Introduction
Neuroblastoma is a type of solid tumor that commonly
occurs in children under 5 years of age, with a current prevalence
of 1 out of 100,000 children (1).
The clinical hallmark of neuroblastoma is its heterogeneity
(2). It most commonly originates
from one of the adrenal glands but it can also develop in the nerve
tissues located in the neck, chest, abdomen and pelvis (2). Common treatments for neuroblastoma
include intensive chemotherapy, surgery, radiotherapy, stem cell
transplantation and immunotherapy (3–5).
Low-risk patients can be cured via surgery, while intermediate and
high-risk patients require intensive chemotherapy and/or other
treatments (4). Vincristine
(VCR), an alkaloid extracted from Catharanthus roseus, is
frequently used in combination chemotherapy (6). For example, VCR is used together
with topotecan for the treatment of advanced bilateral intraocular
retinoblastoma (7). It is also
used together with carboplatin for the treatment of children with
newly diagnosed progressive low-grade gliomas (8).
In contrast to normal somatic cells, cancer cells
exhibit an uncontrolled cell cycle and decreased apoptosis
(9). Most chemotherapeutic agents
used in the clinic target those two features of cancer cells,
inducing their cell cycle arrest and apoptosis. For example, it has
been demonstrated that combined treatment with VCR and cisplatin,
another commonly used anticancer agent, induced apoptosis of the
human retinoblastoma cell lines Y79 and WERI-Rb1, in a
dose-dependent manner (10). In
acute lymphoblastic leukemia cells, VCR induced apoptosis through
activation of caspase-3 and -9 (11). Other anticancer drugs such as
baicalein led to cell cycle arrest at the S phase by downregulating
cell cycle factors including CDK-4, cyclin B1 and D1 in human lung
squamous carcinoma CH27 cells (12). Similarly, VCR, as an anti-tubulin
compound, can also lead to cell cycle arrest at the G2/M
phase by promoting microtubule depolymerization in many tumor cell
lines (13). However, despite the
studies demonstrating the anti-tubulin and apoptotic effect of VCR
on cancer cells, the mechanism involved in the VCR-induced cell
death in neuroblastoma cells is still not clear. Consequently, the
application of VCR in chemotherapies, for neuroblastoma and
lymphoma, still requires a high dosage resulting in high toxicity
(14,15).
The SH-SY5Y cell line originates from human
neuroblastoma. It contains many features representing dopaminergic
neurons (16). SH-SY5Y cells can
also be induced to differentiate into mature neurons (16), which exhibit a distinct morphology
and are easily detected (16).
Therefore, in the present study we used the SH-SY5Y cell line to
analyze the impact of VCR on the cell cycle and changes in
apoptosis. We also investigated the expression of apoptotic and
cell cycle-related factors following VCR treatment. We aimed to
clarify the mechanisms involved in the anticancer function of VCR
and provide important references for VCR application in
neuroblastoma chemotherapy.
Materials and methods
Cell culture
The human neuroblastoma cell line SH-SY5Y was
purchased from the China Center for Type Culture Collection. Cells
were cultured in H-DMEM medium containing 10% FBS at 37°C with 5%
CO2.
MTT assay measurement of cell
proliferation
SH-SY5Y cells at a logarithmic phase were seeded in
96-well plates (at 2×106/l) and incubated for 12 h until
cells formed a monolayer. Wells were randomly chosen for treatment
groups and a control group. For the treatment groups, cells were
incubated with 200 μl of cell culture medium containing 0.001,
0.01, 0.1, 1 or 10 μM of VCR (Sigma-Aldrich, St. Louis, MO, USA).
In the control group, cells were grown in 200 μl cell culture
medium only. Cells were incubated for another 24, 48 and 72 h and
then 20 μl of 5 g/l MTT (0.1 mg/l final concentration) was added to
each well. After 4 h of incubation, the cell culture supernatant
was removed, 150 μl of DMSO was added to each well and the plate
was shaken for 10 min. The absorbance of each well was detected at
490 nm (A value) on an ELISA plate reader. The growth
inhibition rate of VCR-treated cells was calculated as: Growth
inhibition rate % = [(average A value of control group -
average A value of VCR-treated group)/average A value
of control group] × 100%. This experiment was performed in
triplicates.
Flow cytometric measurement of the cell
cycle distribution
SH-SY5Y cells were seeded in 6-well plates (at
2×106/ml) and incubated for 24 h until they were treated
with 0.1 μM VCR for 6, 12, 18 and 24 h. Non-treated cells at 0 h
were used as the control group. Cells were washed twice with
ice-cold PBS and fixed with 70% ice-cold ethanol. After
centrifugation, 100 mg/l RNase and 5 g/l propidium iodide (both
purchased from Sigma-Aldrich) were added to each tube, and cells
were stained in the dark for 30 min. Detection of cell cycle
distribution was then performed on a BD FACSCalibur. FCS Express
Version 3.0 software was used to analyze the data.
Immunofluorescent staining measurement of
the mitotic index and apoptosis
SH-SY5Y cells were grown on coverslips inside the
wells of 6-well plates and were treated with 0.1 μM VCR for 6, 12,
18 and 24 h. Non-treated cells at 0 h were used as the control
group. Cells were incubated in warm PHEM solution at 37°C for 1
min, permeabilized with 0.1% Triton X-100 for 1 min and fixed with
3.7% formaldehyde for 15 min at 37°C. Cells were then washed in PBS
and stained for 20 min with 20 μl of Hoechst 33342 (final
concentration: 10 g/l; Sigma-Aldrich). After washing with TBST,
cells were incubated with FITC-labeled anti-α-tubulin antibody
(1:50 dilution; Sigma-Aldrich) for 1 h at 37°C. After another TBST
wash, fluorescent mounting media were added and the slides were
sealed. Under a fluorescence microscope, chromatin agglutination,
the number of mitotic cells and the number of apoptotic cells were
detected or calculated using Image-Pro Plus 6.0 software. On each
slide, 5 fields were chosen randomly, and the number of mitotic
cells was counted. The mitotic index (MI) was calculated using the
following equation: MI = number of mitotic cells/total number of
cells (17–19).
Real-time (RT)-PCR measurement of
caspase-3 and -9, cyclin B and D mRNA expression
Cells were seeded and treated as described
previously. Total RNA was extracted using the TRIzol reagent
(Invitrogen Life Technologies, Carlsbad, CA, USA) and cDNA was
synthesized using the PrimeScript™ RT kit (TransGen Biotech,
Beijing, China) according to the respective manufacturers'
instructions. The primers used in the RT-PCR reactions are listed
in Table I. The total reaction
volume was 20 μl including 1 μl forward primer, 1 μl reverse
primer, 1 μl cDNA, 4 μl SYBR-Green PCR master mix (Roche
Diagnostics Corporation, Roche Applied Science, Basel, Switzerland)
and 13 μl ddH2O. The following program was used in all
reactions: step 1, 1 cycle at 95°C for 10 min; step 2, 45 cycles of
94°C for 10 sec, 60°C for 15 sec and 72°C for 15 sec. Fluorescence
signal was collected at each cycle in step 2. All RT-PCR reactions
were performed in triplicates. Data were analyzed according to the
instruction manual of the Roche LightCycler 2.0 detection system.
First, ΔCt = CtGene - CtGAPDH. Then, ΔΔCt =
ΔCt treated - ΔCt control. Lastly, 2−ΔΔCt was calculated
as the relative mRNA expression of the target genes.
 | Table IPrimers used in the RT-PCR
reactions. |
Table I
Primers used in the RT-PCR
reactions.
| Gene | Primer sequences | Length (bp) |
|---|
| Cyclin B | Forward
5′-TCGAAAGTGTCGCATCAAACT-3′
Reverse 5′-CACAGAAGATGTGAGAGCAGG-3′ | 65 |
| Cyclin D | Forward
5′-TCCTCCAGGCTCTAGGCTATC-3′
Reverse 5′-CCTAAAACCTCTAGGAGCGTCT-3′ | 136 |
| Caspase 9 | Forward
5′-CACTTCCCCTGAAGACGAGTC-3′
Reverse 5′-GTGGGCAAACTAGATATGGCG-3′ | 111 |
| Caspase 3 | Forward
5′-CATGGAAGCGAATCAATGGACT-3′
Reverse 5′-CTGTACCAGACCGAGATGTCA-3′ | 139 |
| GAPDH | Forward
5′-AAGGTGAAGGTCGGAGTCAAC-3′
Reverse 5′-GGGGTCATTGATGGCAACAATA-3′ | 102 |
Western blot detection of caspase-3 and
-9, cyclin B and D protein expression
Cells were seeded and treated as described
previously. Cells were washed twice in PBS and lysed in lysis
buffer [50 mM Tris-HCl (pH 7.4); 150 mM NaCl; 1 mM ethylene diamine
tetra-acetic acid (EDTA); 5% (v/v) β-mercaptoethanol; 1% Nonidet P
(NP)−40; 0.25% sodio deoxycholate; 5 μg/ml leupeptin; 5 μg/ml
aprotinin; 0.2 mM phenylmethyl sulfonyl fluoride (PMSF)] and
incubated on ice for 60 min. Cell lysates were centrifuged at
12,000 × g, the supernatants were collected and the protein
concentration was measured. Protein (50 μg) was loaded from each
sample on SDS-PAGE using a 12% running gel. Separated proteins were
then transferred to PVDF membranes and blocked with 5% skim milk
for 2 h at 37°C. Blots were then incubated with primary antibodies
(all rabbit IgG) against caspase-3 and -9 (both at a 1:1,000
dilution; purchased from Epitomics, Burlingame, CA, USA), cyclin B
(1:50 dilution), cyclin D (1:200 dilution; both purchased from
Thermo Fisher Scientific, Fremont, CA, USA) or β-actin (1:200
dilution; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA)
overnight at 4°C. After washing in TBST for 3×15 min, the blots
were incubated with HRP-labeled rabbit IgG (1:4,000 dilution; KPL,
Gaithersburg, MD, USA) for 2 h at 37°C. They were washed again in
TBST for 3×15 min, exposed and developed to X-ray films. BandScan
5.0 software was used for protein densitometry measurements
(A value).
Statistical analysis
The SPSS 13.0 software was used for data analysis.
The t-test was used in the comparison between 2 groups and one-way
ANOVA in the comparison between more groups. Results are presented
as means ± SE. P<0.05 was considered to indicate a significant
difference.
Results
VCR inhibits the proliferation of SH-SY5Y
cells
We investigated the impact of VCR on SH-SY5Y cell
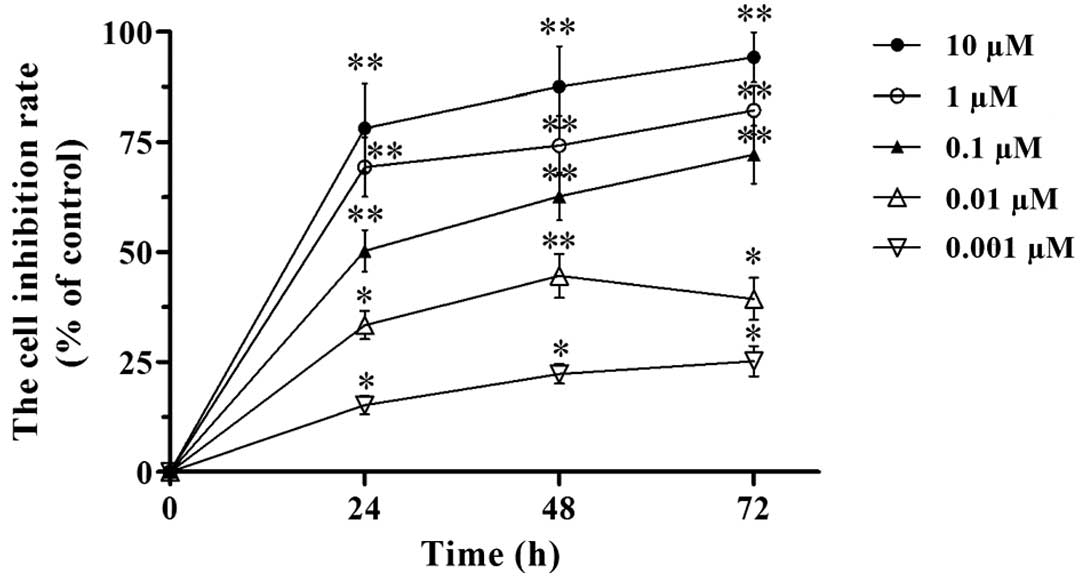
proliferation by the MTT assay. Our results revealed that VCR
inhibited the proliferation of SH-SY5Y cells (Fig. 1). The inhibitory effect increased
with the dose of VCR (from 0.001 to 10 μM). Under the same VCR
dose, the inhibitory effect on cell proliferation increased with
the time of treatment (24, 48 and 72 h time points). Hence, the
inhibition of SH-SY5Y cell proliferation by VCR was dose- and
time-dependent. According to the MTT assay, the IC50 of
VCR in SH-SY5Y cells was 0.113±0.012, 0.078±0.009 and 0.051±0.008
μM at 24, 48 and 72 h, respectively. We therefore chose 0.1 μM of
VCR as the treatment dose in the following experiments.
VCR induces SH-SY5Y cell cycle arrest at
the G2/M phase
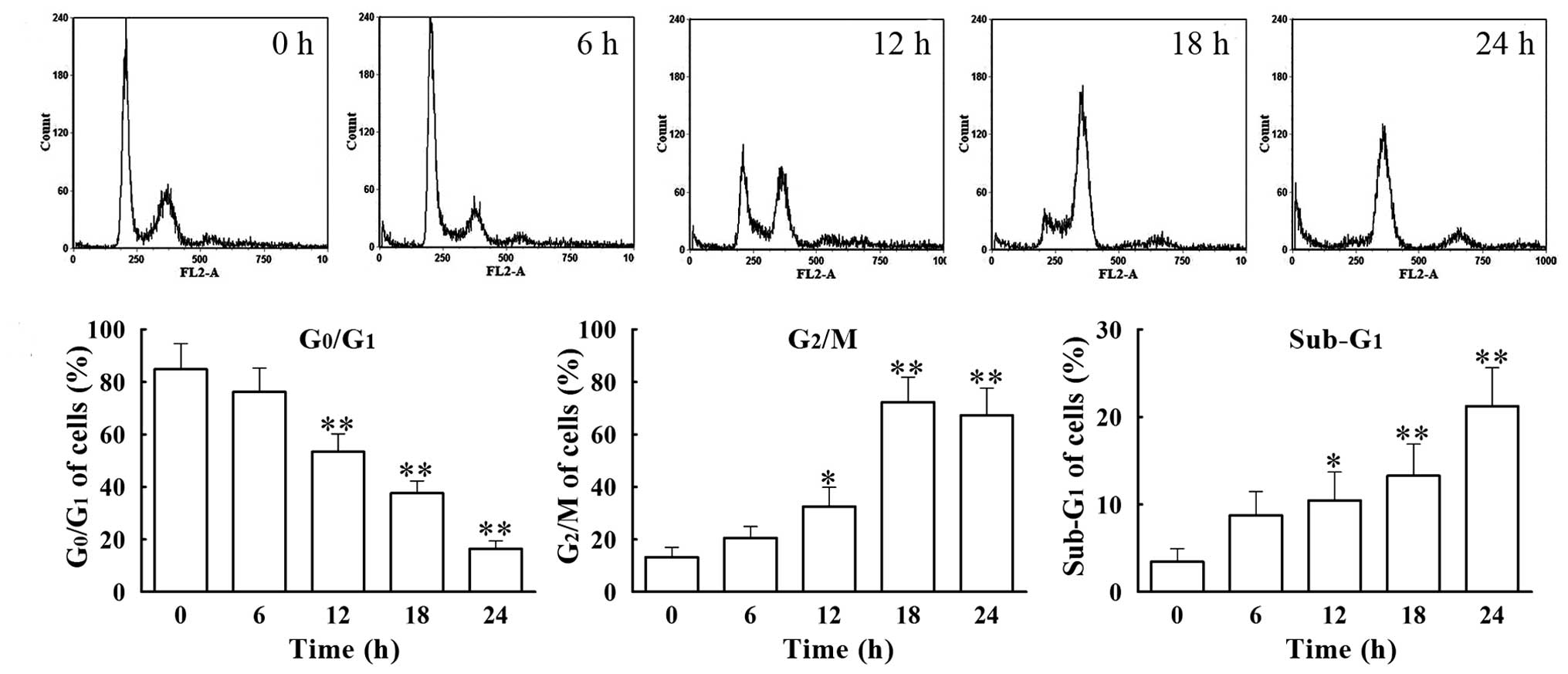
We analyzed the cell cycle distribution of
VCR-treated SH-SY5Y cells by flow cytometry. Cells were treated
with 0.1 μM of VCR for 6, 12, 18 and 24 h. The percentage of
G0/G1 phase cells decreased gradually with
time, from 76.26±9.05% at 6 h to 16.46±2.99% at 24 h, with that of
the group treated for 24 h being significantly lower (P<0.01)
than that in the control group (84.83 ±9.72%) (Fig. 2). Concomitantly, the percentage of
G2/M phase cells increased gradually from 20.60±4.32% at
6 h to 72.34±9.44% at 18 h, with the percentage in the group
treated for 18 h being significantly higher (P<0.01) than in the
control group (13.28±3.76%). Furthermore, the percentage of cells
present at the apoptotic phase (sub-G1 phase) increased
from 5.75±2.74% at 6 h to 21.25±4.36% at 24 h, with the percentage
of cells in the 24 h treated group being significantly higher
(P<0.01) than that in the control group (3.47±1.46%). These data
suggest that VCR induces apoptosis of SH-SY5Y cells following cell
cycle arrest at the G2/M phase.
VCR induces mitotic arrest of SH-SY5Y
cells
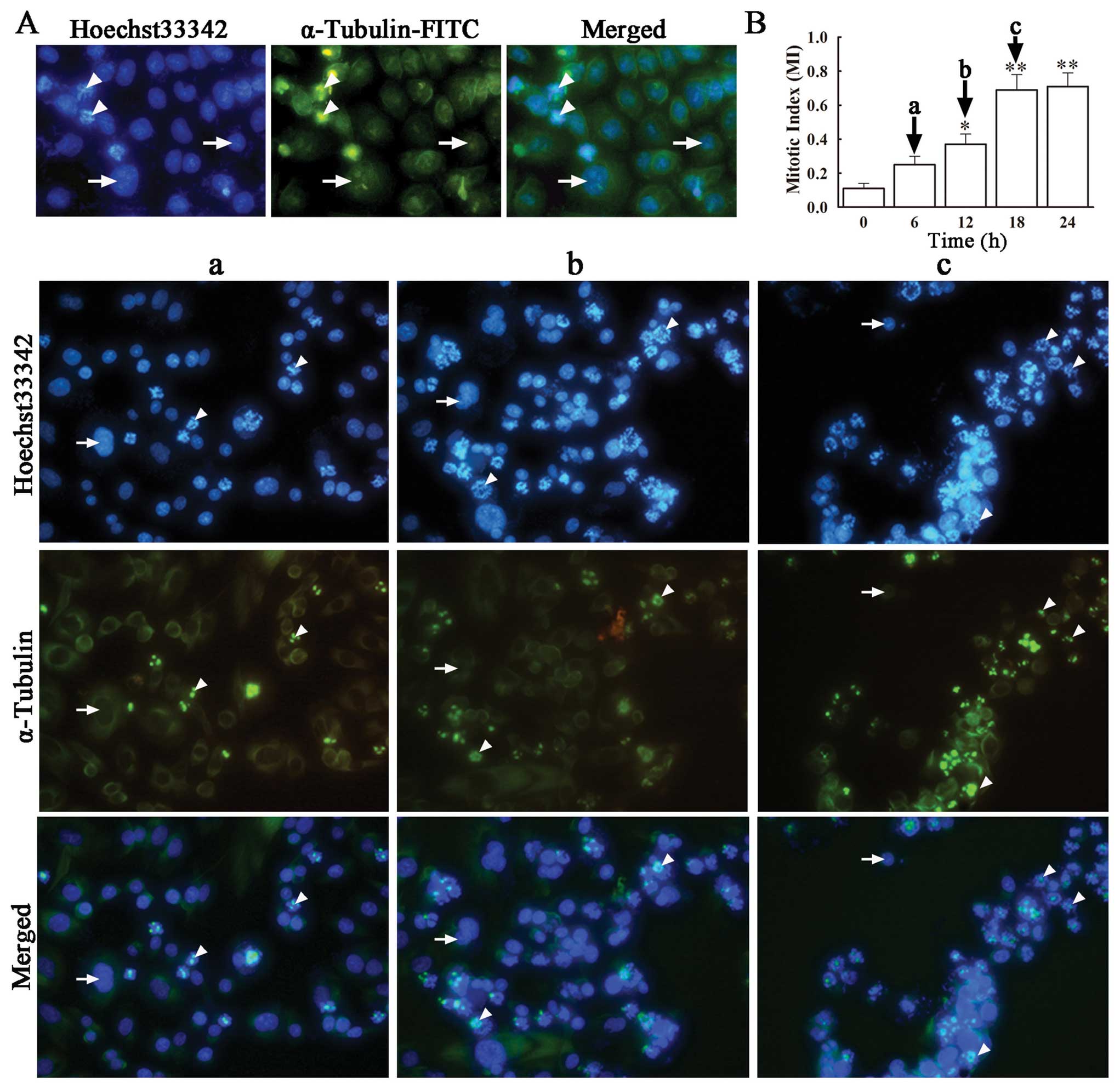
Anti-tubulin compounds act by disrupting spindle
integrity and activating the mitotic checkpoint, which leads to
cell cycle arrest at the M phase and eventually apoptosis (20). In order to confirm whether VCR
induces SH-SY5Y cell cycle arrest at the M phase, we performed
immunofluorescence microscopy for α-tubulin and DNA in VCR-treated
and non-treated cells, determined the number of cells at the M
phase and calculated the respective mitotic index (MI). Staining
with FITC-labeled α-tubulin antibody and Hoechst 33342, revealed an
evenly distributed microtubule network in green and blue-stained
nuclei in untreated SH-SY5Y cells. On the contrary, VCR-treated
SH-SY5Y cells exhibited distinct changes in the microtubular
structure, showing an accumulation of microtubules close to the
nuclei, concomitant with a dramatic decrease in microtubule density
in the rest of the cytoplasm (Fig.
3A). This indicates that VCR treatment disrupted the correct
assembling of tubulin in these cells, caused cell cycle arrest at
the M phase and premature termination of mitosis. In the presence
of VCR, the number of cells in the M phase increased with time,
from a MI of 0.25±0.05 at 6 h to 0.71±0.08 at 18 h, both being
significantly higher than that in the control group (Fig. 3B) (P<0.05, P<0.01).
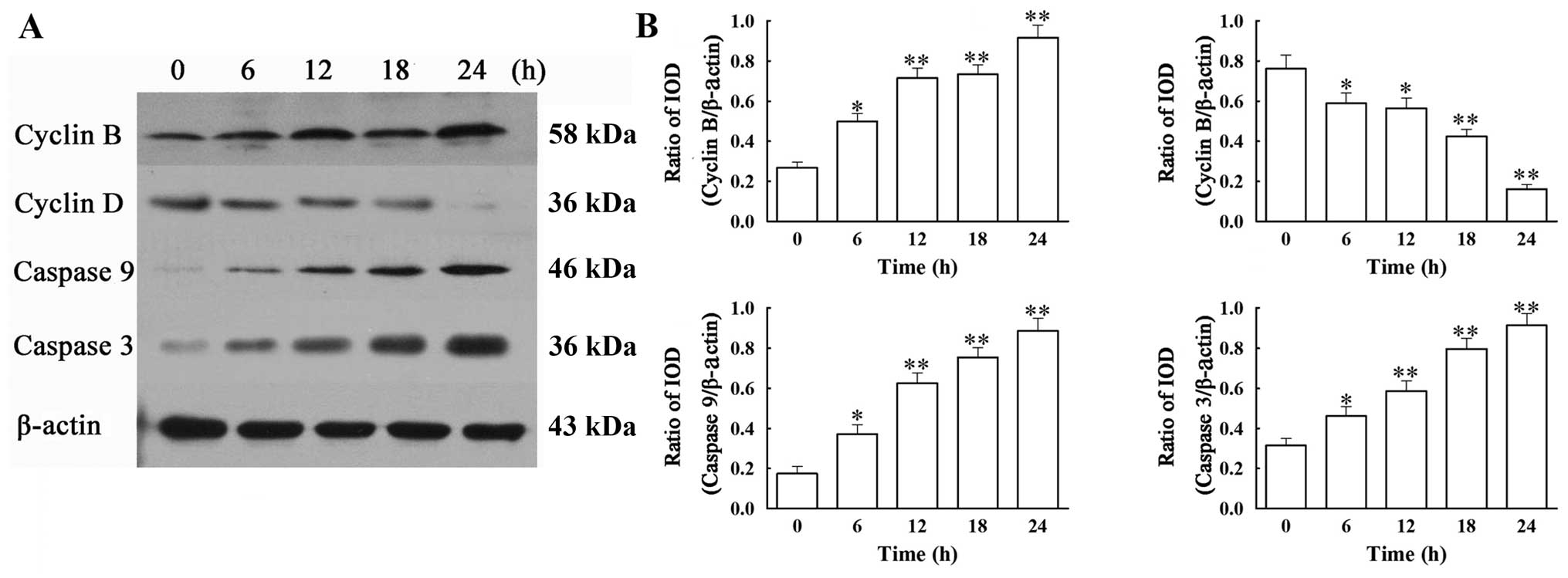
VCR affects the mRNA expression of cyclin
B and D, caspase-3 and -9 in SH-SY5Y cells
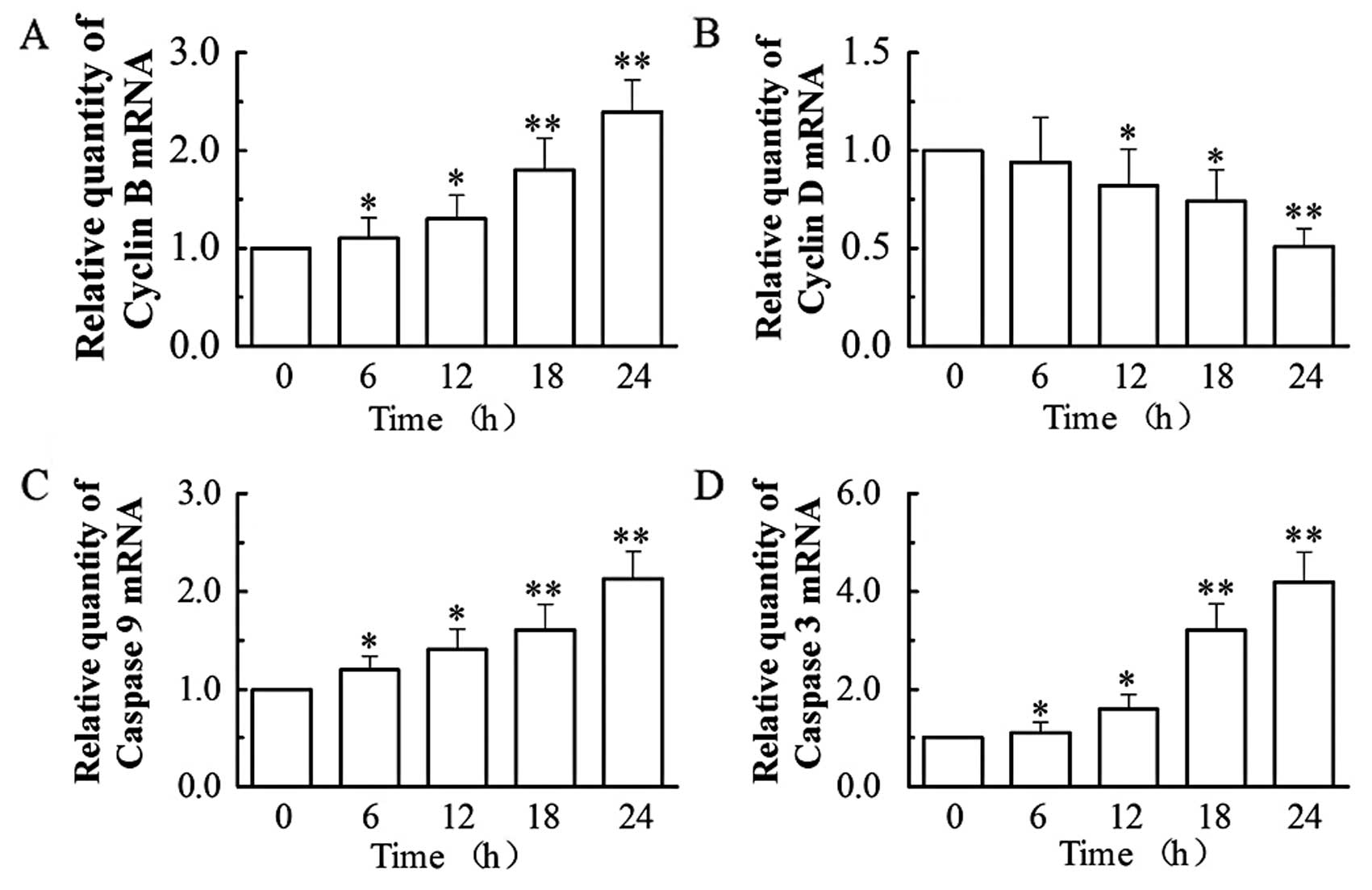
Based on the above findings, we then analyzed by
RT-PCR (by the relative quantification method, 2−ΔΔCt)
the mRNA expression of two apoptotic (caspase-3 and -9) and two
cell cycle (cyclin B and D) regulators (Fig. 4). Our results showed that
treatment with 0.1 μM of VCR dramatically increased the mRNA
expression of cyclin B, caspase-9 and -3, while decreasing the mRNA
expression of cyclin D. At 24 h, VCR increased the mRNA levels of
cyclin B by 2.4-fold, caspase-9 by 2.1-fold and caspase-3 by
4.2-fold (all P<0.01), while it decreased cyclin D mRNA
expression to 51.21% of that of the control group (P<0.01).
VCR affects the protein expression of
cyclin B and D, caspase-3 and -9 in SH-SY5Y cells
We next addressed whether the mRNA changes in the 4
regulators were observed at the protein level. Western blot
analysis revealed that treatment with 0.1 μM of VCR induced a
significant increase in protein expression of cyclin B, caspase-3
and -9 and a significant decrease in that of cyclin D, when
compared with the control group (Fig.
5) (all P<0.01). These results are in agreement with the
changes in the mRNA expression of the corresponding molecules.
Discussion
Neuroblastoma is the most common extracranial solid
tumor of childhood. The survival rate of children with this disease
is only 15% (21). It is highly
heterogeneous and can be classified into 3 risk categories from low
to high (2,4). Due to the fact that most low-risk
patients can be cured through surgery (4), most neuroblastoma studies are
focused on intermediate- and high-risk patients. A clinical study
found that short duration and low dosage of combined chemotherapy
can lead to a similar curative effect, with a 3-year overall
survival rate as high as 96±1% and a 3-year event-free survival as
high as 88±2%, when compared with a long period and high dosage
chemotherapy using the same anticancer agents (14). Among high-risk pediatric patients,
another clinical study demonstrated that combined chemotherapy and
stem cell transplantation can markedly improve the 3-year overall
survival rate compared to chemotherapy alone (21). VCR is often used in intensive
chemotherapy to treat pediatric patients with advanced
neuroblastoma (22,23). However, the mechanisms involved in
VCR-induced cell death in this disease are still not clear. High
doses of VCR can lead to drug resistance and potential side effects
(14,24). Therefore, in the present study we
used a human neuroblastoma cell line, SH-SY5Y, to investigate the
impact of a large range of VCR doses (0.001–10 μM) on SH-SY5Y cell
cycle and apoptosis, as well as the underlying mechanisms. We
demonstrated for the first time that a low dose of VCR (0.1 μM,
determined by the IC50 value calculated through MTT
assay measurement of the impact of different VCR doses on SH-SY5Y
cell proliferation) induces mitotic arrest and apoptosis of SH-SY5Y
cells.
VCR is a microtubule-interfering compound and
thereby a cell cycle-specific agent. VCR disrupts the formation of
mitotic spindles and forces cell cycle arrest at the M phase. Our
results indicate that VCR upregulates the expression of cyclin B
and downregulates the expression of cyclin D in SH-SY5Y cells.
Cyclin B is a mitosis-promoting factor and in normal somatic cells
its expression peaks at the G2-M transition (25). In contrast, cyclin D promotes
G1-S transition (25).
The upregulation of cyclin B by VCR may lead to the observed
increased number of cells at the M phase. The downregulation of
cyclin D is not sufficient to drive the cells to progress from the
M phase into G0/G1, resulting in a cell cycle
arrest at the M phase. At the G2-M transition, the DNA
damage checkpoint is an important control mechanism in eukaryotic
cells, ensuring a DNA repair opportunity before entering into
mitosis (26). When VCR induces
cell cycle arrest at the M phase, the self-repair mechanism will
allow some cells to pass the cell cycle checkpoint after DNA
repair. However, since most cells are not able to pass that
checkpoint, there is activation of apoptotic signaling pathways,
leading cells to apoptosis (27).
VCR can affect cellular metabolism, inhibit
proliferation and induce apoptosis in many types of tumors
(28–30). Our results show that VCR induces
apoptosis in SH-SY5Y cells through a mechanism involving activation
of caspase-3 and -9. The activation of caspase family proteins
plays a key role in apoptosis. An inadequate caspase activity is
usually observed in neuroblastoma and it is an important factor
leading to cancer severity as well as to the development of drug
resistance (31). Caspases are
usually synthesized and maintained as inactive proenzymes (32). Apoptotic signals can activate the
caspase cascade, among which caspase-3 and -9 are the key caspase
proteins. Caspase-3 is an effector or ‘executioner’, while
caspase-9 is an initiator caspase (32). Caspase-9 can directly or
indirectly activate the downstream caspase-3, as well as
endonuclease G, which further leads to DNA fragmentation (32). A previous study has suggested that
because the peripheral nervous system relies on caspase-3 for
induction of apoptosis, this caspase is expected to be a target
gene in the treatment of peripheral nervous system injuries
(33). Our study revealed that
during VCR-induced SH-SY5Y apoptosis, the expression of caspase-3
and -9 dramatically increased with time. Upregulation of both
caspase-3 and -9 can disrupt mitochondrial membrane integrity,
leading to the release of cyctochrome c, which induces apoptosis.
Caspase-3 and -9 have also been reported to be able to specifically
digest ATPase 4b isoform and disrupt the function of calcium pumps,
leading to intracellular calcium overload and apoptosis (34).
In conclusion, our study suggests that a low dose of
VCR can induce cell cycle arrest at the M phase and eventually
apoptosis in SH-SY5Y neuroblastoma cells. This may result from
regulation of the expression of cell cycle-related proteins, cyclin
B and D, as well as apoptotic factors including caspase-3 and -9 by
VCR. These data provide reliable evidence for the application of
VCR in neuroblastoma chemotherapy.
Acknowledgements
This study was supported by an NFSC grant (no.
30872668) and the Medical College of Chinese People's Armed Police
Forces grant (WYM201015, WYM201109).
References
|
1
|
Dome JS, Rodriguez-Galindo C, Spunt SL and
Santana V: Pediatric solid tumors. Clinical Oncology. Abeloff MD,
Armitage JO, Niederhuber JE, Kastan MB and McKenna WG: 4th edition.
Churchill Livingstone, Elsevier; Philadelphia, PA: pp. 2661–2722.
2008
|
|
2
|
Maris JM, Hogarty MD, Bagatell R and Cohn
SL: Neuroblastoma. Lancet. 369:2106–2120. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fish JD and Grupp SA: Stem cell
transplantation for neuroblastoma. Bone Marrow Transplant.
41:159–165. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Haase GM, Perez C and Atkinson JB: Current
aspects of biology, risk assessment, and treatment of
neuroblastoma. Semin Surg Oncol. 16:91–104. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Johnson E, Dean SM and Sondel PM:
Antibody-based immunotherapy in high-risk neuroblastoma. Expert Rev
Mol Med. 9:1–21. 2007. View Article : Google Scholar
|
|
6
|
Coderch C, Morreale A and Gago F:
Tubulin-based structure-affinity relationships for antimitotic
Vinca alkaloids. Anticancer Agents Med Chem. 12:219–225. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Qaddoumi I, Billups CA, Tagen M, et al:
Topotecan and vincristine combination is effective against advanced
bilateral intraocular retinoblastoma and has manageable toxicity.
Cancer. Apr 19–2012.(Epub ahead of print).
|
|
8
|
Packer RJ, Ater J, Allen J, et al:
Carboplatin and vincristine chemotherapy for children with newly
diagnosed progressive low-grade gliomas. J Neurosurg. 86:747–754.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Knudson AG: Two genetic hits (more or
less) to cancer. Nat Rev Cancer. 1:157–162. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Conway RM, Madigan MC, Billson FA and
Penfold PL: Vincristine- and cisplatin-induced apoptosis in human
retinoblastoma. Potentiation by sodium butyrate. Eur J Cancer.
34:1741–1748. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Groninger E, Meeuwsen-De Boer GJ, De Graaf
SS, Kamps WA and De Bont ES: Vincristine-induced apoptosis in acute
lymphoblastic leukaemia cells: A mitochondrial controlled pathway
regulated by reactive oxygen species? Int J Oncol. 21:1339–1345.
2002.
|
|
12
|
Lee HZ, Leung HW, Lai MY and Wu CH:
Baicalein induced cell cycle arrest and apoptosis in human lung
squamous carcinoma CH27 cells. Anticancer Res. 25:959–964.
2005.PubMed/NCBI
|
|
13
|
Blajeski AL, Phan VA, Kottke TJ and
Kaufmann SH: G(1) and G(2) cell-cycle arrest following microtubule
depolymerization in human breast cancer cells. J Clin Invest.
110:91–99. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Baker DL, Schmidt ML, Cohn SL, et al:
Outcome after reduced chemotherapy for intermediate-risk
neuroblastoma. N Engl J Med. 363:1313–1323. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Haim N, Epelbaum R, Ben-Shahar M,
Yarnitsky D, Simri W and Robinson E: Full dose vincristine (without
2-mg dose limit) in the treatment of lymphomas. Cancer.
73:2515–2519. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xie HR, Hu LS and Li GY: SH-SY5Y human
neuroblastoma cell line: in vitro cell model of dopaminergic
neurons in Parkinson's disease. Chin Med J (Engl). 123:1086–1092.
2010.
|
|
17
|
Gupta A, Inaba S, Wong OK, Fang G and Liu
J: Breast cancer-specific gene 1 interacts with the mitotic
checkpoint kinase BubR1. Oncogene. 22:7593–7599. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Madhavan J, Ganesh A and Kumaramanickavel
G: Retinoblastoma: from disease to discovery. Ophthalmic Res.
40:221–226. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Singh VK, Zhou Y, Marsh JA, et al:
Synuclein-gamma targeting peptide inhibitor that enhances
sensitivity of breast cancer cells to antimicrotubule drugs. Cancer
Res. 67:626–633. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jordan MA: Mechanism of action of
antitumor drugs that interact with microtubules and tubulin. Curr
Med Chem Anticancer Agents. 2:1–17. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Matthay KK, Villablanca JG, Seeger RC, et
al: Treatment of high-risk neuroblastoma with intensive
chemotherapy, radiotherapy, autologous bone marrow transplantation,
and 13-cis-retinoic acid. Children's Cancer Group. N Engl J Med.
341:1165–1173. 1999. View Article : Google Scholar
|
|
22
|
Zoubek A, Holzinger B, Mann G, et al:
High-dose cyclophosphamide, adriamycin, and vincristine (HD-CAV) in
children with recurrent solid tumor. Pediatr Hematol Oncol.
11:613–623. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rubie H, Coze C, Plantaz D, et al:
Localised and unresectable neuroblastoma in infants: excellent
outcome with low-dose primary chemotherapy. Br J Cancer.
89:1605–1609. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kotchetkov R, Cinatl J, Blaheta R, et al:
Development of resistance to vincristine and doxorubicin in
neuroblastoma alters malignant properties and induces additional
karyotype changes: a preclinical model. Int J Cancer. 104:36–43.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ford HL and Pardee AB: Cancer and the cell
cycle. J Cell Biochem Suppl. 32–33:166–172. 1999.
|
|
26
|
Cuddihy AR and O'Connell MJ: Cell-cycle
responses to DNA damage in G2. Int Rev Cytol. 222:99–140. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kung AL, Zetterberg A, Sherwood SW and
Schimke RT: Cytotoxic effects of cell cycle phase specific agents:
result of cell cycle perturbation. Cancer Res. 50:7307–7317.
1990.PubMed/NCBI
|
|
28
|
Shi JH, Xu XP, Zhang ZL, Zhang JS, Ge JB
and Cheng WY: Inhibition of activation of nuclear factor-kappaB
enhanced apoptosis of leukemic cells induced by homoharringtonine.
Zhonghua Nei Ke Za Zhi. 42:292–295. 2003.(In Chinese).
|
|
29
|
Shinwari Z, Al-Hindi H, Al-Shail E, et al:
Response of medulloblastoma cells to vincristine and lomustine:
role of TRKC, CTNNB1 and STK15. Anticancer Res. 31:1721–1733.
2011.PubMed/NCBI
|
|
30
|
Sung KH, Lee EH and Kim YZ: Factors
influencing the response to high dose methotrexate-based
vincristine and procarbazine combination chemotherapy for primary
central nervous system lymphoma. J Korean Med Sci. 26:551–560.
2011. View Article : Google Scholar
|
|
31
|
Zhang J, Chatterjee K, Alano CC,
Kalinowski MA, Honbo N and Karliner JS: Vincristine attenuates
N-methyl-N′-nitro-N-nitrosoguanidine-induced poly-(ADP) ribose
polymerase activity in cardiomyocytes. J Cardiovasc Pharmacol.
55:219–226. 2010.PubMed/NCBI
|
|
32
|
Salvesen GS: Caspases: opening the boxes
and interpreting the arrows. Cell Death Differ. 9:3–5. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Simpson MT, MacLaurin JG, Xu D, et al:
Caspase 3 deficiency rescues peripheral nervous system defect in
retinoblastoma nullizygous mice. J Neurosci. 21:7089–7098.
2001.PubMed/NCBI
|
|
34
|
Hajnoczky G, Davies E and Madesh M:
Calcium signaling and apoptosis. Biochem Biophys Res Commun.
304:445–454. 2003. View Article : Google Scholar : PubMed/NCBI
|