Introduction
Intestinal ischemia is a common clinical problem
occurring in many clinical settings such as superior mesenteric
artery occlusion, hemorrhagic shock, cardiac insufficiency with
associated low flow state, necrotizing enterocolitis, and small
bowel transplant. It is associated with significant morbidity and
mortality. Interruption of blood supply to a local area causes
ischemia which rapidly damages metabolically active tissues. The
restoration of blood flow or reperfusion is necessary to maintain
cell function and viability, but alone it elicits a cascade of
adverse reactions that paradoxically injure tissues. The
pathophysiology of ischemia/reperfusion (I/R) injury is complex,
involving many biochemical pathways (1–3).
Local and systemic inflammatory derangements occur after I/R
(4). Damage to the
microcirculation triggers a brisk local, then systemic,
inflammatory response (5,6). Several mechanisms have been proposed
to explain the tissue injury that results from intestinal ischemia.
However, little progress has been made in improving the clinical
outcome for this devastating disease. The development of novel and
effective therapies are imperative in improving patient outcome in
gut I/R injury- related conditions.
Studies in gut I/R patients and animals have
demonstrated that a key aspect of gut I/R injury is the increased
occurrence of apoptotic cell death in the gut (7–9). A
large number of studies have shown that excessive apoptosis has
pathological consequences on the immune system (10–18). Without proper clearance, apoptotic
cells undergo secondary necrosis and have the potential to pose
great harm to the host. Milk fat globule-epidermal growth
factor-factor 8 (MFG-E8), a secretory protein, is a crucial
molecule for apoptotic cell clearance (19–21). Our recent studies have shown that
the administration of either MFG-E8-containing exosomes or
recombinant murine MFG-E8 (rmMFG-E8), reduces apoptosis and
inflammation under various disease conditions (22–24). However, it remains unknown whether
MFG-E8 ameliorates bacterial translocation and promotes tissue
repair after gut I/R. The purpose of this study was to determine
whether MFG-E8 reduces bacterial translocation and promotes tissue
repair in a mouse model of gut I/R.
Materials and methods
Experimental animals
Adult male C57BL/6J mice, purchased from Taconic
(Albany, NY), were used in this study. The mice were housed in a
temperature-controlled room on a 12 h light/dark cycle and fed a
standard Purina rat chow diet. The mice were fasted for 12 h prior
to the procedure. Animal experimentation was carried out in
accordance with the Guide for the Care and Use of Laboratory
Animals (Institute of Laboratory Animal Resources). This project
was approved by the Institutional Animal Care and Use Committee
(IACUC) of the Feinstein Institute for Medical Research.
Experimental model
Ischemia was induced in male C57BL/6J mice (BW,
20–25 g; Taconic) by clamping the superior mesenteric artery (SMA)
for 90 min under general anesthesia using isoflurane. At 90 min
after SMA, the vascular clamp was released to allow reperfusion. At
the beginning of reperfusion mice were resuscitated with a 0.5-ml
intraperitoneal (i.p.) injection of saline and were i.p. treated
with recombinant murine MFG-E8 (rmMFG-E8; R&D Systems,
Minneapolis, MN) at a dose of 0.4 mg/20 g BW in 0.5 ml normal
saline or normal saline (Vehicle). The isoflurane was discontinued
after i.p. injection of rmMFG-E8 or saline. Control animals
underwent the same operative procedure with the exception of the
SMA clamping (Sham). Four hours after reperfusion, animals were
anesthetized and blood and small intestinal samples (non-necrotic
areas; they were selected based on the color of the small intestine
segment) were harvested for various measurements.
Measurement of MFG-E8, Bcl-2, poly
(ADP-ribose) polymerase-1 (PARP-1), and vascular endothelial growth
factor (VEGF) protein levels
MFG-E8, Bcl-2, cleaved PARP-1 and VEGF protein
levels in the small intestine were measured by western blot
analysis. The band densities were normalized by β-actin with the
use of the Bio-Rad Image System. Briefly, 25 μg of protein from gut
samples was fractionated on a Bis-Tris gel and transferred to a
0.22-μm nitrocellulose membrane. Blots were blocked with 5% BSA in
Tris-buffered saline containing 0.1% v/v Tween-20. The membranes
were then incubated overnight at 4°C with the primary antibodies as
obtained from respective vendors: rabbit anti-mouse MFG-E8
polyclonal antibody (1:1,000; R&D Systems), rabbit anti-Bcl-2
antibody (1:500; Santa Cruz Biotechnology, Inc., Santa Cruz, CA),
rabbit anti-cleaved PARP antibody (1:300; Cell Signaling
Technology, Inc., Danvers, MA), and rabbit anti-VEGF antibody
(1:500; Santa Cruz Biotechnology, Inc.). The blots were then
incubated with horseradish peroxidase-linked anti-rabbit
immunoglobulin G (1:10,000; Cell Signaling Technology, Inc.,) for 1
h at room temperature. A chemiluminescent peroxidase substrate
(ECL; Amersham Biosciences, Piscataway, NJ) was applied according
to the manufacturer’s instructions, and the membranes were exposed
briefly to radiography film.
TUNEL assay
The presence of apoptotic cells in the small
intestine was demonstrated using a green fluorescence-tagged
terminal deoxynucleotide transferase dUTP nick-end labeling (TUNEL)
staining kit (Roche Diagnostics, Indianapolis, IN) counterstained
with propidium iodide and examined under a fluorescence microscope.
Apoptotic cells appeared as green fluorescence on a red background
staining.
Histopathology
Samples of the small intestine were fixed in 10%
formalin and embedded in paraffin. Tissue blocks were sectioned at
a thickness of 5 μm, transferred to glass slides, and stained with
hematoxylin and eosin. Morphologic examinations were performed
using light microscopy.
Measurement of myeloperoxidase (MPO)
activity
MPO activity in the small intestine was determined
using the peroxidase-catalyzed reaction. Briefly, tissues were
homogenized in KPO4 buffer containing 0.5%
hexadecyl-trimethyl-ammonium bromide (60°C for 2 h). After
centrifuging, the supernatant was diluted in reaction solution and
DOD was measured at 460 nm to calculate MPO activity.
Bacterial culture
The mesenteric lymph nodes (MLN) and blood samples
were collected for bacterial culture. Briefly, the MLN complex was
harvested and equal amounts of wet tissues were homogenized and
briefly centrifuged to remove gross particulate matters. Serial log
dilutions of tissue homogenates or blood samples were applied. Five
hundred microliters of each dilution was then plated on chocolate
agar plates (Fisher Scientific) and incubated at 37°C for 24 h
under aerobic conditions. The colony-forming units (CFU) were
counted and the results were expressed as CFU per gram of tissue
(MLN) or positive rates (blood).
Statistical analysis
All data are expressed as means ± SE and compared by
the Student’s t-test or one-way ANOVA and the Student Newman-Keuls
test. Differences in values were considered significant at
P<0.05.
Results
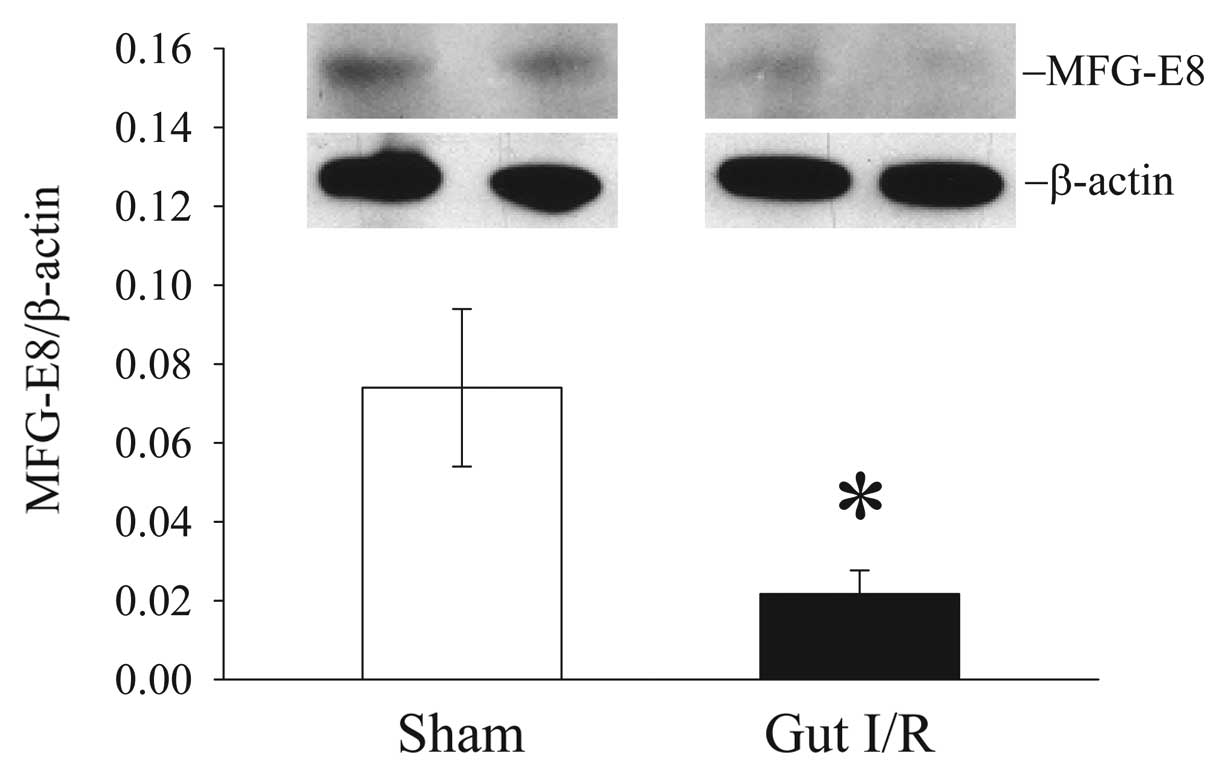
Intestinal levels of MFG-E8 decrease
after gut I/R
To determine whether MFG-E8 levels are altered after
I/R injury, we measured its protein levels in the small intestine 4
h post reperfusion after 90 min ischemia. Intestinal levels of
MFG-E8 protein decreased by 71% after gut I/R (Fig. 1).
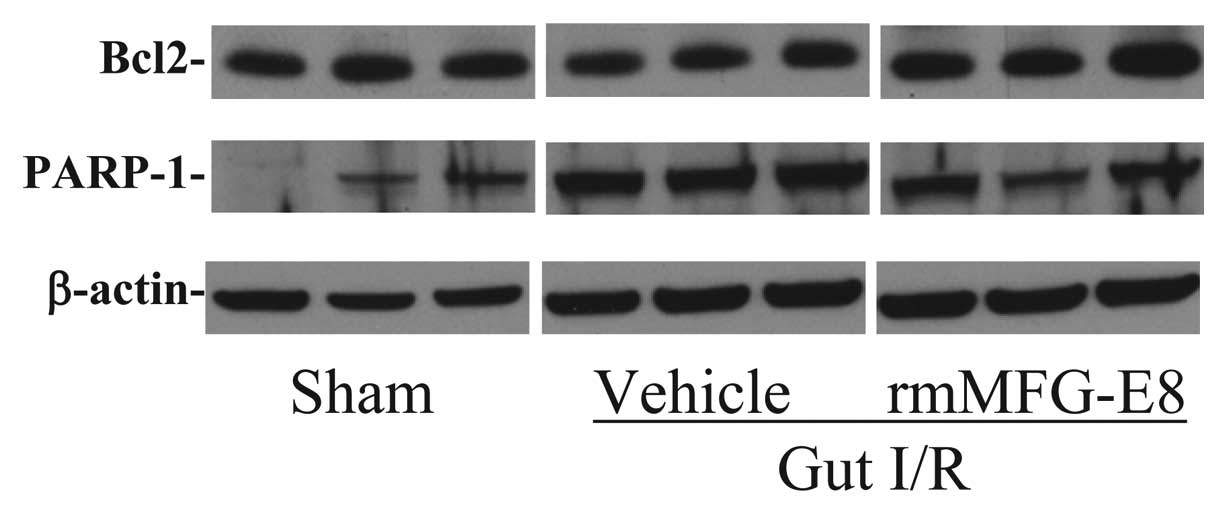
rmMFG-E8 attenuates intestinal apoptosis
after gut I/R
The intestinal expression of Bcl-2, an
anti-apoptosis protein, was markedly decreased after gut I/R
(Fig. 2). Treatment with rmMFG-E8
increased intestinal Bcl-2 levels dramatically, which were similar
to those in the sham animals. On the other hand, the expression of
PARP-1, an indicator of apoptosis, increased dramatically at 4 h
after gut I/R (Fig. 2).
Administration of rmMFG-E8 reduced intestinal levels of PARP-1
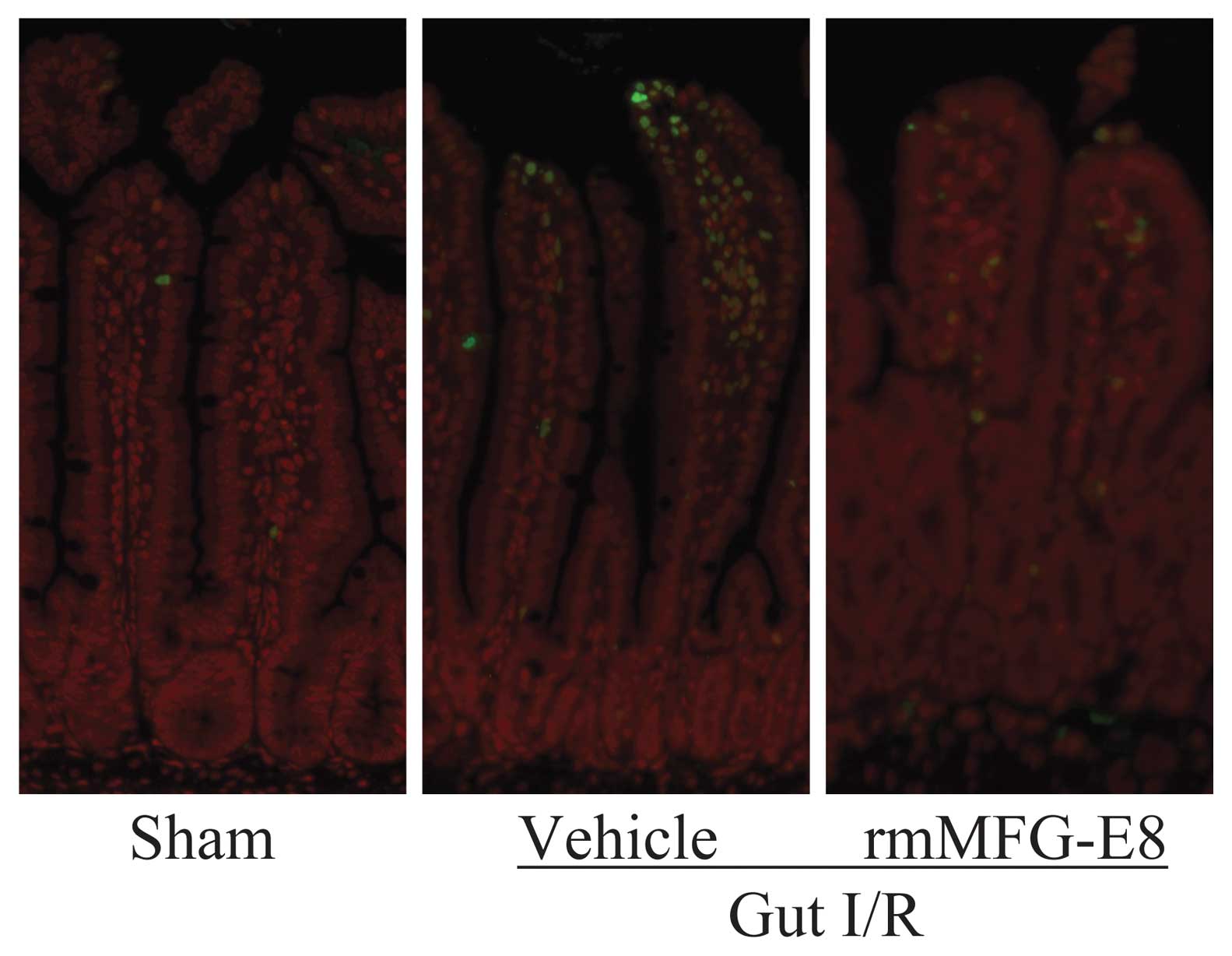
markedly. Consistent with these results, we found an increase in
the number of apoptotic cells in the small intestinal tissue by
TUNEL staining (Fig. 3).
Treatment with rmMFGE8, however, suppressed the number of
detectable apoptotic cells in the small intestine after gut I/R
injury.
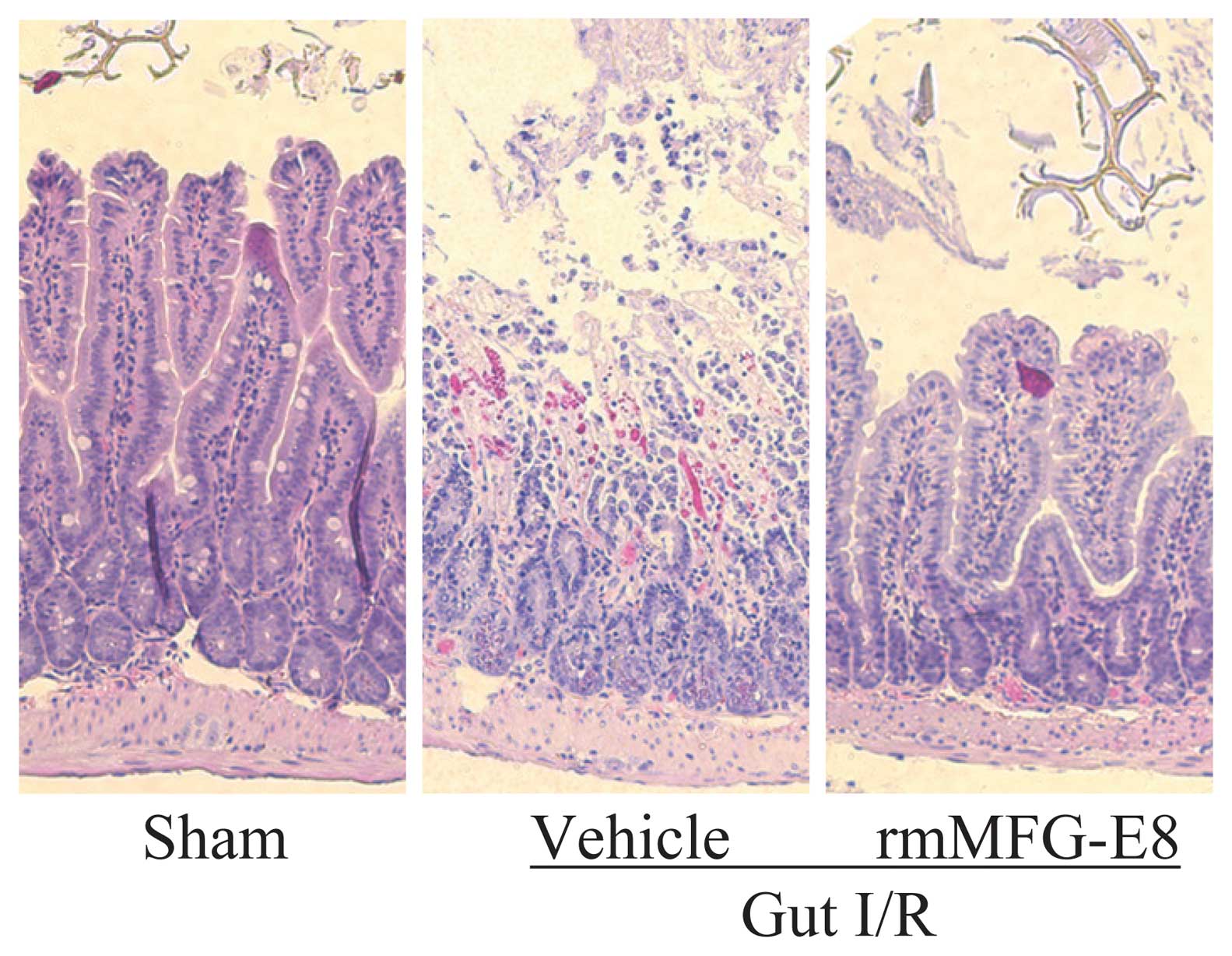
rmMFG-E8 mitigates intestinal injury
after gut I/R
Mucosal destruction, loss of villi and epithelial
cells, hemorrhage, and infiltration of inflammatory cells were
observed microscopically in the rat intestine after I/R as compared
with sham controls (Fig. 4).
Treatment with rmMFG-E8 dramatically improved these microscopic
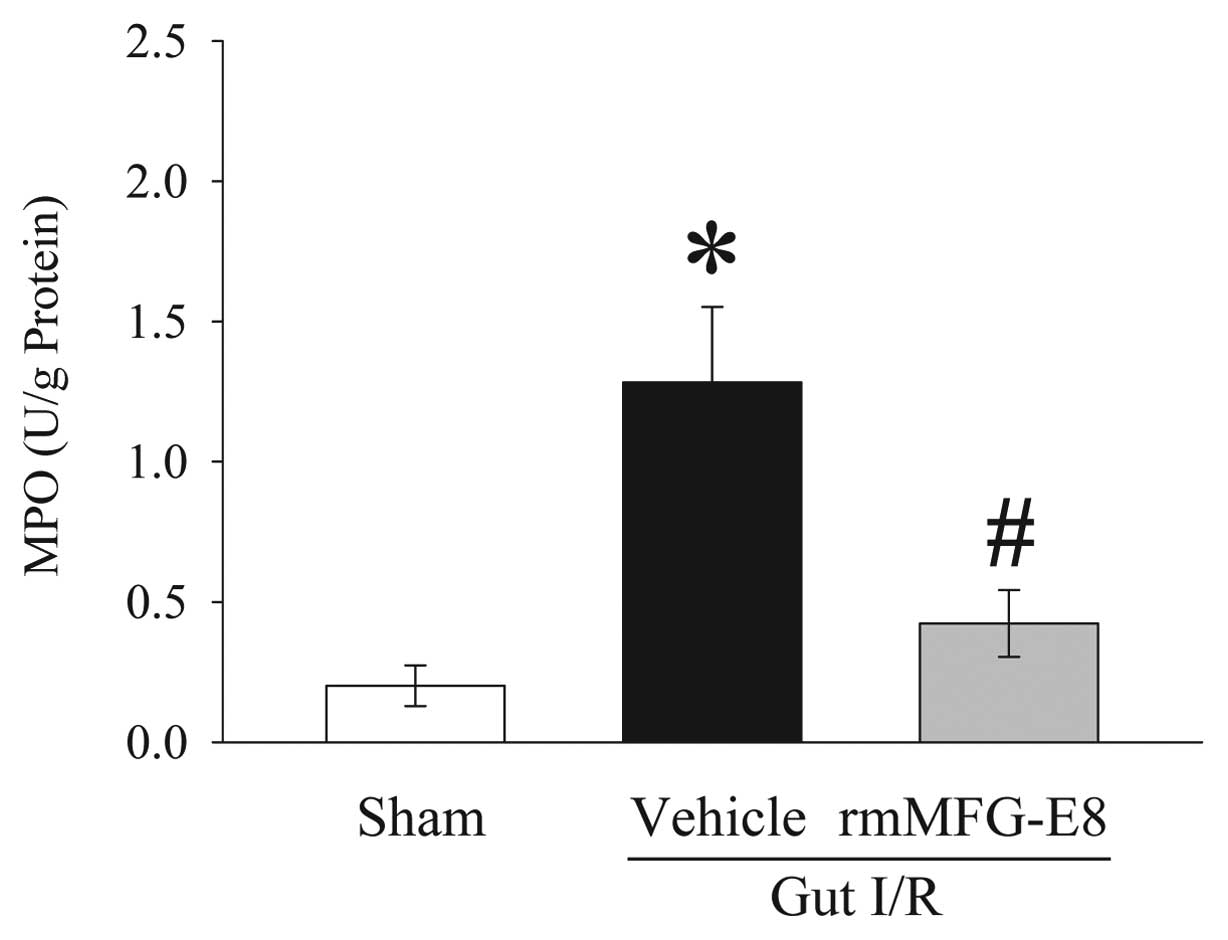
alterations. The level of MPO activity is an indicator of
neutrophil infiltration. As demonstrated in Fig. 5, gut I/R induced a more than
5-fold increase in intestinal MPO activities in vehicle-treated
rats as compared with sham animals. Treatment with rmMFG-E8
significantly inhibited the increase in intestinal MPO activities
by 67% after gut I/R (P<0.05).
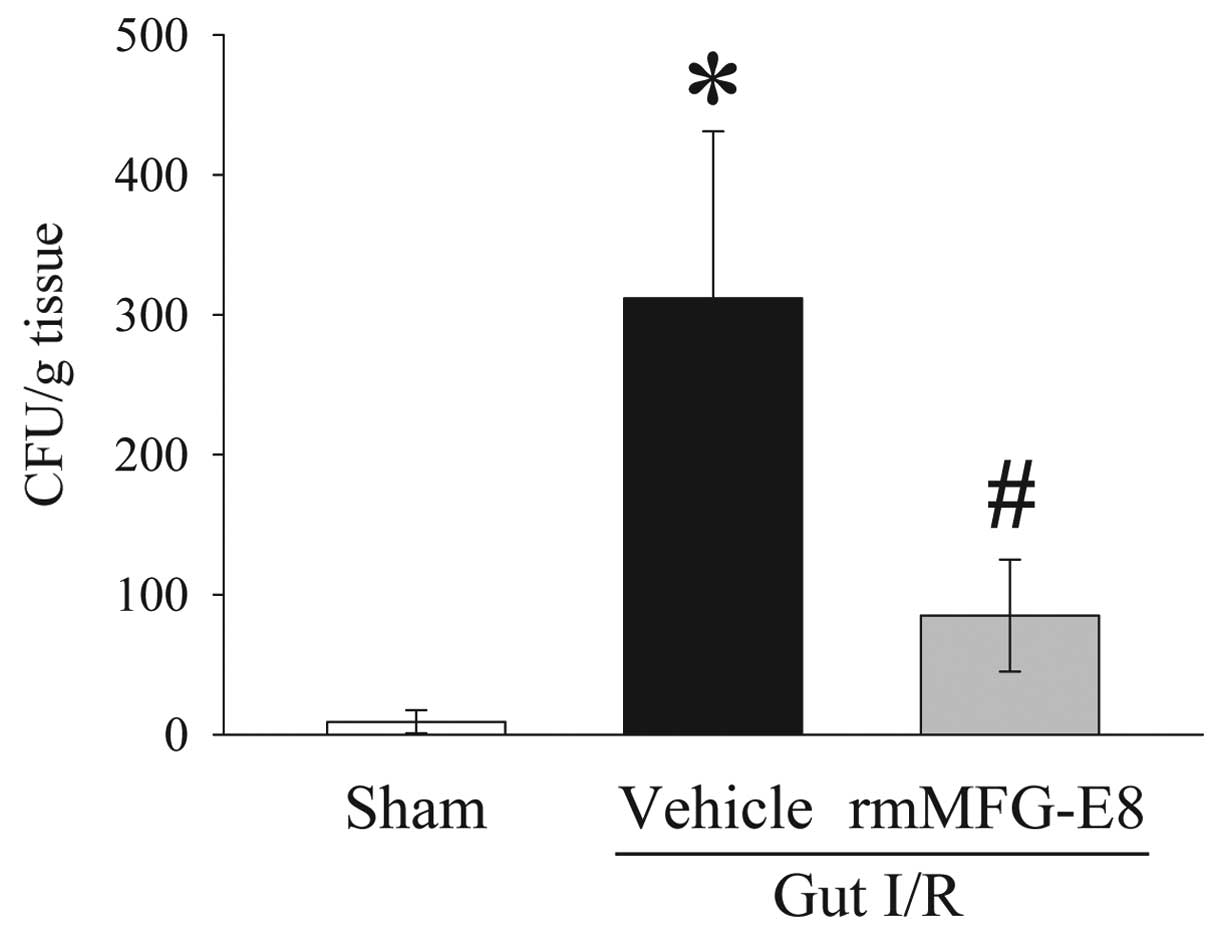
rmMFG-E8 reduces bacterial translocation
after gut I/R
Bacterial translocation to the MLN was minimal in
the sham group, but was extensive in the gut I/R vehicle-treated
group (P<0.05) (Fig. 6).
Treatment with rmMFG-E8 at the time of reperfusion, however,
significantly ameliorated the development of bacterial
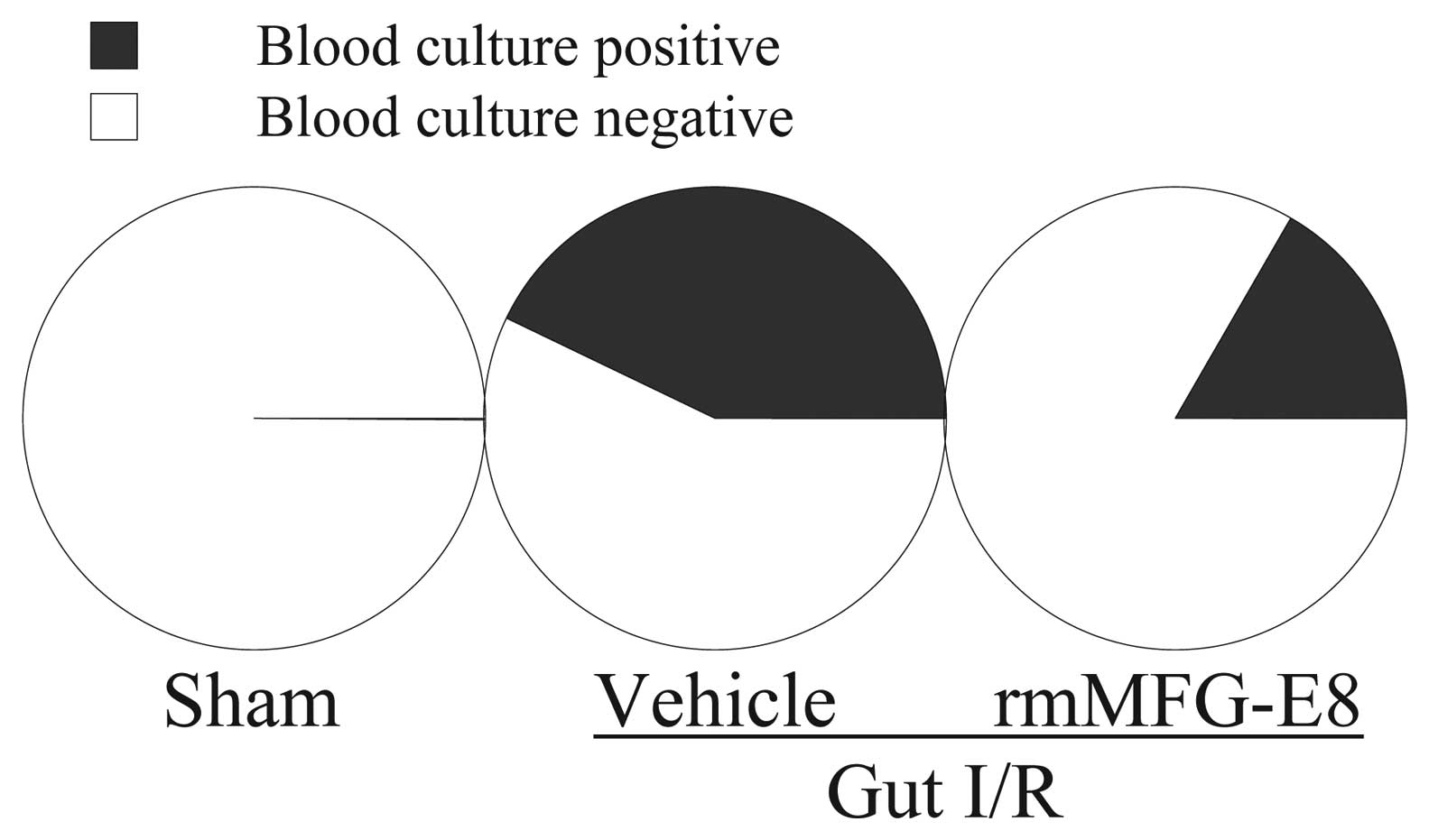
translocation. Moreover, bacteremia was determined by blood
culture. As shown in Fig. 7, 3 of
7 vehicle-treated gut I/R animals developed bacteremia at 4 h post
reperfusion. However, only 1 of 6 rmMFG-E8-treated gut I/R animals
showed a positive blood culture result.
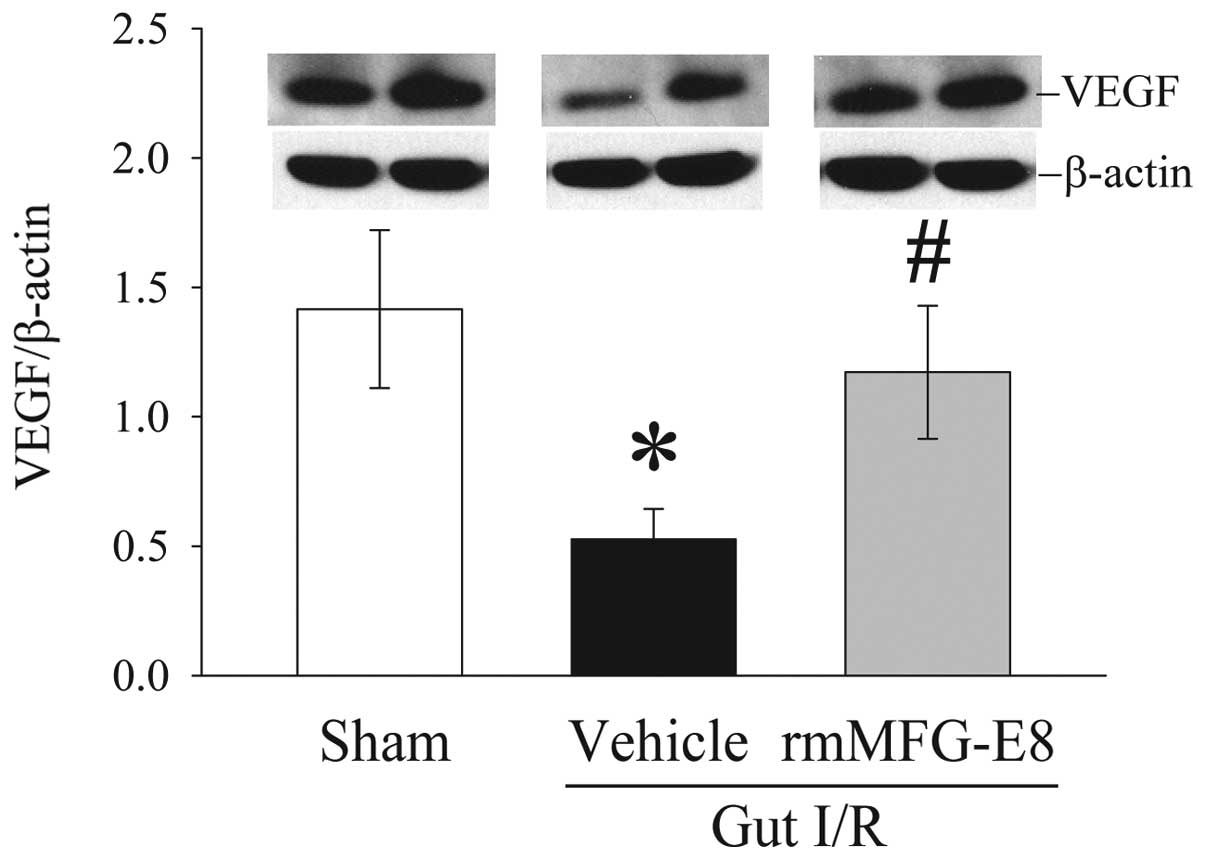
rmMFG-E8 increases intestinal VEGF
expression after gut I/R
Intestinal levels of VEGF decreased by 63% at 4 h
after gut I/R. Administration of rmMFG-E8 at the time of
reperfusion, however, significantly increased VEGF expression in
the gut by 123% at 4 h after reperfusion (P<0.05) (Fig. 8).
Discussion
Gut I/R injury is a serious condition in the
intensive care units and among vascular surgical patients. A key
aspect of I/R injury is the increased occurrence of apoptotic cell
death in the gut (7–9). In the current study, we found that
intestinal levels of MFG-E8 are significantly reduced after I/R
injury, which correlates with increased apoptosis and impaired
barrier function. MFG-E8 is a glycoprotein secreted from the
glandular epithelial cells in milk fat globules during lactation
(25–27). In milk, MFG-E8 acts as an
antiviral protein, inhibiting the symptoms of rotavirus infection
(28). Recent studies have shown
that MFG-E8 is also produced by macrophages and dendritic cells and
has been linked to the opsonization of apoptotic cells
(20,21,29–31). It plays a crucial role in the clearance of
apoptotic cells (19–21). Binding of MFG-E8 to
phosphatidylserine (PS) exposed on the surface of apoptotic cells
opsonizes them for a complete engulfment by macrophages via
αvβ3- or αvβ5-integrins
(32). Without MFG-E8, full
engulfment and the removal of apoptotic cells cannot be completed
(21). In this regard, gut I/R
induces apoptosis in the small intestine, and decreases apoptotic
cell clearance through the downregulation of MFG-E8 at the same
time. The reduced levels of MFG-E8 in the small intestine after I/R
injury may contribute to the increased apoptosis under such a
condition.
The current study also shows that administration of
rmMFG-E8 decreases apoptosis, mitigates bacterial translocation,
inhibits neutrophil infiltration, and promotes tissue repair after
gut I/R. The most noteworthy function of MFG-E8 is its ability to
promote the clearance of apoptotic cells by forming a tether
between phagocytes and apoptotic cells. Excessive apoptosis has
various pathological consequences. Recent studies have shown that
the lack of clearance of apoptotic cells in the spleen potentially
leads to autoimmune diseases (20,21). Accumulated apoptotic cells may
undergo secondary necrosis. These cells leak their dangerous
contents such as cytokines and enzymes, therefore, exaggerating
inflammation and potentiating tissue injury under such conditions.
Administration of rmMFG-E8 enhances apoptotic cell clearance, and
therefore, a secondary (post-apoptotic) necrosis of apoptotic cells
is prevented. Hence, the potential harm from apoptotic cells by
leakage of their dangerous contents due to secondary necrosis is
abrogated.
Organ injury induced by I/R is not necessarily
limited to the ischemic organ. The clinical features of gut
ischemia originate from both local and systemic responses. Gut I/R
injury i s one of the most common causes of gut barrier disruption
(33). Loss of the barrier
function of the gastrointestinal tract has been implicated as a
potential source of multiple organ failure under such a condition.
The gastrointestinal tract not only functions as a site for
nutrient absorption but also acts as a barrier between the
circulation and noxious substances such as intraluminal organisms
entering the circulation (34).
Maintenance of normal epithelial structure and function is
important in preventing transcellular and paracellular movement of
large molecules and bacteria (35). Increased intestinal permeability
has been reported to be associated with an increased risk of
complications, multiple organ failure, or even mortality in
critically ill patients (36–38). Our previous study has shown that
the administration of rmMFG-E8 attenuates lung injury after gut I/R
(24). In this regard, the
restoration of gut barrier function by rmMFG-E8 treatment may also
contribute to attenuated lung injury under certain conditions.
MFG-E8 appears to play an important role in the
maintenance of intestinal homeostasis and the promotion of mucosal
healing. In breast milk fed infants, MFG-E8 is involved in the
uptake of milk fat globules in the gut (25–27). It is also an important milk
mucin-associated defense component that inhibits enteric pathogen
binding and infectivity (39).
Previous studies have shown that MFG-E8 regulates the migration of
enterocytes and intestinal repair (40) and plays a role in VEGF-dependent
neovascularization (41). Various
studies have demonstrated that VEGF promotes angiogenesis during
acute inflammation and ischemia (42,43). VEGF also plays a role in
counteracting the local imbalance of fibrogenesis and fibrolysis,
leading to an accumulation of immature subepithelial matrix in
collagenous colitis (44). Using
intravital microscopy of the rat mesenteric microcirculation to
measure leukocyte-endothelium interactions, Scalia et al
(45) demonstrated that VEGF
inhibits leukocyte-epithelial cell adherence and the effects of
chronic inflammation. In the current study, we found that MFG-E8
treated animals had higher levels of VEGF in the small intestine
after I/R injury. Therefore, increasing VEGF production may be a
novel mechanism for MFG-E8-promoted mucosal healing after I/R
injury.
In summary, using an established animal model of gut
I/R such as a superior mesenteric artery occlusion, we showed that
intestinal levels of MFG-E8 are significantly reduced after I/R
injury, which correlated with increased apoptosis and impaired
barrier function. In addition, administration of rmMFG-E8,
decreases apoptosis, mitigates bacterial translocation, inhibits
neutrophil infiltration, and promotes tissue repair after gut I/R.
Thus, enhancing apoptotic cell clearance by rmMFG-E8 can be a novel
concept in the treatment of gut I/R injury.
Acknowledgements
This study was supported by the
National Institutes of Health grants R01 GM053008, R01 AG028352 and
R01 GM057468 (P.W.).
References
|
1.
|
WA OldenburgLL LauTJ RodenbergHJ EdmondsCD
BurgerAcute mesenteric ischemia: a clinical reviewArch Intern
Med16410541062200410.1001/archinte.164.10.105415159262
|
|
2.
|
J BerlangaP PratsD RemirezR GonzalezP
Lopez-SauraJ AguiarM OjedaJJ BoyleAJ FitzgeraldRJ
PlayfordProphylactic use of epidermal growth factor reduces
ischemia/reperfusion intestinal damageAm J
Pathol161373379200210.1016/S0002-9440(10)64192-212163361
|
|
3.
|
LJ BrandtSJ BoleyAGA technical review on
intestinal ischemia. American Gastrointestinal
AssociationGastroenterology118954968200010.1016/S0016-5085(00)70183-110784596
|
|
4.
|
DV RocourtVB MehtaGE BesnerHeparin-binding
EGF-like growth factor decreases inflammatory cytokine expression
after intestinal ischemia/reperfusion injuryJ Surg
Res139269273200710.1016/j.jss.2006.10.047
|
|
5.
|
HT HassounBC KoneDW MercerFG MoodyNW
WeisbrodtFA MoorePost-injury multiple organ failure: the role of
the gutShock15110200110.1097/00024382-200115010-0000111198350
|
|
6.
|
MP FinkEffect of critical illness on
microbial translocation and gastrointestinal mucosa
permeabilitySemin Respir Infect925626019947886323
|
|
7.
|
RA MatthijsenJP DerikxD KuipersRM van
DamCH DejongWA BuurmanEnterocyte shedding and epithelial lining
repair following ischemia of the human small intestine attenuate
inflammationPLoS ONE4e7045200910.1371/journal.pone.0007045
|
|
8.
|
CY HuangJK HsiaoYZ LuTC LeeLC
YuAnti-apoptotic PI3K/Akt signaling by sodium/glucose transporter 1
reduces epithelial barrier damage and bacterial translocation in
intestinal ischemiaLab
Invest91294309201110.1038/labinvest.2010.17720975661
|
|
9.
|
PR DiE EspositoE MazzonI PaternitiM
GaluppoS CuzzocreaGW0742, a selective PPAR-beta/delta agonist,
contributes to the resolution of inflammation after gut
ischemia/reperfusion injuryJ Leukoc
Biol88291301201010.1189/jlb.011005320430778
|
|
10.
|
A OberholzerC OberholzerRM MinterLL
MoldawerConsidering immunomodulatory therapies in the septic
patient: should apoptosis be a potential therapeutic target?Immunol
Lett75221224200110.1016/S0165-2478(00)00307-211166379
|
|
11.
|
PA EfronK TinsleyDJ MinnichV MonterrosoJ
WagnerP LaineeK LorrePE SwansonR HotchkissLL MoldawerIncreased
lymphoid tissue apoptosis in baboons with bacteremic
shockShock21566571200410.1097/01.shk.0000126648.58732.8c15167687
|
|
12.
|
RS HotchkissPE SwansonBD FreemanKW
TinsleyJP CobbGM MatuschakTG BuchmanIE KarlApoptotic cell death in
patients with sepsis, shock, and multiple organ dysfunctionCrit
Care Med2712301251199910.1097/00003246-199907000-0000210446814
|
|
13.
|
RS HotchkissCM CoopersmithIE
KarlPrevention of lymphocyte apoptosis - a potential treatment of
sepsis?Clin Infect Dis41Suppl
7S465S469200510.1086/43199816237649
|
|
14.
|
RS HotchkissSB OsmonKC ChangTH WagnerCM
CoopersmithIE KarlAccelerated lymphocyte death in sepsis occurs by
both the death receptor and mitochondrial pathwaysJ
Immunol17451105118200510.4049/jimmunol.174.8.511015814742
|
|
15.
|
R MahidharaTR BilliarApoptosis in
sepsisCrit Care
Med28N105N113200010.1097/00003246-200004001-00013
|
|
16.
|
A AyalaXY XinCA AyalaDE SonefeldSM KarrTA
EvansIH ChaudryIncreased mucosal B-lymphocyte apoptosis during
polymicrobial sepsis is a Fas ligand but not an endotoxin-mediated
processBlood911362137219989454767
|
|
17.
|
DE WescheJL Lomas-NeiraM PerlCS ChungA
AyalaLeukocyte apoptosis and its significance in sepsis and shockJ
Leukoc Biol78325337200510.1189/jlb.010501715817707
|
|
18.
|
DE Wesche-SoldatoCS ChungJ Lomas-NeiraLA
DoughtySH GregoryA AyalaIn vivo delivery of caspase-8 or Fas siRNA
improves the survival of septic
miceBlood10622952301200510.1182/blood-2004-10-408615941915
|
|
19.
|
R HanayamaK MiyasakaM NakayaS
NagataMFG-E8-dependent clearance of apoptotic cells, and
autoimmunity caused by its failureCurr Dir
Autoimmun9162172200616394660
|
|
20.
|
R HanayamaM TanakaK MiwaA ShinoharaA
IwamatsuS NagataIdentification of a factor that links apoptotic
cells to phagocytesNature417182187200210.1038/417182a12000961
|
|
21.
|
R HanayamaM TanakaK MiyasakaK AozasaM
KoikeY UchiyamaS NagataAutoimmune disease and impaired uptake of
apoptotic cells in MFG-E8-deficient
miceScience30411471150200410.1126/science.109435915155946
|
|
22.
|
M MiksaR WuW DongP DasD YangP
WangDendritic cell-derived exosomes containing milk fat globule
epidermal growth factor-factor VIII attenuate proinflammatory
responses in
sepsisShock25586593200610.1097/01.shk.0000209533.22941.d016721266
|
|
23.
|
M MiksaR WuW DongH KomuraD AminY JiZ WangH
WangTS RavikumarKJ TraceyP WangImmature dendritic cell-derived
exosomes rescue septic animals via milk fat globule epidermal
growth factor VIIIJ
Immunol18359835990200910.4049/jimmunol.080299419812188
|
|
24.
|
T CuiM MiksaR WuH KomuraM ZhouW DongZ
WangS HiguchiW ChaungSA BlauMilk fat globule epidermal growth
factor 8 attenuates acute lung injury in mice after intestinal
ischemia and reperfusionAm J Respir Crit Care
Med181238246201010.1164/rccm.200804-625OC19892861
|
|
25.
|
S AkakuraS SinghM SpataroR AkakuraJI KimML
AlbertRB BirgeThe opsonin MFG-E8 is a ligand for the alphavbeta5
integrin and triggers DOCK180-dependent Rac1 activation for the
phagocytosis of apoptotic cellsExp Cell
Res292403416200410.1016/j.yexcr.2003.09.01114697347
|
|
26.
|
K OshimaN AokiM NegiM KishiK KitajimaT
MatsudaLactation-dependent expression of an mRNA splice variant
with an exon for a multiply O-glycosylated domain of mouse milk fat
globule glycoprotein MFG-E8Biochem Biophys Res
Commun254522528199910.1006/bbrc.1998.01079920772
|
|
27.
|
MR TaylorJR CoutoCD ScallanRL CerianiJA
PetersonLactadherin (formerly BA46), a membrane-associated
glycoprotein expressed in human milk and breast carcinomas,
promotes Arg-Gly-Asp (RGD)-dependent cell adhesionDNA Cell
Biol16861869199710.1089/dna.1997.16.861
|
|
28.
|
DS NewburgJA PetersonGM Ruiz-PalaciosDO
MatsonAL MorrowJ ShultsML GuerreroP ChaturvediSO NewburgCD
ScallanRole of human-milk lactadherin in protection against
symptomatic rotavirus
infectionLancet35111601164199810.1016/S0140-6736(97)10322-19643686
|
|
29.
|
K MiyasakaR HanayamaM TanakaS
NagataExpression of milk fat globule epidermal growth factor 8 in
immature dendritic cells for engulfment of apoptotic cellsEur J
Immunol3414141422200410.1002/eji.20042493015114675
|
|
30.
|
C TheryA RegnaultJ GarinJ WolfersL
ZitvogelP Ricciardi-CastagnoliG RaposoS AmigorenaMolecular
characterization of dendritic cell-derived exosomes. Selective
accumulation of the heat shock protein hsc73J Cell
Biol147599610199910.1083/jcb.147.3.59910545503
|
|
31.
|
K OshimaN AokiT KatoK KitajimaT
MatsudaSecretion of a peripheral membrane protein, MFG-E8, as a
complex with membrane vesiclesEur J
Biochem26912091218200210.1046/j.1432-1033.2002.02758.x11856354
|
|
32.
|
P VeronE SeguraG SuganoS AmigorenaC
TheryAccumulation of MFG-E8/lactadherin on exosomes from immature
dendritic cellsBlood Cells Mol
Dis358188200510.1016/j.bcmd.2005.05.00115982908
|
|
33.
|
GM SwankEA DeitchRole of the gut in
multiple organ failure: bacterial translocation and permeability
changesWorld J Surg20411417199610.1007/s0026899000658662128
|
|
34.
|
WW SoubaRJ SmithDW WilmoreGlutamine
metabolism by the intestinal tractJPEN J Parenter Enteral
Nutr9608617198510.1177/01486071850090056083900455
|
|
35.
|
ML MarinAJ GreensteinSA GellerRE GordonAH
Aufses JrA freeze fracture study of Crohn’s disease of the terminal
ileum: changes in epithelial tight junction organizationAm J
Gastroenterol785375471983
|
|
36.
|
BJ AmmoriPC LeederRF KingGR BarclayIG
MartinM LarvinMJ McMahonEarly increase in intestinal permeability
in patients with severe acute pancreatitis: correlation with
endotoxemia, organ failure, and mortalityJ Gastrointest
Surg3252262199910.1016/S1091-255X(99)80067-5
|
|
37.
|
PL FariesRJ SimonAT MartellaMJ LeeGW
MachiedoIntestinal permeability correlates with severity of injury
in trauma patientsJ
Trauma4410311035199810.1097/00005373-199806000-000169637159
|
|
38.
|
CJ DoigLR SutherlandJD SandhamGH FickM
VerhoefJB MeddingsIncreased intestinal permeability is associated
with the development of multiple organ dysfunction syndrome in
critically ill ICU patientsAm J Respir Crit Care
Med158444451199810.1164/ajrccm.158.2.97100929700119
|
|
39.
|
RH YolkenJA PetersonSL VonderfechtET
FoutsK MidthunDS NewburgHuman milk mucin inhibits rotavirus
replication and prevents experimental gastroenteritisJ Clin
Invest9019841991199210.1172/JCI1160781331178
|
|
40.
|
HF BuXL ZuoX WangMA EnsslinV KotiW HsuehAS
RaymondBD ShurXD TanMilk fat globule-EGF factor 8/lactadherin plays
a crucial role in maintenance and repair of murine intestinal
epitheliumJ Clin Invest11736733683200718008006
|
|
41.
|
JS SilvestreC TheryG HamardJ BoddaertB
AguilarA DelcayreC HoubronR TamaratO Blanc-BrudeS
HeenemanLactadherin promotes VEGF-dependent neovascularizationNat
Med11499506200510.1038/nm1233
|
|
42.
|
Y WangHK HaiderN AhmadM XuR GeM
AshrafCombining pharmacological mobilization with intramyocardial
delivery of bone marrow cells over-expressing VEGF is more
effective for cardiac repairJ Mol Cell
Cardiol40736745200610.1016/j.yjmcc.2006.02.00416603183
|
|
43.
|
DE VonS MeyerD ThornD MarmeUT HoptO
ThomuschTargeting vascular endothelial growth factor pathway offers
new possibilities to counteract microvascular disturbances during
ischemia/reperfusion of the
pancreasTransplantation82543549200610.1097/01.tp.0000229434.92523.99
|
|
44.
|
T GrigaA TrommW SchmiegelO PfistererKM
MullerF BraschCollagenous colitis: implications for the role of
vascular endothelial growth factor in repair mechanismsEur J
Gastroenterol
Hepatol16397402200410.1097/00042737-200404000-0000515028972
|
|
45.
|
R ScaliaG BoothDJ LeferVascular
endothelial growth factor attenuates leukocyte-endothelium
interaction during acute endothelial dysfunction: essential role of
endothelium-derived nitric oxideFASEB J13103910461999
|