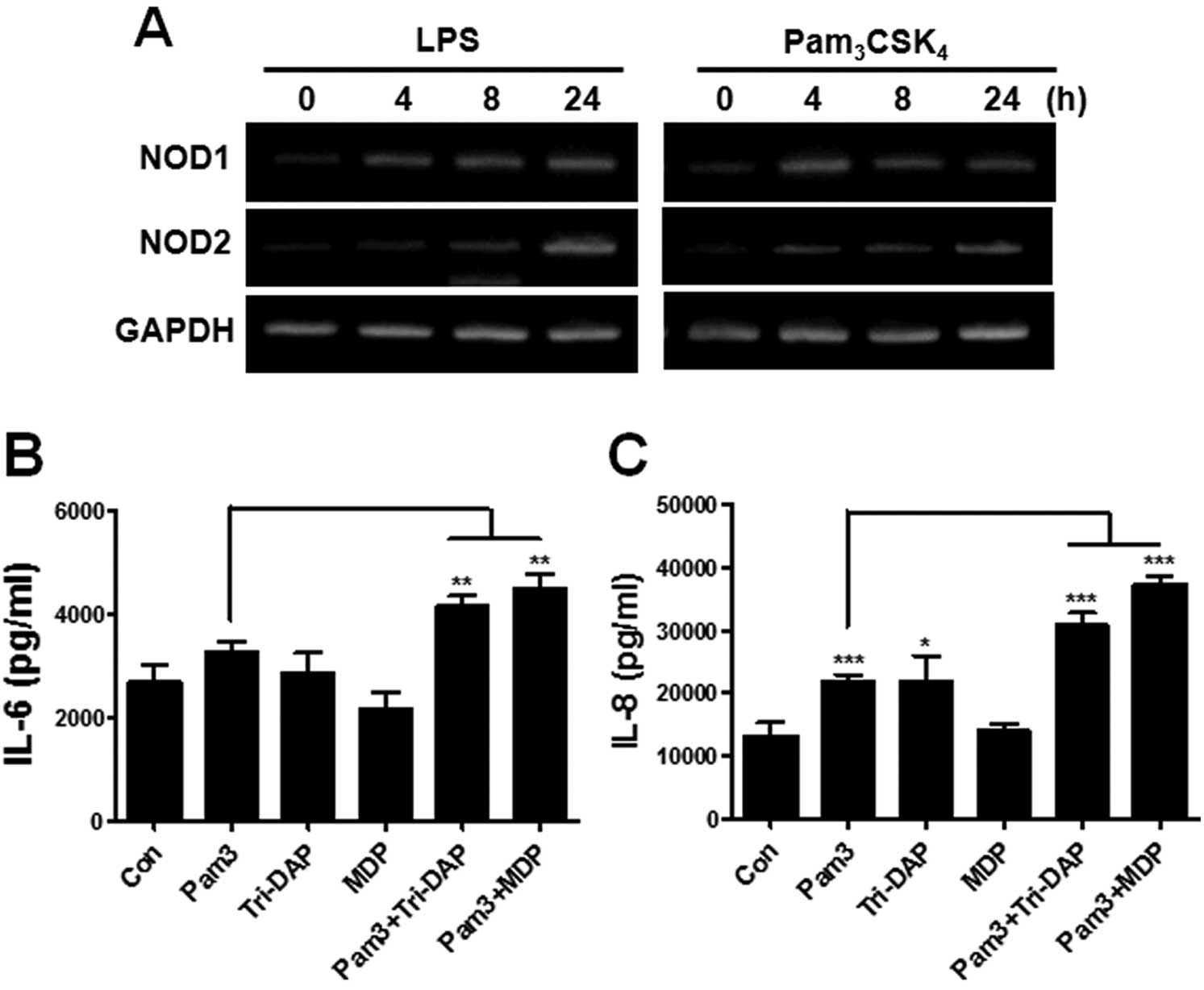
TLR stimulation enhances the gene
expression of NOD1 and NOD2 and augments the production of IL-6 and
IL-8 increased by Tri-DAP and MDP in PDL cells
It has been known that TLRs synergize with NOD1 and
NOD2 to produce cytokines in macrophages and dendritic cells
(20,22). We first examined whether
stimulation by TLRs affects the gene expression of NOD1 and NOD2 in
PDL cells. The treatment of LPS (a TLR4 agonist) and
Pam3CSK4 (a TLR2 agonist) could enhance the
gene expression of NOD1 and NOD2 beginning at 4 h after stimulation
(Fig. 4A). We next investigated
whether activation of TLRs can augment the ability of PDL cells to
produce IL-6 and IL-8 by Tri-DAP and MDP. Dose response experiments
were performed to determine an appropriate dose of
Pam3CSK4 to induce marginal production of
IL-6 and IL-8. Results revealed that 0.1 μg/ml of
Pam3CSK4 led to a minor increase of IL-6 and
IL-8 production in PDL cells (data not shown), and this
concentration was used for further experiments. For the synergism
experiment, the cells were treated with indicated agonists alone or
their combination for 24 h and IL-6 and IL-8 production was
measured from culture supernatants. As shown in Fig. 4, combination treatment of
Pam3CSK4 and Tri-DAP or MDP significantly
augmented IL-6 or IL-8 production in PDL cells, as compared to the
single agonist-treated group (Fig. 4B
and C).
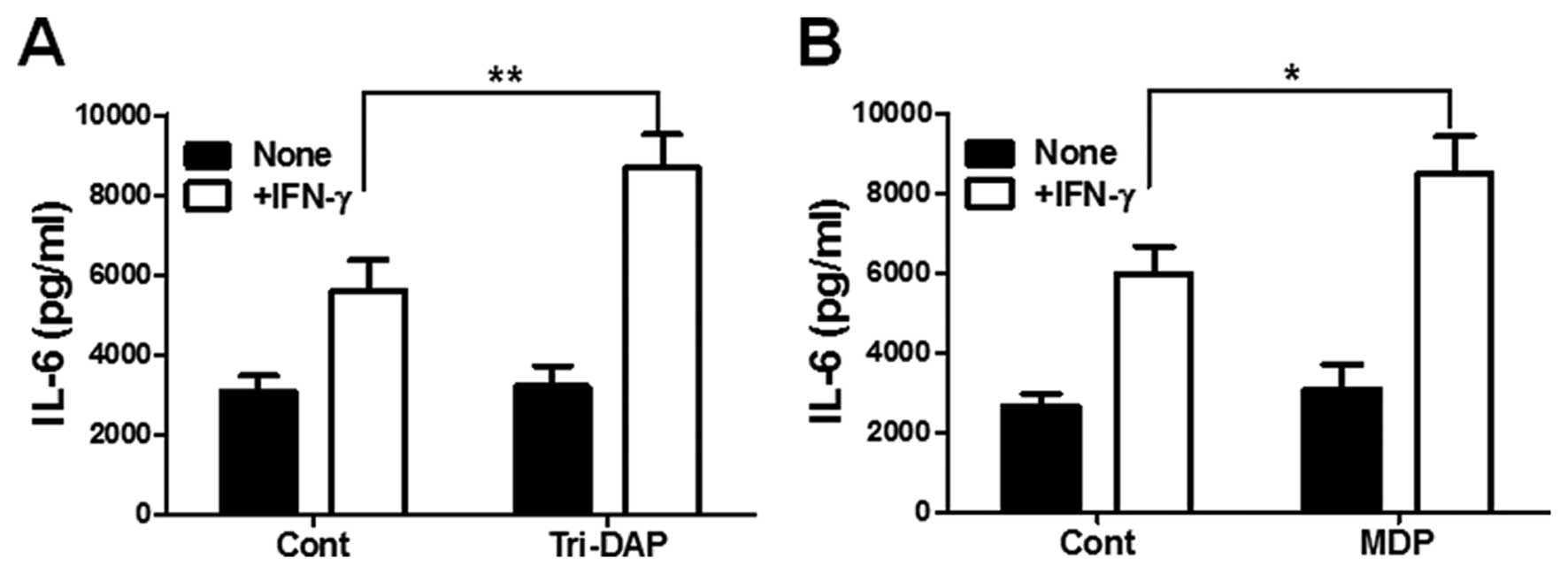
IFN-γ synergizes with Tri-DAP and MDP to
produce IL-6 in PDL cells
Finally, we examined whether IFN-γ can augment
Tri-DAP and MDP-induced cytokine production by PDL cells. IFN-γ
alone could increase IL-6 production in PDL cells (Fig. 5A). In addition, co-stimulaion with
Tri-DAP upregulated IL-6 production in PDL cells, as compared to
IFN-γ or Tri-DAP alone (Fig. 5A).
Furthermore, combination treatment with MDP and IFN-γ also enhanced
IL-6 production by PDL cells, although a low dose of MDP alone (0.1
μg/ml) could not increase IL-6 production (Fig. 5B).
Discussion
Recent studies have demonstrated that NOD1 and NOD2
are expressed in various cell types that exist within the oral
tissues and play a role in triggering immune responses (23–25). In healthy gingival tissues, NOD1
and NOD2 exhibit stronger expression than TLRs (24). NOD1 and NOD2 are also expressed in
various oral epithelial cells and the stimulation with iE-DAP and
MDP upregulates the gene expression of β-defensin 2 (24). In human gingival fibroblasts, both
NOD1 and NOD2 were strongly expressed and their agonists (FK156 for
NOD1, MDP for NOD2) could increase the production of IL-6, IL-8 and
MCP-1 via an NF-κB-dependent pathway (25). In addition, Hirao et al
(23) showed the gene and protein
expression of NOD1 and NOD2 in pulp fibroblasts and that iE-DAP and
MDP could produce IL-8, suggesting that NOD1 and NOD2 are
functionally expressed in pulp fibroblasts. Tang et al
(26) showed the gene and protein
expression and localization of NOD1 and NOD2 in human PDL
fibroblasts. The activation of NOD1 and NOD2 led to the
upregulation of tumor necrosis factor receptor-associated factor 6
(TRAF6) and pro-inflammatory cytokines in human PDL cells (26).
In the present study, we revealed that NOD1 is
strongly expressed in PDL cells and the expression level is
comparable to that in THP-1 cells. NOD2 expression was relatively
weak in PDL cells. The expression level of NOD2 varies between cell
types. The mRNA and protein of NOD2 was markedly expressed in
hepatocytes, oral epithelial cells, and renal tubular epithelial
cells (27–29), but was not expressed or was weakly
expressed in intestinal epithelial cells (30). In addition, NOD2 expression seems
to be regulated by specific treatment. Bacterial flagellin (a TLR5
agonist), E. coli, and IL-1β increased the gene expression
of NOD2 in intestinal epithelial cells (30). Furthermore, in the presence of
histamine, MALP-2 (a TLR2 agonist), peptidoglycan, and β-glucan
also enhanced NOD2 gene expression in keratinocytes (31). In this study, the gene expression
of NOD2 was upregulated by MDP stimulation, suggesting that NOD2
may be inducible in PDL cells. In addition, the activation of NOD1
and NOD2 with Tri-DAP and MDP led to IL-6 and IL-8 production and
the activation of NF-κB and MAPK in PDL cells, indicating that NOD1
and NOD2 may be functionally expressed in PDL cells.
Previous studies showed that NOD1 and NOD2 have the
synergistic or additive effect with TLRs to produce cytokines and
chemokines in immune cells and mesothelial cells (20,21,32). These phenomena were also found in
several epithelial cells. In oral epithelial cells, NOD1 and NOD2
agonists in combination with TLR agonists synergistically enhanced
β-defensin 2 secretion (33).
Moreover, LPS pretreatment enhanced the activation of NF-κB, ERK
and JNK by MDP in hepatocytes (27). Likewise, in this study, NOD1 and
NOD2 agonists (Tri-DAP and MDP) synergized with a TLR2 agonist
Pam3CSK4 to produce IL-6 and IL-8 in PDL
cells. Our results indicate that NOD1 and NOD2 may cooperate with
TLRs to elicit immune responses in PDL cells.
IFN-γ is known to increase the expression of NOD1
and NOD2 in macrophages (34,35). In addition, IFN-γ is essential for
a NOD1 agonist, KF1B-induced nitric oxide production in mesothelial
cells (21). Therefore, we
examined whether IFN-γ augments IL-6 production by NOD1 and NOD2
activation in PDL cells. Results showed that the co-stimulation
with IFN-γ and Tri-DAP or MDP upregulated IL-6 production in PDL
cells, as compared to IFN-γ or the agonist alone-treated group.
These findings suggest that IFN-γ may enhance the innate immune
response mediated by NOD1 and NOD2 signaling in PDL cells.
In conclusion, we reported here that NOD1 and NOD2
are functionally expressed in human PDL cells and the activation of
these receptors can induce innate immune responses such as cytokine
production and the activation of NF-κB and MAPK. In addition, our
results revealed that TLRs can synergize with NOD1 and NOD2 to
produce proinflammatory cytokines/chemokines. Similarly to immune
responses, NOD1 and NOD2 signaling can mediate cellular
physiological functions, such as proliferation and differentiation
(36–38). Future studies should clarify the
function of NOD1 and NOD2 on the cellular physiology of PDL
cells.
Acknowledgements
This study was supported by a grant by the Korea
Science and Engineering Foundation (KOSEF) funded by the Korean
government (MOST) (grant no. R13-2008-010-00000-0).
References
|
1
|
S AkiraS UematsuO TakeuchiPathogen
recognition and innate
immunityCell124783801200610.1016/j.cell.2006.02.01516497588
|
|
2
|
JA HoffmannThe immune response of
DrosophilaNature4263338200310.1038/nature0202114603309
|
|
3
|
B BeutlerC EidenschenkK CrozatJL ImlerO
TakeuchiJA HoffmannS AkiraGenetic analysis of resistance to viral
infectionNat Rev Immunol7753766200710.1038/nri217417893693
|
|
4
|
R MedzhitovRecognition of microorganisms
and activation of the immune
responseNature449819826200710.1038/nature0624617943118
|
|
5
|
L YuL WangS ChenEndogenous toll-like
receptor ligands and their biological significanceJ Cell Mol
Med1425922603201010.1111/j.1582-4934.2010.01127.x20629986
|
|
6
|
G ChenMH ShawYG KimG NunezNOD-like
receptors: role in innate immunity and inflammatory diseaseAnnu Rev
Pathol4365398200910.1146/annurev.pathol.4.110807.09223918928408
|
|
7
|
L FranchiN WarnerK VianiG NunezFunction of
Nod-like receptors in microbial recognition and host defenseImmunol
Rev227106128200910.1111/j.1600-065X.2008.00734.x19120480
|
|
8
|
N InoharaM ChamaillardC McDonaldG
NunezNOD-LRR proteins: role in host-microbial interactions and
inflammatory diseaseAnnu Rev
Biochem74355383200510.1146/annurev.biochem.74.082803.13334715952891
|
|
9
|
N InoharaT KosekiJ LinL del PesoPC LucasFF
ChenY OguraG NúñezAn induced proximity model for NF-κB activation
in the Nod1/RICK and RIP signaling pathwaysJ Biol
Chem27527823278312000
|
|
10
|
SE GirardinR TournebizeM MavrisAL PageX
LiGR StarkJ BertinPS DiStefanoM YanivPJ SansonettiDJ
PhilpottCARD4/Nod1 mediates NF-κB and JNK activation by invasive
Shigella flexneriEMBO Rep2736742200111463746
|
|
11
|
MS HaydenS GhoshSignaling to NF-κBGenes
Dev18219522242004
|
|
12
|
T NishiharaT KosekiMicrobial etiology of
periodontitisPeriodontol
2000361426200410.1111/j.1600-0757.2004.03671.x
|
|
13
|
J SlotsHS ReynoldsRJ
GencoActinobacillus actinomycetemcomitans in human
periodontal disease: a cross-sectional microbiological
investigationInfect Immun29101310201980
|
|
14
|
S SocranskyA HaffajeeM CuginiC SmithR Kent
JrMicrobial complexes in subgingival plaqueJ Clin
Periodontol25134144199810.1111/j.1600-051X.1998.tb02419.x9495612
|
|
15
|
Y YamajiT KubotaK SasaguriS SatoY SuzukiH
KumadaT UmemotoInflammatory cytokine gene expression in human
periodontal ligament fibroblasts stimulated with bacterial
lipopolysaccharidesInfect Immun63357635811995
|
|
16
|
Y SunR ShuCL LiMZ ZhangGram-negative
periodontal bacteria induce the activation of Toll-like receptors 2
and 4, and cytokine production in human periodontal ligament cellsJ
Periodontol8114881496201010.1902/jop.2010.10000420528699
|
|
17
|
Y SunR ShuMZ ZhangAP WuToll-like receptor
4 signaling plays a role in triggering periodontal infectionFEMS
Immunol Med
Microbiol52362369200810.1111/j.1574-695X.2008.00386.x18328075
|
|
18
|
S FujiiH MaedaN WadaY KanoA
AkamineEstablishing and characterizing human periodontal ligament
fibroblasts immortalized by SV40T-antigen and hTERT gene
transferCell Tissue
Res324117125200610.1007/s00441-005-0101-416408200
|
|
19
|
KS KobayashiM ChamaillardY OguraO
HenegariuN InoharaG NuñezRA FlavellNod2-dependent regulation of
innate and adaptive immunity in the intestinal
tractScience307731734200510.1126/science.110491115692051
|
|
20
|
JH ParkYG KimC McDonaldTD KannegantiM
HasegawaM Body-MalapelN InoharaG NúñezRICK/RIP2 mediates innate
immune responses induced through Nod1 and Nod2 but not TLRsJ
Immunol17823802386200710.4049/jimmunol.178.4.238017277144
|
|
21
|
JH ParkYG KimM ShawTD KannegantiY
FujimotoK FukaseN InoharaG NúñezNod1/RICK and TLR signaling
regulate chemokine and antimicrobial innate immune responses in
mesothelial cellsJ
Immunol179514521200710.4049/jimmunol.179.1.51417579072
|
|
22
|
H TadaS AibaKI ShibataT OhtekiH
TakadaSynergistic effect of Nod1 and Nod2 agonists with toll-like
receptor agonists on human dendritic cells to generate
interleukin-12 and T helper type 1 cellsInfect
Immun7379677976200510.1128/IAI.73.12.7967-7976.200516299289
|
|
23
|
K HiraoH YumotoK TakahashiK MukaiT
NakanishiT MatsuoRoles of TLR2, TLR4, NOD2, and NOD1 in pulp
fibroblastsJ Dent
Res88762767200910.1177/002203450934177919734466
|
|
24
|
Y SugawaraA UeharaY FujimotoS KusumotoK
FukaseK ShibataS SugawaraT SasanoH TakadaToll-like receptors, NOD1,
and NOD2 in oral epithelial cellsJ Dent
Res85524529200610.1177/15440591060850060916723649
|
|
25
|
A UeharaH TakadaFunctional TLRs and NODs
in human gingival fibroblastsJ Dent
Res86249254200710.1177/15440591070860031017314257
|
|
26
|
L TangXD ZhouQ WangY WangXY LiDM
HuangExpression of TRAF6 and pro-inflammatory cytokines through
activation of TLR2, TLR4, NOD1, and NOD2 in human periodontal
ligament fibroblastsArch Oral
Biol5610641072201110.1016/j.archoralbio.2011.02.02021457942
|
|
27
|
MJ ScottC ChenQ SunTR BilliarHepatocytes
express functional NOD1 and NOD2 receptors: a role for NOD1 in
hepatocyte CC and CXC chemokine productionJ
Hepatol53693701201010.1016/j.jhep.2010.04.02620615568
|
|
28
|
AA ShigeokaA KamboJC MathisonAJ KingWF
HallJ da Silva CorreiaRJ UlevitchDB McKayNod1 and nod2 are
expressed in human and murine renal tubular epithelial cells and
participate in renal ischemia reperfusion injuryJ
Immunol18422972304201010.4049/jimmunol.090306520124104
|
|
29
|
Y SugawaraA UeharaY FujimotoS KusumotoK
FukaseK ShibataS SugawaraT SasanoH TakadaToll-like receptors, NOD1,
and NOD2 in oral epithelial cellsJ Dent
Res85524529200610.1177/15440591060850060916723649
|
|
30
|
B BegueC DumantJC BambouJF BeaulieuM
ChamaillardJP HugotO GouletJ SchmitzDJ PhilpottN
Cerf-BensussanMicrobial induction of CARD15 expression in
intestinal epithelial cells via toll-like receptor 5 triggers an
antibacterial response loopJ Cell
Physiol209241252200610.1002/jcp.2073916897777
|
|
31
|
M KobayashiR YoshikiJ SakabeK KabashimaM
NakamuraY TokuraExpression of Toll-like receptor 2, NOD2 and
dectin-1 and stimulatory effects of their ligands and histamine in
normal human keratinocytesBr J
Dermatol160297304200910.1111/j.1365-2133.2008.08897.x19016710
|
|
32
|
DA van HeelS GhoshM ButlerK HuntBM
FoxwellD Mengin-LecreulxRJ PlayfordSynergistic enhancement of
Toll-like receptor responses by NOD1 activationEur J
Immunol3524712476200516021603
|
|
33
|
A UeharaH TakadaSynergism between TLRs and
NOD1/2 in oral epithelial cellsJ Dent
Res87682686200810.1177/15440591080870070918573991
|
|
34
|
S TotemeyerM SheppardA LloydD RoperC
DowsonD UnderhillP MurrayD MaskellC BryantIFN-γ enhances production
of nitric oxide from macrophages via a mechanism that depends on
nucleotide oligomerization domain-2J Immunol176480448102006
|
|
35
|
T HisamatsuM SuzukiDK PodolskyInterferon-γ
augments CARD4/NOD1 gene and protein expression through interferon
regulatory factor-1 in intestinal epithelial cellsJ Biol
Chem27832962329682003
|
|
36
|
HS KimTH ShinSR YangMS SeoDJ KimSK KangJH
ParkKS KangImplication of NOD1 and NOD2 for the differentiation of
multipotent mesenchymal stem cells derived from human umbilical
cord bloodPLoS
One5e15369201010.1371/journal.pone.001536921042538
|
|
37
|
T PettersonJ JendholmA ManssonA BjartellK
RiesbeckLO CardellEffects of NOD-like receptors in human B
lymphocytes and crosstalk between NOD1/NOD2 and Toll-like
receptorsJ Leukoc Biol89177187201110.1189/jlb.021006120844241
|
|
38
|
SM CruickshankL WakenshawJ CardonePD
HowdlePJ MurraySR CardingEvidence for the involvement of NOD2 in
regulating colonic epithelial cell growth and survivalWorld J
Gastroenterol1458345841200810.3748/wjg.14.583418855982
|