Introduction
Acute myocardial infarction (AMI) remains a major
cause of chronic heart failure (HF) due to a loss of myocardial
tissue. Although AMI has been postulated to lead to an
irrecoverable loss of cardiomyocytes, bone marrow-derived cells
(BMCs) might be able to differentiate into cardiomyocytes after
AMI. Several experimental studies have confirmed this
cardiomyocytogenic capability of BMCs (1–3),
whereas others have excluded the differentiation of BMCs in
cardiomyocytes (4–8). Clinical trials have demonstrated
beneficial effects of BMC treatment (9–11)
after AMI with improved functional parameters (9–11)
or a complete absence of effects (12). A recent meta-analysis brought the
therapeutic impact of BMCs into question (13). Given these controversial
experimental and clinical results, the differential potential of
BMCs has been critically challenged and the (patho-)physiological
relevance of BMCs in the repair of myocardial damage remains
unclear. In order to gain a better understanding of the role of
BMCs during pathophysiological processes, improving our knowledge
of the physiological role of BMCs in the myocardium is
indispensible. Thus, we examined the role of BMCs in the
physiological aging processes of the heart. For this purpose, we
used a mouse model in which the original bone marrow was replaced
by an enhanced green fluorescent protein (eGFP)-marked stem cell
pool. These labeled cells offer a possibility of clearly
identifying the fate and behavior of potentially differentiated BMC
offspring.
Materials and methods
Bone marrow transplantation and
transgenic mice
Bone marrow transplantation (BMTx) was performed
according to a previously described protocol (6). Briefly, C57BL/6-TgN(ACTbEGFP)1Osb
transgenic mice (Jackson Laboratory, Bar Harbor, ME, USA) served as
bone marrow donors. In this transgenic line, all cells, with the
exception of erythrocytes and hair follicle cells, express eGFP and
appear green in the presence of excitation wavelengths (14). A total of 36 mice were
transplanted. The success of BMTx was monitored by flow cytometric
analysis (FACSCalibur, BD Biosciences, Germany).
In 18 mice, hearts were excised at the age of 4
months to serve as the young control group (group A). To
investigate the aging myocardium, 18 mice were euthanized at the
age of 18 months (group B).
All investigations conformed to the Guide for the
Care and Use of Laboratory Animals published by the US National
Institutes of Health (NIH publication no. 85-23, revised 1996) and
were approved by the appropriate authorities (Regierungspräsidium
Darmstadt, Hessen, Germany).
Perfusion fixation and tissue
sampling
The mice were euthanized by cervical dislocation,
the thoracic aorta was cannulated, and the hearts were retrograde
gravity-perfused at a mean pressure of 100 mmHg with PBS buffer
containing 0.1% adenosine (Fluka, Germany) and 0.05% bovine serum
albumin (Sigma, Germany) for 3 min, followed by fixative (3%
buffered paraformaldehyde solution) for 4 min. Afterwards, the
hearts were quickly excised and the tissue cryopreserved in
Tissue-Tek OCT Compound (Sakura, Japan) at −80°C until
sectioning.
Histological analysis
Serial cryosections of the heart were obtained and
20 representative slices of the whole myocardium were analyzed.
Immunostaining was performed on 6-μm cryosections. To assess
the incorporation of BM-derived cells into the myocardium, the
number of eGFP+ cells in all cryosections was
determined. Sections of spleen served as positive controls. An
anti-eGFP antibody (Abcam, USA) was used to exclude autofluorescent
effects. Staining procedures and picture acquisition were performed
as previously described (15).
All sections were incubated for 2 h at room temperature. Incubation
with the first antibody was followed by treatment with biotinylated
secondary antibody when indirectly labeled antibodies were used.
The directly labeled antibodies were conjugated to Cy3. The last
incubation was carried out with streptavidin-Cy2 (Rockland
Immunochemicals, Inc., USA). Nuclei were stained with DRAQ-5
(Alexis, USA). Omission of the primary antibody served as a
negative control. Pictures were captured with a Leica TCS SP laser
scanning confocal microscope (Leica, Germany) equipped with
appropriate filter blocks using a Silicon Graphics Octane
workstation (Silicon Graphics, USA) and three-dimensional
multichannel image processing software (Bitplane, Germany).
Flow cytometric analysis
The efficacy of BMTx was determined by
fluorescence-based flow cytometry (FCM) of eGFP expression in
peripheral blood leukocyte subpopulations. Briefly, aliquots of
peripheral blood were stained with a panel of APC-conjugated
monoclonal antibodies against CD3, CD4, CD8, CD11b, CD19, and
F4/80. Following erythrocyte lysis and washing steps, acquisition
was performed on a BD FACS Calibur flow cytometer (BD Biosciences,
Germany). Data were analyzed using the CellQuest Software (BD
Biosciences).
For tissue analysis, hearts were minced and digested
in ADS buffer (0.11 M NaCl, 5 mM KCl, 5 mM dextrose, 0.8 mM
MgSO4, 12.5 mM NaH2PO4, 20 mM
HEPES) containing 1 mg/ml collagenase IV and 0.5 mg/ml
hyaluronidase. The resulting cell suspension was filtered through a
70 μm cell strainer (BD Biosciences) and washed twice in
PBS/2% fetal calf serum prior to FCM (EPICS Altra, Beckman
Coulter).
Results
Bone marrow transplantation
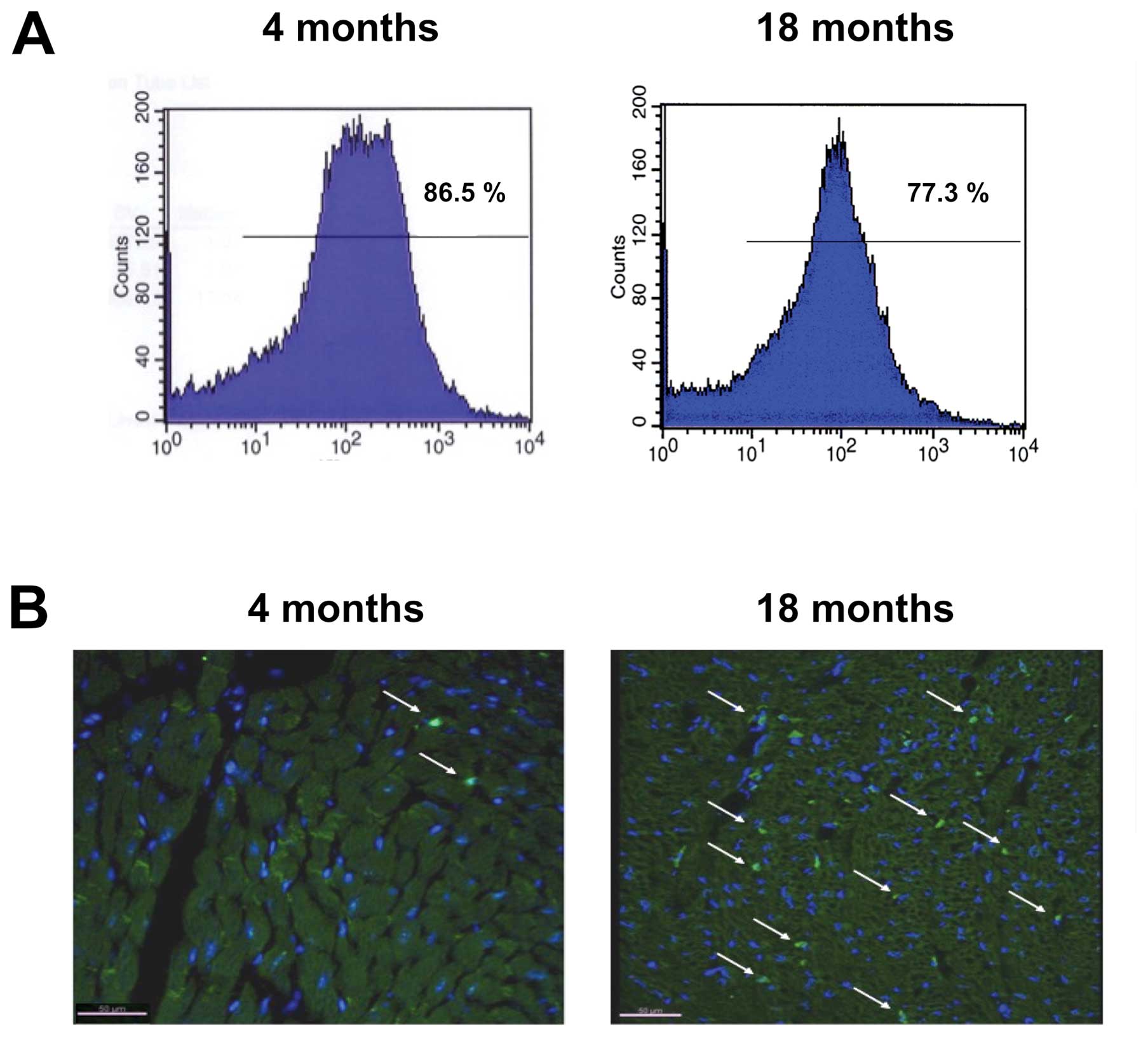
The efficacy of BMTx was assessed by FACS analysis
of the peripheral blood at different time points after
transplantation. Fluorescence intensity showed that 86.5±5.3% of
all nucleated cells in group A and 77.3±4.9% in group B expressed
eGFP after BMTx, indicating successful replacement of the original
stem-cell population (Fig. 1A).
In addition, the proportional leukocyte subpopulations were
compared between transplanted and non-transplanted mice using flow
cytometry. No significant differences were found between groups,
indicating that the white blood cell counts were within the
physiological range at the time of surgery (data not shown).
Quantification and phenotype of
eGFP+ cells
Four weeks after BMTx, group A mice were euthanized.
Immunohistochemical staining of the myocardium revealed only a
small number of eGFP+ cells, which were unexceptional
leukocytes. In total, <1 eGFP+ cells/mm2
was observed in myocardium samples from group A. In contrast,
9.4±2.8 eGFP+ cells/mm2 were counted in group
B samples (Fig. 1B). For
additional quantification, hearts were digested and isolated cells
analyzed for eGFP by flow cytometry. This quantification documented
0.25±0.03% eGFP+ cells in group A and 4.2±1.2% in group
B.
In order to investigate the differentiation of
BM-derived cells within the myocardium, cryosections were
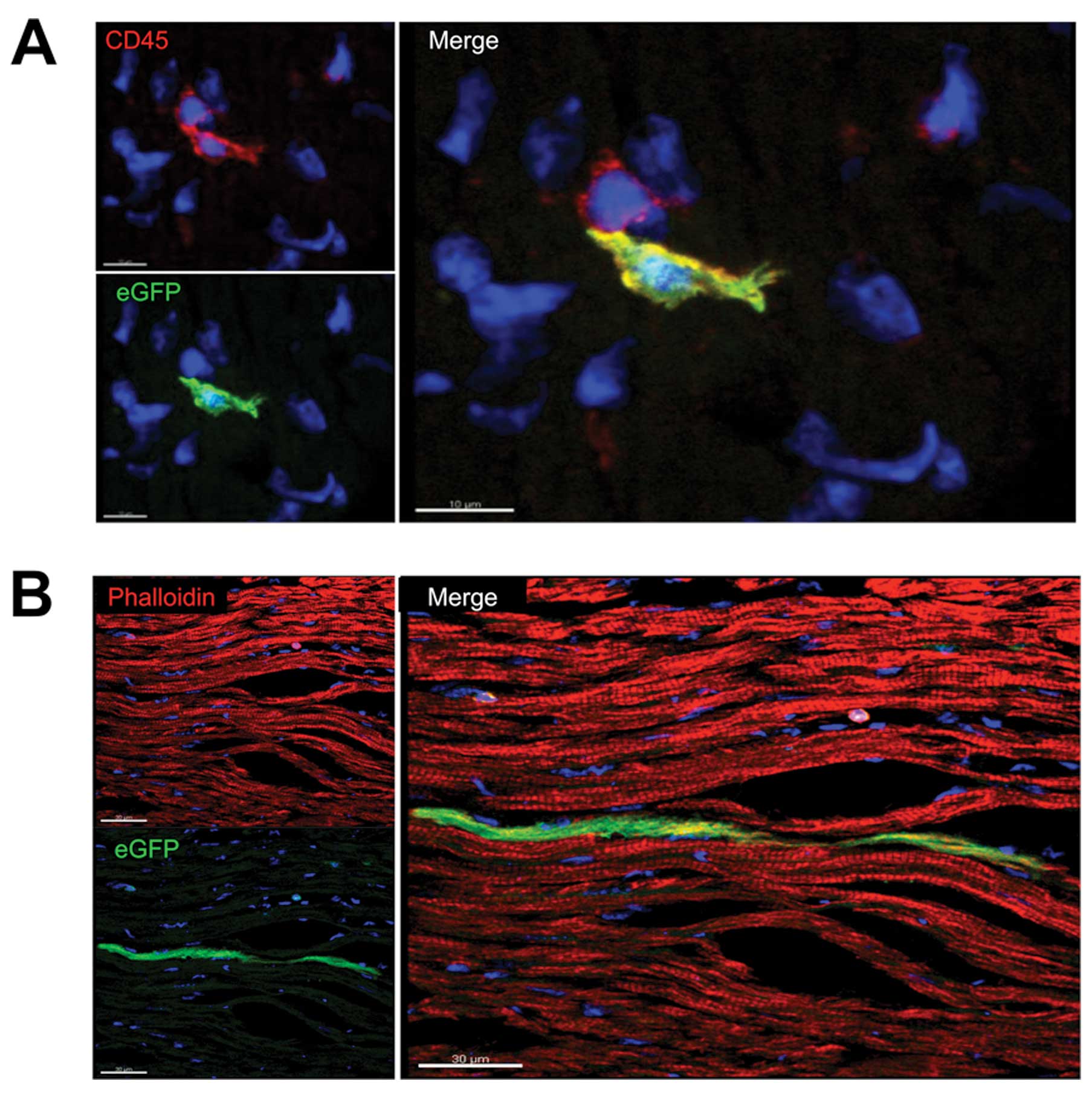
co-stained with cell type-specific markers. Most of the leukocytes
were eGFP+ as demonstrated by positive
immunohistochemical staining with the pan-leukocyte marker CD45
(Fig. 2A). In contrast to group
A, a considerable number of eGFP+ cells did not
positively stain for CD45 in group B, revealing
transdifferentiation into a non-inflammatory cell type.
Cardiomyocytes were characterized by anti-titin
staining. We examined 10 hearts per group, detecting only five
cardiomyocytes that were eGFP+ (group A n=3, group B
n=2; Fig. 2B). Immunohistochemicl
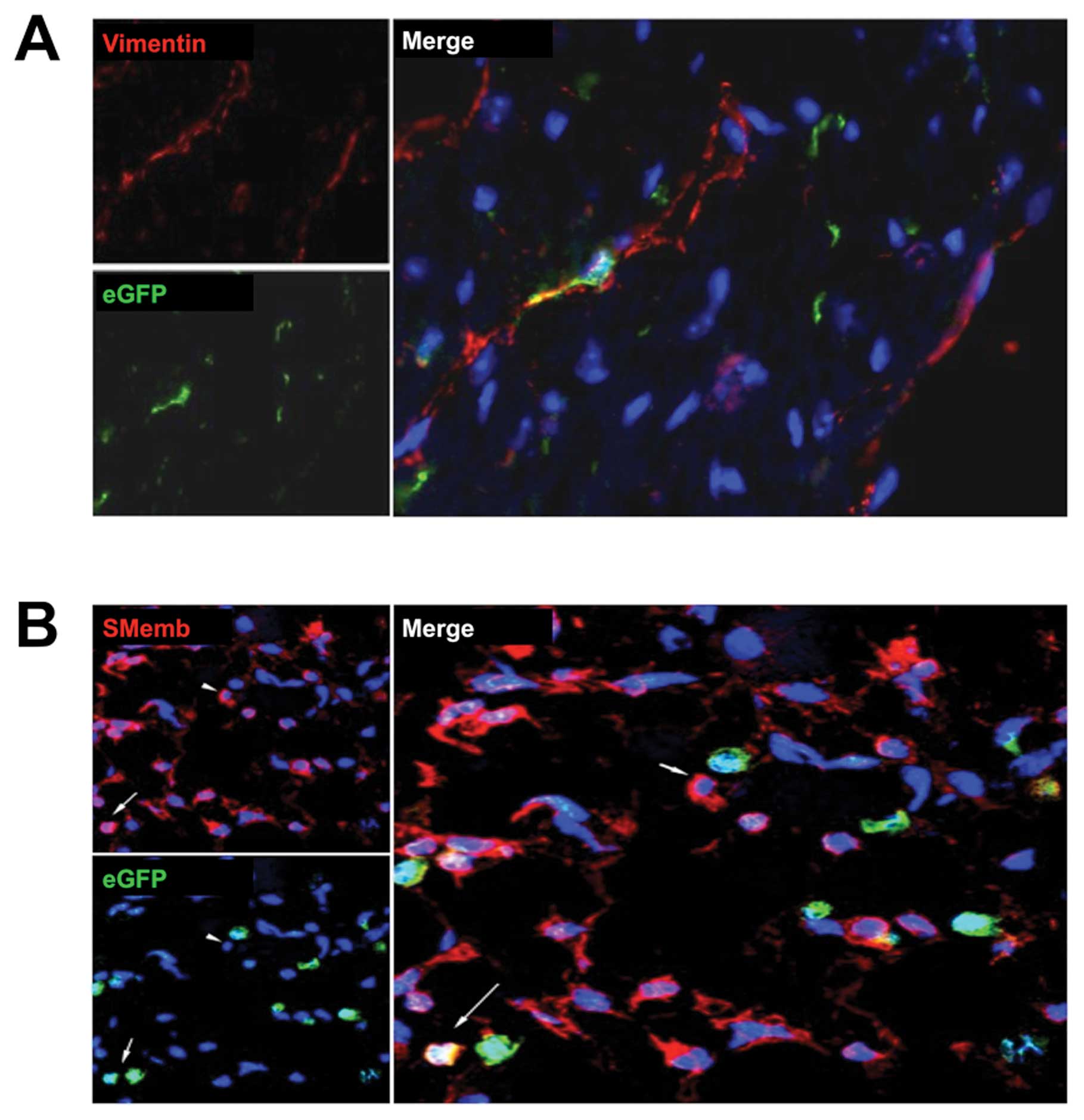
staining for vimentin showed that some cells co-expressed this
fibroblast marker with eGFP, indicating a BM-derived origin in
group B (Fig. 3A). To detect
myofibroblasts, we used the specific marker SMemb. A considerable
number of myofibroblasts were eGFP+, mainly in group B
(Fig. 3B). The occasional
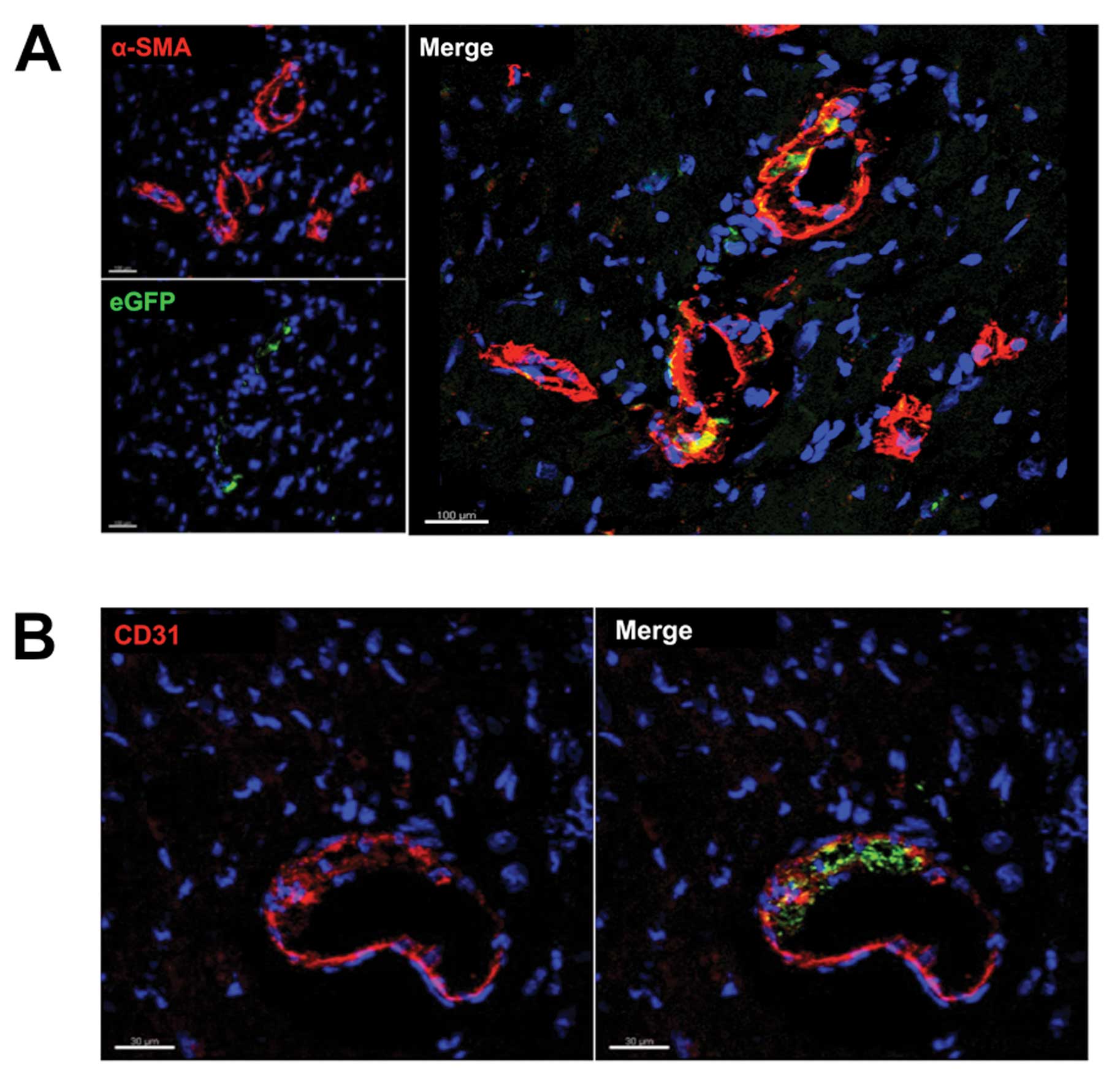
eGFP+ vascular smooth muscle cells were found primarily
in the walls of smaller vessels (Fig.
4A). We also detected bone marrow-derived endothelial cells,
which were represented by positive co-staining for eGFP and the
endothelial cell marker CD31. These cells were also found primarily
in small vessels and capillaries (Fig. 4B). However, the vast majority of
CD31+ cells were negative for eGFP.
Discussion
The adult mammalian heart has long been considered a
terminally differentiated, postmitotic organ. However, this dogma
has been challenged as the role of BMCs in cardiac repair has been
extensively investigated, driven by the goal of developing novel
therapies aimed at regenerating the damaged myocardium. The bone
marrow comprises a wide variety of stem cells, which are able not
only to generate blood cells, but to differentiate into other cell
types, including liver cells, neurons, skeletal muscle and
endothelial cells (16). During
physiological cardiac aging, the heart undergoes several structural
and morphological changes, including a substantial increase in
interstitial and perivascular fibrosis (17), a progressive loss of
cardiomyocytes due to necrosis and apoptosis, hypertrophy of the
remaining cardiomyocytes and an increase in the number of cardiac
fibroblasts (18). These changes
can be designated as ‘age-associated cardiomyopathy’ (19).
In this study, we demonstrated that during the
lifespan of mice, 4% of cells within the myocardium are recruited
from the bone marrow. These BMCs differentiated into
tissue-resident leukocytes or transdifferentiated into fibroblasts
and myofibroblasts. Differentiation into smooth muscle cells and
endothelial cells was rarely observed. In addition, only a
negligibly small number of eGFP+ cardiomyocytes was
detected, indicating that BMC differentiation into these cell types
does not contribute to the regenerative processes of the myocardium
during aging. These findings are in agreement with data published
by Daniel et al (20), who
demonstrated that the differentiation of BMCs into smooth muscle
cell or endothelial cell lineages is an extremely rare event. The
failure of BMCs to transdifferentiate into cardiomyocytes has been
clearly shown in our previous work (6) and by that of several other authors
(4,5,8).
In contrast, we found a remarkable number of
fibroblasts and myofibroblasts of BMC origin. An increased number
of cardiac fibroblasts and myofibroblasts in the aging heart has
been described (18,21), though several authors found a
blunted capacity of fibroblast proliferation during physiological
aging (22,23). Given this discrepancy, the origin
of these fibroblasts has remained controversial; the traditional
view is that activated myofibroblasts are derived from resident
fibroblasts through proliferation and activation. However, tracking
the proliferating cell populations localized proliferating
fibroblast-like cells in the surrounding blood vessels, indicating
that these fibroblasts may be recruited by circulating progenitor
cells (24,25). This suggestion is in accordance
with data demonstrating BMC differentiation in different
cardiovascular pathologies (6,26,27). However, the role of BM-derived
progenitor cells in the aging heart was unclear. For the first
time, our study demonstrates substantial recruitment of BMCs during
physiological cardiac aging and relevant differentiation of BMCs
into fibroblasts and myofibroblasts. The increased number of
BM-derived fibroblasts and myofibroblasts found in our setting may
be due to the aging heart having a reduced capacity for fibroblast
and myofibroblast formation (23,28). In this context, the increased
homing and transdifferentiation of BMCs can be considered a
compensatory mechanism for the progressive loss of different cell
types in the aging heart.
This awareness might be therapeutically relevant
because the age-related increase in post-AMI mortality is at least
partially caused by an impaired response of senescent fibroblasts
to fibrogenic mediators, resulting in unfavorable scar tissue
formation and subsequently disturbed myocardial performance
(29–31). Therefore, therapeutic approaches
that increase the homing and differentiation of BMCs may enhance
the reparative potential of the aged heart after myocardial
damage.
The design of the present study is descriptive and
data were obtained from a rather small sample size. The
considerably small percentage of eGFP+ cells made
quantification of the different cell types and statistical analysis
impossible. Further experimental studies are required to confirm
the hypothesis that BMCs contribute to cell turnover in the heart
during physiological cardiac aging. In addition, the data were
acquired in mice. Due to obvious different physiological properties
(lifespan, cell turnover), the observations made in our study might
not fully translate to human physiology. Nevertheless, our data
provide new insights into the physiological impact of BMCs.
In conclusion, our study demonstrates that BMCs
trans-differentiate into fibroblasts and myofibroblasts in the
aging murine myocardium, suggesting their contribution to the
preservation of myocardial structural integrity while they do not
account for the regenerative processes of the heart.
References
|
1.
|
D OrlicJ KajsturaS ChimentiDM BodineA
LeriP AnversaBone marrow stem cells regenerate infarcted
myocardiumPediatr Transplant7Suppl
3S86S88200310.1034/j.1399-3046.7.s3.13.x
|
|
2.
|
KA JacksonSM MajkaH WangRegeneration of
ischemic cardiac muscle and vascular endothelium by adult stem
cellsJ Clin Invest10713951402200110.1172/JCI1215011390421
|
|
3.
|
D OrlicJ KajsturaS ChimentiMobilized bone
marrow cells repair the infarcted heart, improving function and
survivalProc Natl Acad Sci
USA981034410349200110.1073/pnas.18117789811504914
|
|
4.
|
LB BalsamAJ WagersJL ChristensenT
KofidisIL WeissmanRC RobbinsHaematopoietic stem cells adopt mature
haematopoietic fates in ischaemic
myocardiumNature428668673200410.1038/nature0246015034594
|
|
5.
|
CE MurryMH SoonpaaH ReineckeHaematopoietic
stem cells do not transdifferentiate into cardiac myocytes in
myocardial
infarctsNature428664668200410.1038/nature0244615034593
|
|
6.
|
H MollmannHM NefS KostinBone
marrow-derived cells contribute to infarct remodellingCardiovasc
Res71661671200610.1016/j.cardiores.2006.06.01316854401
|
|
7.
|
JM NygrenS JovingeM BreitbachBone
marrow-derived hematopoietic cells generate cardiomyocytes at a low
frequency through cell fusion, but not transdifferentiationNat
Med10494501200410.1038/nm104015107841
|
|
8.
|
KI OdorferI WalterM KleiterEP SandgrenRG
ErbenRole of endogenous bone marrow cells in long-term repair
mechanisms after myocardial infarctionJ Cell Mol
Med1228672874200810.1111/j.1582-4934.2008.00511.x19210759
|
|
9.
|
F KuetheHR FigullaM HerzauTreatment with
granulocyte colony-stimulating factor for mobilization of bone
marrow cells in patients with acute myocardial infarctionAm Heart
J150115200510.1016/j.ahj.2005.04.03016086558
|
|
10.
|
B AssmusV SchachingerC
TeupeTransplantation of Progenitor Cells and Regeneration
Enhancement in Acute Myocardial Infarction
(TOPCARE-AMI)Circulation10630093017200210.1161/01.CIR.0000043246.74879.CD
|
|
11.
|
V SchachingerS ErbsA ElsasserIntracoronary
bone marrow-derived progenitor cells in acute myocardial
infarctionN Engl J
Med35512101221200610.1056/NEJMoa06018616990384
|
|
12.
|
A SchaeferGP MeyerM FuchsImpact of
intracoronary bone marrow cell transfer on diastolic function in
patients after acute myocardial infarction: results from the BOOST
trialEur Heart J27929935200610.1093/eurheartj/ehi81716510465
|
|
13.
|
E Martin-RendonSJ BrunskillCJ HydeSJ
StanworthA MathurSM WattAutologous bone marrow stem cells to treat
acute myocardial infarction: a systematic reviewEur Heart
J2918071818200810.1093/eurheartj/ehn22018523058
|
|
14.
|
M OkabeM IkawaK KominamiT NakanishiY
Nishimune‘Green mice’ as a source of ubiquitous green cellsFEBS
Lett4073133191997
|
|
15.
|
S SzardienHM NefS VossRegression of
cardiac hypertrophy by granulocyte colony-stimulating
factor-stimulated interleukin1β synthesisEur Heart
J33595605201222106340
|
|
16.
|
F TogelC WestenfelderAdult bone
marrow-derived stem cells for organ regeneration and repairDev
Dyn23633213331200710.1002/dvdy.2125817685479
|
|
17.
|
CR Gazoti DebessaLB Mesiano MaifrinoR
Rodrigues de SouzaAge related changes of the collagen network of
the human heartMech Ageing Dev12210491058200111389923
|
|
18.
|
P AnversaT PalackalEH SonnenblickG
OlivettiLG MeggsJM CapassoMyocyte cell loss and myocyte cellular
hyperplasia in the hypertrophied aging rat heartCirc
Res67871885199010.1161/01.RES.67.4.8712145091
|
|
19.
|
PM TreutingNJ LinfordSE KnoblaughReduction
of age-associated pathology in old mice by overexpression of
catalase in mitochondriaJ Gerontol A Biol Sci Med
Sci63813822200810.1093/gerona/63.8.81318772469
|
|
20.
|
JM DanielW BielenbergP StiegerS WeinertH
TillmannsDG SeddingTime-course analysis on the differentiation of
bone marrow-derived progenitor cells into smooth muscle cells
during neointima formationArterioscler Thromb Vasc
Biol3018901896201010.1161/ATVBAHA.110.20969220576944
|
|
21.
|
G OlivettiM MelissariJM CapassoP
AnversaCardiomyopathy of the aging human heart. Myocyte loss and
reactive cellular hypertrophyCirc
Res6815601568199110.1161/01.RES.68.6.15602036710
|
|
22.
|
ML LindseyDK GoshornCE
SquiresAge-dependent changes in myocardial matrix
metalloproteinase/tissue inhibitor of metalloproteinase profiles
and fibroblast functionCardiovasc
Res66410419200510.1016/j.cardiores.2004.11.02915820210
|
|
23.
|
FI WolfA TorselloV CovacciOxidative DNA
damage as a marker of aging in WI-38 human fibroblastsExp
Gerontol37647656200210.1016/S0531-5565(02)00005-011909682
|
|
24.
|
A LjungqvistG UngeThe proliferative
activity of the myocardial tissue in various forms of experimental
cardiac hypertrophyActa Pathol Microbiol Scand
A8123324019734767222
|
|
25.
|
E MandacheG UngeLE AppelgrenA
LjungqvistThe proliferative activity of the heart tissues in
various forms of experimental cardiac hypertrophy studied by
electron microscope autoradiographyVirchows Arch B Cell
Pathol121121221973
|
|
26.
|
MJ van AmerongenG Bou-GhariosE PopaBone
marrow-derived myofibroblasts contribute functionally to scar
formation after myocardial infarctionJ
Pathol214377386200818095257
|
|
27.
|
G KaniaP BlyszczukS
SteinHeart-infiltrating prominin-1+/CD133+
progenitor cells represent the cellular source of transforming
growth factor beta-mediated cardiac fibrosis in experimental
autoimmune myocarditisCirc Res105462470200919628793
|
|
28.
|
KA CieslikJ TrialML EntmanDefective
myofibroblast formation from mesenchymal stem cells in the aging
murine heart rescue by activation of the AMPK PathwayAm J
Pathol17917921806201110.1016/j.ajpath.2011.06.02221819956
|
|
29.
|
M BujakHJ KweonK ChatilaN LiG TaffetNG
FrangogiannisAging-related defects are associated with adverse
cardiac remodeling in a mouse model of reperfused myocardial
infarctionJ Am Coll
Cardiol5113841392200810.1016/j.jacc.2008.01.01118387441
|
|
30.
|
K ShivakumarDE DostalK BohelerKM BakerEG
LakattaDifferential response of cardiac fibroblasts from young
adult and senescent rats to ANG IIAm J Physiol Heart Circ
Physiol284H1454H1459200310.1152/ajpheart.00766.200212595286
|
|
31.
|
G ErtlS FrantzHealing after myocardial
infarctionCardiovasc
Res662232200510.1016/j.cardiores.2005.01.011
|