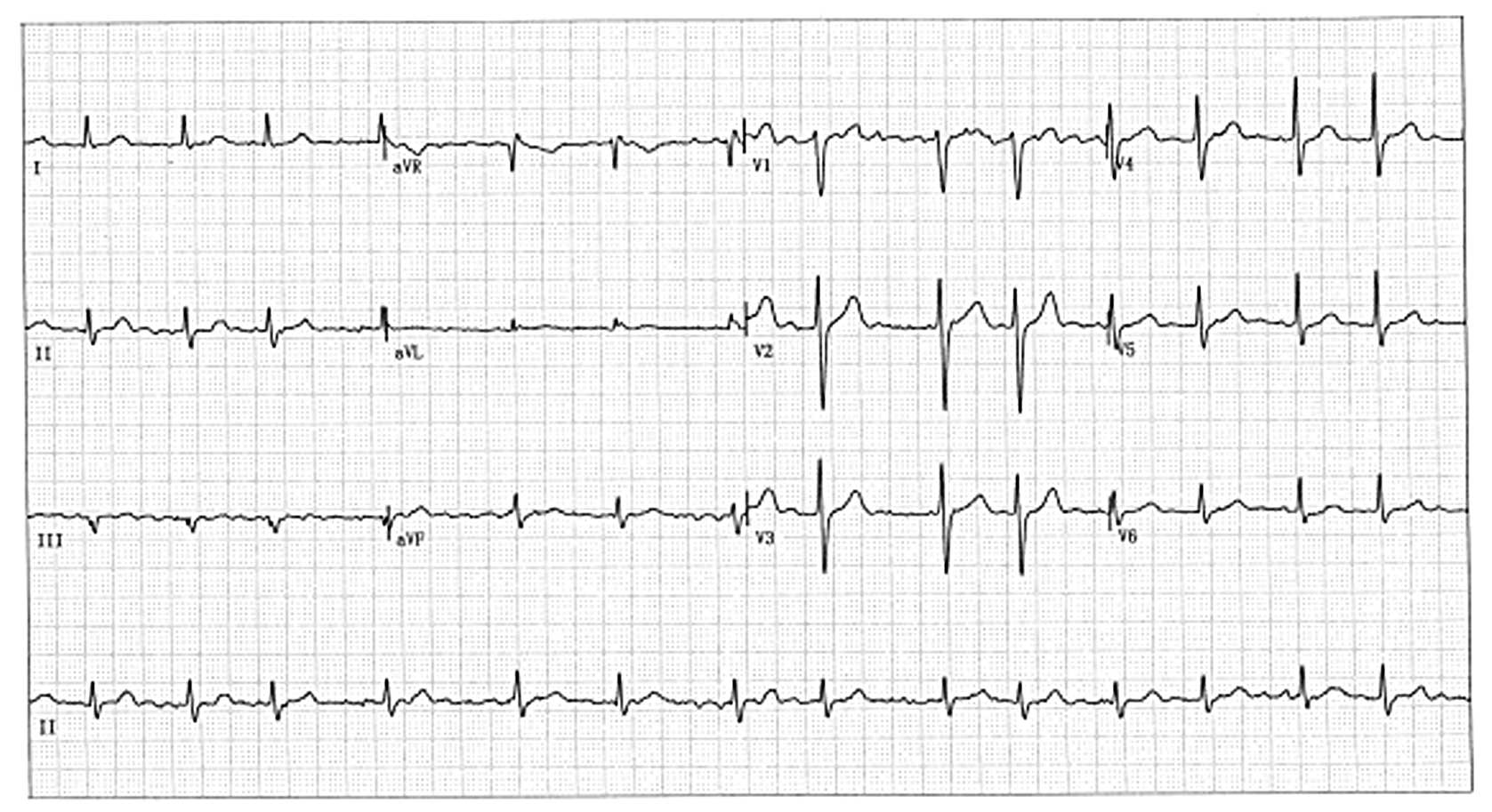
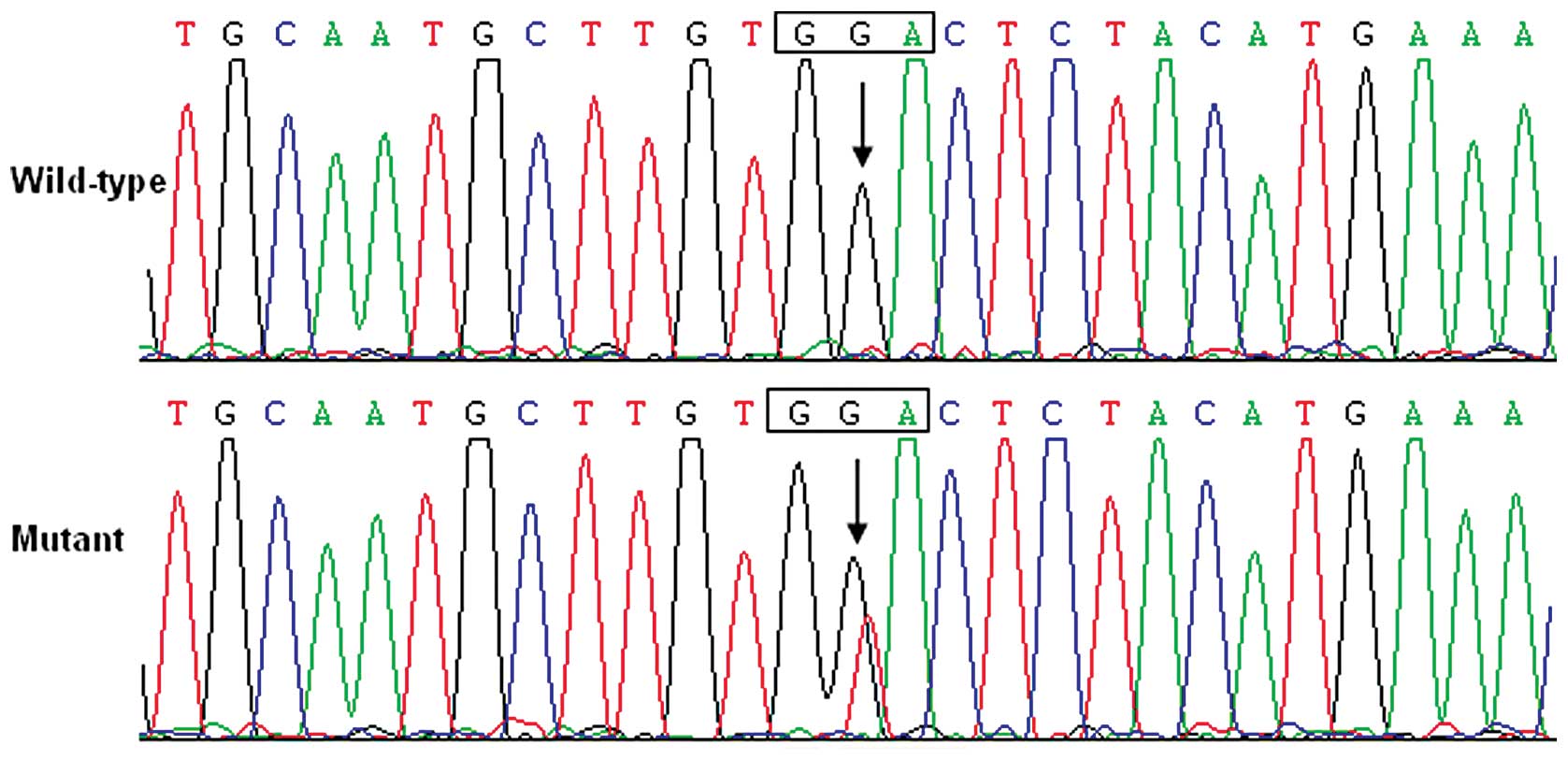
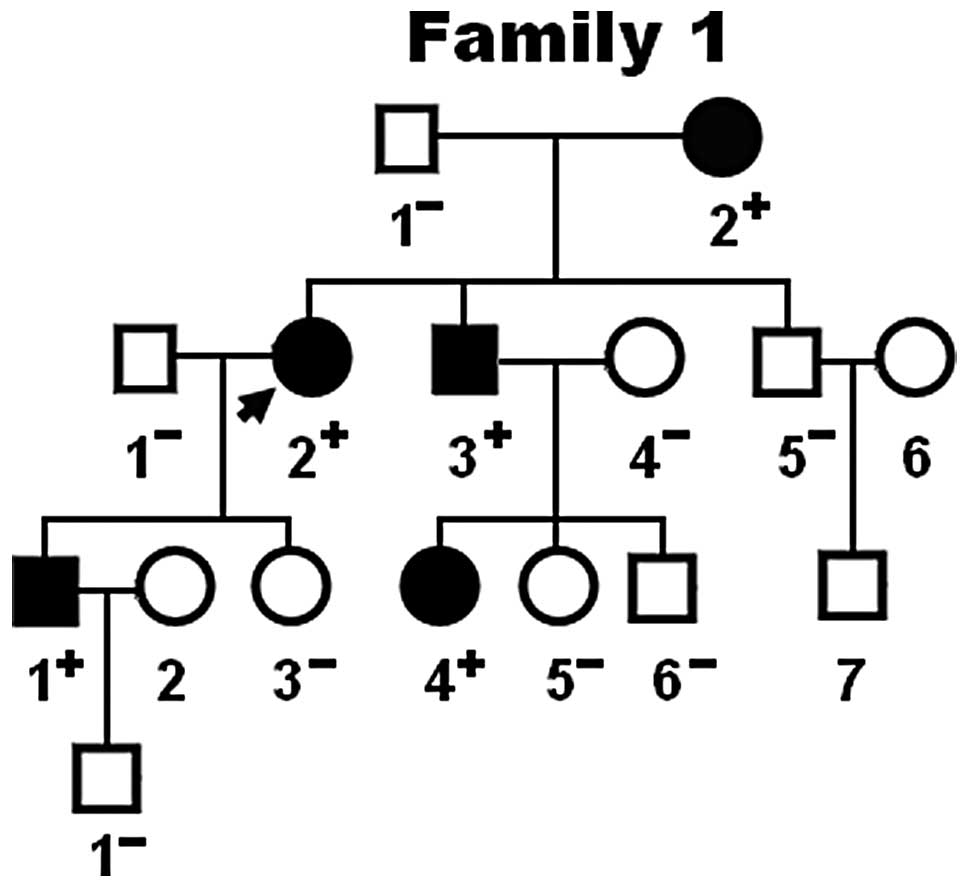
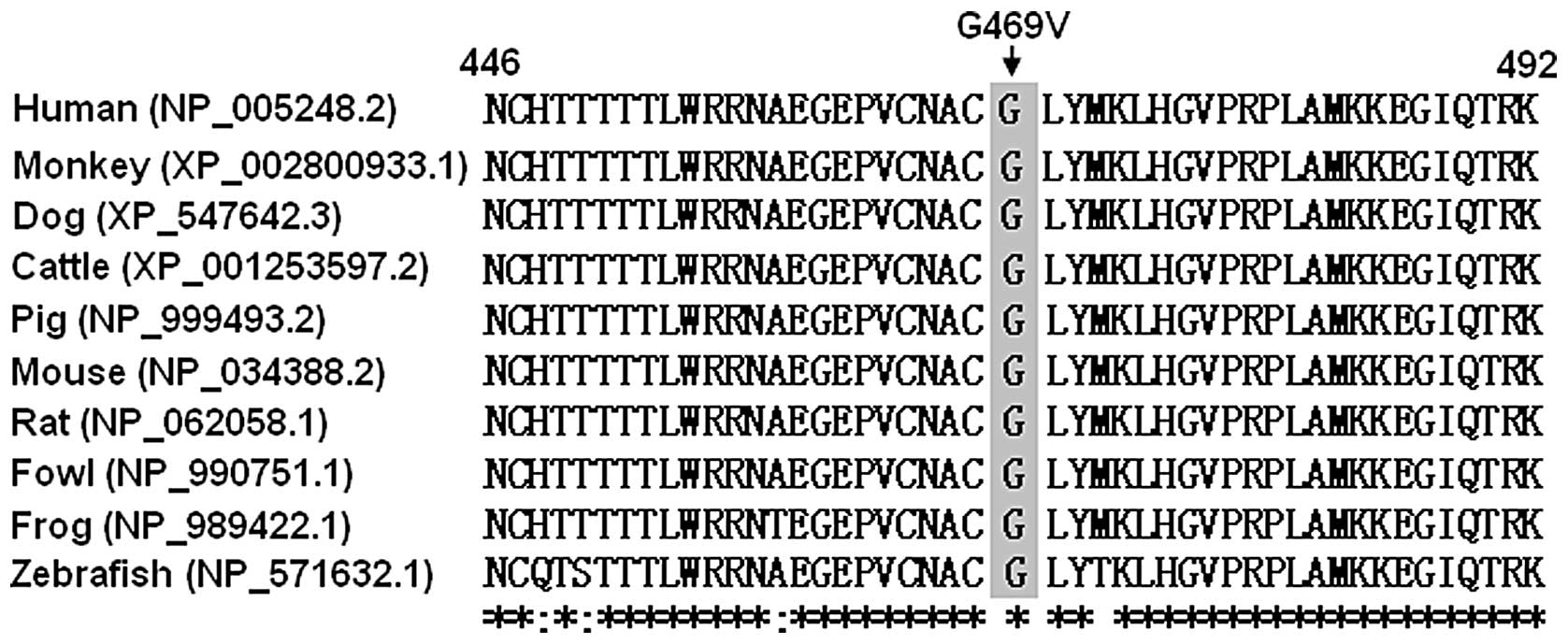
|
1.
|
V FusterLE RydénDS CannomHJ CrijnsAB
CurtisKA EllenbogenJL HalperinGN KayJY Le HuezeyJE LoweAmerican
College of Cardiology Foundation/American Heart Association Task
Force2011 ACCF/AHA/HRS focused updates incorporated into the
ACC/AHA/ESC 2006 guidelines for the management of patients with
atrial fibrillation: a report of the American College of Cardiology
Foundation/American Heart Association Task Force on practice
guidelinesCirculation123e269e3672011
|
|
2.
|
AS GoEM HylekKA PhillipsY ChangLE
HenaultJV SelbyDE SingerPrevalence of diagnosed atrial fibrillation
in adults: national implications for rhythm management and stroke
prevention: the AnTicoagulation and Risk Factors in Atrial
Fibrillation (ATRIA)
StudyJAMA28523702375200110.1001/jama.285.18.2370
|
|
3.
|
DM Lloyd-JonesTJ WangEP LeipMG LarsonD
LevyRS VasanRB D’AgostinoJM MassaroA BeiserPA WolfEJ
BenjaminLifetime risk for development of atrial fibrillation: the
Framingham Heart
StudyCirculation11010421046200410.1161/01.CIR.0000140263.20897.4215313941
|
|
4.
|
PA WolfRD AbbottWB KannelAtrial
fibrillation as an independent risk factor for stroke: the
Framingham
StudyStroke22983988199110.1161/01.STR.22.8.9831866765
|
|
5.
|
EJ BenjaminPA WolfRB D’AgostinoH
SilbershatzWB KannelD LevyImpact of atrial fibrillation on the risk
of death: the Framingham Heart
StudyCirculation98946952199810.1161/01.CIR.98.10.9469737513
|
|
6.
|
JW MagnaniM RienstraH LinMF SinnerSA
LubitzDD McManusJ DupuisPT EllinorEJ BenjaminAtrial fibrillation:
current knowledge and future directions in epidemiology and
genomicsCirculation12419821993201110.1161/CIRCULATIONAHA.111.03967722042927
|
|
7.
|
D DarbarKJ HerronJD BallewA JahangirBJ
GershWK ShenSC HammillDL PackerTM OlsonFamilial atrial fibrillation
is a genetically heterogeneous disorderJ Am Coll
Cardiol4121852192200310.1016/S0735-1097(03)00465-012821245
|
|
8.
|
PT EllinorDM YoergerJN RuskinCA
MacRaeFamilial aggregation in lone atrial fibrillationHum
Genet118179184200510.1007/s00439-005-0034-816133178
|
|
9.
|
DO ArnarS ThorvaldssonTA ManolioG
ThorgeirssonK KristjanssonH HakonarsonK StefanssonFamilial
aggregation of atrial fibrillation in IcelandEur Heart
J27708712200610.1093/eurheartj/ehi72716428254
|
|
10.
|
MJ JunttilaMJ RaatikainenJS PerkiömäkiK
HongR BrugadaHV HuikuriFamilial clustering of lone atrial
fibrillation in patients with saddleback-type ST-segment elevation
in right precordial leadsEur Heart
J28463468200710.1093/eurheartj/ehl47417242012
|
|
11.
|
IE ChristophersenLS RavnE
Budtz-JoergensenA SkyttheS HaunsoeJH SvendsenK ChristensenFamilial
aggregation of atrial fibrillation: a study in Danish twinsCirc
Arrhythm
Electrophysiol2378383200910.1161/CIRCEP.108.78666519808493
|
|
12.
|
YQ YangXL ZhangXH WangHW TanHF ShiWY FangX
LiuFamilial aggregation of lone atrial fibrillation in the Chinese
populationIntern
Med4923852391201010.2169/internalmedicine.49.413021088338
|
|
13.
|
SA LubitzX YinJD FontesJW MagnaniM
RienstraM PaiML VillalonRS VasanMJ PencinaD LevyAssociation between
familial atrial fibrillation and risk of new-onset atrial
fibrillationJAMA30422632269201010.1001/jama.2010.169021076174
|
|
14.
|
CS FoxH PariseRB D’Agostino SrDM
Lloyd-JonesRS VasanTJ WangD LevyPA WolfEJ BenjaminParental atrial
fibrillation as a risk factor for atrial fibrillation in
offspringJAMA29128512855200410.1001/jama.291.23.285115199036
|
|
15.
|
YH ChenSJ XuS BendahhouXL WangY WangWY
XuHW JinH SunXY SuQN ZhuangKCNQ1 gain-of-function mutation in
familial atrial
fibrillationScience299251254200310.1126/science.107777112522251
|
|
16.
|
Y YangM XiaQ JinS BendahhouJ ShiY ChenB
LiangJ LinY LiuB LiuIdentification of a KCNE2 gain-of-function
mutation in patients with familial atrial fibrillationAm J Hum
Genet75899905200410.1086/42534215368194
|
|
17.
|
TM OlsonVV MichelsJD BallewSP ReynaML
KarstKJ HerronSC HortonRJ RodehefferJL AndersonSodium channel
mutations and susceptibility to heart failure and atrial
fibrillationJAMA293447454200510.1001/jama.293.4.44715671429
|
|
18.
|
K HongP BjerregaardI GussakR BrugadaShort
QT syndrome and atrial fibrillation caused by mutation in KCNH2J
Cardiovasc
Electrophysiol16394396200510.1046/j.1540-8167.2005.40621.x15828882
|
|
19.
|
M XiaQ JinS BendahhouY HeMM LarroqueY
ChenQ ZhouY YangY LiuB LiuA Kir2.1 gain-of-function mutation
underlies familial atrial fibrillationBiochem Biophys Res
Commun33210121019200510.1016/j.bbrc.2005.05.05415922306
|
|
20.
|
MH GollobDL JonesAD KrahnL DanisXQ GongQ
ShaoX LiuJP VeinotAS TangAF StewartSomatic mutations in the
connexin 40 gene (GJA5) in atrial fibrillationN Engl J
Med35426772688200610.1056/NEJMoa05280016790700
|
|
21.
|
TM OlsonAE AlekseevXK LiuS ParkLV ZingmanM
BienengraeberS SattirajuJD BallewA JahangirA TerzicKv1.5
channelopathy due to KCNA5 loss-of-function mutation causes human
atrial fibrillationHum Mol
Genet1521852191200610.1093/hmg/ddl14316772329
|
|
22.
|
A LundbyLS RavnJH SvendsenS HaunsSP
OlesenN SchmittKCNE3 mutation V17M identified in a patient with
lone atrial fibrillationCell Physiol
Biochem214754200810.1159/00011374618209471
|
|
23.
|
LS RavnY AizawaGD PollevickJ Hofman-BangJM
CordeiroU DixenG JensenY WuE BurashnikovS HaunsoGain of function in
IKs secondary to a mutation in KCNE5 associated with atrial
fibrillationHeart
Rhythm5427435200810.1016/j.hrthm.2007.12.01918313602
|
|
24.
|
DM Hodgson-ZingmanML KarstLV ZingmanDM
HeubleinD DarbarKJ HerronJD BallewM de AndradeJC Burnett JrTM
OlsonAtrial natriuretic peptide frameshift mutation in familial
atrial fibrillationN Engl J
Med359158165200810.1056/NEJMoa070630018614783
|
|
25.
|
X RenC XuC ZhanY YangL ShiF WangC WangY
XiaB YangG WuIdentification of NPPA variants associated with atrial
fibrillation in a Chinese GeneID populationClin Chim
Acta411481485201010.1016/j.cca.2009.12.01920064500
|
|
26.
|
X ZhangS ChenS YooS ChakrabartiT ZhangT
KeC ObertiSL YongF FangL LiMutation in nuclear pore component
NUP155 leads to atrial fibrillation and early sudden cardiac
deathCell13510171027200810.1016/j.cell.2008.10.02219070573
|
|
27.
|
H WatanabeD DarbarDW KaiserK
JiramongkolchaiS ChopraBS DonahuePJ KannankerilDM RodenMutations in
sodium channel beta1- and beta2-subunits associated with atrial
fibrillationCirc Arrhythm
Electrophysiol2268275200910.1161/CIRCEP.108.77918119808477
|
|
28.
|
P WangQ YangX WuY YangL ShiC WangG WuY
XiaB YangR ZhangFunctional dominant-negative mutation of sodium
channel subunit gene SCN3B associated with atrial fibrillation in a
Chinese GeneID populationBiochem Biophys Res
Commun39898104201010.1016/j.bbrc.2010.06.04220558140
|
|
29.
|
IL ThibodeauJ XuQ LiG LiuK LamJP VeinotDH
BirnieDL JonesAD KrahnR LemeryParadigm of genetic mosaicism and
lone atrial fibrillation: physiological characterization of a
connexin 43-deletion mutant identified from atrial
tissueCirculation122236244201010.1161/CIRCULATIONAHA.110.961227
|
|
30.
|
R BrugadaT TapscottGZ CzernuszewiczAJ
MarianA IglesiasL MontJ BrugadaJ GironaA DomingoLL BachinskiR
RobertsIdentification of a genetic locus for familial atrial
fibrillationN Engl J
Med336905911199710.1056/NEJM1997032733613029070470
|
|
31.
|
PT EllinorJT ShinRK MooreDM YoergerCA
MacRaeLocus for atrial fibrillation maps to chromosome
6q14–16Circulation10728802883200312782570
|
|
32.
|
C ObertiL WangL LiJ DongS RaoW DuQ
WangGenome-wide linkage scan identifies a novel genetic locus on
chromosome 5p13 for neonatal atrial fibrillation associated with
sudden death and variable
cardiomyopathyCirculation11037533759200410.1161/01.CIR.0000150333.87176.C7
|
|
33.
|
PG VoldersQ ZhuC TimmermansPM EurlingsX
SuYH ArensL LiRJ JongbloedM XiaLM RodriguezYH ChenMapping a novel
locus for familial atrial fibrillation on chromosome10p11–q21Heart
Rhythm44694752007
|
|
34.
|
DL HuFK ChenYQ LiuYH ShengR YangXQ KongKJ
CaoHT GuLM QianGATA-4 promotes the differentiation of P19 cells
into cardiac myocytesInt J Mol Med26365372201020664952
|
|
35.
|
BG BruneauThe developmental genetics of
congenital heart
diseaseNature451943948200810.1038/nature0680118288184
|
|
36.
|
S PikkarainenH TokolaR KerkeläH
RuskoahoGATA transcription factors in the developing and adult
heartCardiovasc
Res63196207200410.1016/j.cardiores.2004.03.02515249177
|
|
37.
|
J WangM FangXY LiuYF XinZM LiuXZ ChenXZ
WangWY FangX LiuYQ YangA novel GATA4 mutation responsible for
congenital ventricular septal defectsInt J Mol
Med28557564201121637914
|
|
38.
|
XY LiuJ WangJH ZhengK BaiZM LiuXZ WangX
LiuWY FangYQ YangInvolvement of a novel GATA4 mutation in atrial
septal defectsInt J Mol Med281723201121373748
|
|
39.
|
J WangYF XinXY LiuZM LiuXZ WangYQ YangA
novel NKX2-5 mutation in familial ventricular septal defectInt J
Mol Med27369375201121165553
|
|
40.
|
I Gutierrez-RoelensL De RoyC OvaertT
SluysmansK DevriendtHG BrunnerM VikkulaA novel CSX/NKX2-5 mutation
causes autosomal-dominant AV block: are atrial fibrillation and
syncopes part of the phenotype?Eur J Hum
Genet1413131316200610.1038/sj.ejhg.520170216896344
|
|
41.
|
LH BoldtMG PoschA PerrotM PolotzkiS RolfAS
ParwaniM HuemerA WutzlerC OzcelikW HaverkampMutational analysis of
the PITX2 and NKX2–5 genes in patients with idiopathic atrial
fibrillationInt J Cardiol145316317201020022124
|
|
42.
|
MG PoschLH BoldtM PolotzkiS RichterS RolfA
PerrotR DietzC OzcelikW HaverkampMutations in the cardiac
transcription factor GATA4 in patients with lone atrial
fibrillationEur J Med
Genet53201203201010.1016/j.ejmg.2010.03.00820363377
|
|
43.
|
YQ YangMY WangXL ZhangHW TanHF ShiWF
JiangXH WangWY FangX LiuGATA4 loss-of-function mutations in
familial atrial fibrillationClin Chim
Acta41218251830201110.1016/j.cca.2011.06.01721708142
|
|
44.
|
JQ JiangFF ShenWY FangX LiuYQ YangNovel
GATA4 mutations in lone atrial fibrillationInt J Mol
Med2810251032201121874226
|
|
45.
|
Y ZhangN RathS HannenhalliZ WangT CappolaS
KimuraE Atochina-VassermanMM LuMF BeersEE MorriseyGATA and Nkx
factors synergistically regulate tissue-specific gene expression
and development in
vivoDevelopment134189198200710.1242/dev.0272017164424
|
|
46.
|
E SuzukiT EvansJ LowryL TruongDW BellJR
TestaK WalshThe human GATA-6 gene: structure, chromosomal location,
and regulation of expression by tissue-specific and
mitogen-responsive
signalsGenomics38283290199610.1006/geno.1996.06308975704
|
|
47.
|
X LinZ HuoX LiuY ZhangL LiH ZhaoB YanY
LiuY YangYH ChenA novel GATA6 mutation in patients with tetralogy
of Fallot or atrial septal defectJ Hum
Genet55662667201010.1038/jhg.2010.84
|
|
48.
|
GF ZhengD WeiH ZhaoN ZhouYQ YangXY LiuA
novel GATA6 mutation associated with congenital ventricular septal
defectInt J Mol Med2910651071201222407241
|
|
49.
|
YQ YangXH WangHW TanWF JiangWY FangX
LiuPrevalence and spectrum of GATA6 mutations associated with
familial atrial fibrillationInt J
Cardiol155494496201210.1016/j.ijcard.2011.12.09122257684
|
|
50.
|
M HaïssaguerreP JaïsDC ShahA TakahashiM
HociniG QuiniouS GarrigueA Le MourouxP Le MétayerJ
ClémentySpontaneous initiation of atrial fibrillation by ectopic
beats originating in the pulmonary veinsN Engl J
Med33965966619989725923
|
|
51.
|
MT MommersteegNA BrownOW PrallC de Gier-de
VriesRP HarveyAF MoormanVM ChristoffelsPitx2c and Nkx2–5 are
required for the formation and identity of the pulmonary
myocardiumCirc Res1019029092007
|
|
52.
|
MT MommersteegVM ChristoffelsRH AndersonAF
MoormanAtrial fibrillation: a developmental point of viewHeart
Rhythm618181824200910.1016/j.hrthm.2009.07.01119726237
|
|
53.
|
MT MommersteegWM HoogaarsOW PrallC de
Gier-de VriesC WieseDE CloutVE PapaioannouNA BrownRP HarveyAF
MoormanVM ChristoffelsMolecular pathway for the localized formation
of the sinoatrial nodeCirc
Res100354362200710.1161/01.RES.0000258019.74591.b317234970
|
|
54.
|
Y WatanabeDW BensonS YanoT AkagiM
YoshinoJC MurrayTwo novel frameshift mutations in NKX2.5 result in
novel features including visceral inversus and sinus venosus type
ASDJ Med Genet39807811200210.1136/jmg.39.11.80712414819
|
|
55.
|
S PabstB WollnikE RohmannY HintzK GlänzerH
VetterG NickenigC GrohéA novel stop mutation truncating critical
regions of the cardiac transcription factor NKX2-5 in a large
family with autosomal-dominant inherited congenital heart
diseaseClin Res Cardiol973942200810.1007/s00392-007-0574-0
|
|
56.
|
EJ GruverD FatkinGA DoddsJ KissloBJ
MaronJG SeidmanCE SeidmanFamilial hypertrophic cardiomyopathy and
atrial fibrillation caused by Arg663His beta-cardiac myosin heavy
chain mutationAm J
Cardiol8313H18H199910.1016/S0002-9149(99)00251-910750581
|
|
57.
|
YQ YangX LiuXL ZhangXH WangHW TanHF ShiWF
JiangWY FangNovel connexin40 missense mutations in patients with
familial atrial
fibrillationEuropace1214211427201010.1093/europace/euq27420650941
|
|
58.
|
YQ YangXL ZhangXH WangHW TanHF ShiWF
JiangWY FangX LiuConnexin40 nonsense mutation in familial atrial
fibrillationInt J Mol Med26605610201020818502
|