Introduction
Atrial fibrillation (AF), as a rhythm disorder
characterized by chaotic electrical activity of the atria, is a
frequent cause of cardiovascular morbidity and mortality worldwide.
Its prevalence is 0.4 to 1.0% in the general population and
increases with age (1). The
mechanisms underlying AF remain elusive, and AF is thought to be
maintained either via ectopic foci, multiple wavelets, or
fibrillatory conduction emanating from a small number of stable
rotors (2). Electrical and
structural remodeling have emerged as crucial components in the
persistence of AF (3). Electrical
remodeling, such as changes in major repolarized ion channels,
leads to the shortening of the action potential duration (APD) and
the loss of APD rate-dependent adaptation, while structural
remodeling (fibrosis) leads to an increase in atrial conduction
slowing, re-entry, and thereby inducible AF (3).
Studies using large animal models (rabbits, dogs,
canines and goats) have demonstrated a possible link between
age-related changes in histopathological and electrophysiological
properties and the vulnerability to AF (4–8).
However, time-saving and low-cost animal models, such as rats and
mice, have not been fully characterized to date. Rodent models for
AF have been reported in a limited number of studies, but there are
insufficient data for young vs. old rats (9), young vs. middle-aged rats (10) or mice (11). Moreover, small animal models, in
particular mouse models, provide powerful tools in the
investigation of basic structural and electrical mechanisms of
cardiac arrhythmias. It is believed that sustained fibrillatory
activity does not exclusively depend on critical myocardial mass or
that critical mass is smaller than the mouse atrial surface of
<35 mm2 (12). The
inducibility of AF in the whole mouse heart in vivo has
previously been evaluated using transvenous atrial stimulation,
isolated stimulation and vagal activation by pharmacological
intervention (29).
Transesophageal stimulation is a minimally invasive method for
atrial stimulation without direct atrial manipulation or mechanical
changes during cardiac surgery or catheterization. Moreover, serial
examinations of the same animal under different experimental
conditions can be easily performed. These can facilitate the
characterization of age-dependent electrophysiological properties
in an individual animal.
Therefore, in the present study, adopting 3
sequential time-points (young, middle-aged and aged) for further
investigation in a mouse model, we aimed to evaluate dynamic
alterations associated with atrial fibrosis, and to determine
atrial electrical properties related to vulnerability to AF.
Materials and methods
Animals
For our study, male healthy Kunming mice aged 2
(young), 12 (middle-aged), and 24 months (aged) were obtained from
the Laboratory Animal Center of the Academy of Military Medical
Sciences (Beijing, China) and received humane care in compliance
with the National Institutes of Health Guide for the Care and Use
of Laboratory Animals (NIH publication no. 85–23, revised in 1996).
We chose these ages of mice based on previous study (13). The ages of 2, 12 and 24 months are
equivalent to the human age of ~20, 40 and 70 years, respectively.
The characteristics of the mice are shown in Table I.
 | Table IAge and weight of the mice in the 3
groups. |
Table I
Age and weight of the mice in the 3
groups.
| Young | Middle-aged | Aged |
|---|
| Age (months) | 2 | 12 | 24 |
| Weight (g) | 35.4±4.2 | 52.1±5.4 | 60.5±7.2 |
In vivo global electrophysiological
analysis
Sinus cycle length (SCL) was determined by averaging
3 consecutive R-R intervals. P-wave duration (PWD) was measured by
determining the earliest onset and latest offset of atrial
deflection from 3 simultaneously recorded surface leads.
For the transesophageal electrophysiological study,
a 2-French octapolar mouse electrophysiological catheter [8 0.5 mm
circular electrodes; electrode pair spacing, 0.5 mm (Ciber Mouse;
NuMed Inc., New York, NY)] was used for recording cardiac
electrograms as well as for pacing the heart using consecutive
electrode pairs. This catheter was inserted into the esophagus with
a depth of 3–4 mm and unipolar recordings were obtained from each
ring electrode. The threshold of pacing was examined by a 1 msec
pulse width and a pacing rate 10 beats/min faster than normal. The
electrode catheter was adaptively positioned to the site closest to
the left atrium to ensure that constant atrial capture and
correspondence to the minimum threshold (<1.5 mA) were attained.
Sinus node recovery time (SNRT) was measured after a 30-sec pacing
train with a basic cycle length (BCL) of 100 msec, a stimulus
amplitude of 2-fold diastolic capture threshold and a stimulus
duration of 1 msec. The SNRT was defined as the interval between
the last stimulus in the pacing train and the onset of the first
sinus return beat. Rate-corrected SNRT (CSNRT) was defined as the
SCL subtracted from the SNRT.
Inducibility of atrial tachycardia and fibrillation
(AT/F) was examined by applying 15-sec bursts (25 msec BCL, 2-fold
diastolic capture threshold and 1 msec duration). This series of
bursts was repeated 10 times. AF was defined as a period of rapid
and fragmented atrial electrograms with irregular AV-nodal
conduction and ventricular rhythm for at least 1 sec (14). AT was defined as rapid and regular
rhythm lasting for at least 30 sec.
Histopathological analysis
After in vivo electrophysiological analysis,
the mice were sacrificed and prepared for histopathological
analysis. The aortas were then perfused via the common iliac
bifurcation with pre-cooled PBS for 5 min. The left and right
auricles were quickly cut and snap-frozen in liquid nitrogen for 1
min and stored at −80°C. Sections (5 μm in thickness) of the
paraffin-embedded tissue were stained with picrosirius red (Direct
Red 80; Sigma Aldrich) (15).
Briefly, tissue sections were rinsed in distilled water and
incubated in saturated 0.1% picrosirius red for 90 min. The
sections were then rinsed twice with 0.01 N HCl for 1 min,
dehydrated with an ethanol gradient and prepared for collagen
analysis. Images were captured by a digital charge-coupled device
(CCD) camera (Pro 150ES; Pixera Corporation, Los Gatos, CA), and
connected to a polarized microscope (E600POL; Nikon, Tokyo, Japan)
equipped and modified with a commercially available circular
polarizer (Kenko, Tokyo, Japan). The collagen volume fraction (CVF)
was calculated as described previously (15).
Patch-clamp experiments
To isolate high-quality mouse atrial myocytes, we
adopted a Langendorff-perfused method based on modified intubation
skills (16). In brief, mice were
anesthetized using an intraperitoneal injection of 0.4%
pentobarbital sodium solution at a dose of 50 mg/kg body weight.
The inferior vena cava was exposed under direct vision following an
abdominal median incision. While 4°C heparinized saline at a dose
of 4–5 ml (100 U/ml) was administrated into the inferior vena cava,
the abdominal aorta was dissected for bloodletting. Subsequently,
the thoracic aorta, isolated through thymus dissection following a
left thoracotomy between the second and fourth intercostal space,
was slightly intubated by a self-made catheter (7 F paracentetic
needle with blunt tip, ring-shaped notching 0.5 mm from the distal
ending) at a position of 2 mm distance from the root of the aorta.
A syringe with 3 ml saline was connected to the end of the catheter
for excluding the internal air. The successful intubation was fixed
to the aorta by sealing with 5–0 silk. The heart was then quickly
excised and removed to the Langendorff apparatus for immediate
retrograde perfusion. Atrial myocytes were prepared using enzymatic
digestion and then separated from Kraft-Brühe (KB) solution by
centrifuging at 600 rpm for 3 min. The cells were maintained at
room temperature for the experiments that followed and used within
10 h.
Myocytes were transferred into an experimental
chamber (~3 ml) mounted on the stage of an inverted microscope
(TE2000-S; Nikon) and allowed to adhere to the glass bottom of the
chamber. Cells were perfused with extracellular solution. A
ruptured-patch whole cell voltage clamp was used to measure
transient outward potassium (Ito) and
ultra-rapid delayed rectifier potassium
(Ikur) currents, and membrane capacitance
(Cm), while a current clamp was used for measuring a single cell
action potential (AP). For Ito and
Ikur measurements, the internal solution
contained (in mmol/l) 110 K-aspartate, 20 KCl, 1 MgCl2,
5 Mg-ATP, 10 HEPES, 10 EGTA and 5 Na2-creatine
phosphate, pH 7.3–7.4 (NaOH). To block Ca2+-activated
chloride currents, 0.01 mmol/l niflumic acid was added to the
pipette solution. The external solution contained (in mmol/l) 137
NaCl, 5.4 KCl, 1.8 CaCl2, 1 MgCl2, 0.33
NaH2PO4, 5 HEPES, 10 D-glucose and
4-aminopyridine, pH 7.3–7.4 (NaOH). Ca2+ channels were
blocked with 0.05 mmol/l CdCl2. Atropine of 1 μmol/l was
used to prevent muscarinic receptor activation, and 5 μmol/l E-4031
was added to the external solution to block the rapid delayed
rectifier current (Ikr). In addition, EGTA
was used for chelating free Ca2+, thereby blocking
Ito2 and INa-Ca.
For AP measurements, the internal solution contained (in mmol/l)
110 K-aspartate, 20 KCl, 1.8 MgCl2, 10 HEPES, 5 Mg-ATP,
0.05 EGTA and 5 Na2-creatine phosphate (pH 7.3–7.4,
KOH). The external solution contained (in mmol/l) 137 NaCl, 5.4
KCl, 1 MgCl2, 1.8 CaCl2, 10 D-glucose, 0.33
NaH2PO4 and 5 HEPES (pH 7.3–7.4, NaOH).
Signals were filtered with 2.9 and 10 kHz Bessel filters and
recorded by an Axopatch700 A amplifier with the
Digidata1320A-pClamp 8.2 Data Acquisition System (Axon Instruments,
Foster City, CA). All experiments were conducted at room
temperature (25±1°C).
Statistical analyses
Values are presented as the means ± standard
deviation. Surface ECG and esophageal electrogram were measured by
3 independent observers and compiled for statistical
interpretation. Differences between groups were analyzed using a
two-tailed Student’s t-test, analysis of variance (ANOVA),
Kruskal-Wallis H test, Mann-Whitney U test or Chi-square test,
where appropriate. A two-tailed P<0.05 was considered to
indicate a statistically significant difference. All statistical
analyses were performed with SPSS software.
Results
In vivo global electrophysiology
A total of 32 mice had the transesophageal rapid
pacing procedure performed. No complications due to anesthesia or
surgical preparation of the esophagus were observed in the young
group and middle-aged group. Two out of 10 mice in the aged group
died from either sinus bradycardia or complete atrioventricular
block by rapid atrial pacing and had to be excluded from the
analysis.
The threshold of pacing (0.95±0.38 mA) in the young
group was almost compatible with the middle-aged group (1.36±0.44
mA) and the aged group (1.22±0.21 mA). There was no significant
difference in SCL among the 3 groups [112.73±17.78 sec (young),
110.42±11.64 sec (middle-aged) and 117.34±23.73 sec (aged)]. PWD in
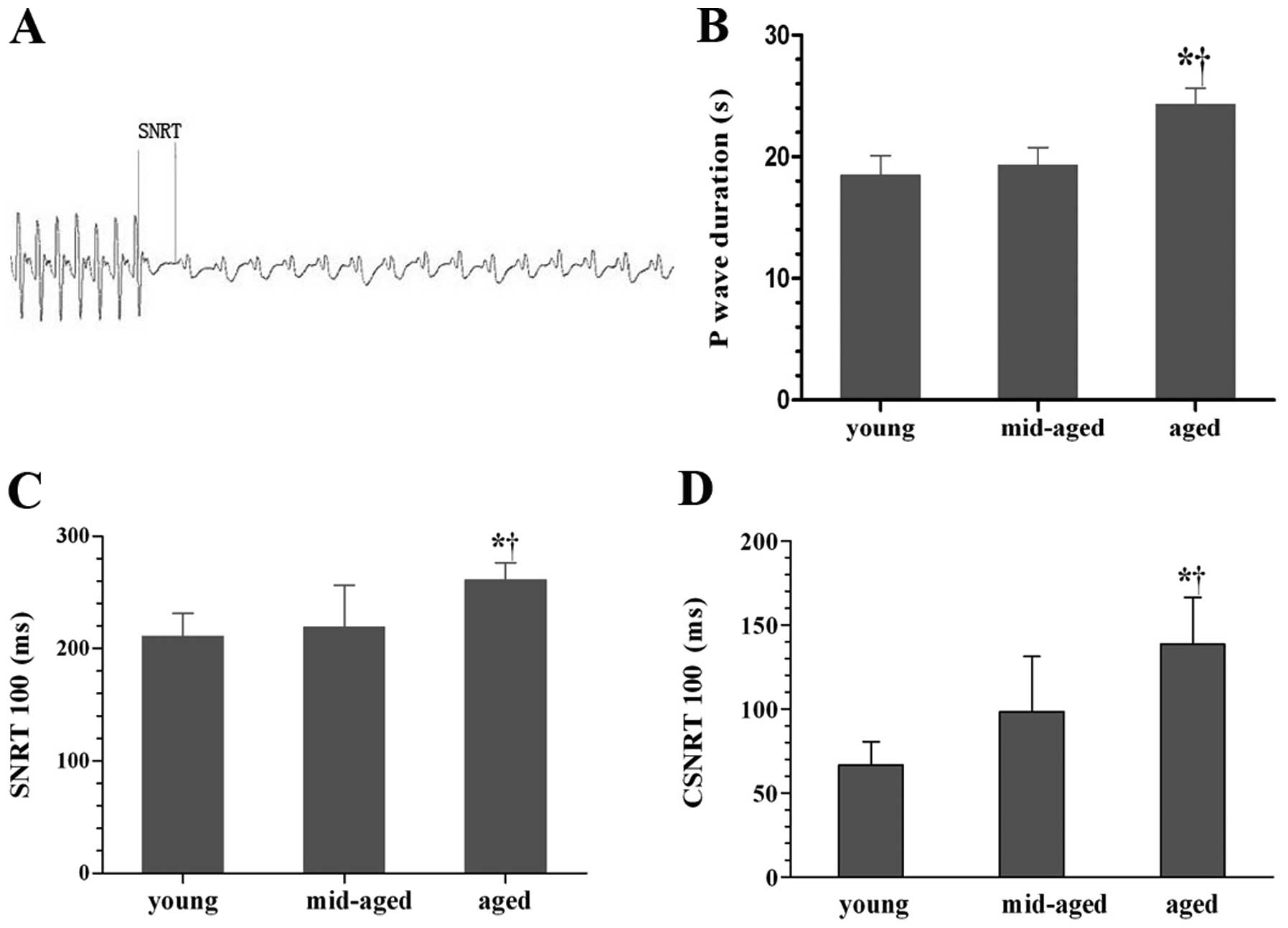
the aged group had a significantly higher prolongation than the
young and middle-aged group (Fig.
1B) (P<0.05). A typical representation of SNRT measurement
is shown in Fig. 1A. SNRT and
CSNRT at a S1S1 stimulation cycle length of 100 msec in the aged
group had a significantly higher prolongation relative to the young
and middle-aged group (P<0.05); however, there was no
significant difference between the young and middle-aged group
(Fig. 1C and D) (P>0.05). The
inducibility of AT/F among the 3 groups is shown in Table II. Although the duration of each
AF episode was not significantly different among the groups, there
was an overall induced rate of AT/F that increased significantly
with age (P<0.05). Vulnerability of the atrial myocardium was
defined as when AT/F was successfully induced in the mouse
model.
 | Table IIComparisons of the inducibility of
AT/F among the 3 groups. |
Table II
Comparisons of the inducibility of
AT/F among the 3 groups.
| Young | Middle-aged | Aged |
|---|
| Induction rate of
AF (%) | 27.3 | 63.6a | 62.5b |
| Median duration for
each AF (S) | 3.04±1.32 | 2.54±0.86 | 2.66±0.22 |
| Induction rate of
AT (%) | 0 | 9.1a | 25b |
| Vulnerability to
AT/F (%) | 27.3 | 72.7a | 87.5b |
Age-related dynamic changes in the atrial
CVF
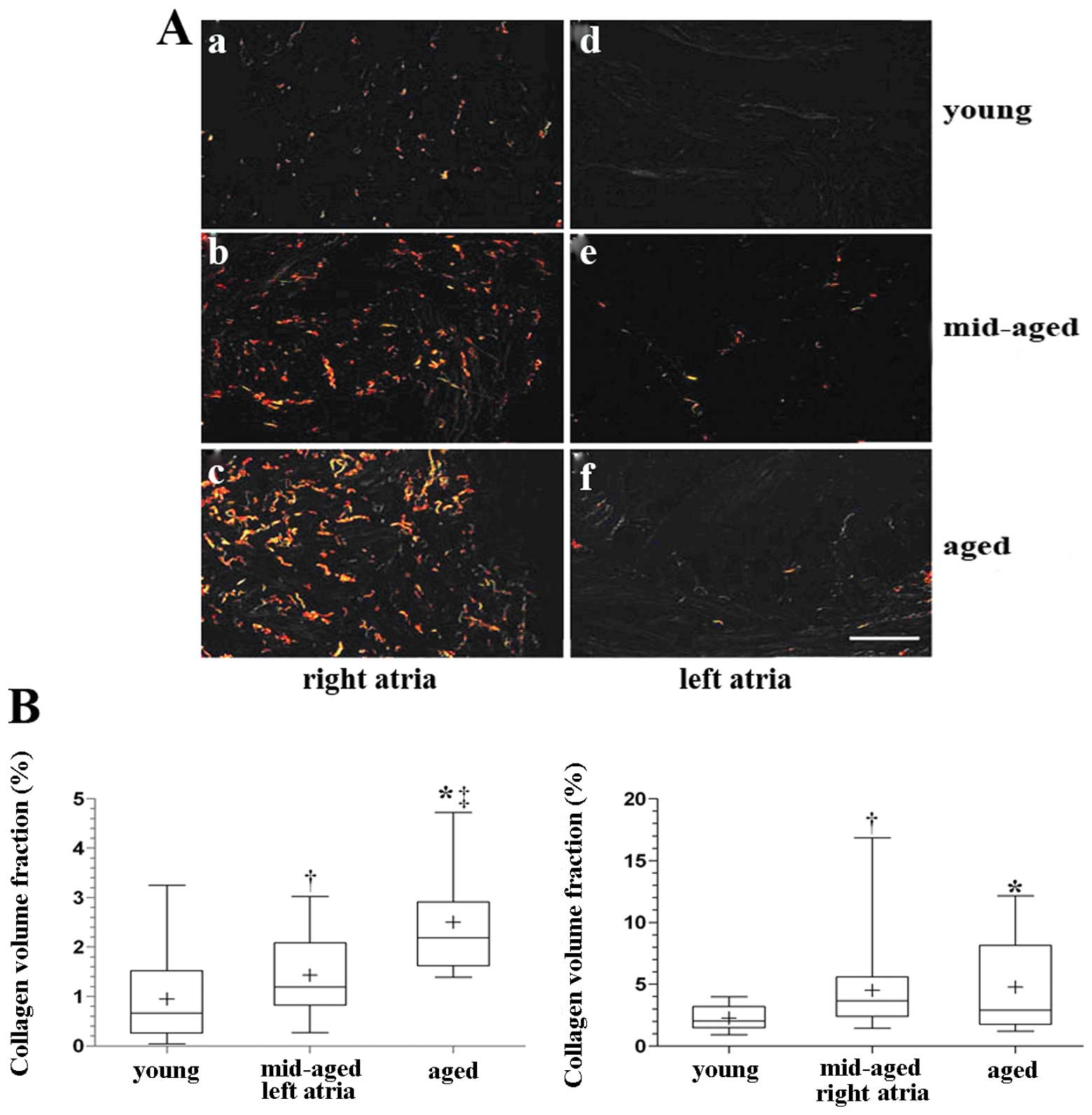
Collagen fibers were much thicker in the right
atrium than the left atrium, and the CVF was higher in the right
atrium among the 3 groups (Fig.
2A). Spearman rank correlation analysis showed that the CVF in
the left atrium significantly correlated with age (P<0.01,
r=0.592) (Fig. 2B). The CVF in
the right atrium was higher in the aged and middle-aged groups than
in the young group; however, but there was no significant
difference between the aged and middle-aged groups. Spearman rank
correlation analysis showed that the CVF in the right atrium
correlated with age (r=0.326, P<0.01); however, this did not
achieve the level of the left atrium (Fig. 2B).
Overall, the right atrium showed significantly
higher fibrosis than the left atrium in all the groups, and the
extent of fibrosis in the left atrium had a higher positive
correlation with age relative to the right atrium.
Patch clamp experiments
Age-related changes in Aps and Cm
In the current clamp mode, APs were elicited by
square current pulses of 400–600 pA amplitude and 3 msec duration.
A steady-state AP was considered as the last of a train of 20 at
the same stimulation rate. The APD at 20, 50 and 90% repolarization
(APD20, APD50, APD90,
respectively) has a tendency towards prolonging followed by
shortening in regard to the age development, whereas the
APD90 in the left relative to the right atrium
demonstrated a significant difference in the aged group (P<0.05)
(Table III).
 | Table IIIComparison of APDs between the left
and right atrium. |
Table III
Comparison of APDs between the left
and right atrium.
| | APD20
(msec) | APD50
(msec) | APD90
(msec) |
|---|
| |
|
|
|
|---|
| Group | No. | Left | Right | Left | Right | Left | Right |
|---|
| Young | 7 | 3.9±0.2 | 4.2±0.3 | 5.0±0.6 | 5.2±0.4 | 22.1±2.6 | 22.5±2.4 |
| Middle-aged | 7 | 5.5±0.5 | 5.9±0.4 | 9.3±1.0 | 9.5±0.9 | 31.9±3.2 | 32.9±3.3 |
| Aged | 7 | 4.9±0.4 | 5.4±0.6 | 6.7±0.6 | 7.5±0.9 | 22.8±2.7 | 27.6±2.8a |
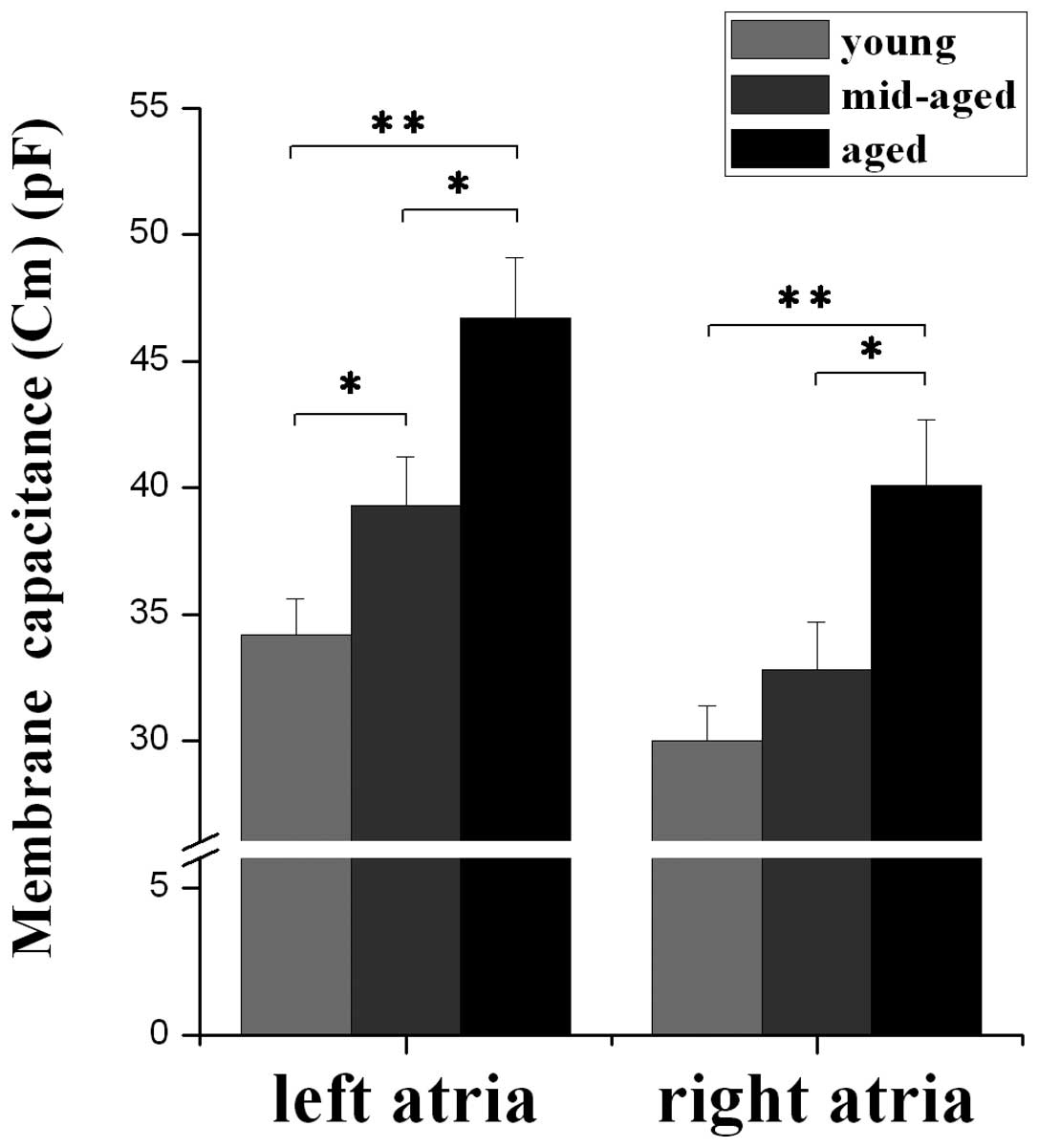
In the whole voltage clamp mode, single atrial
myocyte Cm was elicited by a square pulse of depolarization. Mean
left atrial Cm was 34.2±1.4 pF (young) (n=33), 39.3±1.9 pF
(middle-aged) (n=33) and 46.7±2.4 pF (aged) (n=19), respectively,
while mean right atrial Cm was 30.0±1.4 pF (n=33), 32.8±1.9 pF
(n=33) and 40.1±2.6 pF (n=19), respectively. These results
demonstrate that Cm of the left and right atrium increases with
advancing age, and there is a stronger tendency towards changes in
left atrial Cm with age relative to right atrial Cm (Fig. 3).
Age-related changes of
Ito
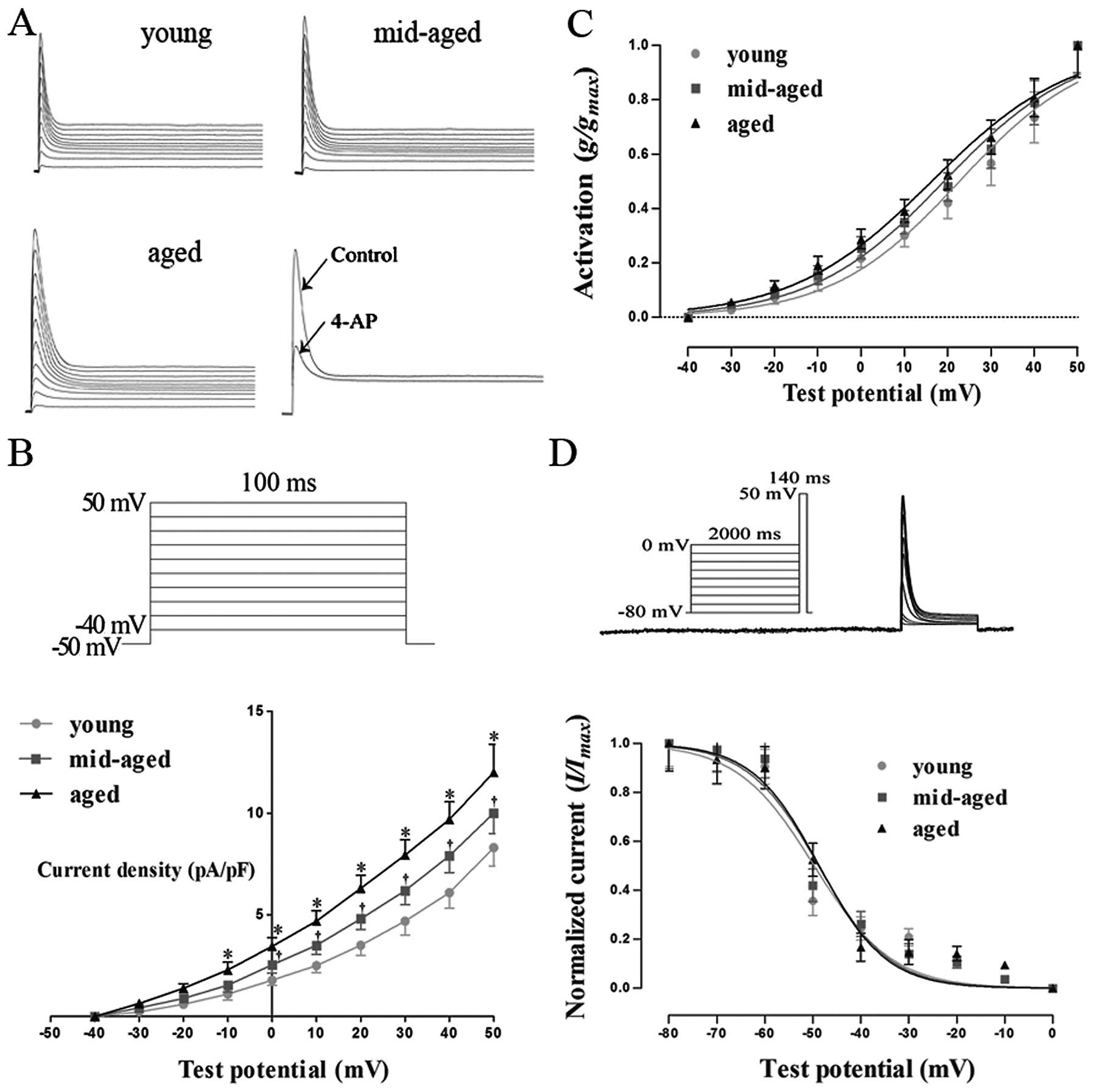
Fig. 4A shows
voltage-dependent Ito elicited by voltage
steps of 100 msec to between −40 and +50 from −50 mV. The holding
potential of −50 mV was used to exclude possible
INa and ICa.T
contamination during current recording. We found that
Ito was activated rapidly and achieved
peak current within minutes after delivering testing potentials,
followed by a rapid decrease in the steady state baseline current
level. Ito increased with testing
potentials in a typically voltage-dependent manner. When 4 mM 4-AP
was added to the external solution, Ito
was blocked by >80% (Fig.
4A).
As shown in Fig.
4B, Ito was activated at −30 mV and current
density was not significantly different among the 3 groups. The
current density of Ito in the aged group
was larger than that in the young and middle-aged groups at −10 mV
(P<0.05), and this was even more significant when the testing
potential was increased. At a test potential of +50 mV, the current
density was 7.8±0.9 pA/pF (n=6), 10.0±1.0 pA/pF (n=6) and 12.0±1.4
pA/pF (n=5) in the young, middle-aged and aged groups,
respectively, indicating that current density increased with age
(P<0.05).
Fig. 4C shows the
activation curve of Ito by depolarization
to +50 from −50 mV. With age, the voltage-dependent
Ito activation curve slightly shifted in a
negative direction. V1/2 and K were
12.8±0.9 and 9.4±0.9 mV (n=6), 11.5±0.6 and 9.5±0.6 mV (n=6), and
10.9±0.8 and 9.2±0.7 mV (n=6) in the young, middle-aged and aged
groups, respectively, indicating that there were no significant
changes in V1/2 and K with age
(P>0.05).
The voltage dependence of the steady state
inactivation relationship was investigated using a standard 2-pulse
protocol: a 2,000 msec preconditioning pulse ranging from −80 to 0
mV followed by a 140 msec test pulse to +50 mV.
V1/2 and K were −49.7±2.1 and
8.1±1.9 mV (n=6), −48.8±1.6 and 7.3±1.5 mV (n=6), and −48.7±1.5 and
7.0±1.4 mV (n=6) in the young, middle-aged and aged groups,
respectively, indicating that there were no significant changes in
V1/2 and K with age (P>0.05)
(Fig. 4D).
Age-related changes of
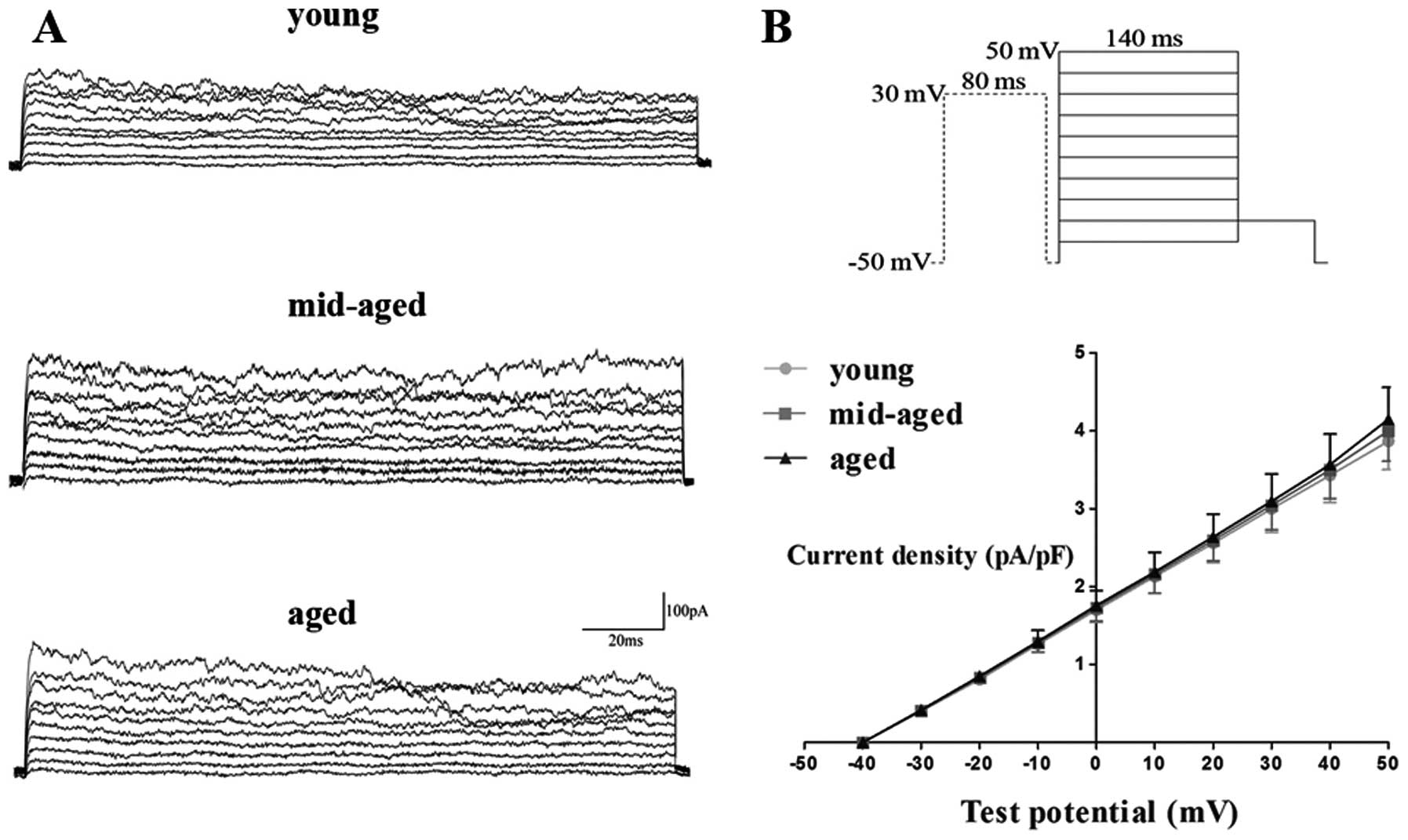
Ikur
Ikur were elicited by a
80-msec prepulse to +30 mV to inactivate
Ito, followed by 140-msec test pulses
between −40 and +50 mV after a 10-msec interval from −50 mV at 0.2
Hz. At a test potential of +50 mV, the current density was 4.1±0.7
pA/pF (n=6), 4.2±1.0 pA/pF (n=6) and 4.2±0.8 pA/pF (n=5) in the
young, middle-aged and aged group, respectively, indicating that
there was no significant difference among the groups (P>0.05)
(Fig. 5B).
Discussion
Main findings
In the present study, we performed measurements of
in vivo global electrical properties by the transesophageal
atrial rapid pacing method and found the following: (i Compared
with the other 2 groups, PWD, as well as the SNRT and CSNRT at a
cycle length of 100 msec were longer in the aged group, indicating
the slowing of intra-atrial conduction and sinus nodal dysfunction.
ii) Although the duration of each AF episode was not significantly
different among the groups, the total inducibility, represented as
the key point for evaluating vulnerability to AT/F, was
significantly increased with aging. As regards changes in collagen
in the left and right atrium, we found that the right atrium showed
significantly higher fibrosis relative to the left atrium in all
the groups, whereas the extent of fibrosis in the left atrium had a
higher positive correlation with age compared to the right atrium.
Furthermore, from the cellular electrophysiological experiments,
the results showed that age-related augmented
Ito currents and no age-related changed
Ikur currents, as well as the subsequent
AP discrepancy in old age, contributed to the dispersion of
repolarization, which further promoted AF.
Age-related abnormal pulse
initiation
Abnormal pulse initiation due to the sinoatrial node
dysfunction is associated with advancing age (17). If the sinus node fails to fire,
then there is a significant tendency towards manifestation of
abnormal impulse initiation in atrial cells. In the present study,
measurements of SNRT and CSNRT at a cycle length of 100 msec showed
that aged mice had a longer SNRT than the young and middle-aged
mice. Recently, Stiles et al (18) demonstrated that the prolongation
of SNRT, regarded as sinus node dysfunction, is a key contributor
which facilitates the progression of paroxysmal AF to permanent AF.
Surprisingly, it has been previously found that although the SCL
increases with age, the SNRT decreases with age (19), which is in contrast to our
results. The likely mechanism for these observations is that
conduction in the sinus node worsens with age and limits the
ability of an extra-stimulus to overdrive and suppress sinus node
automaticity. A previous study found enlargement of the sinus node,
hypertrophy of sinus nodal cells, and remodeling of the
extracellular matrix in aged rats (20). Therefore, they concluded that the
age-dependent decrease in sinus nodal function is due to structural
remodeling of the sinus node. Consequently, decreased automaticity
and conductivity of the sinus node are associated with shortening
of atrial refractoriness and increasing the atrial effective
refractory period (ERP) dispersion, which in turn creates a
substrate for AF initiation and perpetuation (21).
Age-related abnormal pulse
conduction
Abnormal pulse conduction in the aged atrium is a
contributor to the maintenance of AF. Hence, it is important to
comprehend the nature of both the electrical and structural
remodeling that occurs with age. In the current study, surface ECG
showed that PWD was significantly longer in the aged group than in
the young group and had a prolongation tendency in the middle-aged
group, which indicated that the intra-atrial pulse conduction time
was prolonged with increasing age. It has been reported that the
PWD significantly correlates with age in animals (6) and humans (22), and depends mainly on intra-atrial
conduction time and atrial size. The slowing of intra-atrial
conduction, one of the most important requirements for the
initiation of re-entry, is more frequently observed in patients
with paroxysmal AF than in normal subjects (23). Moreover, atrial dilatation
increases the amount of atrial tissue that can accommodate multiple
re-entry circuits (3). Therefore,
P-wave prolongation and atrial dilatation possibly reflect
age-related atrial remodeling that is favorable for AF; thus, aged
mice, compared to young mice, possess a substrate that is more
susceptible to AF.
It is well known that the profound structural
remodeling that occurs with age affects the ability of a premature
beat to penetrate the substrate and initiate propagation. In the
present study, we observed a significant correlation between the
degree of interstitial fibrosis and aging in the left and right
atrium. In a previous study, Burkauskiene et al (24) demonstrated that the diameter and
area of collagen fibers positively correlate with aging, whereas
the amount of collagen fibers does not alter with age. This
indicates that changes in the collagen network may be a
progressively developing process. Another study reported that
spatial distribution of the collagen network was significantly
different in patients with ischemic cardiomyopathy compared with
those without heart disease (25). Taken together, these findings,
including ours, suggest that atrial interstitial fibrosis, which is
increased with age and heart disease, possibly enhances the
heterogeneous slowing of atrial conduction, which may be
responsible for the age-related increase in AF vulnerability. In
general, there is a close correlation between the onset of AF and
structural remodeling in the left atrium (26). However, Nakajima et al
(27) found that although similar
levels of cardiomyocyte apoptosis were present in the right and
left atria of MHC-TGFcys33ser hearts, the extent of
fibrosis was more pronounced in the right atrium than in the left
atrium. They also revealed cardiomyocyte cell activity in left
atrial cardiomyocytes, but not in right atrial cardiomyocytes by
tritiated thymidine incorporation studies. These data support the
notion that cardiomyocyte cell cycle induction can antagonize
fibrosis in the myocardium. In agreement with previous results, the
present study found that the collagen volume of the right atrium
was higher than that in the left atrium in all the groups. Although
we found that the volume of fibers increased with age in the left
and right atrium, less fibrosis was present in the left atrium,
which may be due to faster cell cycle activity in the left
atrium.
Age and predisposition to AF
In our study, we compared the inducibility of atrial
AT/F to evaluate vulnerability to arrhythmias. Our results showed
that the inducibility of atrial AT/F significantly increased with
age, indicating that aging is a key contributor to AF episodes. In
addition, inducible AF was also observed in the young and
middle-aged group, in contrast to the negative results presented in
the study by Guzadhur et al (28). A possible reason is that the
susceptibility of the mouse atrium to the induction of AF appears
to be increased when transesophageal pacing instead of isolated
pacing (28) or transvenous
pacing (29) is performed. It is
possible that relative to isolated or transvenous stimulation,
unipolar transesophageal atrial stimulation affects a larger area
of the atrium, which may involve the vagal nerve. This may result
in a more global inhomogeneous and abnormally delayed activation of
the atrium, which is believed to be the basic mechanism of
induction of atrial arrhythmia by premature depolarization.
Moreover, the atrial stretch is known to enhance atrial
vulnerability and may be augmented in this setting of
transesophageal stimulation. Of note, the present study showed that
PWD was only prolonged in aged group relative to other 2 groups,
not reconciling the high incidence of tachyarrhythmia in the
middle-aged and aged group. The discrepancy may be due to more
electrophysiological remodeling in the middle-aged group, while
structural remodeling was predominant in the aged group.
Age-related cellular electrophysiological
changes conducive to AF
AP changes with age are affected by different types
of inward and outward ion currents. Ito
and Ikur, which are the major ion currents
involved in the repolarization process in mice, contribute to
age-related AP changes and AF initiation and maintenance. In the
present study, a Ca2+-independent transient outward
current (Ito1) was recorded by the
addition of CdCl2 to the external solution to block the
L-type Ca2+ current and the addition of a
Ca2+ chelating agent (EGTA) to the internal solution.
Our results showed that Ito was suppressed
by >80% after the addition of 4-AP. Dun et al (30) found that
Ito current densities of canine right
atria were significantly increased in the aged group compared with
the adult group, whereas inactivation slowing and steady state
inactivation curves showed a trend to shift toward depolarization.
Mansourati et al (31)
also found that Ito was decreased in adult
patients with heart diseases; however, the results did not reflect
the normal electrophysiological properties of humans due to the
existence of pathological factors. We found that
Ito current densities increased with age,
which is in agreement with the results presented in the study by
Crumb et al (32), whereas
the activation and inactivation properties of
Ito were not different among the 3 groups.
As such, the augmentation of Ito may have
contributed to the shortening of atrial APs in the aged group.
Following the inactivation of Ito1, a
slowly inactivating current remains, termed
Isus or Ikur.
There is a general consensus that the KV 1.5 subunit is
largely responsible for Ikur in human
atrial myocytes, similar to other species, such as the mouse and
dog. Since this subunit is much less abundant in the ventricle than
in the atrial myocardium, it appears to be quite specific to atrial
myocytes. Ikur densities are significantly
increased in aged canine right atrial cells compared with adult
cells (30). However, KN-93, a
specific CaMKII inhibitor, has been shown to dramatically inhibit
Ikur in aged right atrial cells compared
with adult right atrial cells, indicating that increased
Ikur in aged RA cells is most likely due
to CaMKII upregulation (33). To
the best of our knowledge, no age-related changes in
Ikur have thus far been observed in human
atrial myocytes. In alignment with human atrial data, the present
study using mice also demonstrated that there were no age-related
changes in Ikur among the different
groups, which may be due to stability after birth.
The abnormal shape of the AP contour derived from a
single isolated cell is conducive to AF. Anyukhovsky et al
(34) found that APD90
averaged across all regions was significantly longer in the aged
compared with adult tissues. Notably, the range of APD90
values was wider in the aged vs. the adult group as a result of an
increase in duration, occurring mainly in right atrial fibers
(34). However, clear-cut
evidence of age-related changes in APs in normal human atria is
lacking, as no age-related changes in APD and ERP have been
obtained (35). In the present
study, we found that APD90 in the left and right atrium
was prolonged with age, followed by a decrease in the aged group,
particularly in the left atrium. In agreement with our report,
Brorson et al (36) used 4
age groups to examine right atrial ERPs and monophasic APs in
healthy male humans in vivo. They found that the middle-aged
group showed a significantly longer right atrial ERP than the other
groups. However, there was no progressive trend in APD with age.
Thus, these results do not support the traditional view that APDs
are prolonged with age. In general, AP is prolonged followed by
shortening with age may due to a decrease in the calcium current in
aged atrial tissue (6).
Limitations
The transesophageal stimulation technique used in
the current study includes difficulties in atrial electrogram
detection during AF, and therefore, the evaluation of the atrial AF
heart rate is not always possible. Additionally, due to the
limitations of experimental devices and technologies, measurements
of atrial ERP and intra-atrial conduction velocity were not
conducted for further insight into the electrophysiological
mechanisms. Finally, it should be acknowledged that the
pathophysiological characteristics of AF in mice may be different
from those in humans.
In conclusion, the results from our study
demonstrate that aging causes structural remodeling, as well as
integrative and cellular electrophysiological changes, facilitating
increased dispersion of repolarization and re-entry formation. This
in turn creates a substrate for AF initiation and perpetuation.
Furthermore, there may be an association between structural and
electrical remodeling, which can exert vicious cycle effects on the
development of AF.
Acknowledgements
The present study was supported by the Tianjin
Municipal Science and Technology Committee (09ZCZDSF04200 and
11JCYBJC12000) and the Natural Science Foundation of China
(81170238 and 81070121).
References
|
1
|
Fuster V, Ryden LE, Cannom DS, et al: 2011
ACCF/AHA/HRS focused updates incorporated into the ACC/AHA/ESC 2006
guidelines for the management of patients with atrial fibrillation:
a report of the American College of Cardiology Foundation/American
Heart Association Task Force on practice guidelines. Circulation.
123:e269–e367. 2011.
|
|
2
|
Jalife J: Deja vu in the theories of
atrial fibrillation dynamics. Cardiovasc Res. 89:766–775. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nattel S, Burstein B and Dobrev D: Atrial
remodeling and atrial fibrillation: mechanisms and implications.
Circ Arrhythm Electrophysiol. 1:62–73. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Spach MS, Miller WT III, Dolber PC,
Kootsey JM, Sommer JR and Mosher CE Jr: The functional role of
structural complexities in the propagation of depolarization in the
atrium of the dog. Cardiac conduction disturbances due to
discontinuities of effective axial resistivity. Circ Res.
50:175–191. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Koura T, Hara M, Takeuchi S, et al:
Anisotropic conduction properties in canine atria analyzed by
high-resolution optical mapping: preferential direction of
conduction block changes from longitudinal to transverse with
increasing age. Circulation. 105:2092–2098. 2002. View Article : Google Scholar
|
|
6
|
Anyukhovsky EP, Sosunov EA, Plotnikov A,
et al: Cellular electrophysiologic properties of old canine atria
provide a substrate for arrhythmogenesis. Cardiovasc Res.
54:462–469. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wongcharoen W, Chen YC, Chen YJ, et al:
Aging increases pulmonary veins arrhythmogenesis and susceptibility
to calcium regulation agents. Heart Rhythm. 4:1338–1349. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wongcharoen W, Chen YC, Chen YJ, Lin CI
and Chen SA: Effects of aging and ouabain on left atrial
arrhythmogenicity. J Cardiovasc Electrophysiol. 18:526–531. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hayashi H, Wang C, Miyauchi Y, et al:
Aging-related increase to inducible atrial fibrillation in the rat
model. J Cardiovasc Electrophysiol. 13:801–808. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xu D, Murakoshi N, Tada H, Igarashi M,
Sekiguchi Y and Aonuma K: Age-related increase in atrial
fibrillation induced by transvenous catheter-based atrial burst
pacing: an in vivo rat model of inducible atrial fibrillation. J
Cardiovasc Electrophysiol. 21:88–93. 2010. View Article : Google Scholar
|
|
11
|
Guzadhur L, Jiang W, Pearcey SM, et al:
The age-dependence of atrial arrhythmogenicity in
Scn5a+/− murine hearts reflects alterations in action
potential propagation and recovery. Clin Exp Pharmacol Physiol.
39:518–527. 2012.PubMed/NCBI
|
|
12
|
Vaidya D, Morley GE, Samie FH and Jalife
J: Reentry and fibrillation in the mouse heart. A challenge to the
critical mass hypothesis. Circ Res. 85:174–181. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fox JG, Davisson MT, Quimby FW, Barthold
SW, Newcomer CE and Smith AL: The Mouse in Biomedical Research.
Normative Biology, Husbandry, and Models. 3. 2nd edition. Elsevier;
London: 2007
|
|
14
|
Schrickel JW, Bielik H, Yang A, et al:
Induction of atrial fibrillation in mice by rapid transesophageal
atrial pacing. Basic Res Cardiol. 97:452–460. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhou X, Yun JL, Han ZQ, et al:
Postinfarction healing dynamics in the mechanically unloaded rat
left ventricle. Am J Physiol Heart Circ Physiol. 300:H1863–H1874.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wolska BM and Solaro RJ: Method for
isolation of adult mouse cardiac myocytes for studies of
contraction and microfluorimetry. Am J Physiol. 271:H1250–H1255.
1996.PubMed/NCBI
|
|
17
|
Lakatta EG and Sollott SJ: The
‘heartbreak’ of older age. Mol Interv. 2:431–446. 2002.
|
|
18
|
Stiles MK, John B, Wong CX, et al:
Paroxysmal lone atrial fibrillation is associated with an abnormal
atrial substrate: characterizing the ‘second factor’. J Am Coll
Cardiol. 53:1182–1191. 2009.PubMed/NCBI
|
|
19
|
Taneja T, Mahnert BW, Passman R,
Goldberger J and Kadish A: Effects of sex and age on
electrocardiographic and cardiac electrophysiological properties in
adults. Pacing Clin Electrophysiol. 24:16–21. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yanni J, Tellez JO, Sutyagin PV, Boyett MR
and Dobrzynski H: Structural remodelling of the sinoatrial node in
obese old rats. J Mol Cell Cardiol. 48:653–662. 2010. View Article : Google Scholar
|
|
21
|
Li G, Liu E, Liu T, et al: Atrial
electrical remodeling in a canine model of sinus node dysfunction.
Int J Cardiol. 146:32–36. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kojodjojo P, Kanagaratnam P, Markides V,
Davies DW and Peters N: Age-related changes in human left and right
atrial conduction. J Cardiovasc Electrophysiol. 17:120–127. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kistler PM, Sanders P, Fynn SP, et al:
Electrophysiologic and electroanatomic changes in the human atrium
associated with age. J Am Coll Cardiol. 44:109–116. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Burkauskiene A, Mackiewicz Z, Virtanen I
and Konttinen YT: Age-related changes in myocardial nerve and
collagen networks of the auricle of the right atrium. Acta Cardiol.
61:513–518. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Burkauskiene A: Age-related changes in the
structure of myocardial collagen network of auricle of the right
atrium in healthy persons and ischemic heart disease patients.
Medicina (Kaunas). 41:145–154. 2005.
|
|
26
|
Abhayaratna WP, Seward JB, Appleton CP, et
al: Left atrial size: physiologic determinants and clinical
applications. J Am Coll Cardiol. 47:2357–2363. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nakajima H, Nakajima HO, Dembowsky K,
Pasumarthi KB and Field LJ: Cardiomyocyte cell cycle activation
ameliorates fibrosis in the atrium. Circ Res. 98:141–148. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Guzadhur L, Pearcey SM, Duehmke RM, et al:
Atrial arrhythmogenicity in aged Scn5a+/DeltaKPQ mice
modeling long QT type 3 syndrome and its relationship to
Na+ channel expression and cardiac conduction. Pflugers
Arch. 460:593–601. 2010.PubMed/NCBI
|
|
29
|
Wakimoto H, Maguire CT, Kovoor P, et al:
Induction of atrial tachycardia and fibrillation in the mouse
heart. Cardiovasc Res. 50:463–473. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dun W, Yagi T, Rosen MR and Boyden PA:
Calcium and potassium currents in cells from adult and aged canine
right atria. Cardiovasc Res. 58:526–534. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mansourati J and Le Grand B: Transient
outward current in young and adult diseased human atria. Am J
Physiol. 265:H1466–H1470. 1993.PubMed/NCBI
|
|
32
|
Crumb WJ Jr, Pigott JD and Clarkson CW:
Comparison of Ito in young and adult human atrial myocytes:
evidence for developmental changes. Am J Physiol. 268:H1335–H1342.
1995.PubMed/NCBI
|
|
33
|
Dun W, Chandra P, Danilo P, Rosen MR and
Boyden PA: Chronic atrial fibrillation does not further decrease
outward currents. It increases them. Am J Physiol Heart Circ
Physiol. 285:H1378–H1384. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Anyukhovsky EP, Sosunov EA, Chandra P, et
al: Age-associated changes in electrophysiologic remodeling: a
potential contributor to initiation of atrial fibrillation.
Cardiovasc Res. 66:353–363. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Spach MS, Dolber PC and Anderson PA:
Multiple regional differences in cellular properties that regulate
repolarization and contraction in the right atrium of adult and
newborn dogs. Circ Res. 65:1594–1611. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Brorson L and Olsson SB: Right atrial
monophasic action potential in healthy males. Studies during
spontaneous sinus rhythm and atrial pacing. Acta Med Scand.
199:433–446. 1976. View Article : Google Scholar
|