Introduction
Successful implantation is very important for the
pregnancy of species. It is not only a complex and intricate
cross-talk between the embryonic and maternal tissues, but also an
absolute requirement for the development of embryos (1,2). A
series of stages are included in the process of implantation: a
normal and functional embryo which develops to the blastocyst
stage, maternal endometrium during the implantation window (ready
to accept the blastocyst), trophoblast adhesion, penetration and
invasion. The coordination of these processes lie with the
regulation and interplay of several factors including
prostaglandins, leukotrienes, cytokines and growth factors, in
which cytokines play a critical role (3).
Cytokines are small multifunctional cell-signaling
glycoproteins that are secreted by numerous cells of the immune
system and other systems of the body and have pleiotropic
regulatory effects on endocrine, hematopoietic, nervous and immune
systems. Cytokines can be classified as proteins, peptides, or
glycoproteins, such as interleukins, interferons and tumor necrosis
factors, which play a crucial role in the initiation, development
and regulation of the immune response (4). However, as molecules, cytokines are
not only limited to their immunomodulatory role, but also involved
in several developmental processes during embryogenesis and
embryo-maternal interactions (5,6).
Entry of the blastocyst into the receptive uterine is able to
encourage trophoblastic cells and uterine epithelium to produce
cytokines that can regulate the endometrial receptivity by
modulating the expression of various adhesion molecules (6,7).
Failure of mammalian implantation and defective placental formation
sometimes result from decreased expression of cytokines and their
signaling (8).
IK cytokine, a 19 kDa factor coded by the IK gene
localized on chromosome 2p15-p14 (9), was first isolated and purified from
the conditioned culture medium of the leukemic cell line K562. It
was reported that IK cytokine inhibited class II major
histocompatibility complex (MHCII) constitutive expression
(10,11). One study demonstrated that IK
cytokine modulated HLA-DR expression on hematopoietic cells and has
an effect on growth factor-dependent CD34+ cell
proliferation and differentiation by regulating HLA-DR expression
(12). It is also accepted that
the ‘semi-foreign’ or semiallogeneic fetus can avoid maternal
immune attack during normal pregnancy. Although the mechanism
underlying the ‘tolerant’ state of the mother throughout pregnancy
has not been clearly explained, the absence of trophoblast MHCII
antigen expression is thought to be an important strategy for
ensuring the maintenance of normal pregnancy (13). IK cytokine has close relationship
with the expression of MHCII. Up to now, there are no relevant
articles addressing whether IK cytokine plays a role in the
fetomaternal immune tolerance during pregnancy. Thus, this study
examined IK cytokine expression in mouse uterus of early pregnancy
confirmed its role in implantation, and provided theoretical
evidence for the mechanism of implantation and preventive and
therapeutic methods of pregnancy failure.
Materials and methods
Materials
Ethics approval for this study was granted from our
local Ethics Committee. Adult mice of the Kunming white strain
(8–10 weeks of age and weighing 25–30 g) were obtained from the
Experimental Animal Center of Chongqing Medical University
[certificate no. SCXK(YU)2007-0001]. The mice were maintained in
specific pathogen-free (SPF) room with a controlled light schedule
(14L:10D) and a controlled temperature range (22–25°C) with free
access to food and water. All animal procedures were approved by
the Ethics Committee, Chongqing Medical University, China. The
adult female mice were mated with fertile males (female:male, 2:1)
overnight (12 h) and vaginal plugs were checked the next morning.
The day of positive vaginal plus was considered as Day 1 of
pregnancy (D1). In addition, some male mice were subjected to
vasoligation. Two weeks later, they were caged with females
(female:male, 2:1) overnight to induce preudo-pregnancy (PD1, PD1 =
the day of vaginal plug positive).
Analysis of IK cytokine expression in
mouse endometrium during early pregnancy
Total-RNA from approximately 50–100 mg mouse
endometrial tissues of D1 to D7 and PD1 to PD7 of pregnancy were
extracted using TRIzol reagent (Invitrogen) following
manufacturer’s instructions. RNA concentration was determined by
spectrophotometric measurement the ratio of
A260/A280. The expression of IK cytokine in
the mouse endometrium of D1 to D7 were detected by real-time PCR,
western blotting and immunohistochemical analysis. The expression
of this protein in mouse endometrium of PD1 to PD7 were examined
using immunohistochemical analysis. The expression of IK cytokine
at implantation site and inter-implantation site of D5 were
detected with western blotting.
Real-time PCR analysis
Primer sequences of IK cytokine (NCBI no. NM_011879)
and β-actin (synthesized by Sangon Biotech Co., Ltd., Shanghai,
China) are as follows: IK cytokine, sense, ACGCAGAATGCTATCC and
antisense, CAGA GCCTCCTTGTTGT; β-actin, sense, CCTGAGGCTCTTTT
CCAGCC and antisense, TAGAGGTCTTTACGGATGTC AACGT. Real-time PCR was
carried out by Bio-Rad CFX 96™ using SYBR-Green (Takara code:
DRR081A). cDNA of 100 ng template was added to 12.5 μl of 2X SYBR
Premix Ex Taq and 0.5 μl of each specific primer, and water to a
final volume of 25 μl. The reactions were performed for 40 cycles
according to the following parameters: denaturation at 95°C for 30
sec; annealing at 95°C for 10 sec; and extension at 56°C for 30
sec. The fluorescence signal was plotted at the end of the
extension phase of each cycle. Melting curve analysis was performed
by the instrument automatically. This experiment was repeated 3
times. To determine a normalized arbitrary value for each gene,
β-actin was used as the reference gene. Relative expression was
calculated according to the equation 2-ΔΔCt and statistically
analyzed by t-test.
Immunohistochemical analysis
Performed according to the SP-9001 Reagent kit
(Zhongshan Goldenbridge Biotechnology Co., Ltd.). The sections were
observed and phototgraphed under a microscope (Olympus BX51). The
intensity of positive expressions were determined by Image
Pro® Plus v.6.0 software. Non-immune serum was used
instead of the primary antibody for negative control.
Western blot analysis
Approximate 100 mg mouse endometrial tissues were
homogenized in 200 μl lysis buffer (Beyotime, China) and 2 μl
chymostatin PMSF (100 mmol/l). Total protein was extracted from the
mouse endometrium of each group using the total protein TriPure
reagent kit (Takara Bio, Inc., China) according to the
manufacturer’s instructions. The protein concentration was examined
by the Bradford assay. Proteins samples (about 50 μl each sample)
were subjected to 8% SDS-PAGE gels and then transferred onto PVDF
membranes by a Bio-Rad electroblot apparatus (Bio-Rad, Beijing,
China). The membrane was incubated in blocking PBS containing 0.05%
Tween-20 (TBST) and 5% nonfat milk for 1.5 h at room temperature
and then was subsequently incubated with monoclonal rabbit anti-IK
cytokine antibody (1:500, Santa Cruz Biotechnology, Inc., IK
(G-13): sc-135485) and β-actin (1:500, Zhongshan Goldenbridge
Biotechnology Co., Ltd., TA-09) at 4°C for 12 h, washed for three
times with PBST (5 min each time), following by incubation with
secondary antibody (goat anti-rabbit IgG) conjugated with
horseradish peroxidase (Zhongsan Biosciences, Inc., Beijing, China)
for 2 h at room temperature, and then washed three times with PBST
(5 min each time). Protein bands were visualized using
diaminobenzidine tetrahydrochloride (DAB). Densitometry analysis of
IK cytokine expression was performed by Quantity One version 4.4.0
software. The β-actin protein was used as the internal control.
Uterine horns injection of IK cytokine
oligodeoxynucleotides
Ninety female mice were given a uterine horn
injection under 3% pentobarbital sodium anesthetic on D3 according
to the procedures described by Zhu et al (14). Among the 90 mice, 60 were injected
with 10 μg antisense IK cytokine oligodeoxynucleotides (IK cytokine
A-ODNs, diluted in 100 μl double-distilled water) in the left of
uterine horns and equal volum of sense IK cytokine
oligodeoxynucleotides (IK cytokine S-ODNs) in the right of the
uterine horns. The remaining 30 mice were injected with equal
volume of distilled water in both uterine horns as control. Fifteen
mice receiving respectively injection of IK cytokine A-ODNs and IK
cytokine S-ODNs in the both horns were killed after 24 h of
injection (D4) and another 15 mice with the same treatment were
sacrificed after 48 h of injection (D5), the tissues of endometrium
were collected for the detection of expression of IK cytokine and
MHCII antigen by immunohistochemical and western blot analysis.
Another 30 receiving respectively injection of IK cytokine A-ODNs
and IK cytokine S-ODNs and 30 mice which were injected in both
horns with distilled water were sacrificed on D7 and the number of
implanted embryos was recorded in each uterine horn. The ODNs were
designed and synthesized by Biosune Co., Ltd. (Shanghai, China).
The sequence of the IK cytokine A-ODNs was,
5′-GTGAGCCCTTCTCTAACCCT-3′-FITC; and the sequence of IK S-ODNs was
5′-AGGGTTAGAGAAG GGCTCAC-3′-FITC. To ensure their long half-lives
in cells, the sequences were thiophosphate-modified. MHCII antigens
(NCBI no. XM_003457013) (synthesized by Sangon Biotech Co., Ltd.)
were as follows: MHCII sense: AAGAA GGAGACTGTCTGGATGC and
antisense: TGAATGATGAA GATGGTGCCC.
Statistical analysis
All statistical data obtained from the experiment
were analyzed using SPSS software (version 11.5), and t-test was
used to analyze the data. Differences were considered significant
at P<0.05.
Results
Expression of IK cytokine mRNA
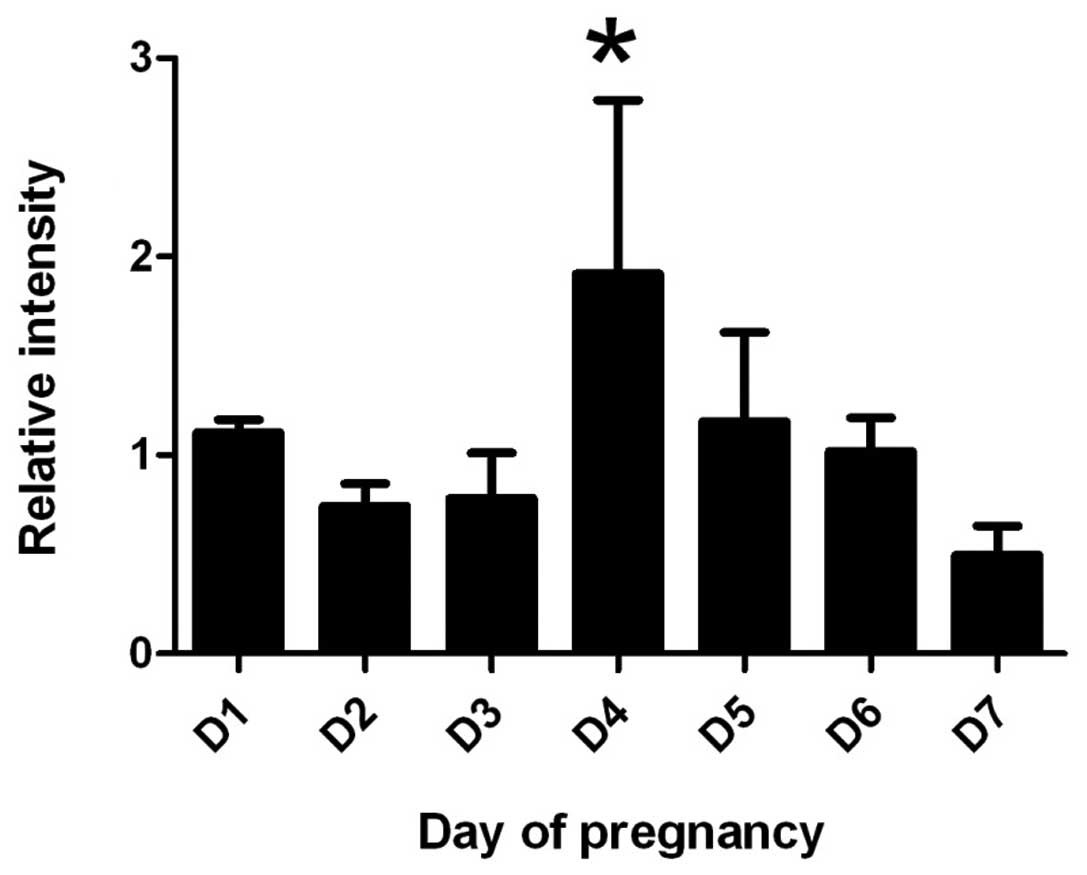
By real-time PCR, the expression of IK cytokine mRNA
increased gradually from D1 to D4 in mouse endometrium and reached
a peak level at D4 (P<0.05), and then decreased gradually from
D5 to D7 (Fig. 1).
Expression of IK cytokine protein
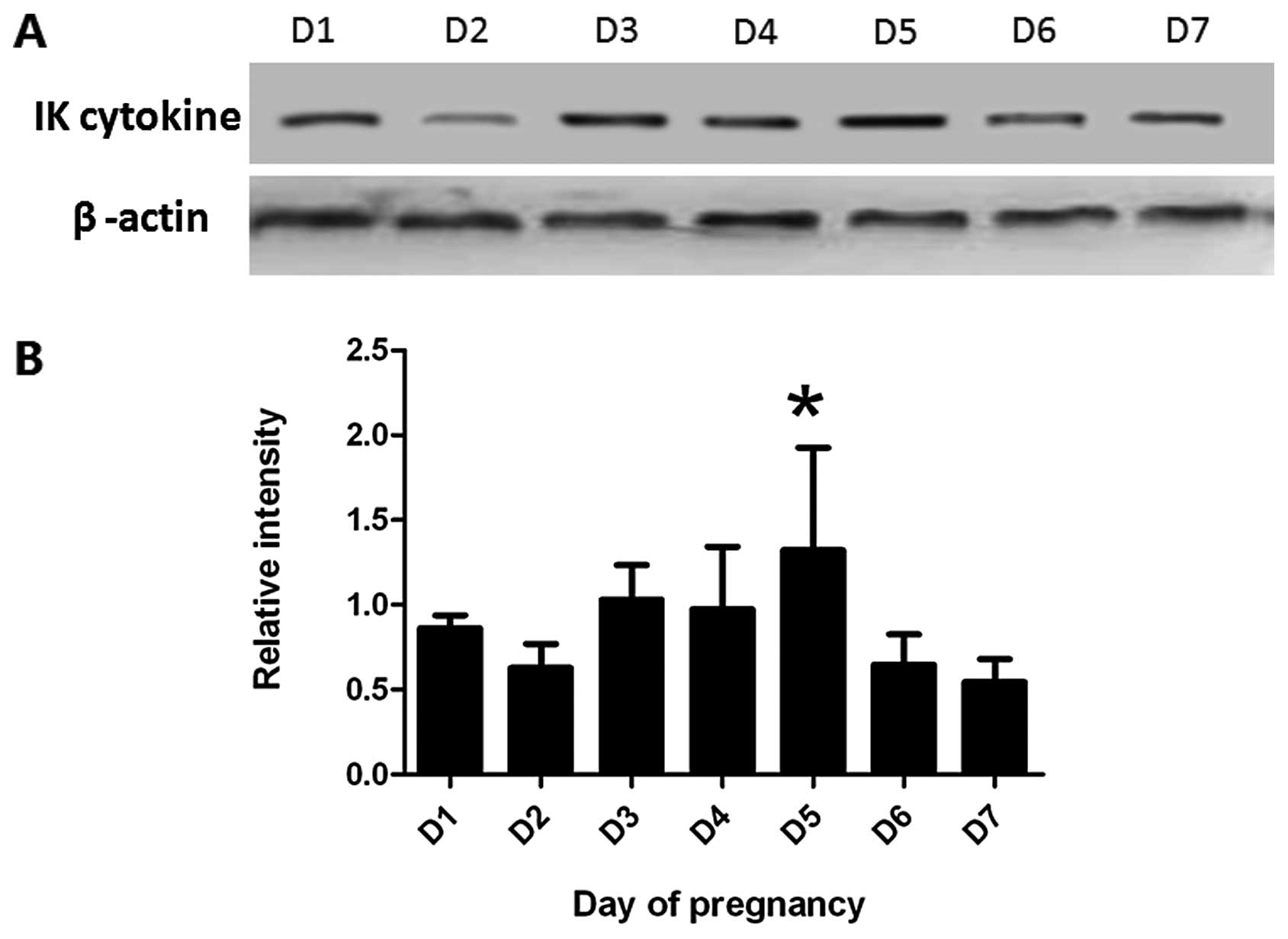
By western blotting, the expression of IK cytokine
protein increased gradually from D1 to D5 in mouse endometrium and
reached a peak level at D5 (P<0.05), and then decreased
gradually from D6 to D7 (Fig. 2).
The change of IK cytokine protein expression coincided with the
mRNA levels detected by real-time PCR.
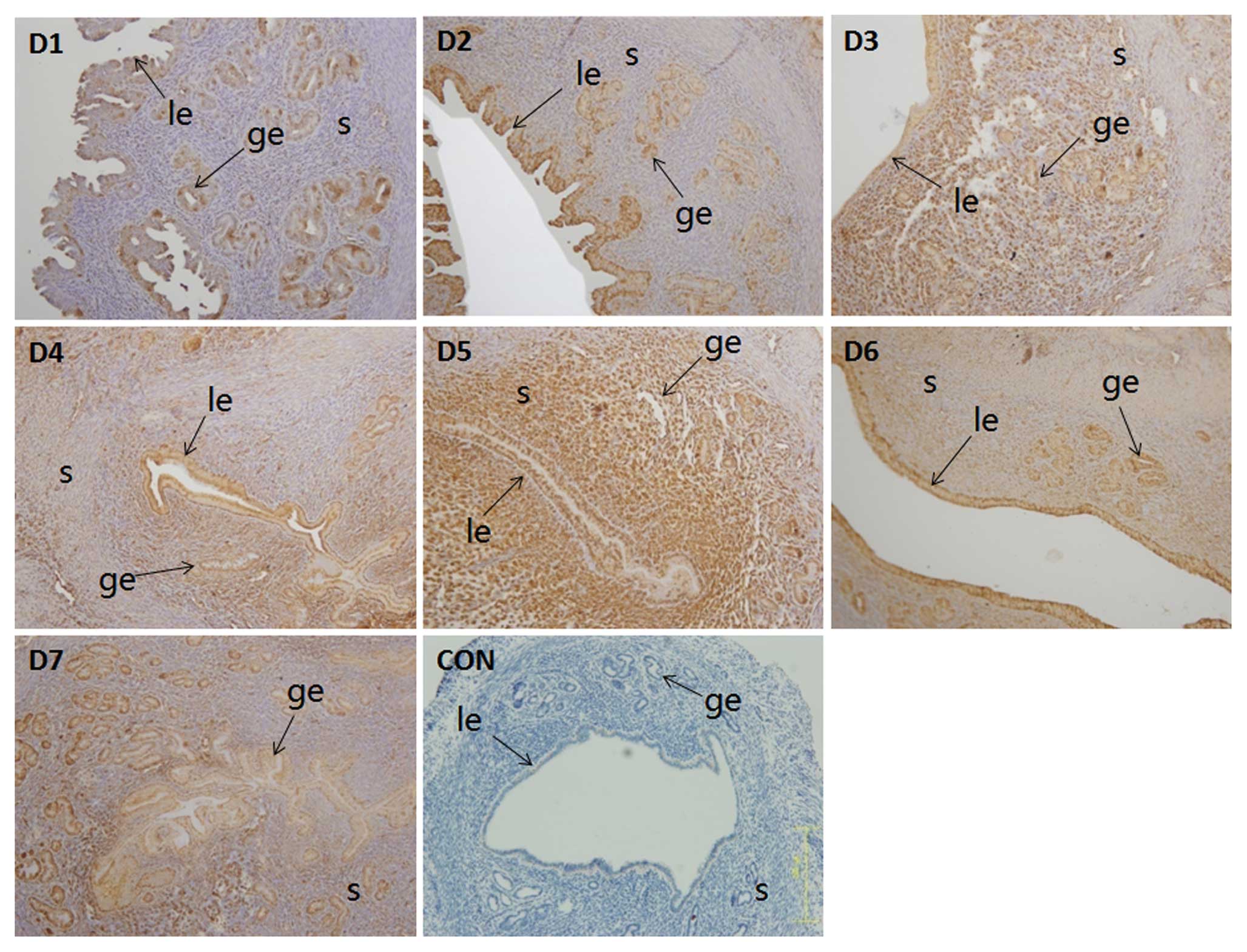
Location of IK cytokine expression
By immunohistochemical analysis, the weak expression
of IK cytokine was observed and mainly localized in the luminal and
the glandular epithelium on D1 and D2. The highest expression
occured on D3 to D5 in the luminal epithelium, glandular epithelium
and stromal cells, following by decreasing gradually from D6 to D7
(Fig. 3). The change of IK
cytokine expression coincided with the mRNA levels detected by
real-time PCR.
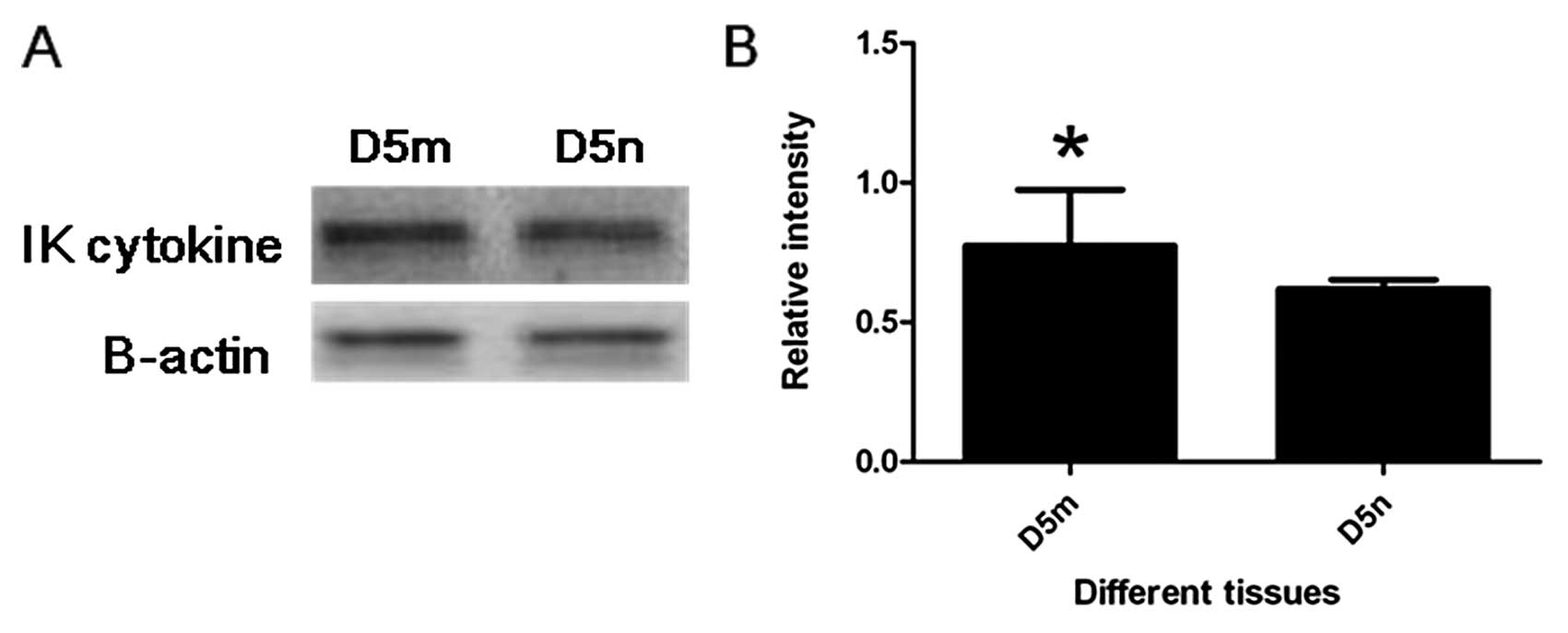
Expression of IK cytokine protein in
endometrium at the implantation site and the inter-implantation
site
By western blotting, IK cytokine expression at the
implantation site was significantly stronger than that at the
inter-implantation site on D5 (P<0.05) (Fig. 4).
Expression of IK cytokine in mouse
endometrium during pseudopregnancy
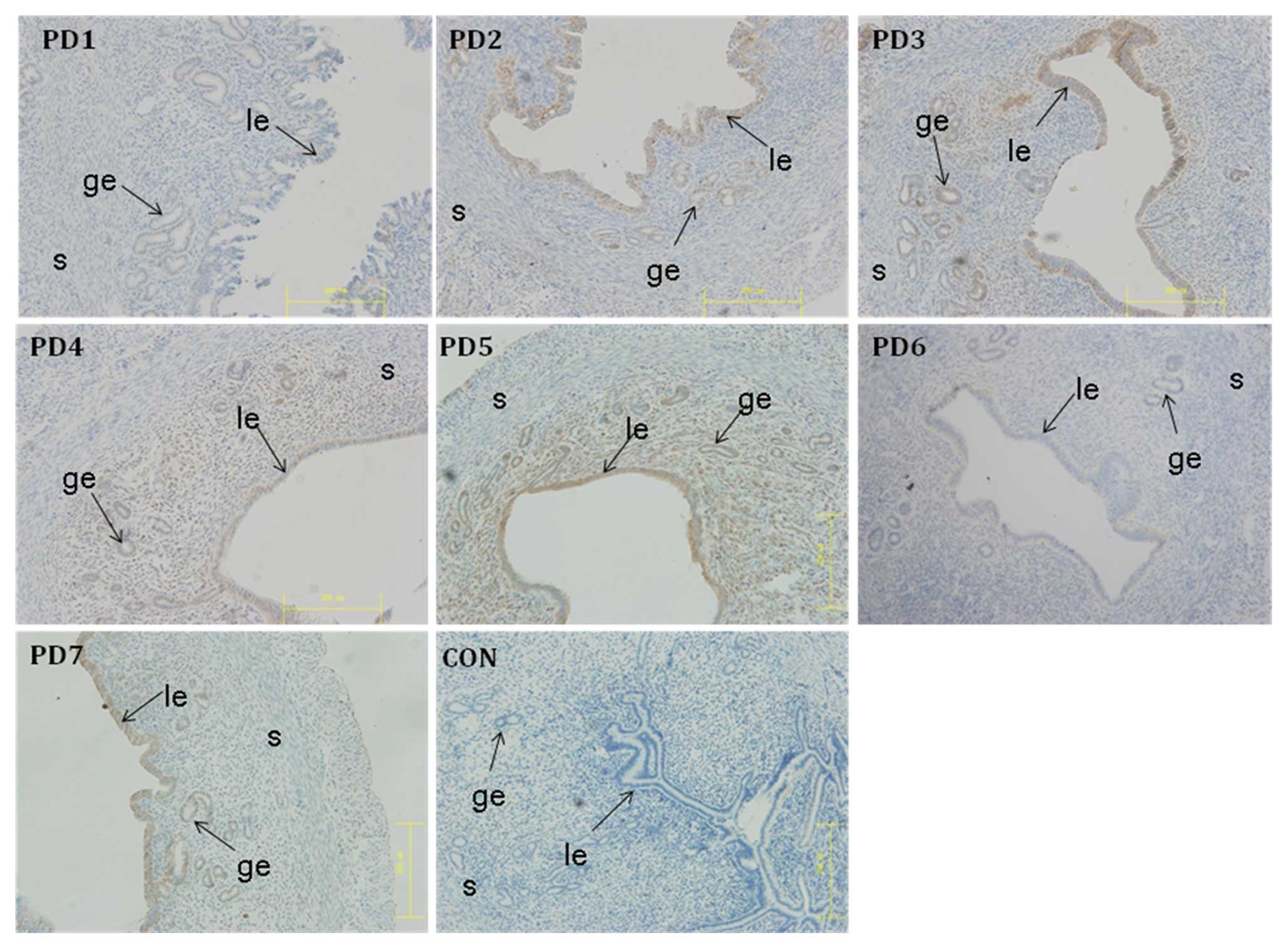
The expression of IK cytokine in mouse endometrium
on PD1 to PD7 were analyzed by immunohistochemical analysis. The
protein was weakly expressed on PD1, increased slightly on PD2 to
PD3, sharply decreased on PD4 in the luminal and the glandular
epithelium, with a remaining low level on PD5 to PD7. The
expression of the protein never reached a peak in the mouse
endomentrium during pseudopregnancy (Fig. 5).
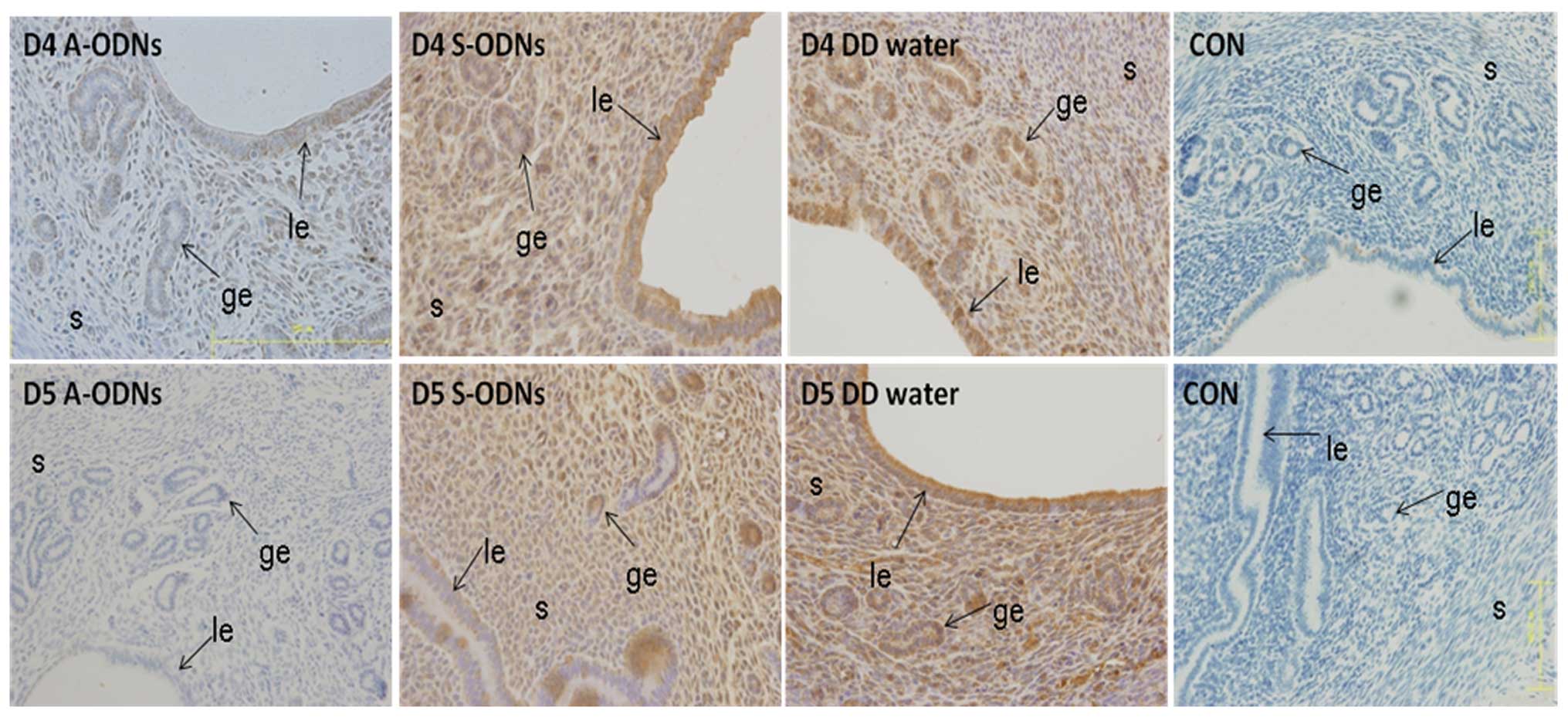
Inhibition of IK cytokine expression in
the endometrium of pregnant mice receiving IK cytokine A-ODNs and
effect on embryo implantation
Mice uterine horns received injection of IK cytokine
A-ODNs, IK cytokine S-ODNs and distilled water on D3. The
expression of IK cytokine assessed by immunohistochemistry was
sharply inhibited on the side of the uterine horns receiving IK
cytokine A-ODNs after 24 and 48 h (i.e. D4 and D5) (P<0.05). In
contrast, the expression of this protein was not changed in the
control groups which were injected with IK cytokine S-ODNs or
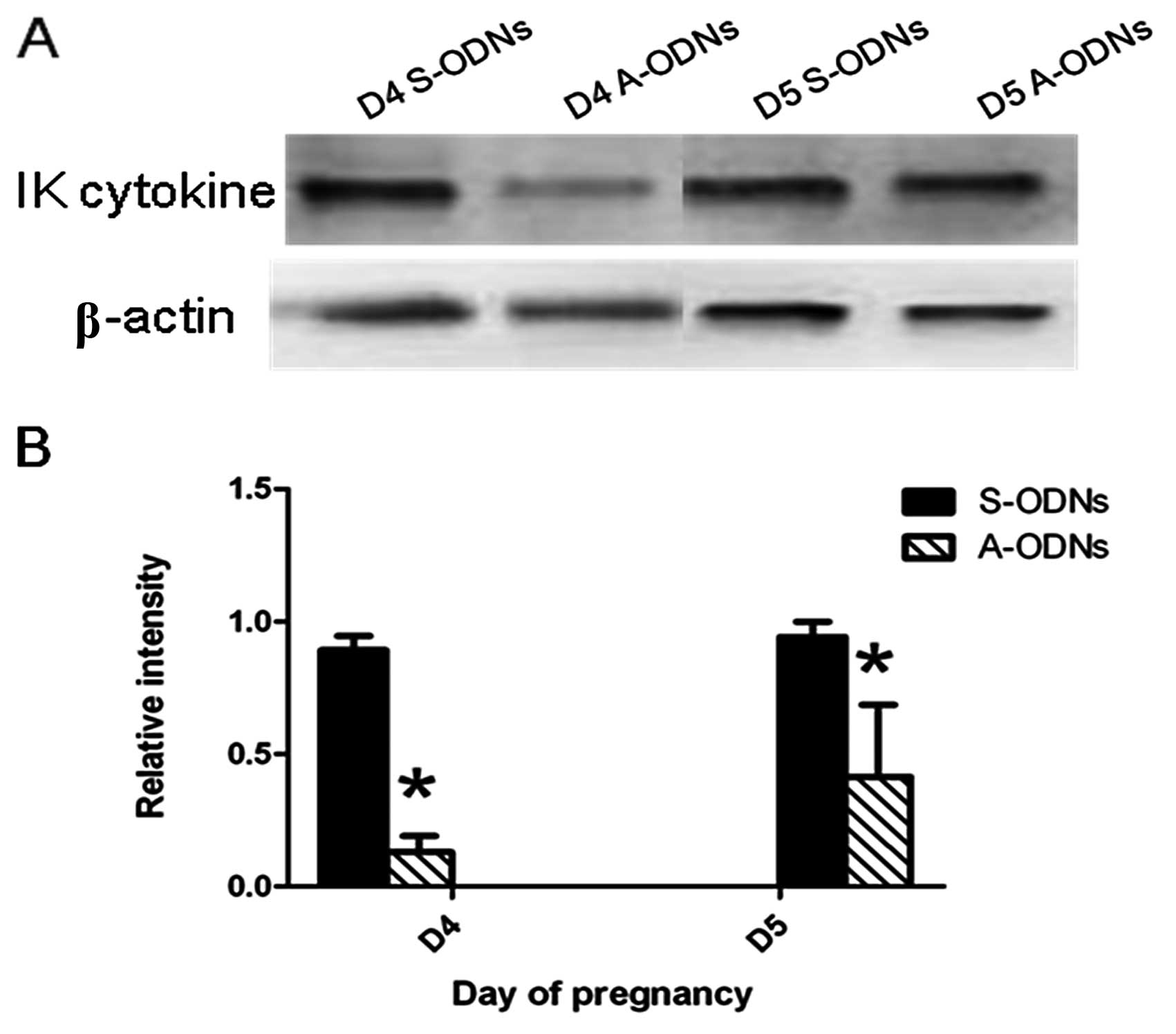
distilled water (Fig. 6). The
results from western blot analysis were similar to those of the
immunohistochemical analysis (Fig.
7). This indicated that the expression of IK cytokine was
indeed inhibited by IK cytokine A-ODNs.
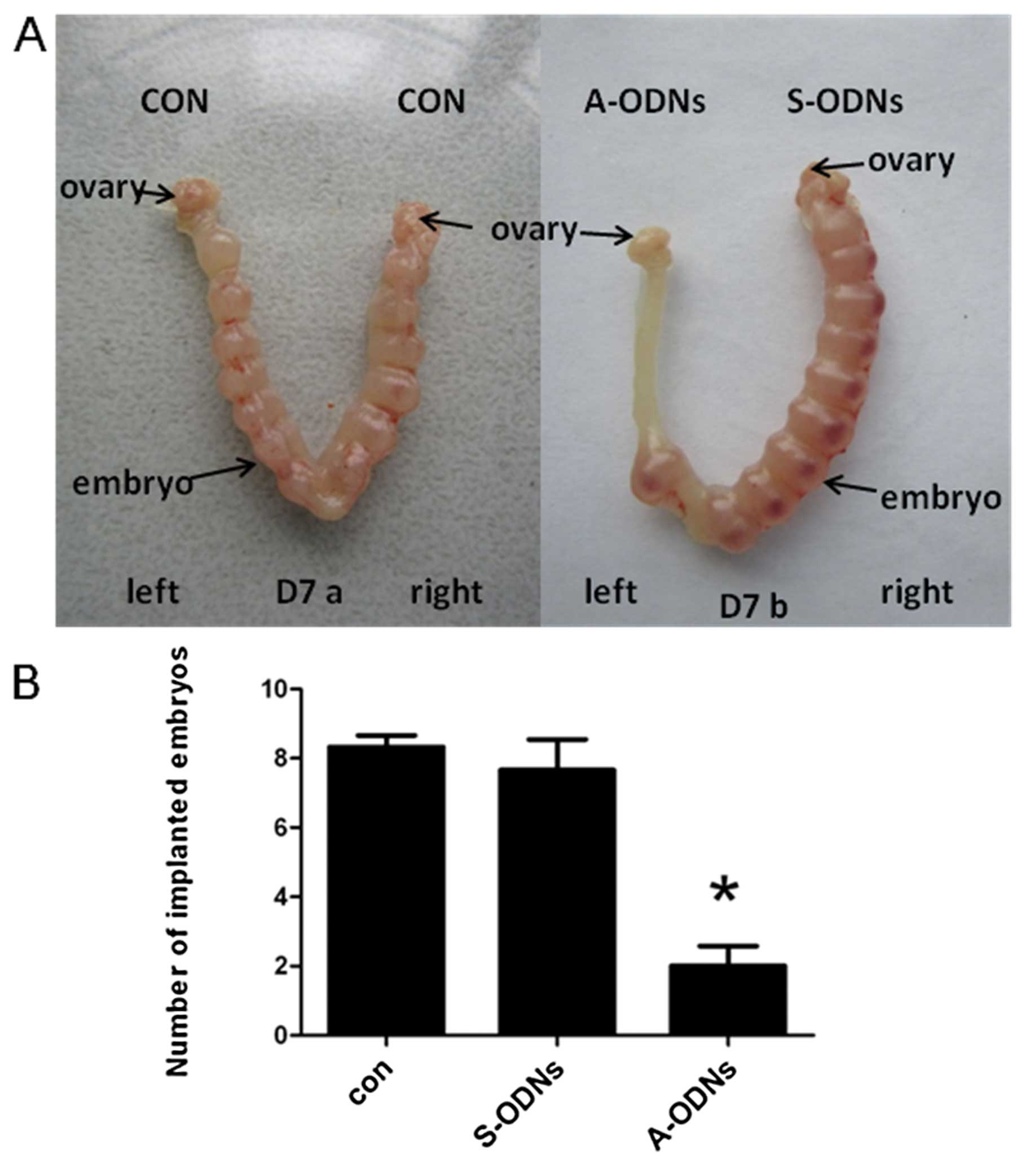
The mice, which received either injection of IK
cytokine A-ODNs or IK cytokine S-ODNs or distilled water in the
uterine horns on D3, were sacrificed on D7 under anesthesia. The
number of implanted embryos in each uterine horn was recorded by
dissection. The number of implanted embryos was sharply reduced in
the A-ODNs-treated group (P<0.05), but no embryo abnormality was
observed. In contrast, no significant change in the number of
implanted embryos was observed on the side of the uterine horns of
mice receiving IK cytokine S-ODNs and those receiving the distilled
water which were used as the controls (Fig. 8A). At the same time, all the
embryos in the S-ODNs-treated horns had a normal appearance and
size as compared with those in the water-treated control group,
implying that both the ODNs itself and the dose used in this study
was non-toxic to embryo implantation.
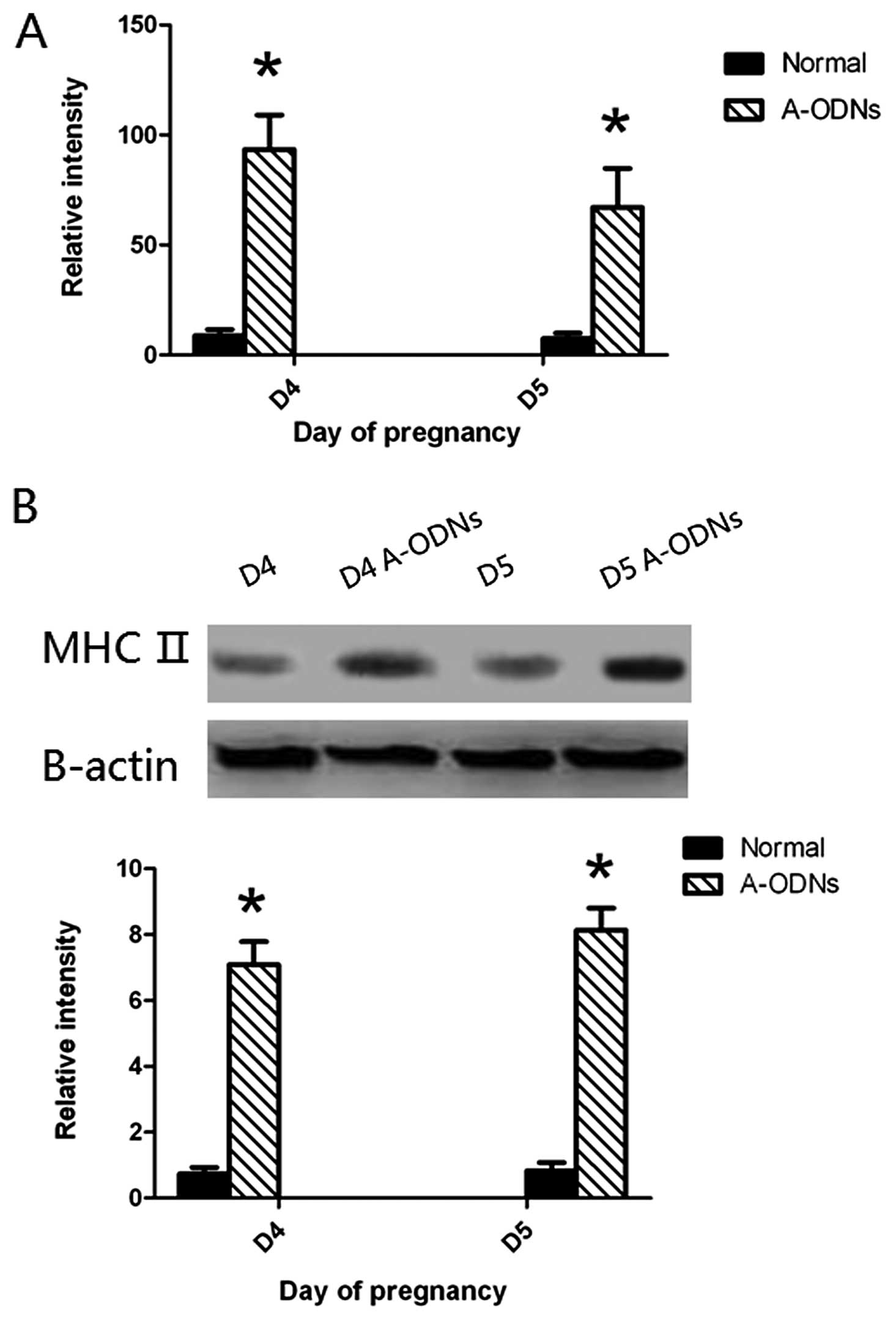
Effect of IK cytokine A-ODNs on the
expression of MHCII in mouse endometrium during early
pregnancy
To understand whether MHCII expression in mouse
endometrium of the implantation window would be influenced after IK
cytokine expression was suppressed by IK cytokine A-ODNs, MHCII
expression were detected by real-time PCR, western blotting before
and after injection of IK cytokine A-ODNs. This result revealed
that MHCII expression in the mouse endometrium was much stronger 24
and 48 h after receiving injection of IK cytokine A-ODNs
(P<0.05) (Fig. 9).
Discussion
Embryonic implantation is a complicated process
which needs the coordination between a blastocyst capable of
implanting and an endometrium receptive to the embryo. A variety of
molecules produced by embryonic and maternal tissues play a
critical role in the regulation of the process (15–17). The endometrium is receptive to the
embryo during only a limited and restricted phase (D4–D5 of
pregnancy) referred to as the window of implantation (18,19). Certain molecules are expressed
during the window of implantation and a number of them were
recognized as biochemical markers of uterine receptivity (20–22). This study indicates that the
expression of IK cytokine in the mouse endometrium during early
pregnancy is time-dependent. The expression of IK cytokine mRNA
increased gradually from D1 to D4 and reached a peak level on D4.
Western blot analysis and immunohistochemistry also showed that its
protein expression increased from D1 to D5 and reached a peak level
on D5, coinciding with the expression of IK cytokine mRNA on
space-time. Additionally, IK cytokine expression at the
implantation sites was much higher than that at the
inter-implantation site. However, the expression IK cytokine
protein in pseudopregnant mouse endometrium was markedly lower than
that in normal pregnant status and no peak level was observed
during the whole pseudopregnant period. It was known that
oligodeoxynucleotide can be effectively absorbed by the uterine
luminal and glandular epithelium and stroma cells (23). Antisense oligodeoxynucleotide is
known to have a half life of 24–48 h in certain tissues (24). Therefore, one may anticipate that
the IK A-ODNs which were injected into the uterine horns on D3 can
survive for the subsequent D4 and D5 and remain effective for
suppression of IK expression. In this study, the performance of
injecting IK cytokine A-ODNs into the uterine horns on D3 led to
suppressed expression of IK cytokine in the endometrium after 24
and 48 h, and in a reduced number of implanted embryos. The
characteristic of IK cytokine expression at the implantation window
and implantation sites as well as the reduction of implanted
embryos due to the inhibition of IK cytokine expression strongly
imply that IK cytokine may be essential for embryo
implantation.
The conception of ‘the fetus as a semiallogeneic
graft’ was proposed by Medawar in the early 1950s because the
mammalian fetus can express paternally derived polymorphic
antigens. As a result, the mammalian fetus is potentially at risk
of attack by the maternal immune system during pregnancy. This kind
of immunological situation also exist in transplantation rejection
reactions (25). However, the
fetus is tolerant of the maternal tissues during the whole period
of gestation. The immunological interplay between the embryonic and
maternal tissues depend on the antigens which are expressed on the
surface of the fetus and the maternal recognition and reaction to
them. A series of mechanisms may participate in modulating maternal
immune tolerance throughout gestation, among which the expression
of the major histocompatibility complex (MHC) antigen was
considered to play a critical role (13). Class II MHC antigens are expressed
constitutively on the surface of antigen-presenting cells (APCs)
such as dendritic cells, macrophages and B cells. It has been
proposed to be critically important for the initiation, development
and modulation of the immune response (11). In addition, MHCII expression can
be induced in the majority of cells by numerous cytokines,
especially by interferon-γ (IFN-γ) (26). It has been reported that aberrant
expression of MHCII antigens was observed in the target organs of
autoimmune diseases and its expression level correlated closely
with the intensity of the immune responses (27,28). The inhibition of MHC antigen
expression in trophoblasts is crucial for the evasion of rejection
by the maternal immune system to the fetus with paternal antigens
and for the maintenance of successful pregnancy (29). Trophoblast cells lack constitutive
expression of MHCII antigens and cannot be induced to express MHCII
molecules by IFN-γ (13,29). Inappropriate MHCII expression has
been reported to be recognized in trophoblasts from the placentas
of women suffering abortion (30–32). These facts demonstrated that the
suppression of MHCII antigens was crucial for the maintenance of
successful pregnancy. It has been confirmed that IK cytokine can
inhibit expression of MHCII antigen (10,11). In the present study, we found that
the expression of IK cytokine in the mouse endometrium reached a
peak level at ‘implantation window’, but a significant reduction of
its protein expression and a remarkable increase of MHCII
expression were observed on D4 and D5 (implantation window) after
treatment with IK A-ODNs on D3. A significant decrease of the
number of implanted embryos was also found. These findings suggest
that the suppression of MHCII antigens by IK cytokine was likely to
inhibit the fetal-maternal immune response, contributing to
maternal immune tolerance of conceptus and the maintenance of
successful pregnancy. When the expression of MHCII is no longer
suppressed because of the inhibition of IK cytokine expression, it
will result in attacking maternal immune system will elicit a
response against the fetus which will influence embryo
implantation. However, embryo implantation has not been completely
inhibited by suppressing the expression of IK cytokine, implying
involvement of certain other mechanisms participating in the
regulation of fetal-maternal immune tolerance, which remains to be
further investigated.
Our data have revealed characterization of IK
cytokine expression in the mouse endometrium during early pregnancy
and its significance an embryo implantation. However, the molecular
mechanism of IK cytokine in modulating fetal-maternal immune
tolerance and embryo implantation remains to be further
examined.
Acknowledgements
This study was supported by grants
from the National Natural Science Foundation of China (nos.
31071278 and 30973195) and the Scientific Research Foundation of
Chongqing Science and Technology Commission (CSTC 2009BA5082).
References
|
1.
|
T Garrido-GómezF DominguezC SimónDioxins:
a novel regulator in embryo implantationSci World J112352412011
|
|
2.
|
B GuoZ TianBC HanXM ZhangZM YangZP
YueExpression and hormonal regulation of Hoxa10 in canine uterus
during the peri-implantation periodReprod Domest
Anim44638642200910.1111/j.1439-0531.2007.01037.x18992082
|
|
3.
|
Z MadejaH YadiR AppsS BoulenouarSJ RoperL
GardnerA MoffettF ColucciM HembergerPaternal MHC expression on
mouse trophoblast affects uterine vascularization and fetal
growthProc Natl Acad Sci
USA10840124017201110.1073/pnas.100534210821300875
|
|
4.
|
JG CannonInflammatory cytokines in
nonpathological statesNews Physiol Sci15298303200011390930
|
|
5.
|
S SaitoCytokine cross-talk between mother
and the embryo/placentaJ Reprod
Immunol521533200110.1016/S0165-0378(01)00112-711600175
|
|
6.
|
M SinghP ChaudhryE AsselinBridging
endometrial receptivity and implantation: network of hormones,
cytokines, and growth factorsJ
Endocrinol210514201110.1530/JOE-10-046121372150
|
|
7.
|
C SimónJC MartínA PellicerParacrine
regulators of implantationBaillieres Best Pract Res Clin Obstet
Gynaecol148158262000
|
|
8.
|
O Guzeloglu-KayisliUA KayisliHS TaylorThe
role of growth factors and cytokines during implantation: endocrine
and paracrine interactionsSemin Reprod
Med276279200910.1055/s-0028-110801119197806
|
|
9.
|
P KriefY Augery-BourgetS PlaisanceMF
MerckE AssierV TanchouM BillardC BoucheixC JasminB AzzaroneA new
cytokine (IK) down-regulating HLA class II: monoclonal antibodies,
cloning and chromosome localizationOncogene93449345619947970704
|
|
10.
|
J VedrenneE AssierR PerenoH
Bouzinba-SegardB AzzaroneC JasminD CharronP KriefInhibitor (IK) of
IFN-gamma induced HLA class II antigens expression also inhibits
HLA class II constitutive expression in the human Raji B cell
lineOncogene1414531461199710.1038/sj.onc.12009719136989
|
|
11.
|
M MuraokaH HasegawaM KohnoA InoueT
MiyazakiM TeradaM NoseM YasukawaIK cytokine ameliorates the
progression of lupus nephritis in MRL/lpr miceArthritis
Rheum5435913600200610.1002/art.2217217075801
|
|
12.
|
LX CaoMC Le Bousse KerdilesD ClayS
OshevskiC JasminP KriefImplication of a new molecule IK in
CD34+ hematopoietic progenitor cell proliferation and
differentiationBlood893615362319979160666
|
|
13.
|
SP MurphyTB TomasiAbsence of MHC class II
antigen expression in trophoblast cells results from a lack of
class II transactivator (CIITA) gene expressionMol Reprod
Dev51112199810.1002/(SICI)1098-2795(199809)51:1%3C1::AID-MRD1%3E3.0.CO;2-L9712312
|
|
14.
|
LJ ZhuMK BagchiIC BagchiAttenuation of
calcitonin gene expression in pregnant rat uterus leads to a block
in embryonic implantationEndocrinology13933033919989421431
|
|
15.
|
S TabibzadehA BabakniaThe signals and
molecular pathways involved in implantation, a symbiotic
interaction between blastocyst and endometrium involving adhesion
and tissue invasionHum
Reprod1015791602199510.1093/HUMREP/10.6.1579
|
|
16.
|
A MakrigiannakisV MinasMechanisms of
implantationReprod Biomed Online175822007
|
|
17.
|
T Garrido-GómezF DominguezC
SimónProteomics of embryonic implantationHandb Exp
Pharmacol19867782010
|
|
18.
|
S YoshiokaH FujiwaraT NakayamaK KosakaT
MoriS FujiiIntrauterine administration of autologous peripheral
blood mononuclear cells promotes implantation rates in patients
with repeated failure of IVF-embryo transferHum
Reprod2132903294200610.1093/humrep/del312
|
|
19.
|
LC GiudicePotential biochemical markers of
uterine receptivityHum Reprod14Suppl
2S3S16199910.1093/humrep/14.suppl_2.3
|
|
20.
|
LA SalamonsenG NieE DimitriadisL RobbJK
FindlayGenes involved in implantationReprod Fertil
Dev134149200110.1071/RD0004611545164
|
|
21.
|
A TapiaC VilosJC MarínHB CroxattoL
DevotoBioinformatic detection of E47, E2F1 and SREBP1 transcription
factors as potential regulators of genes associated to acquisition
of endometrial receptivityReprod Biol Endocrinol279142011
|
|
22.
|
V MinasD LoutradisA MakrigiannakisFactors
controlling blastocyst implantationReprod Biomed
Online10205216200510.1016/S1472-6483(10)60942-X15823225
|
|
23.
|
JX YuanLJ XiaoCL LuXS ZhangT LiuM ChenZY
HuF GaoIncreased expression of heat shock protein 105 in rat uterus
of early pregnancy and its significance in embryo
implantationReprod Biol
Endocrinol723200910.1186/1477-7827-7-2319284651
|
|
24.
|
RW WagnerGene inhibition using antisense
oligodeoxynucleotidesNature372333335199410.1038/372333a07969490
|
|
25.
|
YW LokeA KingImmunology of human placental
implantation: clinical implications of our current understandingMol
Med Today3153159199710.1016/S1357-4310(97)01011-39134528
|
|
26.
|
V SteimleCA SiegristA MottetB
Lisowska-GrospierreB MachRegulation of MHC class II expression by
interferon-γ mediated by the transactivator gene
CIITAScience2651061091994
|
|
27.
|
J GuardiolaA MaffeiControl of MHC class II
gene expression in autoimmune, infectious, and neoplastic diseases
(Review)Crit Rev Immunol1324726819938110378
|
|
28.
|
PL CheahLM LooiCT ChuaSF YapS
FlemingEnhanced major histocompatibility complex (MHC) class II
antigen expression in lupus nephritisMalays J
Pathol19115120199710879251
|
|
29.
|
JA PeymanRepression of major
histocompatibility complex genes by a human trophoblast ribonucleic
acidBiol Reprod602331199910.1095/biolreprod60.1.239858482
|
|
30.
|
I AthanassakisY AifantisA MakrygiannakisE
KoumantakisS VassiliadisPlacental tissue from human miscarriages
expresses class II HLA-DR antigensAmer J Reprod
Immunol34281287199510.1111/j.1600-0897.1995.tb00954.x8595127
|
|
31.
|
JA LataRS TuanKJ ShepleyMM MulliganLG
JacksonJB SmithLocalization of major histocompatibility complex
class I and II mRNA in human first-trimester chorionic villi by in
situ hybridizationJ Exp
Med17510271032199210.1084/jem.175.4.10271552281
|
|
32.
|
S Chatterjee-HasrouniPK LalaMHC antigens
on mouse trophoblast cells: Paucity of Ia antigens despite the
presence of H-2K and DJ Immunol1272070207319816946147
|