Introduction
Aldose reductase (AR), a member of the aldo-keto
reductase (AKR) superfamily, is responsible for conversion of
glucose to sorbitol of the polyol pathway in glucose metabolism
(1). AR is a cytosolic enzyme and
is widely distributed in various tissues and organs such as eye
lens, retina, kidney, and reproductive organs. During the past
several decades the studies of AR focus on the pathology of long
term diabetic complications, including nephropathy, as it plays a
pivotal role in the glucose metabolism under hyperglycemia
(2–4). However, recent studies indicate that
AR functions as not only a metabolizing glucose enzyme but also an
enzyme catalyzing the reduction of a wide array of substances
including various endogenous and exogenous aldehydes and their
glutathione (GSH)-conjugates, phospholipids and steroids (5–7).
In addition, it has been shown that AR is associated with cardiac
disorders, inflammation, mood disorders and cancers (8–10).
Our previous studies also demonstrated that AR was one of the
responsive genes for transforming growth factor-β1 (TGF-β1) in
cultured mesangial cells. AR has also been confirmed to play a role
in extracellular matrix (ECM) deposition and MsC proliferation
mediated by TGF-β1 which is a crucial growth factor in
glomerulonephritis and glomerulosclerosis, and other growth
factors, including platelet-derived growth factor (PDGF) (11).
Abnormal proliferation of MsCs contributes to the
pathogenesis of renal fibrosis and glomerulosclerosis (12). PDGF is generally approved to be a
major mitogen for MsCs and has a high transcription level during
both experimental and human glomerulonephritis. Besides, PDGF
receptor (PDGFR) mRNA and protein expression are upregulated.
Several study reports showed the involvement of PDGF and PDGFR in
the proliferation and migration of MsCs (13). Activation of the intrinsic
tyrosine kinase activity of PDGFR facilitates recruitment of
several SH2 domain-containing molecules and associated proteins
including the p85 subunit of phosphoinositide 3-kinase (PI3K),
RasGAP and PLCγ1. In glomerulonephritis, MsCs are activated,
followed by increased production and release of PDGF into the
extracellular space, which activates proliferation of MsCs again as
a feedback loop (14,15). Specific antagonism of PDGF
suppresses MsC proliferation in vitro, and in experimental
glomerulosclerosis (16). Since
the effects of ARI on the inhibition of MsC proliferation induced
by PDGF have been shown, it is possible that AR could be a
potential target for alleviating MsC proliferation, and then ECM
deposition and glomerulosclerosis.
Although PDGF has a critical role in regulating cell
proliferation in several cell types, the related signaling pathways
vary among different cells (17).
It is known that PDGF binds to a tyrosine kinase receptor which
activates a number of downstream pathways, including the
mitogen-activated protein kinase (MAPK) family members, PI3K/Akt
and many other kinases in MsC (18,19). ARI was recently reported to reduce
the phosphorylation of MAPK1/2 in metaplasia of airway epithelial
cells and PI3K/Akt in vascular smooth muscle cells (VSMCs)
(20,21). However, the PI3K/Akt signaling
pathway is one of the most common downstream pathways of PDGF in
regulating cell proliferation. Overexpression of dominant negative
Akt resulted in complete inhibition of PDGF-induced DNA synthesis
in MsC. On the other side, inhibition of MAPK only partially
attenuated DNA synthesis (22).
In our current study, we confirmed the involvement of the PI3K/Akt
pathway in modulating AR-induced inhibition of MsC
proliferation.
The mammalian cell cycle is a tightly controlled
nuclear event positively regulated by cyclin-dependent kinases
(CDKs) and their cyclin-regulatory subunits, and negatively by
cyclin-dependent kinase inhibitors (CKIs). Among the CKIs both
p21Cip1 and p27Kip1 contain binding domains
for CDK which may intercept the ability of CDK to form active
complexes with cyclins, leading to interference with the
proliferation of MsC (23,24).
In this study, we investigated the mechanism by
which ARI inhibits PDGF-induced MsC proliferation. We demonstrated
that ARI was coupled to attenuation of PI3K/Akt path way activity
in response to PDGF. In addition, our results indicate that ARI
arrested PDGF-induced MsC proliferation in the G1 phase through
mediating the levels of p21Cip1 and
p27Kip1.
Materials and methods
Cell culture and reagents
Rat MsCs were obtained by culturing glomeruli
isolated from the kidneys of 200–250 g Sprague-Dawley rats by
conventional sieving methods as previously described (25). The cells were cultured in
RPMI-1640 medium containing 10% FBS (Gibco-BRL), 100 U/ml
penicillin, 100 μg/ml streptomycin, and 0.3 g/ml sodium pyruvate at
37°C in an atmosphere containing 5% CO2. All experiments
were performed using cells between passages 4 and 10. When the
cells reached 60–70% confluence, the medium was changed to fresh
serum-free RPMI-1640 containing 10 μM zopolrestat. After 24 h, the
cells were stimulated with 20 ng/ml PDGF-BB for the further
investigation. The RPMI-1640 medium was purchased from Invitrogen
(San Diego, CA, USA). The ARI zopolrestat was a gift from Pfizer.
PDGF-BB was purchased from Sigma Chemical (St. Louis, MO, USA). The
wild (HA-Akt) and dominant-negative (HA-Akt-K179A) were generously
provided by Dr Boudewijn Burgering (University Medical Center,
Utrecht, Netherlands).
MTT assay
The MTT reduction assay was used as a qualitative
index of cell viability. After incubation with different compounds
as described above, 20 μl MTT (5 mg/ml) (Invitrogen Corp.,
Carlsbad, CA, USA) was added and cells were cultured for an
additional 4 h. Subsequently, cells were lysed using
dimethylsulfoxide (150 μl/well) (Pierce Biotechnology, Inc.,
Rockford, IL, USA). When the formanzan crystals were completely
dissolved, the optical density (OD) was measured at 490 nm using an
ELx800 multiwell plate reader (Bio-Tek Instruments, Winooski, VT,
USA).
DNA synthesis assay
A colorimetric immunoassay kit Cell proliferation
ELISA, BrdU (colorimetric) (Boehringer Mannheim GmbH, Mannheim,
Germany) was used for quantification of cell proliferation. This
assay is based on the measurement of BrdU incorporation during DNA
synthesis. Briefly, the cells were seeded in 96-well plates;
pre-incubated for 24 h with zopolrestat (10 μM), and then stimulate
with PDGF for another 24 h. They were then labeled with BrdU for 3
h at 37°C, washed, and fixed and stained with anti-BrdU antibody
for 90 min at 37°C. After three washes, the substrate,
tetramethylbenzidine, was added, followed by incubation for 30 min.
A blocking solution (1 M H2SO4) was then
added, and the absorbance of the samples was measured at 450 nm
with a reference wavelength of 690 nm using an ELx800 multiwell
plate reader (Bio-Tek Instruments).
Flow cytometry
Cell cycle analysis was performed using flow
cytometry. After 24 h of treatment with different compounds, cells
were washed twice with phosphate-buffered saline (PBS) and
resuspended in 70% ethanol for 1 h at 4°C. Fixed cells were
collected by centrifugation, treated with RNase (25 μg/ml) at 37°C
for 30 min and stained with propidium iodide (50 μg/ml) at 4°C for
30 min in the dark. The number of cells in the G1, S and G2/M
phases was analyzed by flow cytometry using a FACSCalibur Flow
Cytometer (Becton-Dickinson, San Jose, CA, USA).
Western blot analyses
Cells were cultured and treated as described above,
grown to 60–70% confluence, replaced with serum-free medium for 24
h and then subjected to PDGF (20 ng/ml) in the presence or absence
of zopolrestat (10 μM). Cell lysis was performed on ice with fresh
lysis buffer [1 M Tris (pH 8.0), 2 M NaCl, 10% NaN3, 10%
SDS, 10% NP-40, and 1% sodium deoxycholate]. All lysates were
centrifuged at 15,000 × g for 10 min at 4°C followed by
bicinchoninic acid assay (BCA assay; Pierce Biotechnology, Inc.) to
determine protein concentrations. Protein (40 μg) was loaded onto 8
or 10% sodium dodecyl sulfate polyacrylamide gel (SDS-PAGE) and
electrophoretically transferred to polyvinylidene fluoride (PVDF).
Membranes were blocked for a minimum of 1 h at room temperature in
5% bovine serum albumin (BSA) in Tris-buffered saline with 1 ml
Tween-20 per liter. The membranes were incubated overnight at 4°C
with primary antibodies for p21 (1:500), p27 (1:1,000; all from
Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA), mouse
anti-β-actin (1:1,000; Sigma Chemical), phospho-Akt (1:1,000), Akt
(1:1,000) (all from Cell Signaling Technology), PDGF β-receptor
subunit (1:1,000; Santa Cruz Biotechnology, Inc.). After being
incubated with the respective secondary antibody, immune complexes
were detected with ECL Plus (Amersham Biosciences, Piscataway, NJ,
USA) on Kodak X-ray film.
Statistical analysis
All the experiments were repeated at least three
times independently. Differences were assessed using ANOVA. All
values are expressed as mean ± SD, and statistical significance was
defined at P<0.05.
Results
Effect of zopolrestat on cell
proliferation induced by PDGF in rat MsC
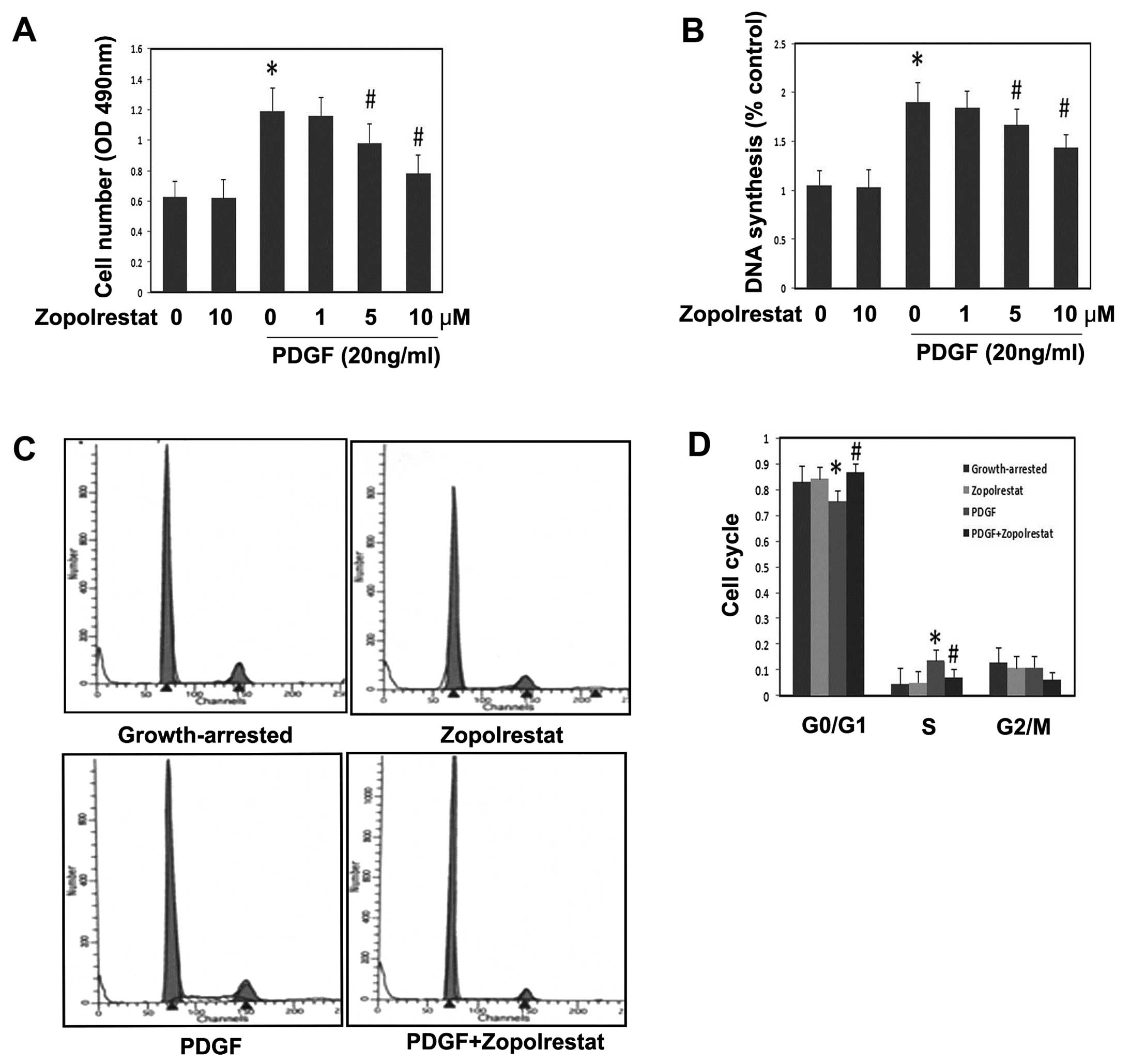
Mesangial cell proliferation was measured by the MTT
assay. Growth-arrested MsCs were treated with or without the ARI
zopolrestat in different doses for 24 h prior to stimulation with
PDGF (20 ng/ml) for another 24 h. The results show that PDGF
significantly induced MsC proliferation (Fig. 1A) (P<0.05 vs. control).
Pretreatment with zopolrestat alone had no effects on MsC
proliferation (Fig. 1A). However,
it inhibited PDGF-induced MsC proliferation in a dose-dependent
manner (Fig. 1A) (P<0.05 vs.
PDGF group). To further assess the effect of zopolrestat on DNA
synthesis in MsC proliferation, a BrdU cell proliferation assay was
performed. PDGF (20 ng/ml) resulted in an increase in the amount of
DNA synthesis, but zopolrestat decreased the DNA synthesis in a
dose-dependent manner (Fig. 1B).
In contrast, zopolrestat alone did not affect DNA synthesis in
MsCs. Our results indicate that zopolrestat treatment inhibited the
PDGF-stimulated BrdU incorporation into DNA in MsCs.
In order to further evaluate the effect of ARI
zopolrestat treatment upon cell cycle profiles, we then performed
flow cytometry. The data showed that PDGF decreased the proportion
of cells in the G1 phase from 82.8 to 75.4%, (Fig. 1C) (P<0.05 vs. control) and
increased that in the S phase from 4.5 to 13.6%, indicating that
PDGF could promote cell cycle progression. In contrast, the
pretreatment of zopolrestat increased the number of cells in the G1
phase (from 75.4 to 86.7%, P<0.05) and decreased that in the S
phase (from 13.6 to 7.25%, P<0.05). These results suggest that
zopolrestat could block PDGF-induced cell cycle progression by
inhibiting the G1-S phase transition and arresting cells in G1.
Effects of zopolrestat on the activation
of PI3K induced by PDGF in rat MsCs
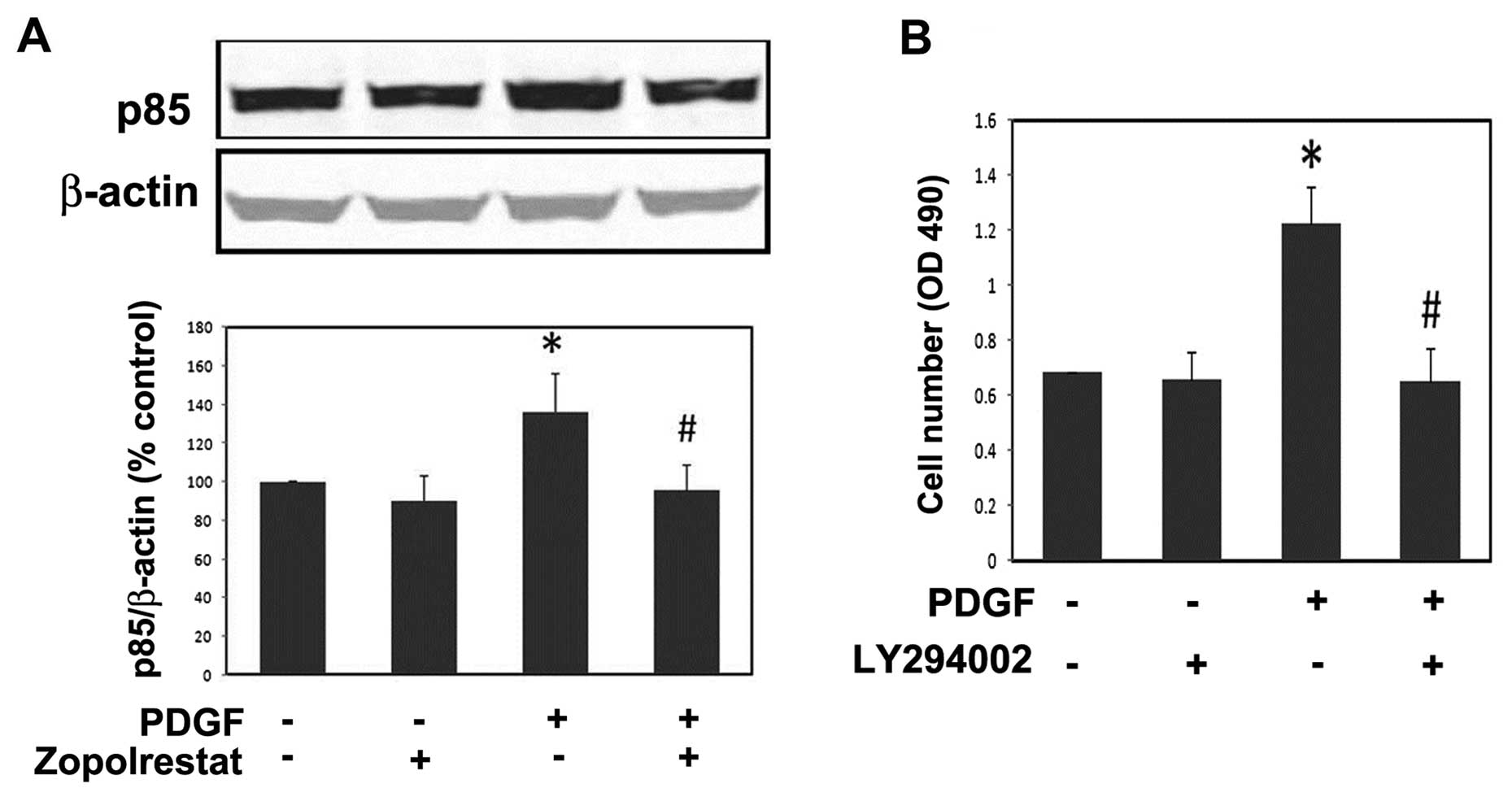
Previous studies showed that PI3K activity is
necessary for PDGF-induced MsC proliferation (18). Therefore, we examined the effect
of ARI on PI3K activity in MsC. PDGF-activated PI3K activity was
assessed by measuring the levels of the p85 regulatory subunit of
PI3K. PDGF obviously increased the protein expression of the p85
subunit, while pre-incubation of ARI significantly reduced the p85
subunit activity (Fig. 2A). We
then observed that LY294002 (Calbiochem), a specific inhibitor of
PI3K, could suppress cell proliferation induced by PDGF (Fig. 2B). We conclude from the above
results that PI3K is involved in the inhibition of ARI on
PDGF-induced MsC proliferation, and that further studies are
warranted.
Effects of zopolrestat on the
phosphorylation levels of Akt kinase induced by PDGF in rat
mesangial cell
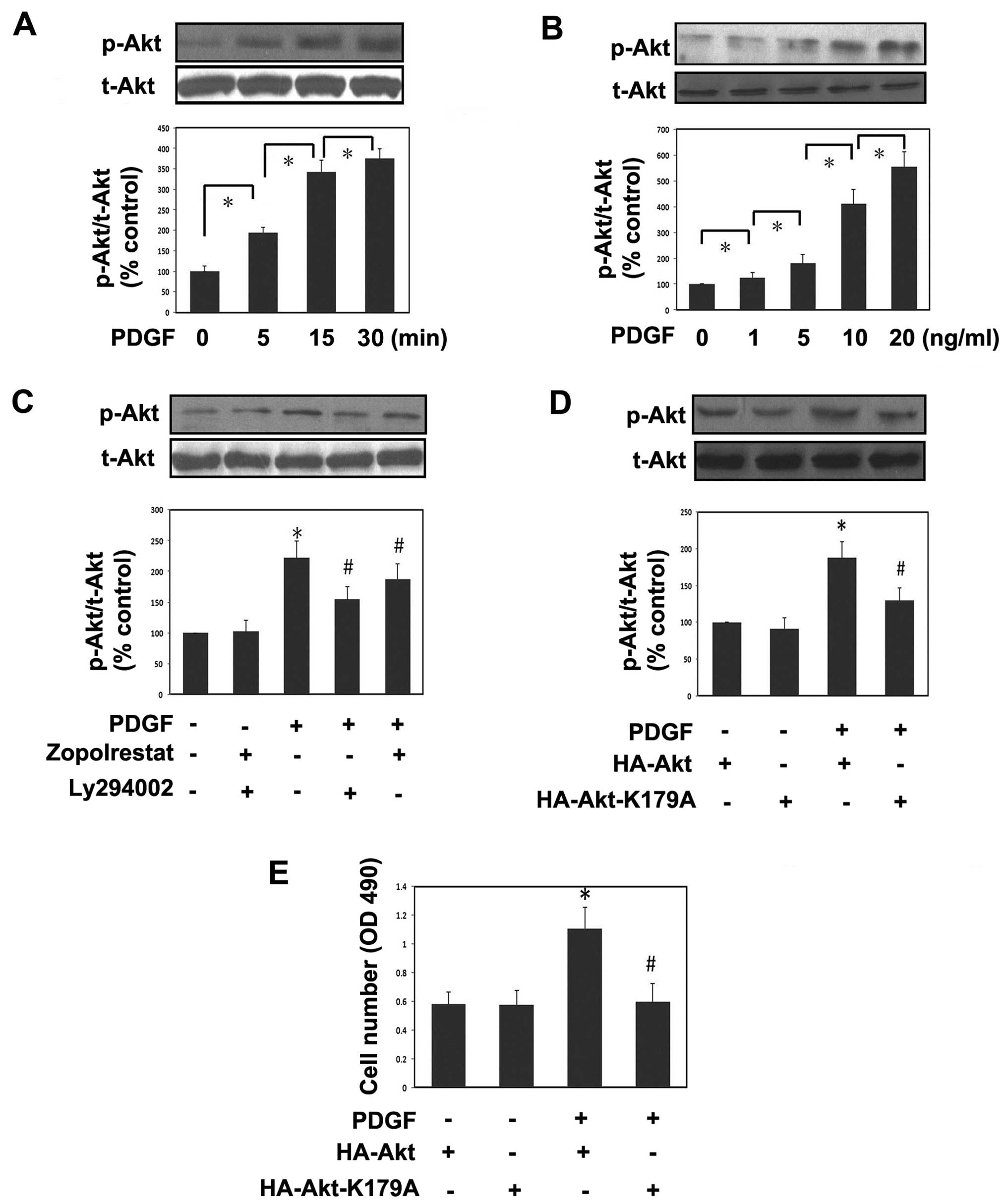
Since the activation of the PI3K/Akt pathway is a
key step in the proliferation process of a variety of cell types
including MsCs (18,22), western blot analysis was next
performed to evaluate the phosphorylation levels of this signaling
pathway. The phosphorylation levels of Akt were rapidly induced
with time and reached their highest level at about 15–30 min after
PDGF stimulation (Fig. 3A). The
representative western blots demonstrate that PDGF led to Akt
pathway activation in a dose-dependent manner (Fig. 3B). Pre-incubation of the ARI
zopolrestat markedly attenuated the induction of PDGF on
phospho-Akt, whereas zopolrestat alone had no effects on Akt
phosphorylation levels. Meanwhile, we found that LY294002 could
downregulate the phosphorylation levels of Akt (Fig. 3C). To closely confirm the role of
Akt in PDGF-induced cell proliferation, a dominant-negative mutant
of Akt, HA-Akt-K179A, was transfected into MsCs. This mutant
significantly reduced the phosphorylation levels of Akt induced by
PDGF (Fig. 3D). In agreement with
these findings, we found that HA-Akt-K179A significantly reduced
cell proliferation induced by PDGF, indicating a role of Akt in
this effect. These results show that the effects of AR inhibition
on the PDGF-induced stimulation of mesangial cell proliferation are
mediated by the Akt signaling pathways.
Effect of zopolrestat on protein
expression of p21Cip1 and p27Kip1 induced by
PDGF in rat MsC
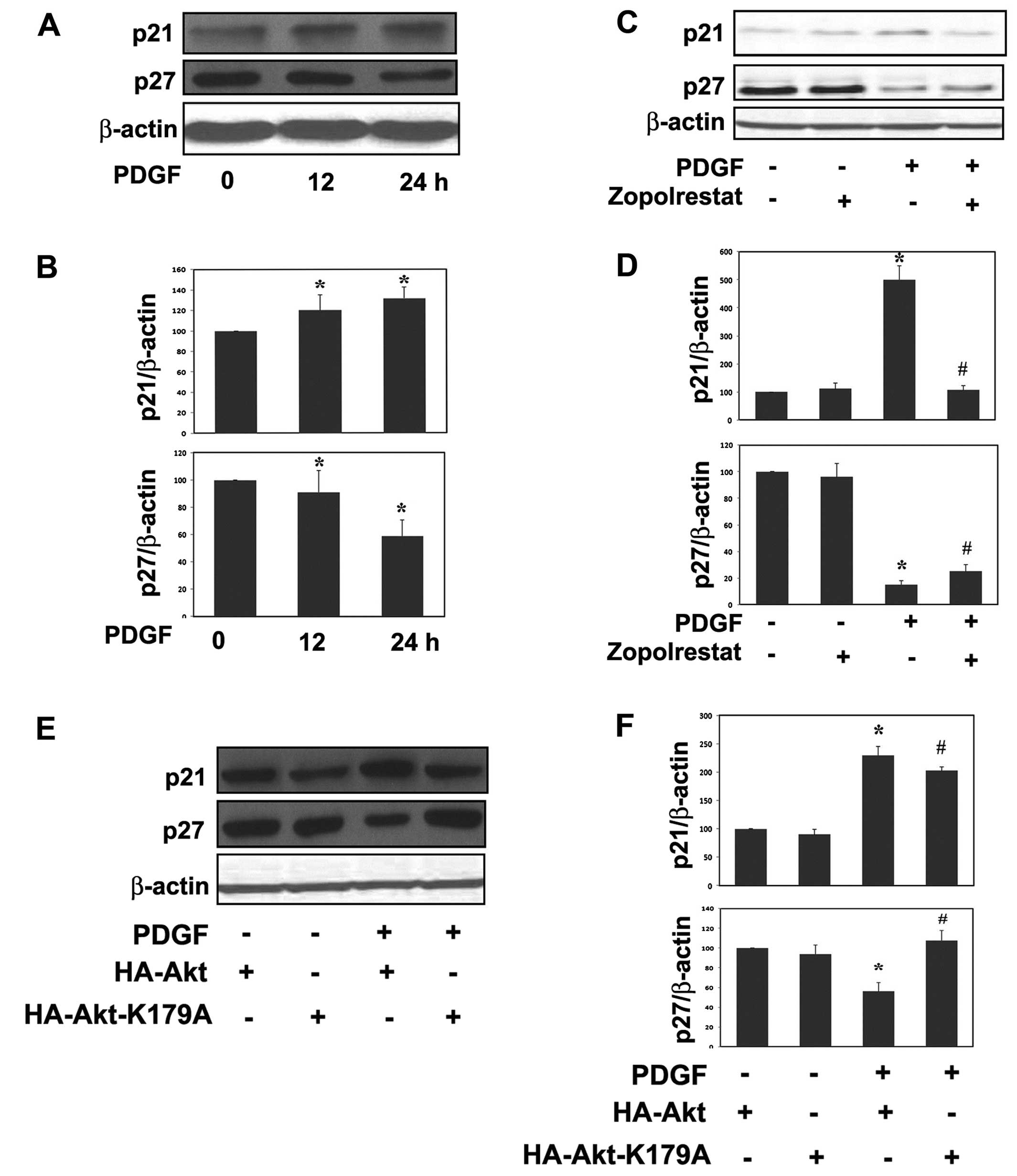
The levels of the G1 phase CKI p21Cip1
and p27Kip1 in quiescent control and proliferating MsCs
were determined by western blot analysis (Fig. 4). Growth-arrested MsCs were
treated with or without ARI zopolrestat (10 μM) 24 h prior to
stimulation with PDGF (20 ng/ml). Much more appreciable expression
of p27Kip1 than p21Cip1 was observed in
quiescent control mesangial cells (Fig. 4A). No change in their protein
expression levels was observed in zopolrestat-treated MsCs.
PDGF-treated cells showed an apparent increase in levels of
p21Cip1 in a time-dependent manner. The increase of
p21Cip1 was obviously suppressed by co-treatment with
zopolrestat or by HA-Akt-K179A mutant cells. In contrast,
incubation with PDGF caused an apparent decrease in detectable
levels of p27Kip1 in a time-dependent manner; however,
the reduction was restored by ARI zopolrestat or by HA-Akt-K179A
mutant cells.
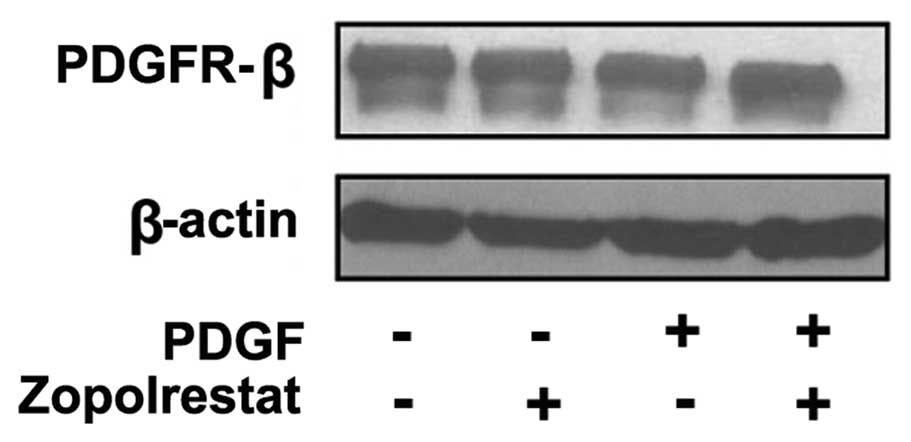
Effects of zopolrestat on PDGFR-β
receptor protein expression
To test whether zopolrestat affects the
PDGF-mediated mitogenic signaling cascade at the level of the PDGF
receptor, we examined the effects of the ARI zopolrestat on the
protein expression of the PDGFR-β receptor. Detectable levels of
PDGFR-β receptor protein expression were expressed in quiescent
control cells and no change in their levels was observed in
PDGF-treated MsCs with or without pretreatment of zopolrestat
(Fig. 5).
Discussion
In the present study, we demonstrated that ARI
partially inhibited the rat MsC proliferation stimulated by PDGF
through modulating the levels of p21Cip1 and
p27Kip1. We also confirmed that ARI suppressed the Akt
signal pathway in response to PDGF. From these results it was
suggested that ARI may has a beneficial role in inhibiting the
deterioration of renal function featured with MsC proliferation and
ECM deposition.
AR, the rate-limiting enzyme in the polyol pathway,
was first found to be implicated in the etiology of the diabetic
complications apart from its physiological role as osmo-regulator
in the kidney and supplier for sperm energy (25,26). However, recent reports have
demonstrated that, under normal glucose levels, AR may be in
involved in various pathological processes other than hyperglycemia
as it is a broad-specificity aldo-keto reductase with wide species
and tissue distribution (8). Our
previous studies showed that AR is one of the responsive genes for
TGF-β1, regulates TGF-β1-induced production of fibronectin and type
IV collagen, and participates in PDGF-induced mesangial cell
proliferation in cultured rat mesangial cells (11,27). The details of this
anti-proliferative mechanism of ARI in mesangial cells are not
clear. In this study, we studied the mechanism by which ARI
inhibits PDGF-induced MsC proliferation under normal glucose
concentrations. Our study confirmed the role of AR in regulating
MsC proliferation induced by PDGF, which is consistent with other
reports in different cell types induced by distinct growth-related
factors (10,25,28).
In our previous studies, we observed that MsC with
AR overexpression proliferated faster than the controls, which
indicated the important role of AR in MsC proliferation. We next
determined the role of ARI, the specific AR inhibitor, in MsC
proliferation. Consistent with the increased proliferation due to
AR overexpression, pretreatment of ARI significantly inhibited MsC
proliferation, which confirmed the regulation of AR on MsC
proliferation (11). However, ARI
did not show obvious inhibition for serum-induced MsC
proliferation, which is likely due to the fact that there are other
growth factors besides PDGF in serum. Previous reports showed that
ARI had no effect on PDGF-induced rat aortic smooth muscle cell
proliferation under normal glucose conditions (29). Taken together, the effect of ARI
on PDGF-induced cell proliferation is cell type-dependent. In this
study we further elucidated the molecular mechanism by which ARI
arrested PDGF-induced MsC proliferation. It was confirmed that
PDGF-induced cell proliferation was significantly suppressed by
pretreatment of ARI while ARI itself had no effects on MsC
proliferation. Our results suggest that AR plays a role in
PDGF-induced cell proliferation and ARI may be available to be used
to protect renal function against mesangial cell abnormally
proliferative associated disorders. In addition, our data showed
that ARI had no effects on PDGFR-β receptor protein expression
which is consistent with previous reports (30,31).
The PI3K pathway has been reported to mediate
PDGF-induced MsC proliferation (18). One of the major downstream
signaling pathways of PI3K is the serine/threonine kinase Akt/PKB.
Akt kinase activity is thought to be stimulated by a variety of
extracellular signaling factors, including many growth factors and
cytokines. The role of Akt related to inhibition of apoptosis has
been extensively reported. For example, angiopoietin-1 inhibits
endothelial cell apoptosis via the Akt/survivin pathway (32). Similarly, in RGC-5 retinal
ganglion cells imatinib diminishes PDGF-induced Akt phosphorylation
to induce apoptosis (33).
However, in our study we confirmed the Akt kinase-mediated
PDGF-induced DNA synthesis, and more importantly that pretreatment
of ARI suppressed it, which is consistent with a previous report in
colon cancer cells (34). Our
data showed that PDGF activates Akt phosphorylation in a time- and
dose-dependent manner, and Akt phosphorylation was significantly
reduced after co-treatment with AR or being transfected with
HA-Akt-K179A. We demonstrated that ARI blocked Akt phosphorylation
triggered by PDGF in MsCs. These data directly link AR to the
induction of Akt without hyperglycemia in rat MsC. Although the
precise role of Akt in regulating proliferation is unclear, our
results indicate that during the process of AR-modulated cell
proliferation the altered expression of p21Cip1 and
p27Kip1 levels that were observed in PDGF-stimulated
cells are coupled to increased levels of Akt kinase
phosphorylation. In this study, we confirmed that the AR modulated
cell proliferation through the Akt pathway mediating PDGF signaling
in MsC, which is in agreement with reports in other studies
(14,35,36).
Cell cycle progression CDK inhibitors, in particular
those members of the Cip/Kip family contribute to cell-cycle
regulatory functions. The Cip/Kip family comprise of
p21Waf1/Cip1 (p21), p27Kip1 (p27) and
p57Kip2 (p57), which inhibit cyclin-CDK complexes both
in the G1 and S phases of the cell cycle (37,38). Former reports have demonstrated
that p21Cip1 and p27Kip1 are critically
involved in cell cycle arrest in MsC (23). In our experiments PDGF induced the
expression of p21Cip1, and this was apparently blocked
by ARI or after being transfected with HA-Akt-k179A, which was in
agreement with the previous study (39). Increased expression of
p21Cip1 during proliferation is observed because it acts
as a scaffold to facilitate the assembly of cyclins and CDKs
required for DNA synthesis (24).
Our data indicate that ARI negatively regulates p21Cip1
expression in MsC. On the other hand, it has been reported that the
onset of MsC proliferation is associated with a reduction in
p27Kip1 levels, and in addition, p27Kip1
safeguards against inflammatory glomerular injury in anti-GBM
glomerulonephritis (40). To
confirm this observation, we found that PDGF greatly downregulated
p27Kip1 protein level, and the effect was reversed by
pretreatment of ARI or after being transfected with HA-Akt-k179A.
It is suggested that p27Kip1 is regulated by ARI in a
positive manner. In our experiments PDGF showed contrary effects on
p21Cip1 and p27Kip1 expression. It is
interesting to note that overexpression of YB-1 led to rat MsC
proliferation by decreasing p21Cip1 expression, but
increasing p27Kip1 expression (41). The underlying mechanism of the p21
and p27 diverse roles in response to different growth factors in
the same cell type remains to be explored.
Accumulating evidence has suggested that under
normal glucose conditions AR may be upregulated by factors other
than hyperglycemia and therefore participate in pathological
conditions in non-diabetic disorders. For instance, growth factors
cause oxidative stress via generation of reactive oxygen species
(ROS) which forms toxic lipid aldehydes such as
4-hydroxy-trans-2-nonenal (HNE) by lipid peroxidation. The final
products of HNE catalyzed by AR, glutathionyl-1,4-dihydroxynonane
(GS-DHN), transduce inflammatory signaling via a cascade of protein
kinases, such as PI3K (8,9). Growth factors-stimulated PI3K may
utilize its major downstream Akt kinase signaling pathway for
induction of DNA synthesis in MsCs. Our observation would support
the importance of the AR/PI3K/Akt/cell cycle protein cascade for
the PDGF-induced proliferation of MsCs.
In summary, our data demonstrate that ARI inhibits
PDGF-stimulated MsC proliferation via modulation of the PI3K/Akt
pathway which results in alterations in the levels of
p21Cip1 and p27Kip1. Further studies to
elucidate the precise molecular mechanisms by which AR mediates
PDGF-induced cell proliferation are currently underway. Future
investigation will be needed to determine how AR affects the
PI3K/Akt signal pathway and the expression level of p21 and p27.
Understanding this mechanism is potentially important in developing
therapeutic strategies to ameliorate mesangial cell proliferative
disorders as well as to prevent renal fibrosis.
Abbreviations:
|
AKR
|
aldo-keto reductase;
|
|
AR
|
aldose reductase;
|
|
ARI
|
aldose reductase inhibitor;
|
|
CDK
|
cyclin-dependent kinase;
|
|
CKI
|
cyclin-dependent kinase inhibitor;
|
|
ECM
|
extracellular matrix;
|
|
GSH
|
glutathione;
|
|
GS-DHN
|
glutathionyl-1,4-dihydroxynonane;
|
|
HNE
|
4-hydroxy-trans-2-nonenal;
|
|
MAPK
|
mitogen-activated protein kinase;
|
|
MsC
|
mesangial cell;
|
|
PDGF
|
platelet-derived growth factor;
|
|
PDGFR
|
platelet-derived growth factor
receptor;
|
|
PI3K
|
phosphoinositide 3-kinase;
|
|
ROS
|
reactive oxygen species;
|
|
TGF-β1
|
transforming growth factor-β1;
|
|
VSMC
|
vascular smooth muscle cell
|
Acknowledgements
This study was supported by the
National Natural Scientific Foundation of China (NSFC30170430) and
the Science and Technology Commission of the Shanghai Municipality
(11ZR1402400). We thank Pfizer for generously providing
zopolrestat. We are grateful to Dr Boudewijn Burgering for kindly
providing us with plasmids.
References
|
1.
|
JM JezTG FlynnTM PenningA new nomenclature
for the aldo-keto reductase superfamilyBiochem
Pharmacol54639647199710.1016/S0006-2952(97)84253-09310340
|
|
2.
|
P Anil KumarG Bhanuprakash ReddyFocus on
molecules: aldose reductaseExp Eye Res85739740200716997295
|
|
3.
|
M LorenziThe polyol pathway as a mechanism
for diabetic retinopathy: attractive, elusive, and resilientExp
Diabetes Res200761038200710.1155/2007/6103818224243
|
|
4.
|
C Yabe-NishimuraAldose reductase in
glucose toxicity: a potential target for the prevention of diabetic
complicationsPharmacol Rev50213319989549756
|
|
5.
|
A PladzykKV RamanaNH AnsariSK
SrivastavaAldose reductase prevents aldehyde toxicity in cultured
human lens epithelial cellsExp Eye
Res83408416200610.1016/j.exer.2006.01.01916631166
|
|
6.
|
KV RamanaAB ReddyR TammaliSK
SrivastavaAldose reductase mediates endotoxin-induced production of
nitric oxide and cytotoxicity in murine macrophagesFree Radic Biol
Med4212901302200710.1016/j.freeradbiomed.2007.01.03317382209
|
|
7.
|
R RamasamyIJ GoldbergAldose reductase and
cardiovascular diseases, creating human-like diabetic complications
in an experimental modelCirc
Res10614491458201010.1161/CIRCRESAHA.109.21344720466987
|
|
8.
|
P AlexiouK PegklidouM ChatzopoulouI
NicolaouVJ DemopoulosAldose reductase enzyme and its implication to
major health problems of the 21st centuryCurr Med
Chem16734752200910.2174/09298670978745836219199934
|
|
9.
|
KV RamanaSK SrivastavaAldose reductase: a
novel therapeutic target for inflammatory pathologiesInt J Biochem
Cell Biol421720201010.1016/j.biocel.2009.09.00919778627
|
|
10.
|
R TammaliKV RamanaSS SinghalS AwasthiSK
SrivastavaAldose reductase regulates growth factor-induced
cyclooxygenase-2 expression and prostaglandin E2 production in
human colon cancer cellsCancer
Res6697059713200610.1158/0008-5472.CAN-06-2105
|
|
11.
|
Q CheT JiangYF LinH LiN ZhangEffects of
aldose reductase transfection on the proliferation of rat mesangial
cells in vitroZhonghua Bing Li Xue Za Zhi344174202005(In
Chinese).
|
|
12.
|
WG CouserPathogenesis of glomerular damage
in glomerulonephritisNephrol Dial Transplant13Suppl
1S10S15199810.1093/ndt/13.suppl_1.10
|
|
13.
|
RJ JohnsonJ FloegeWG CouserCE AlpersRole
of platelet-derived growth factor in glomerular diseaseJ Am Soc
Nephrol411912819938311852
|
|
14.
|
F DasL MahimainathanN
Ghosh-ChoudhuryTGFbeta intercepts nuclear glycogen synthase kinase
3beta to inhibit PDGF-induced DNA synthesis in mesangial cellsFEBS
Lett58152595267200710.1016/j.febslet.2007.10.01417961557
|
|
15.
|
J FloegeF EitnerCE AlpersA new look at
platelet-derived growth factor in renal diseaseJ Am Soc
Nephrol191223200810.1681/ASN.200705053218077793
|
|
16.
|
RE GilbertDJ KellyT McKayPDGF signal
transduction inhibition ameliorates experimental mesangial
proliferative glomerulonephritisKidney
Int5913241332200110.1046/j.1523-1755.2001.0590041324.x
|
|
17.
|
RP DirksHP BloemersSignals controlling the
expression of PDGFMol Biol Rep22124199510.1007/BF00996300
|
|
18.
|
GG ChoudhuryC KaramitsosJ HernandezA
GentiliniJ BardgetteHE AbboudPI-3-kinase and MAPK regulate
mesangial cell proliferation and migration in response to PDGFAm J
Physiol273F931F93819979435682
|
|
19.
|
A Cove-SmithBM HendryThe regulation of
mesangial cell proliferationNephron Exp
Nephrol108e74e79200810.1159/00012735918431073
|
|
20.
|
M CampbellER TrimbleModification of PI3K-
and MAPK-dependent chemotaxis in aortic vascular smooth muscle
cells by protein kinase CbetaIICirc
Res96197206200510.1161/01.RES.0000152966.88353.9d15591231
|
|
21.
|
UC YadavL Aguilera-AguirreKV RamanaI
BoldoghSK SrivastavaAldose reductase inhibition prevents metaplasia
of airway epithelial cellsPLoS
One5e14440201010.1371/journal.pone.001444021203431
|
|
22.
|
GG ChoudhuryAkt serine threonine kinase
regulates platelet-derived growth factor-induced DNA synthesis in
glomerular mesangial cells: regulation of c-fos AND p27(kip1) gene
expressionJ Biol Chem2763563635643200110.1074/jbc.M100946200
|
|
23.
|
CB MarshallSJ ShanklandCell cycle and
glomerular disease: a minireviewNephron Exp
Nephrol102e39e48200610.1159/00008840016179806
|
|
24.
|
SJ ShanklandG WolfCell cycle regulatory
proteins in renal disease: role in hypertrophy, proliferation, and
apoptosisAm J Physiol Renal Physiol278F515F529200010751212
|
|
25.
|
A BhatnagarJ RuefS LiuS SrivastavaSK
SrivastavaRegulation of vascular smooth muscle cell growth by
aldose reductaseChem Biol Interact130–132627636200111306081
|
|
26.
|
HT HoSK ChungJW LawAldose
reductase-deficient mice develop nephrogenic diabetes insipidusMol
Cell Biol2058405846200010.1128/MCB.20.16.5840-5846.200010913167
|
|
27.
|
T JiangQ CheY LinH LiN ZhangAldose
reductase regulates TGF-beta1-induced production of fibronectin and
type IV collagen in cultured rat mesangial cellsNephrology
(Carlton)11105112200610.1111/j.1440-1797.2006.00553.x16669970
|
|
28.
|
KV RamanaD ChandraS SrivastavaA
BhatnagarSK SrivastavaAldose reductase mediates the mitogenic
signals of cytokinesChem Biol Interact143–144587596200312604244
|
|
29.
|
Y KasuyaJ NakamuraY HamadaAn aldose
reductase inhibitor prevents the glucose-induced increase in
PDGF-beta receptor in cultured rat aortic smooth muscle
cellsBiochem Biophys Res
Commun261853858199910.1006/bbrc.1999.111110441515
|
|
30.
|
K IshizawaN DorjsurenY
Izawa-IshizawaInhibitory effects of adiponectin on platelet-derived
growth factor-induced mesangial cell migrationJ
Endocrinol202309316200910.1677/JOE-08-046919460854
|
|
31.
|
HO SchocklmannS LangM KralewskiA HartnerA
LudkeRB SterzelDistinct structural forms of type I collagen
modulate cell cycle regulatory proteins in mesangial cellsKidney
Int5811081120200010.1046/j.1523-1755.2000.00268.x10972675
|
|
32.
|
A PapapetropoulosD FultonK
MahboubiAngiopoietin-1 inhibits endothelial cell apoptosis via the
Akt/survivin pathwayJ Biol
Chem27591029105200010.1074/jbc.275.13.910210734041
|
|
33.
|
SK BiswasY ZhaoL SandirasegaraneImatinib
induces apoptosis by inhibiting PDGF- but not insulin-induced PI
3-kinase/Akt survival signaling in RGC-5 retinal ganglion cellsMol
Vis1515991610200919693287
|
|
34.
|
KV RamanaR TammaliSK SrivastavaInhibition
of aldose reductase prevents growth factor-induced G1-S phase
transition through the AKT/phosphoinositide 3-kinase/E2F-1 pathway
in human colon cancer cellsMol Cancer
Ther9813824201010.1158/1535-7163.MCT-09-0795
|
|
35.
|
D MitchellK RodgersJ HanlyLipoxins inhibit
Akt/PKB activation and cell cycle progression in human mesangial
cellsAm J
Pathol164937946200410.1016/S0002-9440(10)63181-114982847
|
|
36.
|
B VenkatesanN Ghosh-ChoudhuryF
DasResveratrol inhibits PDGF receptor mitogenic signaling in
mesangial cells: role of PTP1BFASEB
J2234693482200810.1096/fj.08-10948818567737
|
|
37.
|
SJ ShanklandCell-cycle control and renal
diseaseKidney Int52294308199710.1038/ki.1997.3359263984
|
|
38.
|
CJ SherrJM RobertsInhibitors of mammalian
G1 cyclin-dependent kinasesGenes
Dev911491163199510.1101/gad.9.10.11497758941
|
|
39.
|
SS GhoshTW GehrS GhoshPPARgamma ligand
attenuates PDGF-induced mesangial cell proliferation: role of MAP
kinaseKidney
Int645262200310.1046/j.1523-1755.2003.00054.x12787395
|
|
40.
|
A YoshimuraT NemotoY SugenoyaEffect of
simvastatin on proliferative nephritis and cell-cycle protein
expressionKidney Int
Suppl71S84S87199910.1046/j.1523-1755.1999.07121.x10412745
|
|
41.
|
Q FengS HuangA ZhangY-box protein 1
stimulates mesangial cell proliferation via activation of
ERK1/2Nephron Exp Nephrol113e16e25200910.1159/00022807919590238
|