Introduction
Hepatocellular carcinoma (HCC) is one of the most
common malignant tumors worldwide, particularly in Asian countries
due to the prevalence of hepatitis B infection. Despite under-going
multimodal treatment including partial hepatectomy, thermal
ablation, radiation, chemotherapy, systemic chemo-therapy and liver
transplantation (1,2), most of these patients eventually die
from progressive tumors. Thus, an understanding of the molecular
mechanisms involved in the tumor progression, development and
growth regulation of HCC is urgently needed.
SPARC, also named osteonectin, is a secreted
multi-functional matricellular glycoprotein and rich in cysteine.
It is involved in important biological functions, including cell
adhesion, migration, tissue repair and remodeling. SPARC is
differentially expressed in varous tumors including breast cancer
(3), melanoma (4), gliomas (5), prostate cancer (6) and colorectal cancer (7). However, the significance remains
unclear and the detailed functions and molecular mechanisms are not
known. Lau et al (8)
observed that SPARC is related to HCC angiogenesis and tumor
progression. Exogenous SPARC increases cell survival under stress
initiated by serum withdrawal through a decrease in apoptosis and
rapidly induces AKT phosphorylation, an effect that is blocked by a
neutralizing SPARC antibody (5).
Cell growth and proliferation are associated with AKT
phosphorylation and activation. Furthermore, AKT activation is also
essential for anti-apoptotic effects since AKT is involved in the
regulation of tumor cell growth (9).
microRNAs (miRs) are small, non-coding RNA molecules
that regulate gene expression (10), by translational inhibition or
cleavage of their target mRNAs through base-pairing to partially or
fully complementary seed sites. Aberrant miRNA expression,
including expression of miR-21, miR-122a, miR-148, miR-185,
miR-199a and miR-151, has been observed in HCCs and has been shown
to regulate cell growth, apoptosis, migration, or invasion in
different study cohorts (11–15). miR-29a is involved in renal
fibrosis by suppressing collagen expression (16). miR-29a also appears to hinder
elastin expression in fibroblasts and smooth muscle cells (17), which prevents and slows the
development of fibrosis. miR-29a suppresses interferon-α receptor
and regulates the threshold for infection-associated thymic
involution (18). In acute
myeloid leukemia cells, upregulation of the nuclear oncogene Ski is
associated with low miR-29a levels because forced expression of
miR-29a downregulates Ski (19).
Given that miR-29a is involved in myogenesis, osteoblastic
differentiation, sclerosis and cardiac fibrosis (14,19–22), there is also evidence showing that
miR-29a is significantly downregulated in patients with HCC
(14). Downregulation was mainly
observed in the serum of fibrosis patients, targeting the
pro-apoptotic factors Bcl-2 and Mcl-1. However, the detailed
regulatory role of miR-29a in HCC tumorigenesis and metastasis is
not fully understood and requires further investigation.
In this present study, we found that miR-29a
expression was dramatically decreased in the majority of examined
HCC tissues, and overexpression of miR-29a dramatically induced
cell growth inhibition, which was associated with SPARC signaling
in the AKT pathway.
Materials and methods
Cell lines and tissue specimens
Human HCC cell lines (PLC, MHCC-97H, MHCC-97L and
SMMC-7721), the normal human hepatic cell line LO2, and HEK-293T
cells were all cultured in Dulbecco’s modified Eagle’s medium
(DMEM; Hyclone, Logan, UT, USA) supplemented with 10% fetal bovine
serum (FBS; Gibco, Austria) in a humidified 37˚C incubator with 5%
CO2. A total of 110 patients with newly diagnosed HCC
joined this study at the Liver Cancer Institute and Zhongshan
Hospital, Fudan University (Shanghai, China) between 2003 and 2006.
Samples of HCC and corresponding adjacent non-tumor liver tissues
were collected after resection of the HCC. All of the clinical
samples were collected from patients after obtaining informed
consent according to an established protocol approved by the Ethics
Committee of Fudan University.
Plasmids, lentivirus production and
transduction
To construct a plasmid expressing miR-29a, the
pri-miR-29a sequence was amplified with the primers
AAAGGATCCGCCATAGAAACC CAGTTTC and AAAACGGCTCCAAGGGATGAATGTAA TTG,
from human genomic DNA and then subcloned into the BamHI and
MluI sites of the pWPI 1.1 vector to generate pWPI-miR-29a.
The wild-type SPARC-3′UTR sequence that contained putative binding
site for miR-29a was PCR-amplified from oligo(dT)-primed HEK 293T
cDNA with the primers ccgCTCGAGATCCACTCCTTCCACAGTACCG and
ttGCGGCCGCGTGTGGTCTGCCTGCTAGA, and the 2.4-kb product was then
subcloned into the XhoI and NotI sites of the
psiCHECK-2 vector, immediately downstream of the Renilla
luciferase gene. The resulting plasmid was named
psi-SPARC-3′UTR-WT. The plasmid psi-SPARC-3′UTR-MUT encodes a
mutated sequence in the complementary site for the seed region of
miR-29a, which was generated via site-directed mutagenesis
(QuickChange XL site-directed mutagenesis kit; Stratagene, Cedar
Creek, TX, USA) and psi-SPARC-3′UTR-WT as a template. The SPARC
mutant site position was as follows: the first site position
UCUGCCUGGAGACAAGGUGCUAA became UCUGCCUGGAGACAACCCCGCAA. The second
site position AGUGAAUACAUUAACGGUGCUAA became
AGUGAAUACAUUAACCCCCGCAA. Virus particles were harvested 60 h after
pWPI-miR-29a transfection of the packaging plasmid psPAX2 and
pMD2.G into HEK-293T cells using the FuGENE 6 reagent (Roche
Diagnostics). PLC and MHCC-97L cells were infected with recombinant
lentivirustransducing units and 8 μg/ml Polybrene (Sigma).
RNA extraction and quantitative
RT-PCR
Total RNA was extracted from frozen tumor specimens
and cell lines using TRIzol reagent (Invitrogen, Carlsbad, CA, USA)
according to the manufacturer’s instructions. Total RNA (500 ng)
was reverse-transcribed using PrimeScript® RT Master Mix
(Takara) reverse transcriptase according to the manufacturer’s
instructions. Real-time PCR analyses were performed with FastStart
Universal SYBR-Green Master (Roche Diagnostics). For qRT-PCR of
miR-29a, 50 ng of total RNA was reverse-transcribed with miR-29a
(ABI ID, 000412)- or U6 (ABI ID, 1973)-specific stem-loop primers
and subjected to TaqMan miRNA assays using an ABI PRISM®
7900HT sequence detection system (from Applied Biosystems, Foster
City, CA, USA).
Luciferase reporter assays
HEK293T cells plated in a 96-well plate were
co-transfected with 200 ng of pWPI.1 or pWPI-miR-29a and 40 ng of
psi-SPARC-3′UTR-WT or psi-SPARC-3′UTR-MUT. For the antagonism
experiment, HEK293T cells were cultured in 96-well plates and
co-transfected with 200 nM anti-miR-C (control) or anti-miR-29a, as
well as 40 ng of psi-SPARC-3′UTR-WT or psi-SPARC-3′UTRMUT.
Luciferase activity was measured 48 h after transfection and
analyzed using the Dual-Luciferase reporter assay system (Promega).
Transfections were performed in duplicate and repeated in at least
three independent experiments.
Cell proliferation assays
Cell proliferation was determined using the WST-1
(Roche Diagnostics) assay. Cells (3×103/well) were
plated into the wells of a 96-well plate, each containing 90 μl of
culture medium. After 24, 48, 72 and 96 h, 10 μl WST-1 was added,
the cells were incubated for 2 h at 37˚C, and then the absorbance
was measured at a wavelength of 450 nm. Three independent
experiments were performed.
Oligonucleotide (AMO) transfection
The small interfering RNA sequence targeting human
SPARC was AUUGCUGCA CACCUUCUCA. The unspecific control siRNA (NC)
was not homologous to any human genome sequences and synthesized by
GenePharma Co., Ltd. (Shanghai, China). The anti-miR-29a was a
2′O-methyl-modified oligonucleotide with a sequence that was
complementary to the mature miR-29a and was designed as an
inhibitor of miR-29a. The anti-miR-C was used as a negative control
in the antagonism experiments. Transfection of plasmid DNA or
co-transfection of RNA duplexes with plasmid DNA was performed
using the FuGENE 6 reagent. All transfections were performed in
triplicate.
Western blotting
Cells were harvested and lysed with RIPA lysis
buffer supplemented with protease inhibitors and phosphatase
inhibitors (Roche Diagnostics). Protein extracts were separated by
10% SDS-PAGE and transferred to a polyvinylidene fluoride membrane
(Millipore). The membrane was blocked with 5% non-fat milk and
incubated with rabbit anti-SPARC polyclonal antibody, rabbit
anti-p-AKT (S473) p-mTOR (S2448) or P-ERK (T202, Y204) polyclonal
antibodies, rabbit anti-AKT polyclonal antibody, or GAPDH rabbit
antibody, all of which were from Cell Signaling Technology, Inc.
The protein bands were detected with Chemiluminescent HRP substrate
(Millipore).
Statistical analyses
Data are expressed as the mean ± standard error of
the mean from at least three independent experiments. Kaplan-Meier
estimate of the survival rate was contributed by SPSS software. All
statistical tests were two-sided, and a P-value <0.05 was
considered to indicate a statistically significant result.
Results
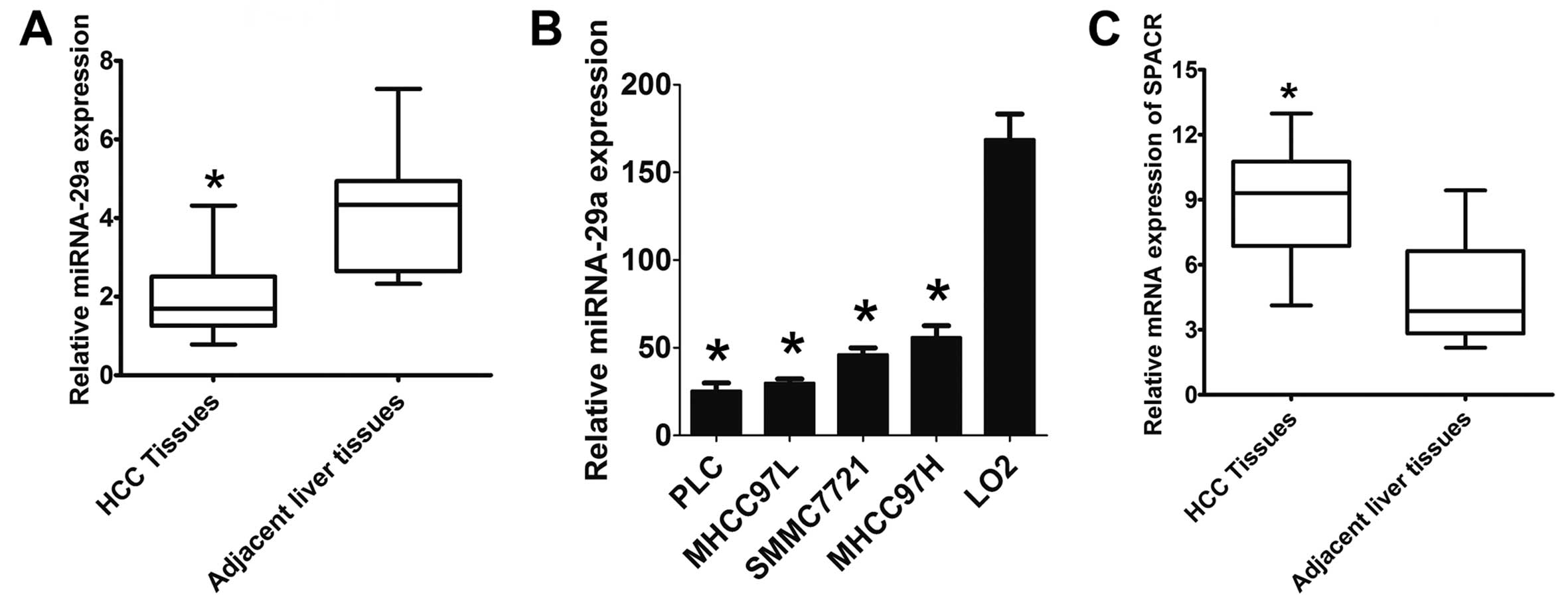
miR-29a is downregulated in HCC
In a previous study, we observed differentially
expressed miRNAs in metastatic versus non-metastatic liver tissues
from patients with HCC (12).
Here, we found that miR-29a was downregulated in HCC tissues from
110 patients compared to corresponding adjacent non-tumor liver
tissues using a specific TaqMan probe RT-PCR assay (Fig. 1A). There was a significant
difference in miR-29a expression levels between HCC and the
adjacent benign tissues (P<0.01). Next, we further analyzed the
expression level of miR-29a in 4 human HCC cell lines compared to
the LO2 normal immortalized liver cell line and found that miR-29a
was expressed at significantly lower levels in HCC cells,
especially in PLC and MHCC-97L, when compared with the LO2 cells
(Fig. 1B). The SPARC mRNA levels
were significantly higher in the HCC tissues than that in the
adjacent non-tumor liver tissues (P<0.01) (Fig. 1C).
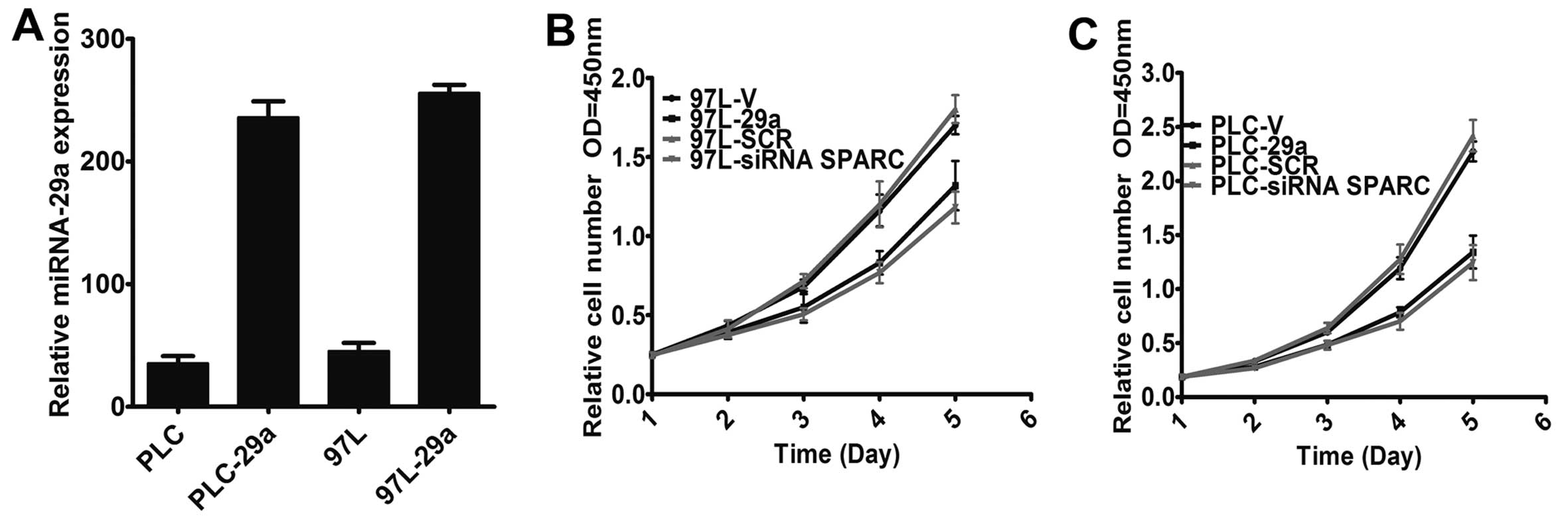
Overexpression of miR-29a suppresses HCC
cell growth
To gain insight into the biological role of miR-29a
in the regulation of cell growth in HCC, we constructed a
lentivirus vector encoding the miR-29a precursor and established
two stably infected cell lines, denoted PLC-29a and 97L-29a, after
lentivirus infection. The expression level of miR-29a was then
detected by TaqMan PCR after overexpression of miR-29a. The data
showed that the expression levels of miR-29a in PLC-29a and 97L-29a
were markedly increased, while miR-29a expression was not affected
in the empty vector-infected cells (Fig. 2A). To explore the role of miR-29a
in HCC cell growth, we analyzed the regulatory effect of miR-29a on
cell growth and found that both PLC-29a and 97L-29a cells grew at
significantly lower rates than matched cells infected with the
empty vector (Fig. 2B and C)
(P<0.01).
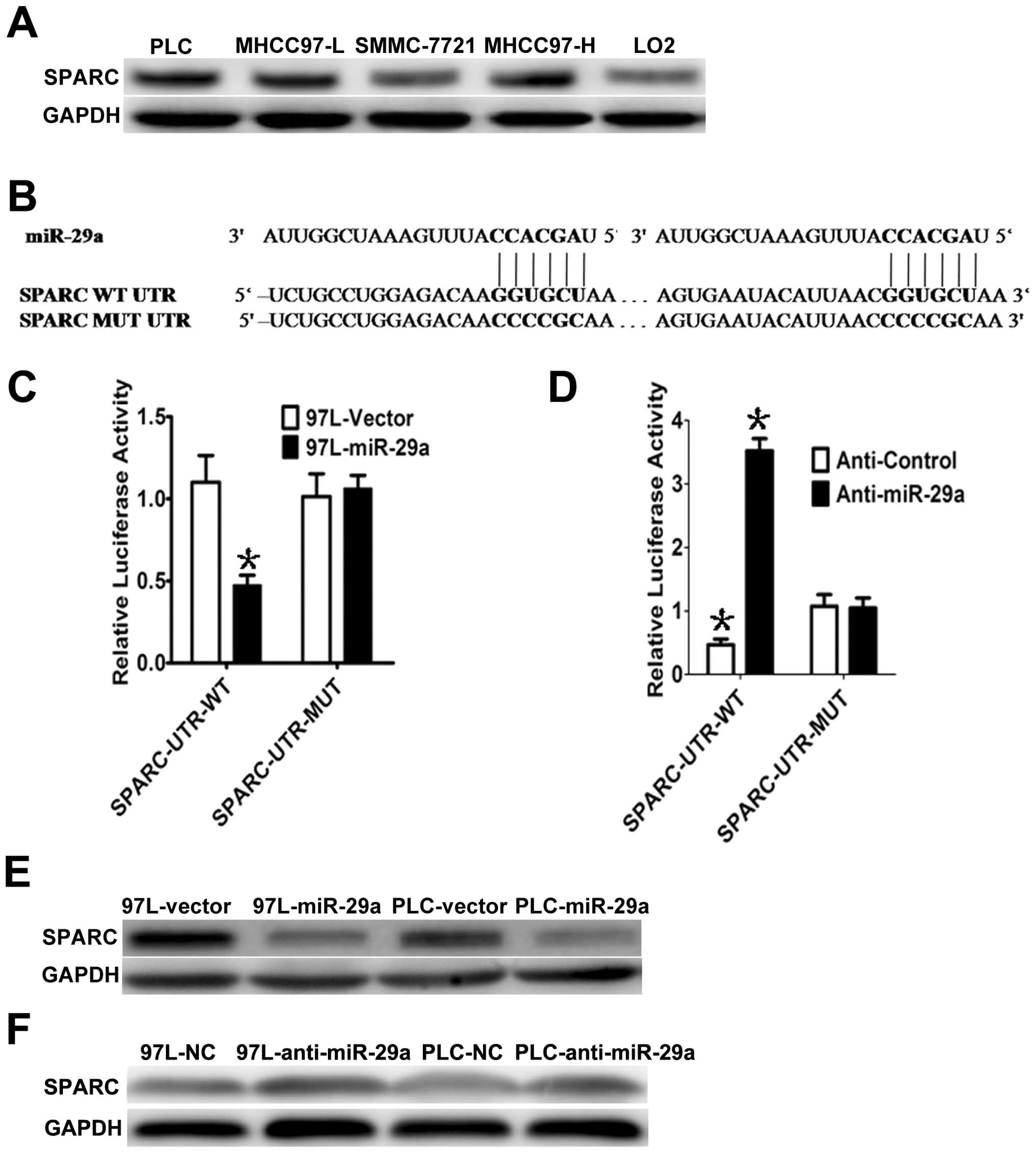
SPARC is a direct target of miR-29a
To explore the possible molecular mechanism by which
miR-29a suppresses cell growth, we analyzed SPARC mRNA, which is a
predicted target of miR-29a. First, we measured SPARC protein
levels in HCC cells and found that SPARC was highly expressed in
the 4 HCC cell lines, but lowly expressed in normal liver LO2 cells
(Fig. 3A). Interestingly, miR-29a
and SPARC expression levels were inversely correlated in the LO2
cells and the 4 HCC cell lines. To determine whether SPARC is a
direct target of miR-29a, a Dual-Luciferase reporter system was
employed. Using miRNA target prediction, we found that the 3′UTR of
SPARC contains two miR-29a-binding sites (Fig. 3B). To assess miRNA binding to the
3′UTR, we constructed lucif-erase reporters with the SPARC-3′UTR.
Co-transfection of the reporter containing the SPARC-3′UTR-WT
construct with miR-29a significantly suppressed the Renilla
luciferase activity in 97L-29a cells compared to the control group
(empty vector), while the mutant SPARC-3′UTR reporter (i.e.,
mutated in the seed sequence binding region) displayed no response
to high levels of miR-29a in 97L-29a cells (Fig. 3B and C). Similar results were also
observed in the PLC-29a cells. Subsequently, we co-transfected
anti-miR-29a and SPARC-3′UTR-WT or SPARC-3′UTR-MUT in
miR-29a-overexpressing MHCC-97L (97L-29a) cells and observed that
the anti-miR-29a inhibitor rescued the luciferase activities of the
reporter containing the wild-type SPARC-3′UTR (Fig. 3D), but the mutant did not. In
accordance with these results, we observed a clear decrease in
endogenous SPARC protein in PLC and MHCC-97L cells with miR-29a
overexpression, whereas no obvious changes were detected in the PLC
and MHCC-97L cells infected with the vector (Fig. 3E). In addition, suppression of
miR-29a by antisense miR-29a led to higher expression of SPARC
(Fig. 3F). These results suggest
that miR-29a downregulates SPARC expression, by direct targeting of
its 3′UTR.
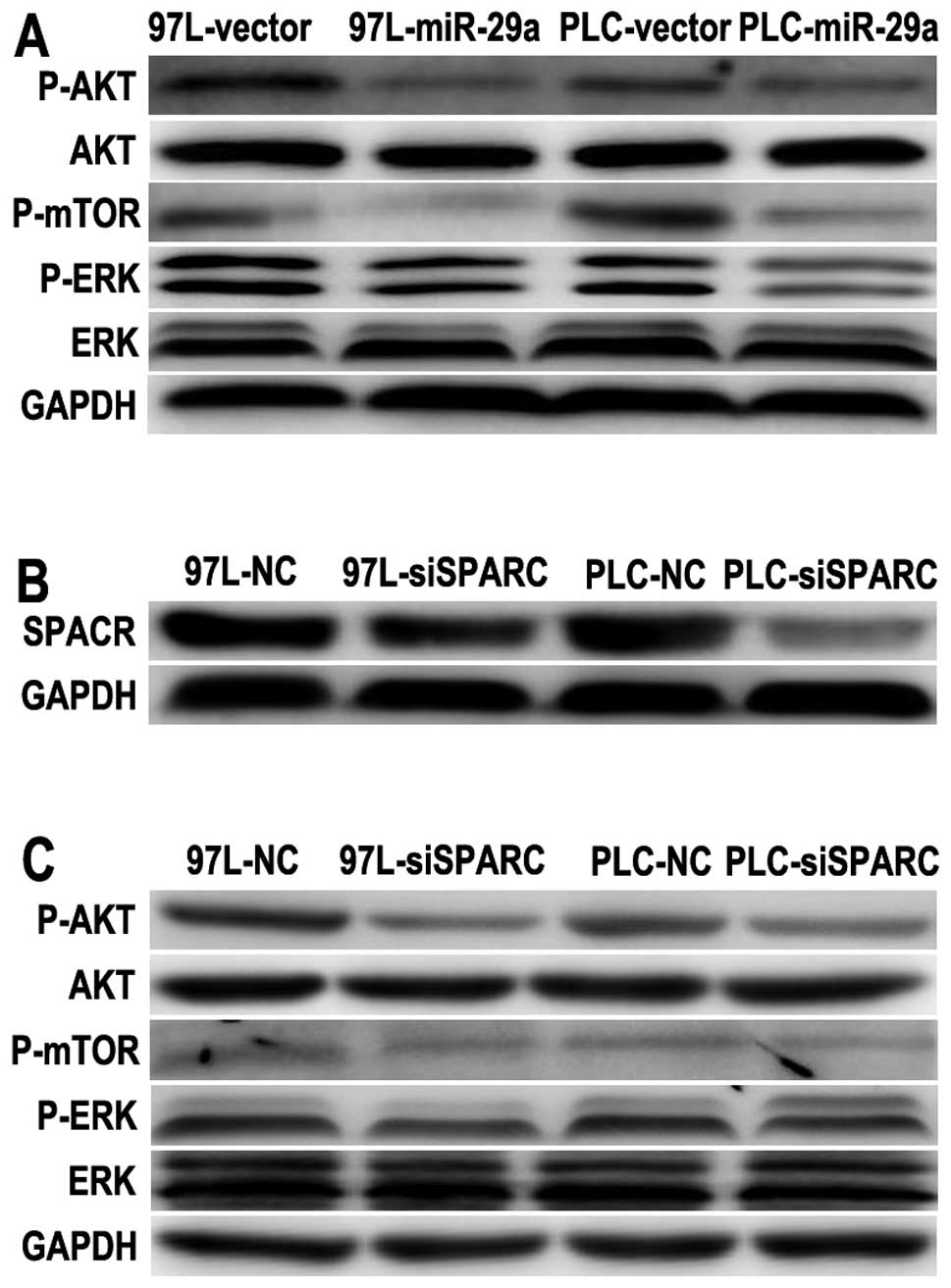
miR-29a inhibits cell growth via the
SPARC-AKT pathway
Since SPARC is a positive regulator of AKT and
mediates cell survival (5), and
miR-29a can directly target SPARC mRNA and suppress HCC growth, the
effectors downstream of SPARC required further investigation. As
the downstream effector, AKT activation is sufficient to promote
cell proliferation and survival. We hence examined the regulatory
effect on and changes in P-AKT levels in PLC and MHCC-97L cells by
miR-29a overexpression. The expression of P-AKT (S473), P-ERK (T202
and Y204) and P-mTOR (S2448) was significantly reduced in the
PLC-29a and 97L-29a cells (Fig.
4A). These observations suggest that the AKT/mTOR pathway was
inhibited in miR-29a-overexpressing HCC cells. To determine the
role of SPARC in the AKT/mTOR pathway further, SPARC was knocked
down to examine the importance of endogenous SPARC expression.
SPARC expression was greatly reduced after SPARC siRNA transfection
in PLC and MHCC-97L cells (Fig.
4B). Silencing of SPARC significantly reduced both its protein
levels and the cell growth rate (Fig.
2B), which was similar to the phenotype induced by miR-29a
overexpression. Thus, we investigated the AKT/mTOR pathway, and
found it was also suppressed after SPARC silencing in both MHCC-97L
and PLC cells (Fig. 4C). These
results suggest that SPARC silencing resulted in a similar effect
on the AKT/mTOR pathway as miR-29a overexpression.
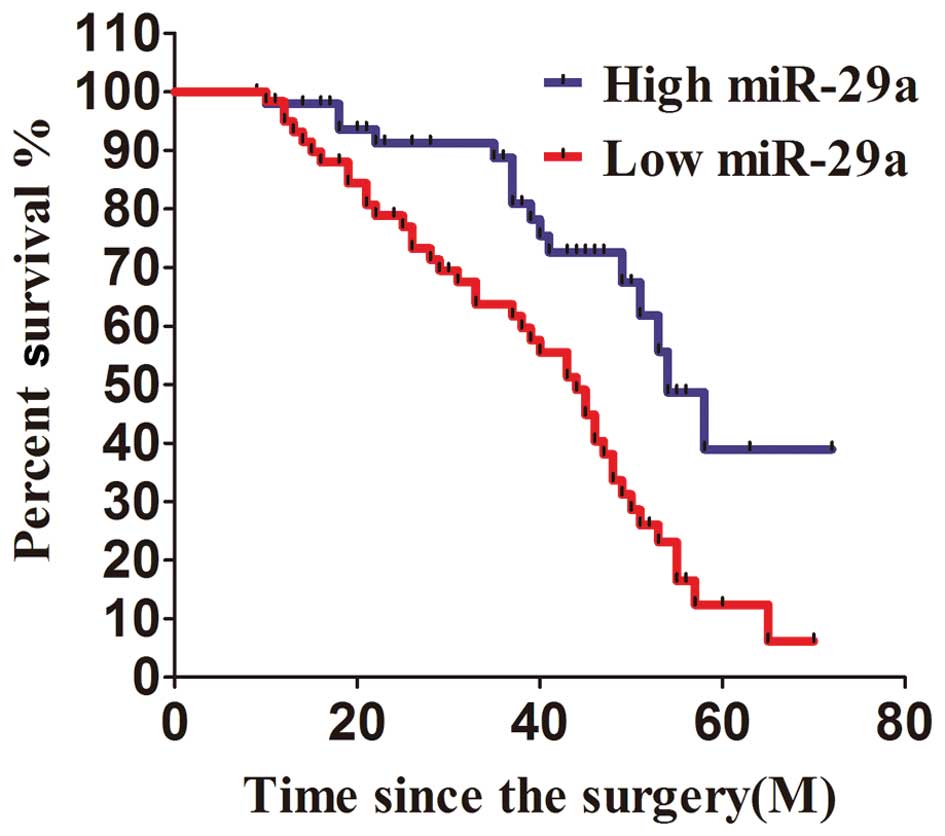
miR-29a expression is associated with
patient survival
All of the 110 patients enrolled in this study were
divided into two groups according to the level of miR-29a
expression. The 60 patients with miR-29a expression less than the
median value were considered to have low expression of miR-29a,
while 50 patients with miR-29a expression higher than the median
level measured by PCR were considered to have high expression of
miR-29a. After analysis with Kaplan-Meier method, we found that the
patients with relatively high expression of miR-29a survived at a
significantly higher rate than those with low expression of miR-29a
in a 6-year survival surveillance (Fig. 5). Patients with higher miR-29a
expression than the median level had a more favorable survival rate
as compared with those with less than the median. Between the
metastasis and no metastasis groups the difference in miR-29a
expression was not significant (P=0.068).
Discussion
Evidence shows that miRNAs have important roles in
mRNA stability, directly contribute to mRNA degradation and gene
expression (23). In this study,
we found that miR-29a expression was reduced in HCC tissues
compared to normal tissues and reduced in 4 HCC cell lines compared
to a normal liver cell line. Hence, we further investigated the
molecular mechanisms involved in miR-29a action. To this end, we
constructed an expression vector and established two cell models
stably overexpressing miR-29a, which were used for cell growth
regulation. Overexpression of miR-29a significantly inhibited the
proliferation rate of both MHCC-97L and PLC cells. These data agree
with previous reports indicating that miR-29 sensitizes HCC cells
to apoptosis triggered by various stimuli (14).
There are lines of evidence indicating that miR-29a
plays an important role in liver fibrosis and in sensitizing HCC
cells to apoptosis (14,24), but some reports also indicate that
miR-29a promotes HCC cell invasion and metastasis by downregulating
the tumor suppressor PTEN (25).
These discrepancies may be due to differences among the samples,
such as the sample source, diverse pathogenic attributes and
miscellaneous pathological characteristics. Because one miRNA can
target dozens of mRNAs that impact several molecules involved in
various signaling pathways, the dominant influence of an miRNA on
the regulation of cellular functions may depend on the relative
importance of the targets that are involved in the signaling
pathways (9). Indeed, the roles
of an miRNA may oscillate between repression and activation in
coordination with other regulators that catalyze the
phosphorylation of the targets, and the roles of the miRNA also
depend on the relative importance of the targeted molecule in the
regulatory pathway (10).
SPARC is an extracellular glycoprotein which is
involved in promoting cell motility and invasion in several
carcinoma cell types, including breast, prostate (6), melanoma (4) and glioblastomas (5). SPARC overexpression in this study
was observed in the HCC cell lines compared to the immortalized
normal liver cell LO2, or in HCC tissues from 110 patients compared
to the adjacent tissues. To elucidate the targets of miR-29a in
HCC, we analyzed SPARC as a potential target of miR-29a. Using a
luciferase reporter assay, we found that the 3′UTR from SPARC mRNA
was significantly targeted by miR-29a. Mutation of the miR-29a
binding sequence in the SPARC-3′UTR abrogated the effects of
miR-29a. Use of an miR-29a antisense oligonucleotide rescued the
activities of the luciferase reporter. Furthermore, in both HCC
samples and HCC cell lines, miR-29a expression inversely correlated
with SPARC expression. All of these data indicated that SPARC mRNA
was targeted by miR-29a in HCC cells. Interestingly, the effect of
SPARC siRNA on HCC cell growth was similar to miR-29a
overexpression in both PLC and MHCC-97L cells. Thus, we believe
that SPARC is the target by which miR-29a inhibits HCC cell
proliferation.
SPARC can mediate cell survival through the
activation of AKT (5). Activated
AKT is sufficient to promote cell proliferation and survival since
activated AKT phosphorylates MDM2 and inactivates p53. The role of
the PI3K-Akt cascade in the regulation of proliferation, survival,
and differentiation of many different cell types has been well
established. Cumulative evidence indicates that abnormal activation
of the PI3K/Akt/mTOR signaling pathway, which can promote cell
growth, frequently occurs in HCC. In our study, we noted that
overexpression of miR-29a reduced the level of AKT phosphorylation,
but anti-miR-29a enhanced the level of P-AKT. The expression of
miR-29a appears to be an independent prognostic factor for overall
survival since a higher expression level of miR-29a was associated
with increased patient survival.
Taken together, our results demonstrate that
overexpression of miR-29a significantly inhibits HCC cell
proliferation in vitro by inhibiting SPARC, a direct and
functional target of miR-29a, which markedly promotes HCC cell
proliferation. These findings may facilitate the development of
potential therapeutics against HCC.
References
|
1.
|
KW BurakNM KnetemanAn evidence-based
multidisciplinary approach to the management of hepatocellular
carcinoma (HCC): the Alberta HCC algorithmCan J
Gastroenterol24643650201021157578
|
|
2.
|
SF LiAM HawxbyR KanagalaH WrightA
SebastianLiver transplantation for hepatocellular carcinoma:
indications, bridge therapy and adjuvant therapyJ Okla State Med
Assoc1051216201222458042
|
|
3.
|
C BouzinO FeronTargeting tumor stroma and
exploiting mature tumor vasculature to improve anti-cancer drug
deliveryDrug Resist
Updat10109120200710.1016/j.drup.2007.03.00117452119
|
|
4.
|
SR AlonsoL TraceyP OrtizA high-throughput
study in melanoma identifies epithelial-mesenchymal transition as a
major determinant of metastasisCancer
Res6734503460200710.1158/0008-5472.CAN-06-348117409456
|
|
5.
|
Q ShiS BaoJA MaxwellSecreted protein
acidic, rich in cysteine (SPARC), mediates cellular survival of
gliomas through AKT activationJ Biol
Chem2795220052209200410.1074/jbc.M40963020015469933
|
|
6.
|
R ThomasLD TrueJA BassukPH LangeRL
VessellaDifferential expression of osteonectin/SPARC during human
prostate cancer progressionClin Cancer Res611401149200010741745
|
|
7.
|
SK ChanOL GriffithIT TaiSJ
JonesMeta-analysis of colorectal cancer gene expression profiling
studies identifies consistently reported candidate biomarkersCancer
Epidemiol Biomarkers
Prev17543552200810.1158/1055-9965.EPI-07-261518349271
|
|
8.
|
CP LauRT PoonST CheungWC YuST FanSPARC and
Hevin expression correlate with tumour angiogenesis in
hepatocellular carcinomaJ
Pathol210459468200610.1002/path.206817029219
|
|
9.
|
CY JiaHH LiXC ZhuMiR-223 suppresses cell
proliferation by targeting IGF-1RPLoS
One6e27008201110.1371/journal.pone.002700822073238
|
|
10.
|
LH WuHH LiCY JiaMicroRNA-223 regulates
FOXO1 expression and cell proliferationFEBS
Lett58610381043201210.1016/j.febslet.2012.02.05022569260
|
|
11.
|
F MengR HensonH Wehbe-JanekK GhoshalST
JacobT PatelMicroRNA-21 regulates expression of the PTEN tumor
suppressor gene in human hepatocellular
cancerGastroenterology133647658200710.1053/j.gastro.2007.05.02217681183
|
|
12.
|
A BudhuHL JiaM ForguesIdentification of
metastasis-related microRNAs in hepatocellular
carcinomaHepatology47897907200810.1002/hep.2216018176954
|
|
13.
|
J HouL LinW ZhouIdentification of miRNomes
in human liver and hepatocellular carcinoma reveals miR-199a/b-3p
as therapeutic target for hepatocellular carcinomaCancer
Cell19232243201110.1016/j.ccr.2011.01.00121316602
|
|
14.
|
Y XiongJH FangJP YunEffects of microRNA-29
on apoptosis, tumorigenicity, and prognosis of hepatocellular
carcinomaHepatology51836845201020041405
|
|
15.
|
R LiN QianK TaoN YouX WangK DouMicroRNAs
involved in neoplastic transformation of liver cancer stem cellsJ
Exp Clin Cancer Res29169201010.1186/1756-9966-29-16921176238
|
|
16.
|
B WangR KomersR CarewSuppression of
microRNA-29 expression by TGF-beta1 promotes collagen expression
and renal fibrosisJ Am Soc
Nephrol23252265201210.1681/ASN.201101005522095944
|
|
17.
|
P ZhangA HuangJ FerruzziInhibition of
microRNA-29 enhances elastin levels in cells haploinsufficient for
elastin and in bioengineered vessels - brief reportArterioscler
Thromb Vasc Biol32756759201210.1161/ATVBAHA.111.23811322095981
|
|
18.
|
AS PapadopoulouJ DooleyMA LintermanThe
thymic epithelial microRNA network elevates the threshold for
infection-associated thymic involution via miR-29a mediated
suppression of the IFN-alpha receptorNat
Immunol13181187201210.1038/ni.2193
|
|
19.
|
S TeichlerT IllmerJ RoemhildD OvcharenkoT
StieweA NeubauerMicroRNA29a regulates the expression of the nuclear
oncogene
SkiBlood11818991902201110.1182/blood-2010-09-30625821685371
|
|
20.
|
AK PandeyG VermaS VigS SrivastavaAK
SrivastavaM DattamiR-29a levels are elevated in the db/db mice
liver and its overexpression leads to attenuation of insulin action
on PEPCK gene expression in HepG2 cellsMol Cell
Endocrinol332125133201110.1016/j.mce.2010.10.00420943204
|
|
21.
|
C DesjobertMH RenalierJ BergaletMiR-29a
down-regulation in ALK-positive anaplastic large cell lymphomas
contributes to apoptosis blockade through MCL-1
overexpressionBlood11766276637201110.1182/blood-2010-09-30199421471522
|
|
22.
|
JL MottS KuritaSC CazanaveSF BronkNW
WerneburgME Fernandez-ZapicoTranscriptional suppression of
mir-29b-1/mir-29a promoter by c-Myc, hedgehog, and NF-kappaBJ Cell
Biochem11011551164201010.1002/jcb.2263020564213
|
|
23.
|
S HuangX HeThe role of microRNAs in liver
cancer progressionBr J
Cancer104235240201110.1038/sj.bjc.660601021102580
|
|
24.
|
C RoderburgGW UrbanK BettermannMicro-RNA
profiling reveals a role for miR-29 in human and murine liver
fibrosisHepatology53209218201110.1002/hep.2392220890893
|
|
25.
|
G KongJ ZhangS ZhangC ShanL YeX
ZhangUpregulated microRNA-29a by hepatitis B virus X protein
enhances hepatoma cell migration by targeting PTEN in cell culture
modelPLoS One6e19518201110.1371/journal.pone.001951821573166
|