Introduction
Colorectal cancer (CRC) is the third leading cause
of cancer-related death for both genders throughout the world
(1). Cancer cells can metastasize
to distinct organs from the primary site (2). Moreover, metastasis of cancer cells
is a major cause of death in cancer patients (3,4).
In particular, colon cancer cells mainly metastasize to the lymph
nodes, liver, and lung (4,5).
The processes and critical steps of metastasis include
proliferation of primary cancer cells, local invasion,
intravasation, cancer cell survival in blood flow, extravasation,
attachment to secondary organs, and metastatic growth in a new
environment (6). In this
mechanism of metastasis for many types of cancer, chemokine
receptors and their corresponding chemokine ligands play an
important role (7).
One of the chemokine receptors that promotes
metastasis is CXC chemokine receptor 4 (CXCR4) (7,8).
CXCR4 is a G protein-coupled human chemokine receptor, which has a
seven-transmembrane domain. CXCR4 is mostly expressed in various
cancer types including CRC, breast cancer, prostate cancer, and
ovarian cancer as well as immune cells including B lymphocytes and
T lymphocytes (9). The expression
of CXCR4 affects the metastatic behavior of CRC cells, which is
associated with poor patient prognosis and lymphatic and distant
dissemination (10–12). CXCR4-overexpressing cancer cells
have an increased ability of migration in vitro and
metastasis to other organs in vivo (13,14). On the other hand, CXCR4-knockdown
cancer cells have decreased invasive ability (15). CXCR4 modulates cellular biology
(i.e., cell growth and migration) through several signaling
pathways including G protein signaling, ERK, JNK, and JAK/STAT
signaling by binding its ligand, CXC chemokine ligand 12 (CXCL12
also known as SDF-1α) (16,17). Stromal cell-derived factor-1α
(SDF-1α) is one of the small pro-inflammatory chemoattractant
cytokines and binds to CXCR4 and CXCR7 (18,19). SDF-1α is expressed in primary
tumors including breast cancer, pancreatic cancer, ovarian cancer
and in primary sites of metastatic cancer (i.e., lung, liver, and
bone) (18).
CXCR4/SDF-1α influences a variety of behaviors in
cancer cells, for example, migration, metastasis, growth/survival,
angiogenesis, and malignant progression as well as trafficking of
stem cells (19–21). In mechanisms of
CXCR4/SDF-1α-mediated tumor metastasis, CXCR4-expressing cancer
cells are attracted to a target organ that secretes SDF-1α by
sensing a chemokine gradient. Upon receiving this chemokine signal,
CXCR4-expressing cancer cells migrate to the secondary organ
through multiple processes (6,7,18).
Recently, macrophage migration-inhibitory factor
(MIF) has become known as a new ligand of CXCR4 and it has
chemokine-like functions. Moreover, MIF binds to CXCR2, CXCR7,
CD44, and CD74 (22).
Inflammatory cells including B lymphocytes and T lymphocytes and
various tumor cells have shown expression of MIF. MIF plays various
roles as a critical mediator of acute and chronic inflammatory
disease and is also involved in tumor progression and development
(23). MIF regulates cell
proliferation and survival in monocytes, T cells, and fibroblasts
in an autocrine manner. Moreover, it regulates tumor cell
proliferation and angiogenesis in a paracrine manner (24). In recent reports, it was shown
that MIF regulates tumor cell metastasis in rhabdomyosarcoma and is
associated with invasive ability of drug-resistant human colon
cancer cells that express CXCR4 (25,26).
Although the mechanism of CXCR4/SDF-1α-mediated
metastasis-related cancer cell behaviors (i.e., invasion, cell
proliferation and adhesion) is well known, the function of
CXCR4/MIF in colon cancer metastasis remains largely unknown. To
confirm the effect of SDF-1α and to compare the effect of MIF with
the effect of SDF-1α in cancer metastatic behaviors, we performed
experiments adding human recombinant SDF-1α or MIF to
CXCR4-expressing colon cancer cells. Here, we investigated the
effect of SDF-1α or MIF on cell cycle, proliferation, adhesion, and
migration in CXCR4-expressing colon cancer cells.
Materials and methods
CRC cell lines and culture
conditions
Thirty-two human CRC cell lines were obtained from
the Korean Cell Line Bank (Seoul, Korea) and were grown in
RPMI-1640 medium with 10% fetal bovine serum (FBS), except for
Caco-2 which was grown in Minimum Essential Medium with 10% FBS and
WiDr in Dulbecco’s modified Eagle’s medium (all were from
Invitrogen Life Technologies, Carlsbad, CA, USA) with 10% FBS. Each
medium contained 100 U/ml of penicillin and 0.1 mg/ml of
streptomycin. Cells were grown in humidified incubators at 37°C, in
5% CO2 and 95% air.
RNA isolation and cDNA synthesis
Cells were collected by trypsinization and suspended
in easy-BLUE (Intron Biotechnology, Gyeonggi, Korea). According to
the manufacturer’s instructions, total RNA was isolated. For cDNA
synthesis, 2 μg of total RNA and 1 μl of the random
primers were mixed and heated at 70°C for 10 min. Then, they were
incubated on ice for 5 min. A mixture containing 4 μl of 5X
FS buffer, 2 μl of 0.1 M DTT, 1 μl of 2.5 mM dNTP, 1
μl of Superscript II reverse transcriptase (Invitrogen Life
Technologies) and 1 μl of distilled water was added, and
reverse transcription reaction was carried out. The reaction
conditions were 1 h for 30 min at 42°C and 15 min at 80°C. Finally,
80 μl of distilled water was added to dilute the cDNA.
Reverse transcriptase-PCR (RT-PCR)
Cells were collected by trypsinization and suspended
in easy-BLUE. According to the manufacturer’s instructions, total
RNA was isolated and cDNA synthesis was performed. For PCR
amplification, cDNA-specific primers for CXCR4, SDF-1α, MIF, and
β-actin, as a quantitative control, were used. The primer sequences
for CXCR2 were forward, 5′-AGGCACAGTGAAGACATCGG-3′ and reverse,
5′-CAGCAGGCTCAGCAGGAATA-3′ (27);
for CXCR4 forward, 5′-AGGGGATCAGTATATACACTT-3′ and reverse,
5′-TGCCCACAATGCCAGTTAAG-3′ (28);
for CXCR7 forward, 5′-TGGGTGGTCAGTCTCGT-3′ and reverse,
5′-CCGGCAGTAGGTCTCAT-3′ (29);
for SDF-1α forward, 5′-AGAGCCAACGTCAAGCATCT-3′ and reverse,
5′-CGTCTTTGCCCTTTCATCTC-3′ (30);
for MIF forward, 5′-CTCTCCGAGCTCACCCAGCAG-3′ and reverse, 5′-CGC
GTTCATGTCGTAATAGTT-3′ (31) and
for β-actin forward, 5′-GACCACACCTTCTACAATGAG-3′ and reverse,
5′-GCA TACCCCTCGTAGATGGG-3′ (32). PCR amplification was carried out
in a programmable thermal cycler (PCR System 9700; Applied
Biosystems, Foster City, CA, USA). The amplified DNA fragments were
fractionated in a 1.5% agarose gel and stained with ethidium
bromide.
Protein isolation and western
blotting
Cells were rinsed three times with PBS and lysed in
PRO-PREP protein extraction solution (Intron Biotechnology) and
placed on ice for 30 min. The lysates were centrifuged at 13000 x g
for 20 min at 4°C and then the supernatant was collected. The
protein concentration was determined using the SMART Micro BCA
protein assay kit (Intron Biotechnology). Twelve micrograms of
protein was resolved by 4X SDS sample buffer and boiled at 95°C for
5 min. Protein was loaded on a 4–12% Bis-Tris gel at 100 V for ∼3 h
and transferred to a polyvinylidene fluoride (PVDF) membrane (all
were from Invitrogen Life Technologies) by electroblotting at 270
mA constant current for 1 h 30 min at 4°C. For blocking, the
membrane was incubated in 1.7% non-fat dry milk and 0.5% Tween
20-TBS buffer containing 1 mM of MgCl2 for 1 h at room
temperature. Primary antibodies against CXCR4 (Abcam, Cambridge,
UK) (1:3,000), MIF (Santa Cruz Biotechnology, Inc., Santa Cruz, CA,
USA) (1:1,000) and β-actin (Applied Biological Materials, Inc.,
Richmond, Canada) (1:5,000) were introduced to the membrane and
incubated at room temperature for 1 h. Peroxidase-conjugated mouse
or rabbit IgG antibody (Jackson ImmunoReasearch, Baltimore, MD,
USA) (1:5,000) was used as a secondary antibody and incubated at
room temperature for 1 h. Chemiluminescent working solution,
WEST-ZOL (Intron Biotechnology), was decanted to the membrane. The
membrane was exposed to Fuji RX film for 1–5 min.
Recombinant human proteins and
AMD3100
Recombinant human proteins, rhSDF-1α and rhMIF, were
purchased from R&D systems (Minneapolis, MN, USA) and 100 ng/ml
of SDF-1α or 50 ng/ml of rhMIF was used in the proliferation assay,
cell cycle analysis, cell counting, adhesion assay, and migration
assay. AMD3100 (1 μg/ml) (Sigma-Aldrich, St. Louis, Mo,
USA), which is a CXCR4 antagonist, was used for the inhibition of
CXCR4.
Cell cycle analysis
For the cell cycle analysis, 4×105
cells/well were seeded on 6-well plates. Cells were starved with
serum-free media for 24 h and then rhSDF-1α (100 ng/ml) or rhMIF
(50 ng/ml) was added. After 12–72 h, cells were washed in cold PBS
and collected by trypsinization. They were fixed in 70% ethanol and
incubated at −20°C for 48 h. After fixation, cells were washed with
cold PBS and stained with propidium iodide (PI) (100 μg/ml)
(Sigma-Aldrich) and RNase A (10 mg/ml) (Intron Biotechnology) for
30 min in ice. Cells were introduced to a fluorescence-activated
cell sorter (FACSCanto II; BD Biosciences, Franklin Lakes, NJ, USA)
to determine the proportion of cell cycle phases.
Cell counting
Cells (3.6×105/well) were seeded on
6-well plates and incubated in growth medium for 24 h. Cells were
starved with serum-free media for 24 h. For CXCR4 inhibition, the
cells were pretreated with AMD3100 (1 μg/ml) for 2 h and
then rhSDF-1α (100 ng/ml) or rhMIF (50 ng/ml) was added for 24–72
h. The cells were stained with 0.4% Trypan blue and cell counting
was performed using a Countess® cell counting chamber
slide and Countess automated cell counter (Invitrogen Life
Technologies). This experiment was repeated three times.
Adhesion assay
A 96-well plate was coated with 20 μg/ml of
human fibronectin (Gibco-BRL) and incubated overnight at 4°C.
Before the adhesion assay, this plate was blocked in RPMI-1640 with
0.5% BSA for 1 h. Cells were starved with serum-free media for 24 h
and then 0.5×105 cells/well were added onto the
fibronectin-coated plate with rhSDF-1α (100 ng/ml) or rhMIF (50
ng/ml). In the case of CXCR4 inhibition, pretreatment with AMD3100
(1 μg/ml) for 30 min was carried out before seeding the
cells. After 1 h of incubation at 37°C, the adherent cells were
washed in RPMI-1640 with 0.1% BSA, fixed with 96% ethanol and
stained with 0.1% crystal violet. After washing with PBS, 0.2%
Triton-X in distilled water was added to the wells. The absorbance
was measured using an ELISA reader (Molecular Devices Co.,
Sunnyvale, CA, USA) at 595 nm.
Migration assay
The migration assay was performed using a
polycarbonate membrane Transwell plate with 8-μm pore
filters (24-well plate; Corning, Tewksbury, MA, USA). The inserts
of the Transwell plate were rehydrated with warm RPMI-1640 at 37°C
for 2 h. Cells were starved with serum-free media for 24 h and
5×104 cells/well were placed on the insert in the
serum-free media. In the case of CXCR4 inhibition, pretreatment
with AMD3100 (1 μg/ml) for 30 min was carried out before
placing the cells. In the lower well, rhSDF-1α (100 ng/ml) or rhMIF
(50 ng/ml) in RPMI-1640 was added, and the Transwell plate was
incubated at 37°C for 48 h. Cells on the upper surface of the
insert were removed using a cotton swab. Migrated cells on the
lower surface of the insert were washed in PBS three times and
fixed with 100% ethanol and then stained with 0.1% crystal violet.
The number of migrated cells was evaluted by photography and
counted under an inverted microscope in three random fields. The
migration assay was performed in triplicate wells.
Statistical analysis
All data were analyzed with the SPSS software
version 19.0 and expressed as means ± standard deviation. One-way
analysis of variance (one-way ANOVA) was used to determine whether
there were any significant changes in a time-dependent manner. For
the post hoc test, Tukey was used. Comparisons between the two
groups were carried out with the Student’s two-tailed t-test.
P<0.05 was considered to indicate a statistically significant
result.
Results
Expression of CXCR2, CXCR4, CXCR7,
SDF-1α, and MIF in CRC cell lines
In order to investigate the mRNA levels of CXCR2,
CXCR4, CXCR7, MIF, and SDF-1α in 32 human CRC cell lines, RT-PCR
was carried out. The mRNA expression of CXCR2 was observed in
several cell lines including SNU-503 and HCT116. The mRNA
expression of CXCR4 in 10 cell lines including Caco-2 and SW480 was
highly detected. The mRNA expression of CXCR7 in 8 cell lines
including Caco-2 and SNU-1047 was highly detected. The mRNA
expression of MIF in most of the cell lines was moderately or
highly detected. The mRNA expression of SDF-1α was highly detected
only in several cell lines including SNU-503 and Caco-2 (Fig. 1A). We performed western blotting
to determine the CXCR4 and MIF protein expression in 17 CRC cell
lines. The protein levels of CXCR4 and MIF were moderately or
highly detected in most of the CRC cell lines (Fig. 1B).
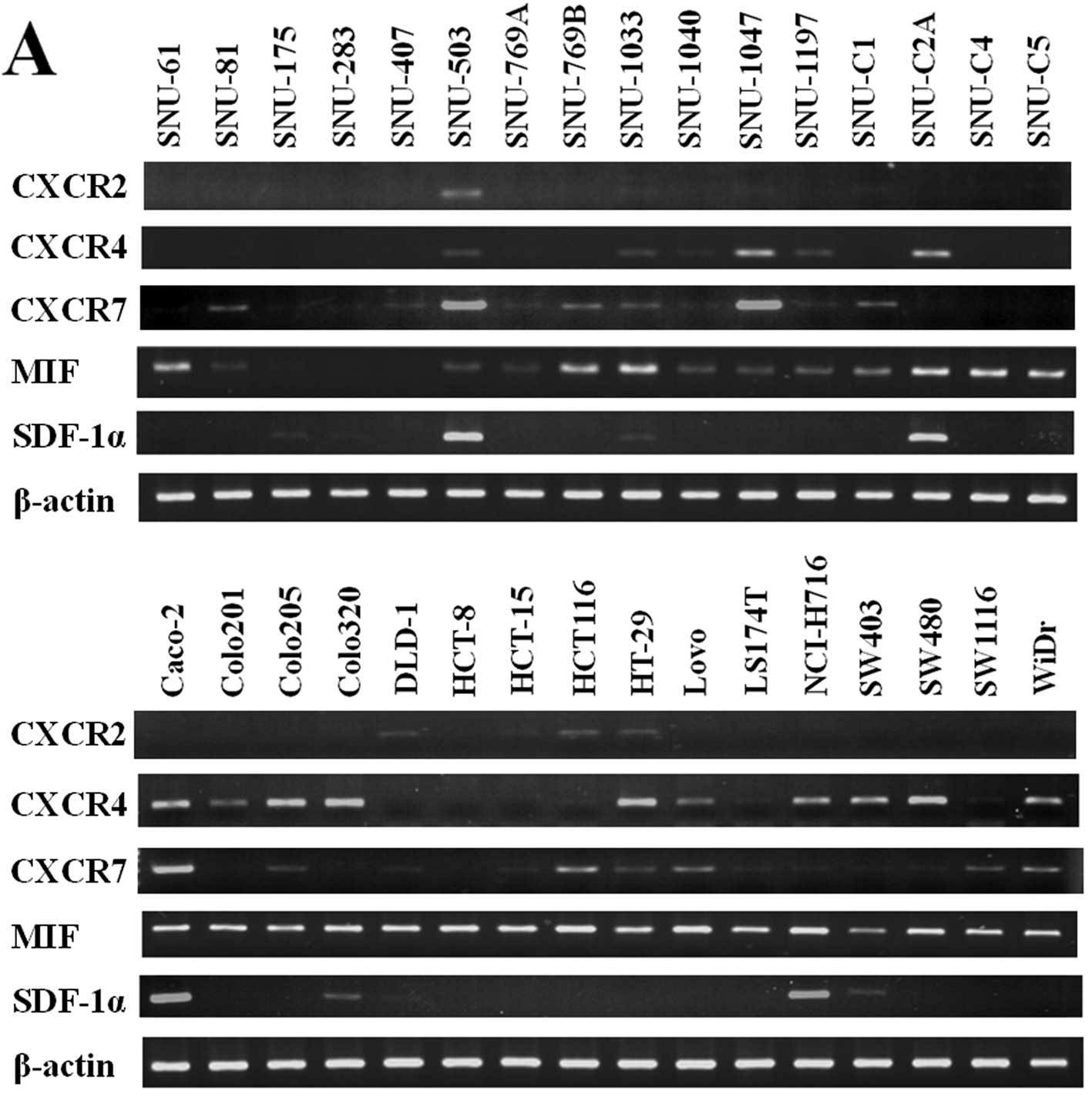 | Figure 1Analysis of CXCR2, CXCR4, CXCR7, MIF,
and SDF-1α expression levels by RT-PCR and western blotting. (A)
The mRNA expression levels of CXCR2, CXCR4, CXCR7, MIF, and SDF-1α
in 32 human CRC cell lines were analyzed by RT-PCR. (B) The protein
expression levels of CXCR4, MIF, and β-actin in 17 human CRC cell
lines were analyzed by western blotting. |
SDF-1α or MIF treatment and their effects
on the cell cycle of CXCR4-expressing SW480 colon cancer cells
In order to investigate the effect of SDF-1α or MIF
on the cell cycle of CXCR4-expressing colon cancer cells, SW480
cells were treated with human recombinant SDF-1α (100 ng/ml) or MIF
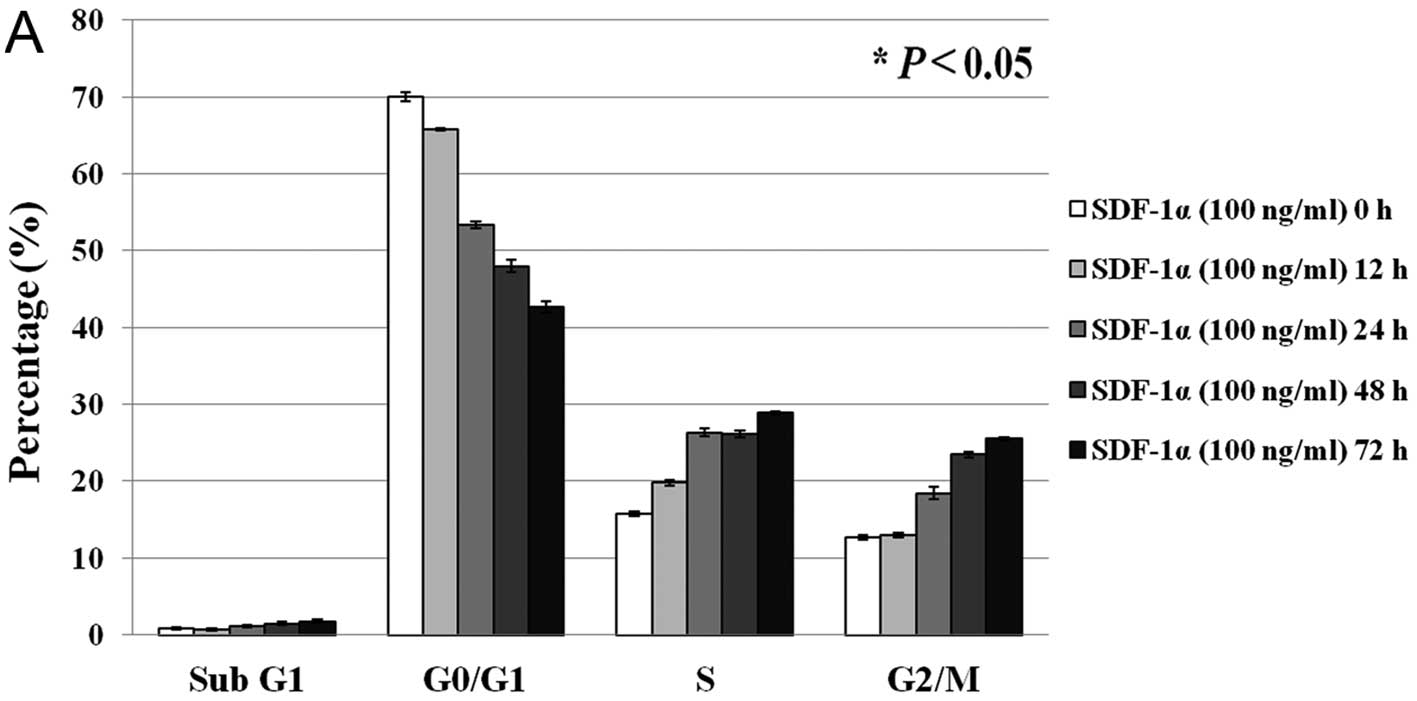
(50 ng/ml). When SW480 cells were treated with SDF-1α, the
percentage of cells in the G0/G1 phase decreased (from 70 to 43%)
and the percentage of cells in the S and G2/M phases increased
(from 16 to 29% and from 13 to 26%, respectively) in a
time-dependent manner (P<0.05 in all phases) (Fig. 2A). Similarly, when SW480 cells
were treated with MIF, the percentage of cells in the G0/G1 phase
decreased (from 70 to 43%) and the percentage of cells in the S and
G2/M phases increased (from 16 to 31% and from 13 to 23%,
respectively) in a time-dependent manner (P<0.05 in all phases)
(Fig. 2B).
SDF-1α or MIF treatment and the effects
on the cell proliferation of CXCR4-expressing SW480 colon cancer
cells
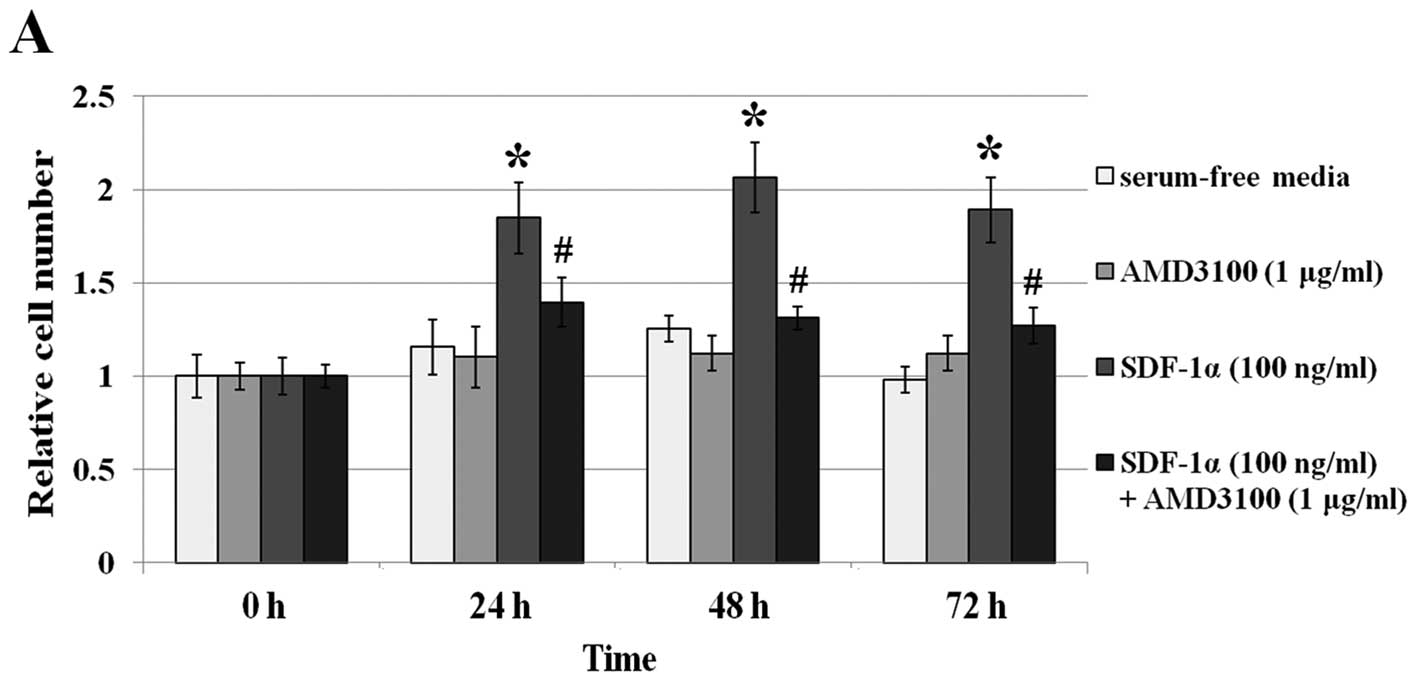
In order to investigate the effect on cell viability
of SDF-1α or MIF in CXCR4-expressing colon cancer cells, we carried
out cell proliferation assays. In serum-free media, SDF-1α (100
ng/ml) or MIF (50 ng/ml) caused an increase in cell proliferation.
When SW480 cells were treated with SDF-1α or MIF, approximately a
1.8- and 2-fold increase in cell numbers was observed at 48 and 72
h, respectively (P<0.05) (Fig.
3). When the cells were pretreated with AMD3100 (1
μg/ml) only, no effect on the viability of the two cell
lines compared to the serum-free medium group was noted
(P>0.05). Although SDF-1α or MIF was added to SW480 cells, cell
proliferation did not increase with AMD3100 pretreatment compared
to the serum-free medium group (P>0.05).
SDF-1α or MIF treatment and their effects
on the adhesion to fibronectin in CXCR4-expressing SW480 colon
cancer cells
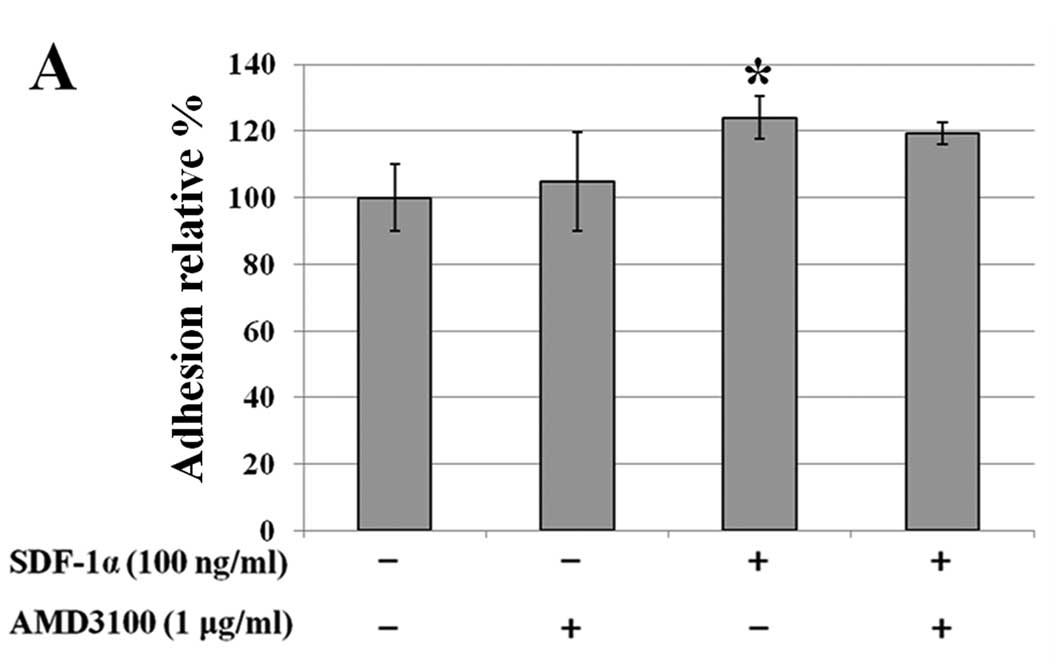
We investigated the effects of SDF-1α or MIF on
adhesion to fibronectin, which is part of the extracellular matrix
(ECM), in CXCR4-expressing colon cancer cells. Starved cells were
seeded onto a fibronectin-coated 96-well plate with or without
SDF-1α (100 ng/ml) and with or without MIF (50 ng/ml). In SW480
cells, SDF-1α or MIF caused increased adhesion to fibronectin (∼22
and 30%, respectively). Pretreatment with AMD3100 decreased these
effects when MIF was added (P<0.05) (Fig. 4).
SDF-1α or MIF treatment and their effects
on the migratory ability of CXCR4-expressing SW480 colon cancer
cells
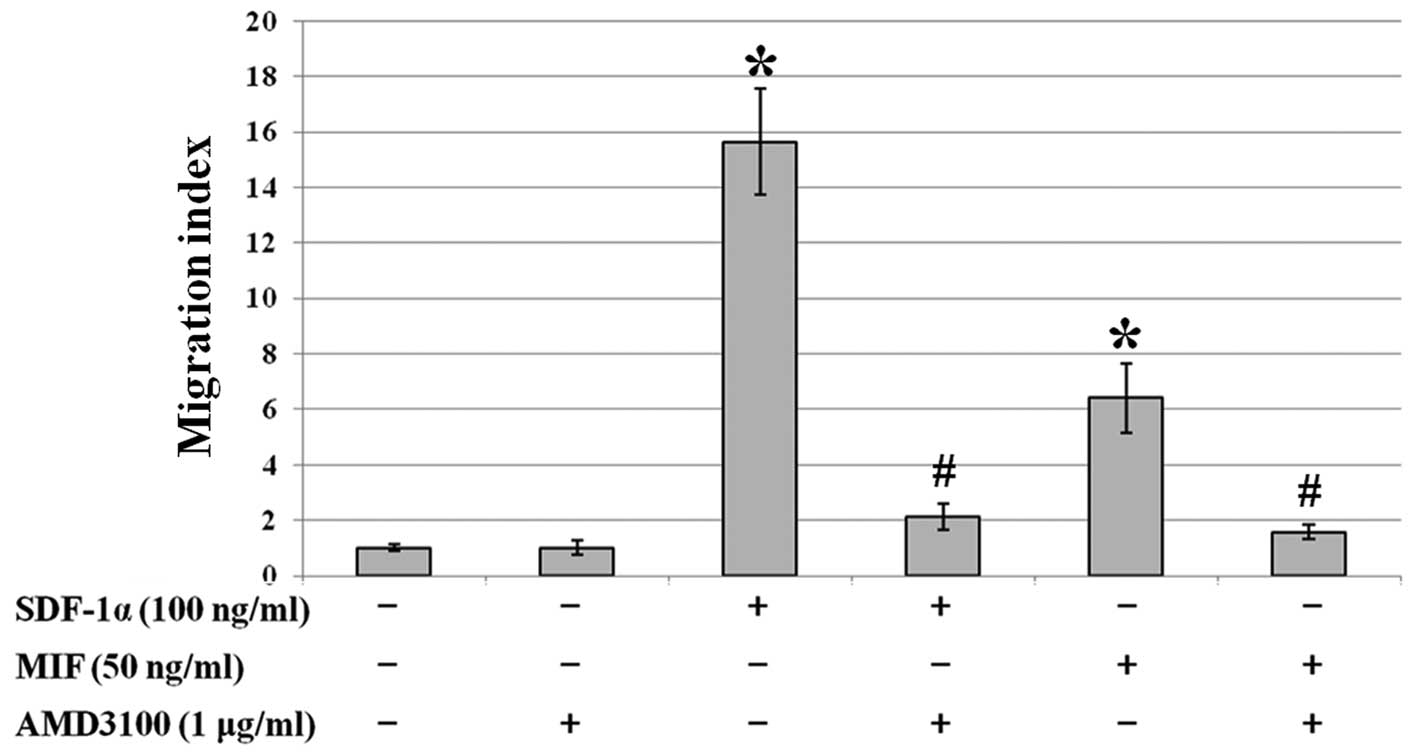
The migratory ability of cancer cells is related to
cancer metastasis. In order to determine the effect of SDF-1α or
MIF on the migration of CXCR4-expressing SW480 colon cancer cell
line, we carried out a migration assay with Transwell plates.
SDF-1α (100 ng/ml) and MIF (50 ng/ml) were applied separately to
the lower wells and serum-free media were used as a negative
control.
The migratory ability of the SW480 cells was
enhanced ∼16- and 6-fold by SDF-1α and MIF, respectively
(P<0.05). Only cells pretreated with AMD3100 (1 μg/ml)
showed no significant effect. Moreover, pretreatment with AMD3100
attenuated the SDF-1α- or MIF-induced increase in migration
(Fig. 5).
Discussion
CXCR4 is G protein-coupled human chemokine receptor
which has a seven-transmembrane domain. At first, CXCR4 was studied
in the co-receptor of T and dendritic cells for HIV entry (33). Since then, CXCR4 and its ligand,
SDF-1α have been studied in regards to many behaviors of cancer
cells. CXCR4 mainly regulates cell migration, invasion, cell
growth/survival, malignant progression, and angiogenesis by binding
its ligand, SDF-1α, in many cancer cells including ovarian cancer,
breast cancer, and rhabdomyosarcoma (7,34).
In 2007, Bernhagen et al (22) showed that MIF interacts with CXCR2
and CXCR4 in Jurkat T cells. CXCR4/MIF has been studied in
drug-resistant human colon cancer cells and CXCR4/MIF-mediated JNK
and AKT signaling pathways in mouse fibroblasts and T cells
(25,35). AMD3100 is a prototype non-peptide
antagonist of CXCR4. AMD3100 binds to CXCR4 through the
interactions of three acidic anchor-point residues in CXCR4 and
blocks other agonists from binding to CXCR4 (36).
Behavior of metastatic cancer cells are related to
cell survival, resistance to apoptosis, adhesion to extracellular
matrix molecules, and cell migration/invasion. The function of
CXCR4/SDF-1α in these metastatic features has been intensely
studied in many types of cancer cells (11,37). However, CXCR4/MIF has rarely been
studied particularly in colon cancer cells. In this study, we
hypothesized that SDF-1α or MIF, which interacts with CXCR4,
affects the metastatic behaviors of CXCR4-expressing colon cancer
cells and performed experiments to confirm the effects of SDF-1α or
MIF on CXCR4-expressing colon cancer cells.
Many cancer cells including breast, ovarian, and
prostate cancer overexpress CXCR4 (9). We also found that the mRNA or
protein of CXCR4 was expressed in 17 out of 32 (53%) and 17 out of
17 (100%) CRC cell lines, respectively. CXCR2 and CXCR7 are also
receptors for SDF-1α and MIF (19,22,26). Therefore, we confirmed their mRNA
level by performing RT-PCR. We used SW480 cells, which do not
express mRNA of CXCR2 and CXCR7, in order to confirm the effect of
MIF or SDF-1α on CXCR4-expressing colon cancer cells. MIF is
induced in the serum and tumor specimens of patients with CRC and
it is overexpressed in solid tumors including prostate and breast
cancer (38,39). We observed that the mRNA or
protein of MIF was expressed in 29 out of 32 (90%) and 17 out of 17
(100%) CRC cell lines, respectively. The SDF-1α expression has been
frequently noted in primary tumors and at the sites of metastasis,
which include lymph nodes, bone marrow, liver, and lung (40). In this study, the mRNA expression
of SDF-1α was rarely observed in 9 out of 32 (28%) CRC cell lines
except in SNU-503, SNU-C2A, Caco-2 and NCI-H716.
Cell proliferation/survival is an important part of
the mechanism of cancer metastasis. The transition of the cell
cycle phase is related to cell division and proliferation/survival
(41). In peripheral blood
CD34+ cells, SDF-1α significantly increased the
percentage of cells in S and G2/M phases (42). In addition, SDF-1α decreased cells
in the sub-G1 phase and increased cell proliferation in serum-free
conditions in CXCR4-expressing pancreatic cancer cells (43). The effect of MIF on cell cycle in
colon cancer cells has been rarely studied. Therefore, we
investigated the effects of SDF-1α or MIF on cell cycle and cell
proliferation. In general, cells incubated in serum-free media are
arrested in the cell cycle at the G0/G1 phase. In this report,
SW480 cells were starved with serum-free media and the result at
the 0 h, the start point, showed a high percentage of cells in the
G0/G1 phase. The percentage of SW480 cells in S and G2/M phases
increased in a time-dependent manner after the cells were treated
separately with SDF-1α or MIF.
In CXCR4-expressing pancreatic cancer cells, the
cell proliferation was enhanced by the addition of SDF-1α in
serum-free conditions (43). MIF
activates AKT signaling, which is related to cell survival and
induces the suppression of apoptosis in fibroblasts and breast
cancer cells (44). In this
study, treatment with SDF-1α or MIF showed an increase in cell
proliferation by carrying out cell counting. When CXCR4 was blocked
by AMD3100, the effect of the increased cell proliferation by
SDF-1α or MIF was inhibited.
Colon cancer cells can disseminate to the liver and
lymph nodes through lymphatic vessels or blood vessels. In these
processes, colon cancer cells are detached from the primary site
and invade lymphatic vessels or blood vessels and migrate to
secondary sites (6). When colon
cancer cells are metastasized, they attach to various ECM molecules
(i.e., E-selectin, integrin, and fibronectin) (6). CXCR4/SDF-1α was found to increase
cell adhesion to elements of the ECM including fibronectin and
collagen type IV by activating the ERK signaling pathway in ovarian
cancer cells (45). However, the
effect of SDF-1α or MIF on CXCR4-expressing colon cancer cell
adhesion has rarely been reported. From the results of the adhesion
assay in this study, SDF-1α or MIF induced an increase
(approximately 22–30%) in cell adhesion to fibronectin in SW480
cells. Blockage of CXCR4 by AMD3100 did not inhibit the effect of
SDF-1α on cell adhesion to fibronectin but decreased the effect of
MIF. Further experiments are necessary to confirm the effect of
SDF-1α or MIF on cell adhesion to other ECM molecules (i.e.,
laminin, Matrigel, and collagen) in CXCR4-expressing colon cancer
cells.
As previously reported, migration/invasion of cancer
cells is related to metastasis, and chemokine receptors and their
ligands are involved in the mechanisms of metastasis (7). CXCR4/SDF-1α regulates cell
trafficking, migration, and invasion in stem cells and many cancer
types including breast and prostate cancer (18,21). CXCR4/MIF was found to promote the
invasive ability of drug-resistant colon carcinoma HT-29 cells in
an autocrine manner. When CXCR4 or MIF was blocked by its inhibitor
(each AMD3100 and ISO-1), the aggressive phenotype of
drug-resistant HT-29 cells was abolished (25). We observed that SDF-1α or MIF
caused an increase in cell migration in the CXCR4-expressing colon
cancer cell line, SW480. When the cells were pretreated with
AMD3100, the effect of increased migration by SDF-1α or MIF was
blocked.
Based on the finding of this study, we conclude that
not only the CXCR4/SDF-1α axis but also the CXCR4/MIF axis induces
metastatic behavior of colon cancer cells and thus can be a
therapeutic target in the metastasis of colon cancer cells.
Moreover, further studies concerning signaling pathways and other
metastatic features are required.
Abbreviations:
|
CXCR4
|
CXC chemokine receptor 4;
|
|
SDF-1α
|
stromal cell-derived factor-1α;
|
|
CXCL12
|
CXC chemokine ligand 12;
|
|
MIF
|
macrophage migration-inhibitory
factor;
|
|
ECM
|
extracellular matrix;
|
|
CRC
|
colorectal cancer
|
Acknowledgements
This study was supported by the
Mid-Career Researcher Program (MEST R01-2008-000-20108-0) and the
Priority Research Centers Program (2009-0093820) through the
National Research Foundation of Korea Grant funded by the MEST.
References
|
1.
|
J FerlayHR ShinF BrayD FormanC MathersDM
ParkinEstimates of worldwide burden of cancer in 2008: GLOBOCAN
2008Int J Cancer12728932917201010.1002/ijc.2551621351269
|
|
2.
|
GP GuptaJ MassagueCancer metastasis:
building a
frameworkCell127679695200610.1016/j.cell.2006.11.00117110329
|
|
3.
|
DA AugustRT OttowPH SugarbakerClinical
perspective of human colorectal cancer metastasisCancer Metastasis
Rev3303324198410.1007/BF000514576394125
|
|
4.
|
JR HeadrickDL MillerDM NagorneySurgical
treatment of hepatic and pulmonary metastases from colon cancerAnn
Thorac Surg71975980200110.1016/S0003-4975(00)02522-411269484
|
|
5.
|
S WelterJ JacobsT KrbekC PoettgenG
StamatisPrognostic impact of lymph node involvement in pulmonary
metastases from colorectal cancerEur J Cardiothorac
Surg31167172200710.1016/j.ejcts.2006.11.00417150367
|
|
6.
|
S GoutJ HuotRole of cancer
microenvironment in metastasis: focus on colon cancerCancer
Microenviron16983200810.1007/s12307-008-0007-219308686
|
|
7.
|
T KakinumaST HwangChemokines, chemokine
receptors, and cancer metastasisJ Leukoc
Biol79639651200610.1189/jlb.110563316478915
|
|
8.
|
M O’HayreC SalangaT HandelS
AllenChemokines and cancer: migration, intracellular signalling and
intercellular communication in the microenvironmentBiochem
J409635649200818177271
|
|
9.
|
F BalkwillCancer and the chemokine
networkNat Rev Cancer4540550200410.1038/nrc1388
|
|
10.
|
J KimH TakeuchiST LamChemokine receptor
CXCR4 expression in colorectal cancer patients increases the risk
for recurrence and for poor survivalJ Clin
Oncol2327442753200510.1200/JCO.2005.07.07815837989
|
|
11.
|
CC SchimanskiS SchwaldN SimiantonakiEffect
of chemokine receptors CXCR4 and CCR7 on the metastatic behavior of
human colorectal cancerClin Cancer
Res1117431750200510.1158/1078-0432.CCR-04-119515755995
|
|
12.
|
N YoshitakeH FukuiH YamagishiExpression of
SDF-1α and nuclear CXCR4 predicts lymph node metastasis in
colorectal cancerBr J Cancer98168216892008
|
|
13.
|
KE LukerGD LukerFunctions of CXCL12 and
CXCR4 in breast cancerCancer
Lett2383041200610.1016/j.canlet.2005.06.02116046252
|
|
14.
|
Y KangPM SiegelW ShuA multigenic program
mediating breast cancer metastasis to boneCancer
cell3537549200310.1016/S1535-6108(03)00132-612842083
|
|
15.
|
MCP SmithKE LukerJR GarbowCXCR4 regulates
growth of both primary and metastatic breast cancerCancer
Res6486048612200410.1158/0008-5472.CAN-04-184415574767
|
|
16.
|
H ZhengG FuT DaiH HuangMigration of
endothelial progenitor cells mediated by stromal cell-derived
factor-1alpha/CXCR4 via PI3K/Akt/eNOS signal transduction pathwayJ
Cardiovasc
Pharmacol50274280200710.1097/FJC.0b013e318093ec8f17878755
|
|
17.
|
JM BusilloJL BenovicRegulation of CXCR4
signalingBiochim Biophys
Acta1768952963200710.1016/j.bbamem.2006.11.00217169327
|
|
18.
|
J WangR LobergRS TaichmanThe pivotal role
of CXCL12 (SDF-1)/CXCR4 axis in bone metastasisCancer Metastasis
Rev25573587200610.1007/s10555-006-9019-x17165132
|
|
19.
|
X SunG ChengM HaoCXCL12/CXCR4/CXCR7
chemokine axis and cancer progressionCancer Metastasis
Rev29709722201010.1007/s10555-010-9256-x20839032
|
|
20.
|
S LiekensD ScholsS HatseCXCL12-CXCR4 axis
in angiogenesis, metastasis and stem cell mobilizationCurr Pharm
Des1639033920201010.2174/13816121079445500321158728
|
|
21.
|
M KuciaR RecaK MiekusTrafficking of normal
stem cells and metastasis of cancer stem cells involve similar
mechanisms: pivotal role of the SDF 1-CXCR4 axisStem
Cells23879894200510.1634/stemcells.2004-034215888687
|
|
22.
|
J BernhagenR KrohnH LueMIF is a noncognate
ligand of CXC chemokine receptors in inflammatory and atherogenic
cell recruitmentNat Med13587596200710.1038/nm156717435771
|
|
23.
|
JP BachB RinnB MeyerR DodelM BacherRole of
MIF in inflammation and
tumorigenesisOncology75127133200810.1159/00015522318791328
|
|
24.
|
RA MitchellR BucalaTumor growth-promoting
properties of macrophage migration inhibitory factor (MIF)Semin
Cancer Biol10359366200010.1006/scbi.2000.032811100884
|
|
25.
|
AF DesseinL StechlyN JonckheereAutocrine
induction of invasive and metastatic phenotypes by the MIF-CXCR4
axis in drug-resistant human colon cancer cellsCancer
Res7046444654201010.1158/0008-5472.CAN-09-382820460542
|
|
26.
|
M TarnowskiK GrymulaR LiuHuman
rhabdomyosarcomas secrete MIF that modulates metastatic behavior of
tumor cells and inhibits recruitment of cancer-associated
fibroblastsMol Cancer
Res813281343201010.1158/1541-7786.MCR-10-0288
|
|
27.
|
Z LiuL YangJ XuX ZhangB WangEnhanced
expression and clinical significance of chemokine receptor CXCR2 in
hepatocellular carcinomaJ Surg
Res166241246201110.1016/j.jss.2009.07.01420018298
|
|
28.
|
JP SalimNP GoettePR LevDysregulation of
stromal derived factor 1/CXCR4 axis in the megakaryocytic lineage
in essential thrombocythemiaBr J
Haematol1446977200910.1111/j.1365-2141.2008.07428.x19006565
|
|
29.
|
K ZhengHY LiXL SuChemokine receptor CXCR7
regulates the invasion, angiogenesis and tumor growth of human
hepatocellular carcinoma cellsJ Exp Clin Cancer
Res2931201010.1186/1756-9966-29-3120380740
|
|
30.
|
S BrandJ DambacherF BeigelCXCR4 and CXCL12
are inversely expressed in colorectal cancer cells and modulate
cancer cell migration, invasion and MMP-9 activationExp Cell
Res310117130200510.1016/j.yexcr.2005.07.00616125170
|
|
31.
|
S WadaS FujimotoY MizueJ
NishihiraMacrophage migration inhibitory factor in the human ovary:
presence in the follicular fluids and production by granulosa
cellsBiochem Mol Biol Int4180581419979111941
|
|
32.
|
HD HemmatiI NakanoJA LazareffCancerous
stem cells can arise from pediatric brain tumorsProc Natl Acad Sci
USA1001517815183200310.1073/pnas.203653510014645703
|
|
33.
|
A Granelli-PipernoB MoserM PopeEfficient
interaction of HIV-1 with purified dendritic cells via multiple
chemokine coreceptorsJ Exp
Med18424332438199610.1084/jem.184.6.24338976200
|
|
34.
|
F BalkwillThe significance of cancer cell
expression of the chemokine receptor CXCR4Semin Cancer
Biol14171179200410.1016/j.semcancer.2003.10.00315246052
|
|
35.
|
H LueM DeworL LengR BucalaJ
BernhagenActivation of the JNK signalling pathway by macrophage
migration inhibitory factor (MIF) and dependence on CXCR4 and
CD74Cell
Signal23135144201110.1016/j.cellsig.2010.08.01320807568
|
|
36.
|
MM RosenkildeLO GerlachJS JakobsenRT
SkerljGJ BridgerTW SchwartzMolecular mechanism of AMD3100
antagonism in the CXCR4 receptorJ Biol
Chem27930333041200410.1074/jbc.M30954620014585837
|
|
37.
|
M KuciaK JankowskiR RecaCXCR4-SDF-1
signalling, locomotion, chemotaxis and adhesionJ Mol
Histol35233245200410.1023/B:HIJO.0000032355.66152.b815339043
|
|
38.
|
N TakahashiJ NishihiraY SatoInvolvement of
macrophage migration inhibitory factor (MIF) in the mechanism of
tumor cell growthMol Med470771419989932108
|
|
39.
|
XX HeK ChenJ YangMacrophage migration
inhibitory factor promotes colorectal cancerMol
Med15110200919009023
|
|
40.
|
AF ChambersAC GroomIC
MacDonaldDissemination and growth of cancer cells in metastatic
sitesNat Rev Cancer2563572200210.1038/nrc86512154349
|
|
41.
|
GK SchwartzMA ShahTargeting the cell
cycle: a new approach to cancer therapyJ Clin
Oncol2394089421200510.1200/JCO.2005.01.559416361640
|
|
42.
|
JJ LatailladeD ClayC DupuyChemokine SDF-1
enhances circulating CD34(+) cell proliferation in synergy with
cytokines: possible role in progenitor
survivalBlood957567682000
|
|
43.
|
F MarchesiP MontiBE LeoneIncreased
survival, proliferation, and migration in metastatic human
pancreatic tumor cells expressing functional CXCR4Cancer
Res6484208427200410.1158/0008-5472.CAN-04-134315548713
|
|
44.
|
H LueM ThieleJ FranzMacrophage migration
inhibitory factor (MIF) promotes cell survival by activation of the
Akt pathway and role for CSN5/JAB1 in the control of autocrine MIF
activityOncogene2650465059200710.1038/sj.onc.121031817310986
|
|
45.
|
X ShenS WangH WangM LiangL XiaoZ WangThe
role of SDF-1/CXCR4 axis in ovarian cancer metastasisJ Huazhong
Univ Sci Technolog Med
Sci29363367200910.1007/s11596-009-0320-019513623
|