Introduction
RNA interference (RNAi) refers to the
sequence-specific degradation of RNA that follows the cellular
introduction of homologous, short interfering RNA (siRNA) (1–4).
RNAi has emerged as a powerful tool for probing the function of
genes of a known sequence both in vitro and in vivo.
Advances in vector design permit the effective expression of siRNA
in human cells by transferring short hairpin RNA (shRNA) expression
cassettes. Recent studies reported the ability of RNAi to decrease
replication of human immunodeficiency virus type 1 (HIV-1) in
lymphocytes using siRNA targeting viral (such as tat, gag, rev,
env, nef) (5–11) and host (such as CCR5, CD4)
(12–14) proteins. Thus, RNAi might
potentially be used as a form of genetic therapy for HIV-1 and
associated infections. Another type of short RNA, microRNA (miRNA),
has also been reported (15–17). miRNAs are transcribed from the
genome as the primary miRNA and digested by Drosha RNase III enzyme
to produce the precursor-miRNA in the nucleus. The precursor miRNA
is then transported to the cytoplasm and digested by Dicer to
become mature miRNA. The miRNA is incorporated into RISC and binds
to the target RNA, similar to siRNA (18–22). Since the miRNA has mismatches with
the target sequence, translation of the target RNA is suppressed. A
large number of miRNAs was recently identified in humans. It is
estimated that tens of thousands of miRNAs are coded in the genome,
and that these miRNAs control protein expression (18,23). miRNAs are important for
maintaining cell functions. New short-strand RNAs have also been
found (24–33). For example, a piwi-interacting RNA
binding a piwi subfamily protein is thought to be involved in
silencing retrotransposons in fetal male germ cells via DNA
methylation of their regulatory regions (34), whereas endogenous siRNAs from
naturally occurring double-stranded RNAs are thought to regulate
protein-coding transcripts and retrotransposons in mammalian
oocytes (35). Most small RNAs
are coded in the non-coding region of the genome, and the more
complex the organism, the larger the non-coding region. Further
investigation of the functions of the small RNAs is essential for a
better understanding of biological systems.
HIV-1 is the most common pathogenic AIDS virus.
HIV-1 infects the immune cells by integrating the viral genome into
the host cell genome. Viral proteins are expressed by the
integrated genome through the host cell genetic expression
mechanisms. Viral particles that bud on the cell membrane are then
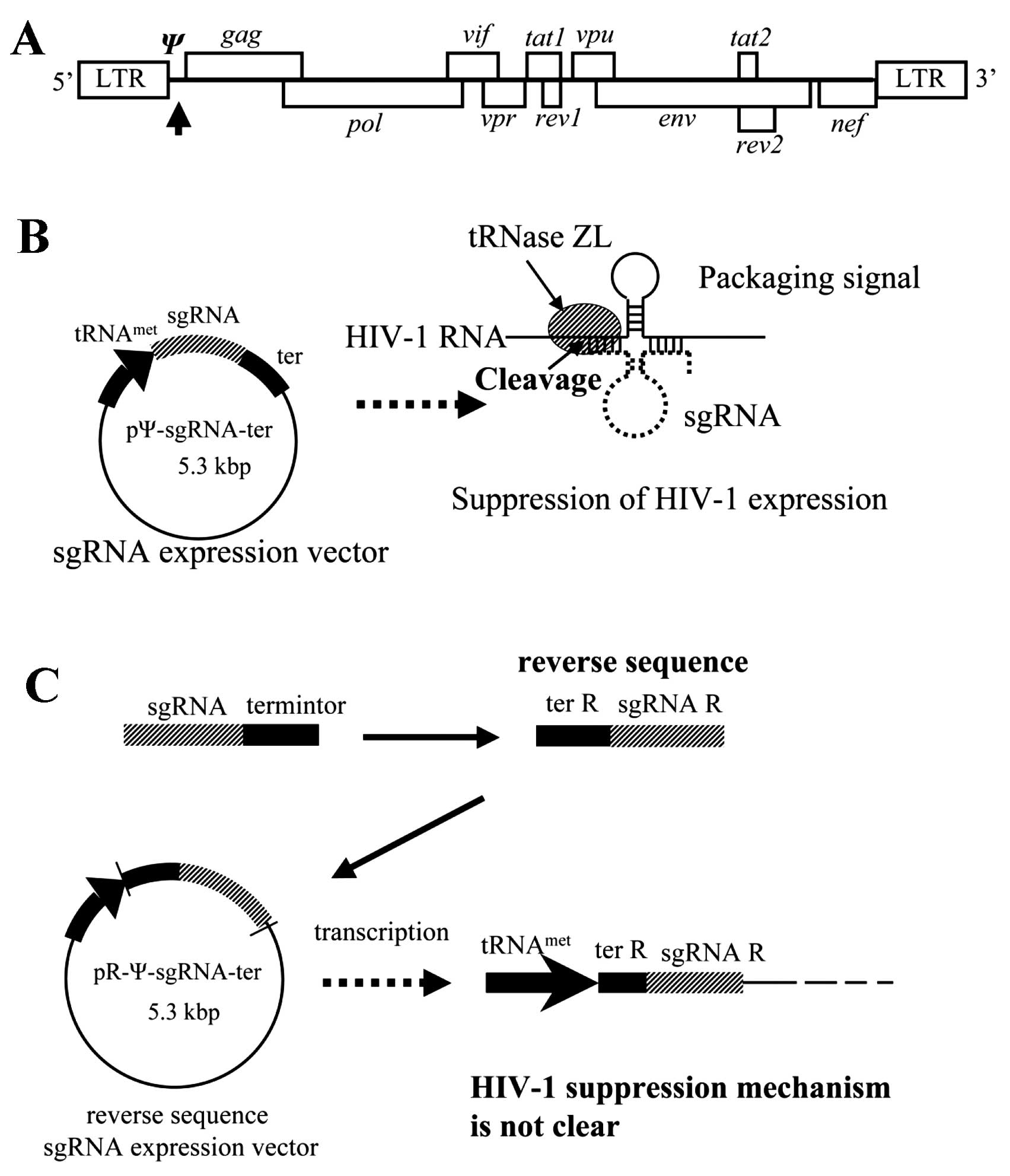
released from the cells. A packaging signal (Ψ) on HIV-1 mRNA is
required for incorporation of the HIV-1 RNA genome into a viral
particle when the HIV-1 virus forms on a host cell membrane.
To suppress HIV-1 replication, we constructed a
small-guide RNA (sgRNA) expression vector that targets the SL3 of Ψ
and evaluated its effect on HIV-1 replication (36–38). The sgRNA is approximately
30-nucleotides (nt) long and forms a tRNA-like structure with the
target RNA in the nucleus, where it cleaves the target RNA by
activating the tRNA processing enzyme tRNAZL (38–42).
A plasmid vector with the reverse sequence of the
sgRNA and terminator was unintentionally constructed. This
reverse-sequence sgRNA expression vector, pR-Ψ-sgRNA-ter, also
inhibited HIV-1 replication (Fig.
1) (43). The R-Ψ-sgRNA
expressed by the pR-Ψ-sgRNA-ter vector is 200 nt long, and, to
suppress HIV-1 replication, must contain the reverse sequence of
the sgRNA (sgRNA-R), terminator (ter-R), and also the plasmid
sequence. The HIV-1 suppressing mechanism of R-Ψ-sgRNA, however,
remains unclear. Thus, to elucidate the mechanism of HIV-1
suppression by R-Ψ-sgRNA, we evaluated the potential involvement of
RNAi or miRNA.
Materials and methods
Cell culture and transfections
HeLa CD4+ cells were grown in RPMI-1640
medium (Sigma, St. Louis, MO, USA) supplemented with 10% (v/v)
heat-inactivated fetal bovine serum (FBS), 50 U/ml penicillin, and
50 U/ml streptomycin at 37°C in a 5% CO2 atmosphere.
Transfection was carried out using the FuGENE™ 6 reagent (Roche
Diagnostics, Indianapolis, IN, USA) or DMRIE-C (Invitrogen,
Carlsbad, CA, USA) according to the manufacturer’s protocol.
Plasmid construction
To construct pR-Ψ-sgRNA-ter, an oligonucleotide
consisting of sgRNA and terminator sequences was ligated into the
KpnI sites of pL6 vector (38,44) of pSV2neo (L6) (38) with the
tRNAiMet promoter (45). One side of the oligonucleotide was
designed not to be digested with KpnI when the
oligonucleotide was ligated to KpnI sites of pL6 vector.
pR-Gag-sgRNA-ter was constructed in the same way as pR-Ψ-sgRNA-ter.
A PCR product to construct pR-Ψ-sgRNA-ter-1 was amplified with
specific primers (forward, 5′-GGAATTCCAATTCGC AACCTGTGGTAGC-3′ and
reverse, 5′-GAAGATCTTCC CCCAAAAAAATAAAGCATTTTTTTCACT-3′) to
pR-Ψ-sgRNA-ter. The forward primer had a KpnI restriction
site. The reverse primer had a BglII restriction site. The
PCR product was ligated to the KpnI and BamHI sites
of the pL6 vector. pL6-ter served as a negative control.
Luciferase assay
The pNL4-3lucΔenv (pNL-luc) was an HIV-1 NL4-3-based
vector containing a firefly luciferase gene as a reporter (46). The nef gene along with some of the
envelope gene sequences of the HIV-1 NL4-3 genome were deleted, and
the firefly luciferase gene was inserted. pNL-luc (0.1 μg) and
either pL6 plasmid pR-Ψ-sgRNA-ter, pR-Gag-sgRNA-ter,
pR-Ψ-sgRNA-ter-1, pter-R+sgRNA-R+ter, psgRNAR, pter-R or pL6-ter
serving as a negative control (0.1 μg) were co-transfected with the
transfection reagent, FuGENE 6, into HeLa CD4+ cells. A
PicaGene kit (Toyo Ink Co., Ltd., Tokyo, Japan) and a Luciferase
Assay System (Promega, Madison, WI, USA) were used for the
luciferase assay. Two days after transfection, the transfected
culture cells were treated with cell lysis buffer. Cell lysate was
recovered into 1.5-ml tubes to remove the unused components by
centrifugation. Lysate was reacted with luminous substrate and the
level of fluorescence was measured on a luminometer (Lumat LB 9507;
Berthold Technologies, Bad Wildbad, Germany). The amount of firefly
luciferase activity was normalized with reference to the protein
concentration of the cell lysate. The protein assay was quantitated
using the BCA Protein Assay Reagent kit (Thermo Scientific Inc.,
Rockford, IL, USA), which is based on bicinchoninic acid (BCA).
RNA synthesis and digestion
The DNA template used to synthesize R-Ψ-sgRNA was
amplified with specific primers (forward,
5′-GACTCGTAATACGACTCACTATAGGCAG AACAGCAGAGTGGCG-3′ and reverse,
5′-AATAAAGCAT TTTTTTCACTGCATTCTAGT-3′) to the pR-Ψ-sgRNA-ter by
PCR. The forward primer had a T7 promoter sequence to transcribe
the R-Ψ-sgRNA. The amplified DNA template was incubated with T7 RNA
polymerase (Invitrogen) and nucleotide triphosphates for 3 h at
37°C and the synthesized R-Ψ-sgRNA was treated with DNase I (Takara
Bio Co., Ltd., Shiga, Japan) to remove the DNA template. R-Ψ-sgRNA
was confirmed by 2.0% denatured formaldehyde agarose gel
electrophoresis. R-Ψ-sgRNA was incubated with recombinant human
Dicer enzyme of BLOCK-iT™ Dicer RNAi kits (Invitrogen) and purified
according to the manufacturer’s protocol. Digested RNA fragments
were confirmed by 15% polyacrylamide gel electrophoresis.
Northern blot analysis
Small RNA (<200 nt) was extracted from
pR-Ψ-sgRNA-ter and siRNA-Dicer (0, 10, 20, 30 nM) co-transfected
HeLa CD4+ cells using a mirVana™ miRNA Isolation kit,
according to the manufacturer’s instructions (Ambion, Foster City,
CA, USA). siRNA corresponding to Dicer
[5′-UGCUUGAAGCAGCUCUGGA(dTdT)-3′] was purchased from Invitrogen.
Small RNA 5 μg samples were loaded onto a 15% (w/v)
polyacrylamide/7 M urea gel. After transfer to a Hybond-N™ nylon
membrane (GE Healthcare Bio-Sciences Corp., Piscataway, NJ, USA),
synthetic LNA/DNA oligonucleotides
(5′-biotin-TGGATCCCCGGGTACGTCTCC-3′) complementary to the antisense
strand of the R-Ψ-sgRNA region (54–75 nt). The membranes were
prehybridized for 1 h in North2South hybridization buffer (Pierce)
at 55°C and hybridized overnight to the 5′-biotin labeled LNA/DNA
probe (30 ng/ml of hybridization buffer). Four posthybridization
washes were carried out at 20 min each at 65°C with 2× SSC, 1× SSC
is 0.15 M NaCl plus 0.015 M sodium citrate-0.1% sodium dodecyl
sulfate (SDS). Detection of LNA/DNA/RNA hybrids was carried out
using the North2South chemiluminescent detection system (Thermo
Scientific Inc.)
RNA sequence analysis
A poly(A) tail was added to the 3′ terminal ends of
the R-Ψ-sgRNA fragments with poly(A) polymerase (Ambion, Austin TX,
USA) and subsequently ligated to a 5′ adapter
(5′-ACGGAATTCCTCACTaaa-3′; uppercase letters represent DNA, the
lowercase letters represent RNA) to the 5′ terminals with T4 RNA
ligase (New England Biolabs, Ipswich, MA, USA). Reverse
transcription was performed with the poly(A)-specific reverse
transcription (RT) primer consisting of a continuous T sequence and
an additional sequence to convert RNA to DNA. The RT primer was as
follows: 5′-CTGATCTAGAGGT ACCGGATCCTTTTTTTTTTTTTTTTTTTT-3′.
ReverTra-α (Toyobo Co., Ltd., Osaka, Japan) was used for this
reaction. The additional sequence of the RT primer was used to
adjust the annealing temperature of the following PCR. The reverse
transcripts were amplified with PCR primers specific to the 5′
adapter sequence (5′-CAGCCAACGGAATTCCTCACTAAA-3′) and the RT primer
by PCR. The PCR products were inserted into the cloning vector,
pGEM-Easy vector (Promega), for sequence analysis. Sequence
analysis was performed with an ABI PRISM 310 Genetic Analyzer (PE
Applied Biosystems, Tokyo, Japan) according to the manufacturer’s
protocol.
HIV-1 p24 assay
For the HIV-1 p24 assay, HeLa CD4+ cells
were plated onto 12-well plates (Iwaki Glass, Tokyo, Japan). After
24 h, the medium was replaced with fresh medium and the cells were
transfected with small RNA fragments of R-Ψ-sgRNA [(0.5, 0.25 and
0.05 μg) and HIV-1 pNL4-3 (0.05 μg)]. The transfection agents were
DMRIE-C (Invitrogen) and FuGENE 6 (Roche Diagnostics). Two days
later, HIV-1 gag p24 antigen levels were measured using a
chemiluminescent enzyme immunoassay (Lumipulse; Fujirebio, Tokyo,
Japan) (47).
Results and Discussion
Inhibition of HIV-1 replication with the
reverse sequence sgRNA expression vector
Recently, we demonstrated the inhibition of HIV-1
gene products in cultured cells by inducing HIV-1 mRNA cleavage
using a modified 5′-half-tRNAArg (sgRNA) and mammalian
tRNA 3′-processing endoribonuclease (tRNase ZL) (38). The sgRNA/target HIV-1RNA complex
formed a pre-tRNA-like structure with 5′-half-tRNA and a stable
hairpin (3′-half-tRNA) structure resembling the T stem-loop region
(Fig. 1A and B). The
tRNAmet-sgRNA transcript was expressed at high levels
and localized in the nucleus. The greatest inhibitory effect on
HIV-1 expression was achieved using sgRNA targeting the HIV-1
packaging (Ψ) signal gene (36–38). To construct the sgRNA expression
plasmid, a pL6 vector, dpSV/neo with a human methionine tRNA
promoter (38,45), was used to insert the sgRNA and
terminator sequences. During this process, pR-Ψ-sgRNA-ter, which
has the reverse sequence of the sgRNA and the terminator, was
unintentionally constructed (Fig.
1C). A transient-expression assay was used to test the ability
of pR-Ψ-sgRNA-ter to inhibit HIV-1 expression. HeLa CD4+
cells were co-transfected with the sgRNA plasmids and an
HIV-1NL4-3 based-vector containing a
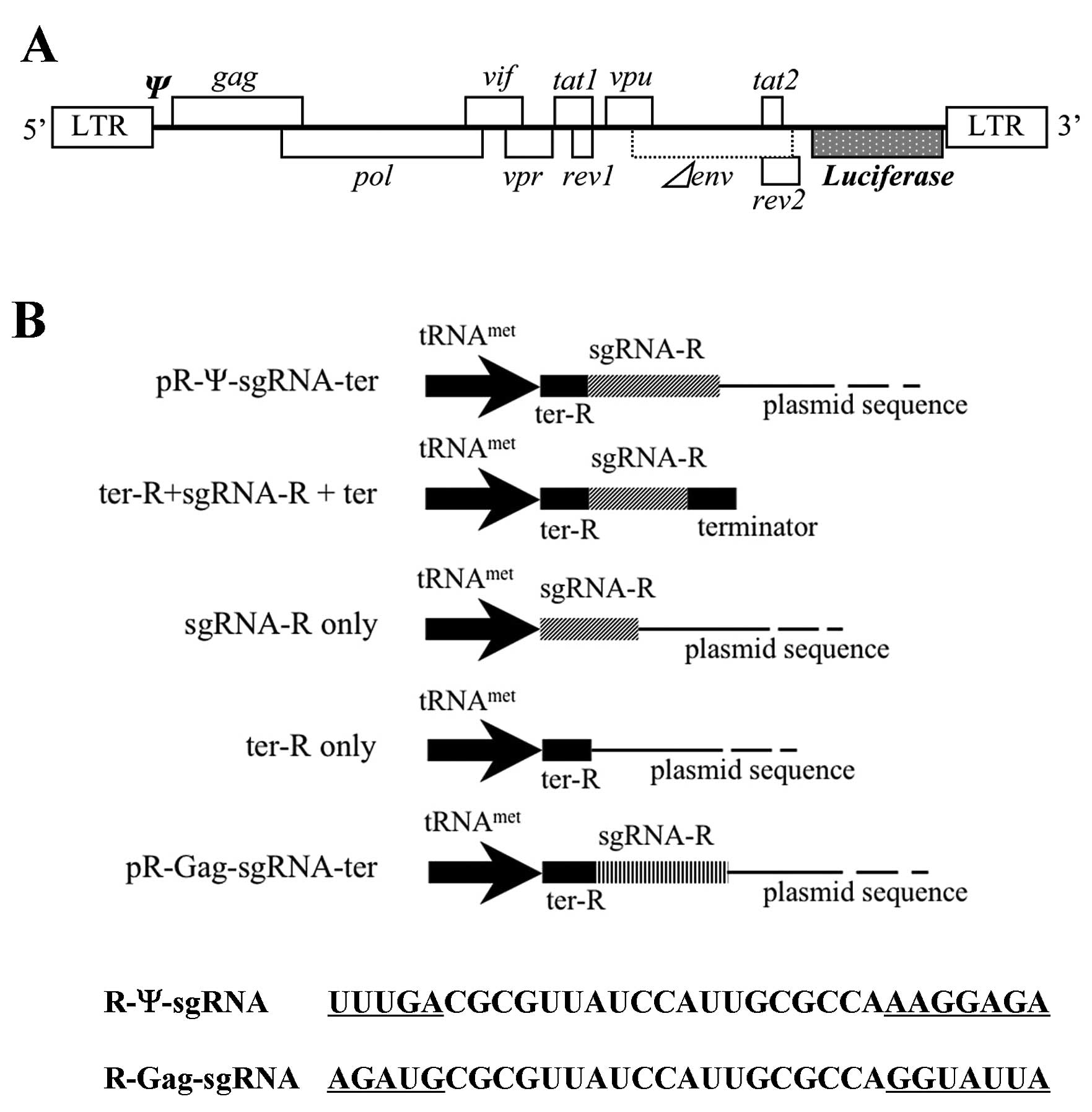
luciferase-expression marker (pNL-luc) (Fig. 2A) (46), and then HIV-1 suppression was
assessed with a single-cycle infectivity assay.
HIV-1NL4-3 based-luciferase activity was recorded using
the control plasmid vector pL6-ter with only the tRNAmet
promoter and terminator sequences, rather than the sgRNA-expression
plasmid. R-Ψ-sgRNA-ter showed good inhibition of HIV-1 expression
in cultured cells (Fig. 2C). The
contribution of the R-Ψ-sgRNA-ter to the anti-HIV-1 effect was
examined by three types of modified R-Ψ-sgRNA-ter plasmids
(R-Ψ-sgRNA-ter that did not contain ter-R+sgRNA-R+ter, sgRNA-R or
ter-R) as defective pR-Ψ-sgRNA-ters (Fig. 2B). We also constructed a control
R-sgRNA, pR-Gag-sgRNA-ter containing the reverse sequence of the
sgRNA targeting the HIV-1 gag gene (gag initiation codon site)
(Fig. 2B). The luciferase assay
indicated that the modified R-Ψ-sgRNA-ter plasmids without the
sgRNA-R, ter-R, or plasmid sequence (ter-R+sgRNA-R+ter) and the
control sgRNA, R-Gag-sgRNA-ter did not suppress HIV-1 expression
(Fig. 2C). These results suggest
that the R-Ψ-sgRNA-ter sequence is critical to HIV-1 specific
expression.
Length of R-Ψ-sgRNA and its inhibitory
effect on HIV-1 replication
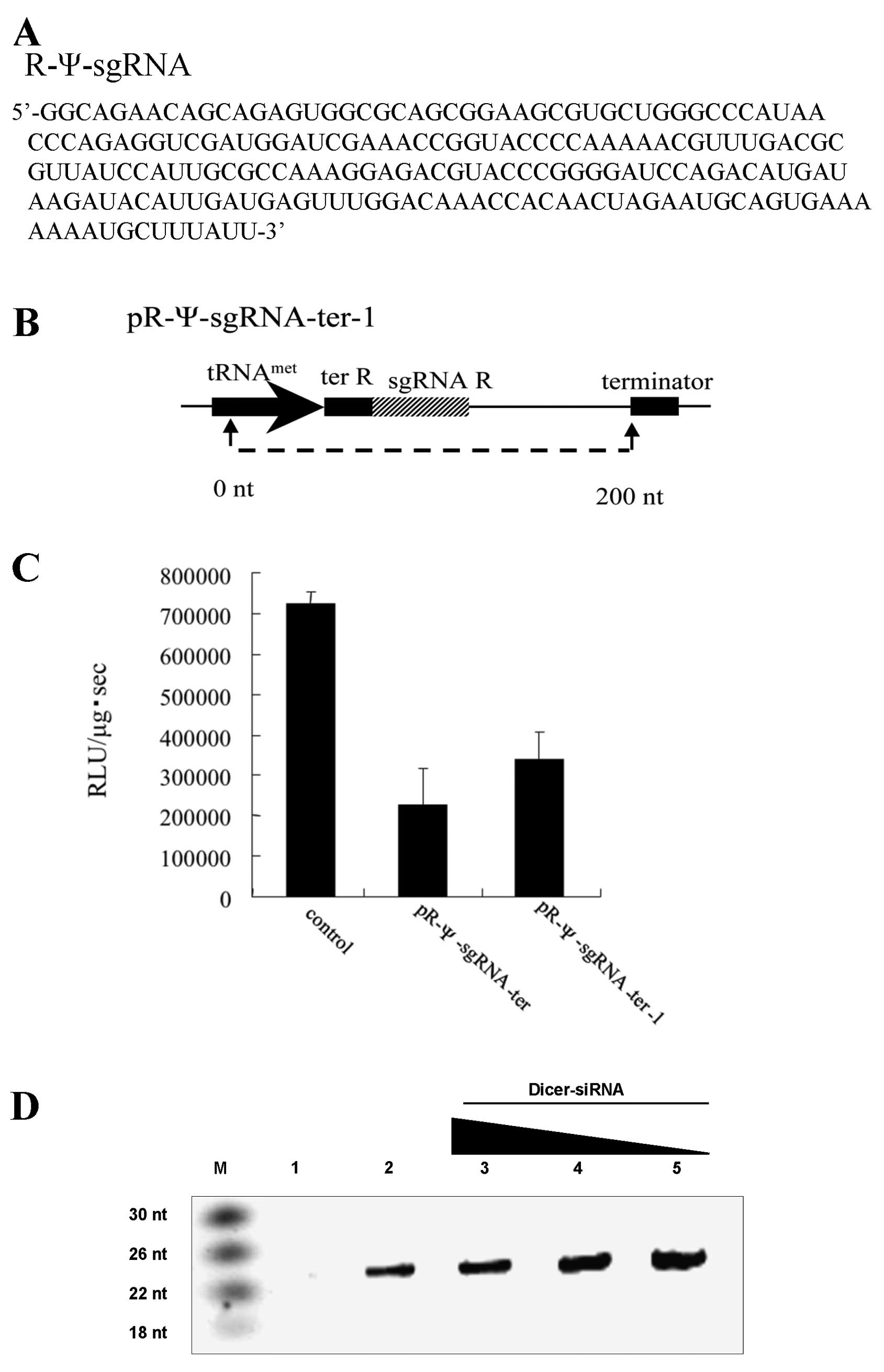
The pR-Ψ-sgRNA-ter termination signal sequence was
also reversed, and pR-Ψ-sgRNA-ter did not have a terminator.
Therefore we could not predict the length of the R-Ψ-sgRNA
transcribed from pR-Ψ-sgRNA-ter. To determine the length of the
R-Ψ-sgRNA transcribed from pR-Ψ-sgRNA-ter, an RNA sequence analysis
was performed. The total RNA of pR-Ψ-sgRNA-ter transfected cells
was treated with poly(A) polymerase to add a poly(A) tail to the 3′
terminal end of R-Ψ-sgRNA. A PCR primer specific to the transcribed
tRNAmet promoter sequence and RT primer specific to the
poly(A) tail were used for RT-PCR. A cloning vector was used to
insert the PCR products, and the sequences were analyzed. RNA
sequence analysis revealed that the R-Ψ-sgRNA-ter was 200 nt long
(Fig. 3A and B). We confirmed
that R-Ψ-sgRNA contained sgRNA-R, ter-R, and a plasmid sequence
following sgRNA-R. A plasmid vector expressing the R-Ψ-sgRNA-ter-1
(200 nt) sequence was constructed with pL6 vector, and a luciferase
assay indicated that the R-Ψ-sgRNA-ter-1 (200 nt) suppressed HIV-1
expression (Fig. 3C).
Size-selected RNAs were electrophoresed in polyacrylamide gels and
transferred to nylon membranes. We then hybridized the membranes
with a synthetic LNA/DNA oligonucleotide
(5′-biotin-TGGATCCCCGGGTACGTCTCC-3′) complementary to the antisense
strand of the R-Ψ-sgRNA region (54–75 nt) and detected a 24 bp
signal (Fig. 3D, lane 2) not seen
in the control-ter-R cells (Fig.
3D, lane 1). Furthermore, HeLa CD4+ cells were
transfected with pR-Ψ-sgRNA-ter and Dicer-siRNA (334–352 nt, 10–30
nM) and the expected small RNA decreased in a dose-dependent manner
(Fig. 3D, lanes 3–5). These
results suggest that the R-Ψ-sgRNA-ter-1 (200 nt) sequence is
required for HIV-1 suppression.
Sequence analysis of the digested RNA
fragments
To search for the double-stranded region recognized
by Dicer, an RNA sequence analysis was performed. A poly(A) tail
and 5′ adapter were added to the digested RNA fragments and the RNA
was converted to DNA by RT-PCR. A cloning vector was used to insert
the DNA fragments and the sequences were analyzed. Most of the RNA
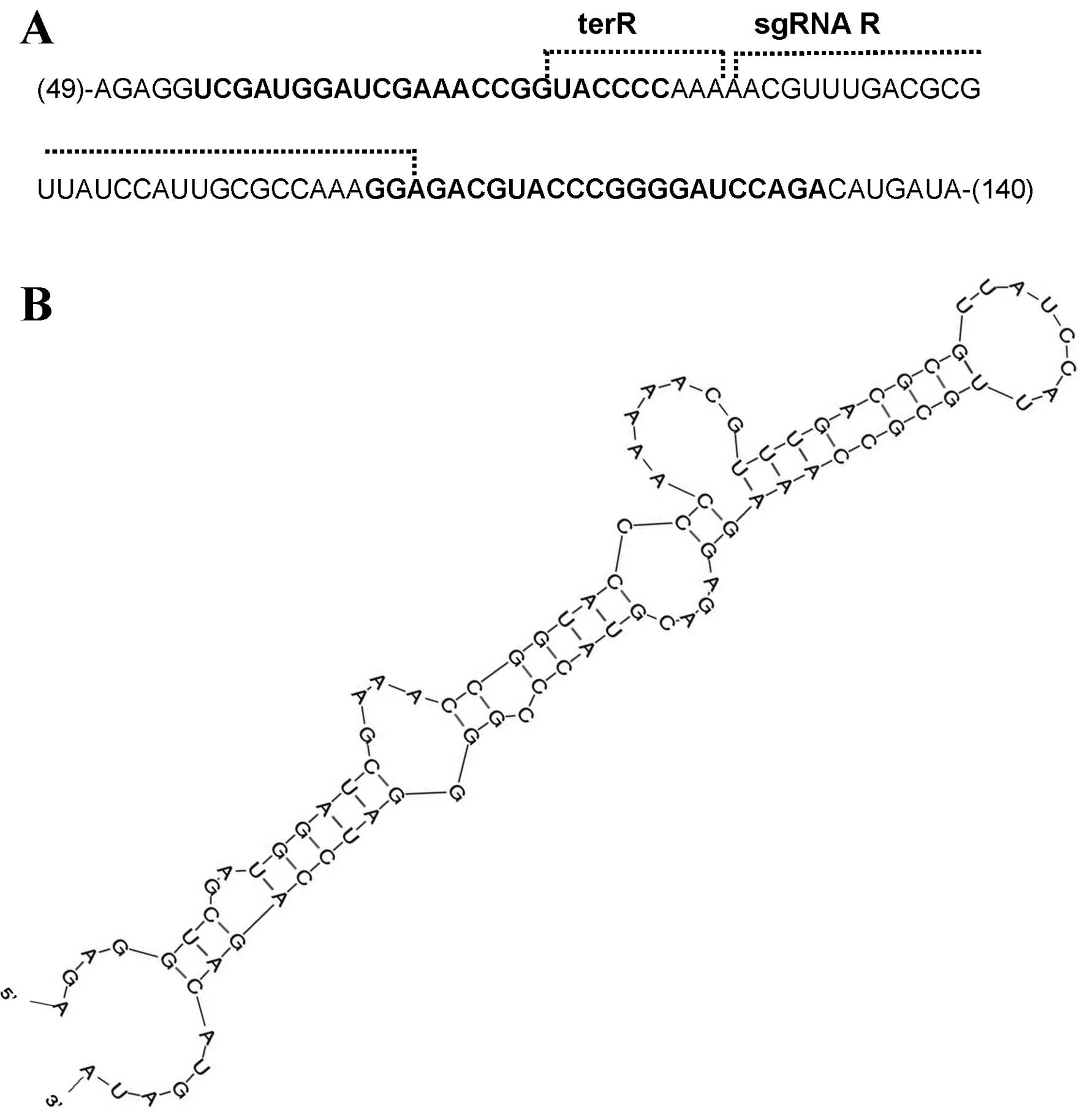
fragment sequences existed in two regions of the R-Ψ-sgRNA: the
region from ter-R to the anterior sequence of ter-R, which was
transcribed initially by the tRNA promoter, and the region from
sgRNA-R to the plasmid sequence following sgRNA-R (Fig. 4A). In addition, the sgRNA-R
sequences obtained from the sequence analysis were not present in
the sgRNA-R region of the pR-Gag-sgRNA-ter targeting gag gene.
These results, therefore, seemed to be related to the use of
defective pR-Ψ-sgRNA-ter and pR-Gag-sgRNA-ter in the assay
(Fig. 2).
The RNA secondary structure of R-Ψ-sgRNA was
predicted using the Mfold program (48) and the double-stranded region was
searched based on the RNA sequence data obtained from the sequence
analysis. The double-stranded region was located between 48 to 140
nt of the R-Ψ-sgRNA, and the presence of sequences of short-strand
RNA fragments in this region was confirmed (Fig. 4B). Furthermore, miRNAs recognize
HIV-1 NL4-3 target mRNAs by their hybridization pattern as
predicted using the miRNA target scanning algorithm program
(49). The miRNA region was
searched based on the 24 nt small RNA shown in Fig. 3D, and the presence of miRNA in the
double-stranded region (nucleotides 48–140) of R-Ψ-sgRN was
confirmed (data not shown). The double-stranded region had
mismatches, indicating that the short-strand RNA fragments include
miRNA, but not siRNA.
Digestion of R-Ψ-sgRNA with Dicer
We hypothesized that the secondary structure of the
R-Ψ-sgRNA had double-stranded regions that may be recognized by the
Dicer RNase III enzyme, which produces the 24 nt long RNA fragments
that are important for RNAi and miRNA. To examine whether the HIV-1
suppression mechanism of R-Ψ-sgRNA involved RNAi or miRNA,
R-Ψ-sgRNA was synthesized in vitro and digested with Dicer
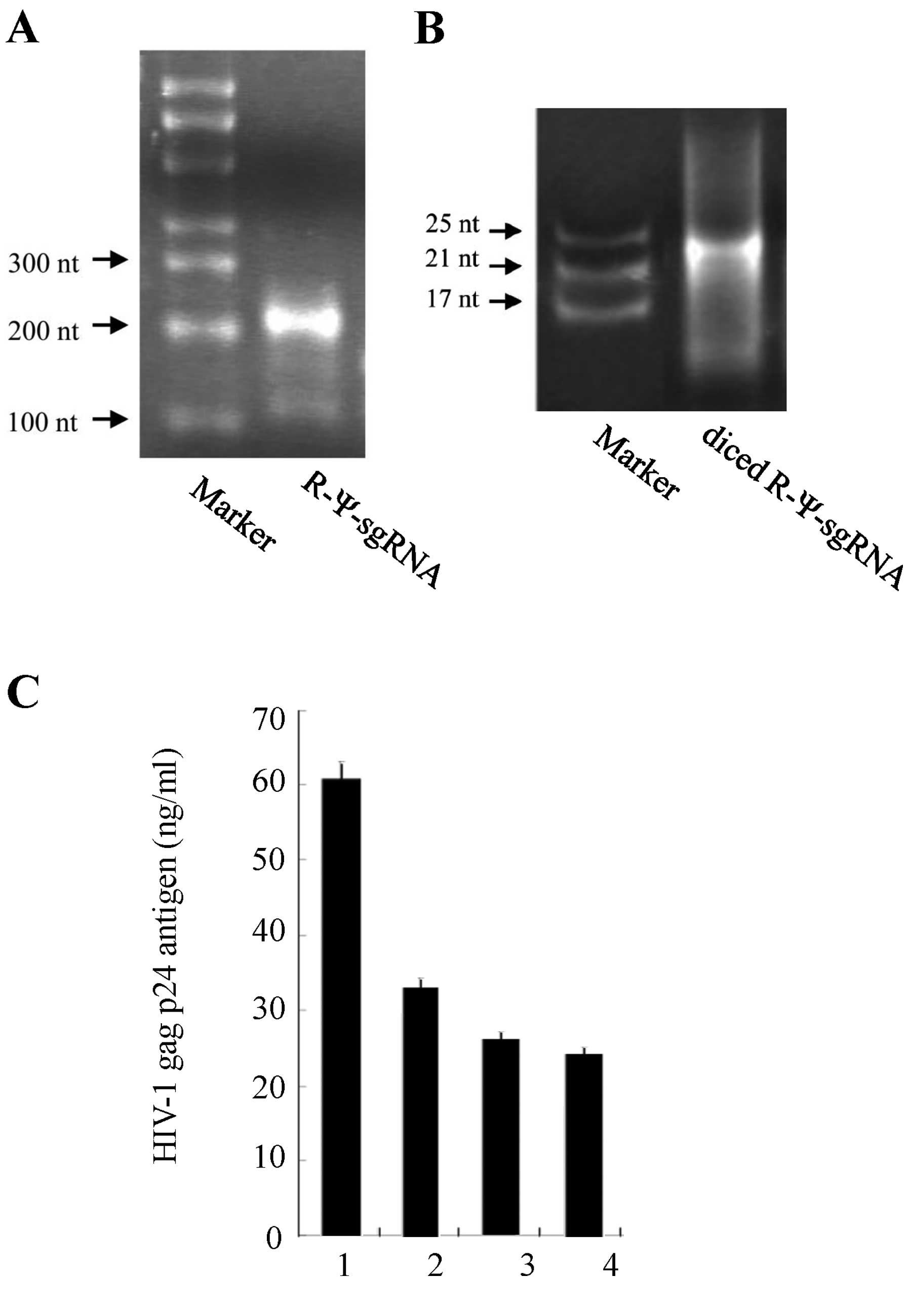
(50). The size of the digested
RNA fragments was confirmed with 15% polyacrylamide gel
electrophoresis (Fig. 5A and
B).
Short-strand RNA fragments inhibit HIV-1
replication
To test the inhibitory activity of the short-strand
RNA fragments on HIV-1 replication in a transient assay, an HIV-1
expression plasmid (pNL4-3) and the short-strand RNA fragments were
transfected into HeLa CD4+ cells. The virus production
in the culture supernatant was monitored with the HIV-1 p24 antigen
(HIV-1 gag gene product) assay. The results of the HIV-1 p24 assay
indicated that these RNA fragments had anti-HIV activity and
suppressed HIV-1 replication. Furthermore, our analysis revealed
that the short-strand RNA fragments dose-dependently inhibited
HIV-1 replication (Fig. 5C).
These results suggest that the short-strand RNA fragments have a
miRNA-like effect on the HIV-1 virus.
In conclusion, the short-strand RNA fragments (24 nt
long) of R-Ψ-sgRNA cleaved using the Dicer enzyme exhibited
anti-HIV-1 activity. The sequences of these RNA fragments were
analyzed to determine the critical sequence for the anti-HIV-1
activity. These findings suggest that R-Ψ-sgRNA acts as a miRNA to
inhibit HIV-1.
Acknowledgements
The authors thank Dr I.S.Y. Chen for providing
pNL4-3lucΔenv and Makoto Abe and Yuji Yokota for their technical
assistance. We also thank Dr Y. Habu for the valuable discussion.
This study was supported in part by a Grant-in-Aid for AIDS
research from the Ministry of Health, Labor, and Welfare, Japan, by
a Grant from the Strategic Research Foundation Grant-aided Project
for Private Universities from the Ministry of Education, Culture,
Sport, Science and Technology, Japan (MEXT), and by a Grant-in-Aid
for Science Research (C) from Japan Society for the Promotion of
Science (JSPS), Japan.
Abbreviations:
|
HIV-1
|
human immunodeficiency virus type
1
|
|
miRNA
|
microRNA
|
|
nt
|
nucleotide
|
|
RISC
|
RNA-induced silencing complex
|
|
RNAi
|
RNA interference
|
|
sgRNA
|
small guide RNA
|
|
siRNA
|
short interfering RNA
|
|
tRNase ZL
|
tRNA 3′-processing
endoribonuclease
|
|
Ψ
|
HIV-1 packaging signal
|
|
tRNAmet
|
methionine tRNA
|
|
Dicer
|
RNase III-family enzyme
|
References
|
1
|
Fire A, Xu S, Montgomery MK, Kostas SA,
Driver SE and Mello CC: Potent and specific genetic interference by
double-stranded RNA in Caenorhabditis elegans. Nature.
39:806–811. 1998. View
Article : Google Scholar
|
|
2
|
Elbashir SM, Harborth J, Lendeckel W,
Yalcin A, Weber K and Tuschl T: Duplexes of 21-nucleotide RNAs
mediate RNA interference in cultured mammalian cells. Nature.
411:494–498. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sharp PA: RNA interference. Genes Dev.
15:485–490. 2001.PubMed/NCBI
|
|
4
|
Hannon GJ: RNA interference. Nature.
418:244–251. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Coburn GA and Cullen BR: Potent and
specific inhibition of human immunodeficiency virus type 1
replication by RNA interference. J Virol. 76:9225–9231. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jacque JM, Trioques K and Stevenson M:
Modulation of HIV-1 replication by RNA interference. Nature.
418:379–380. 2002. View Article : Google Scholar
|
|
7
|
Lee NS, Dohjima T, Bauer G, Li H, Li MJ,
Ehsani A, Salvaterra P and Rossi J: Expression of small interfering
RNAs targeted against HIV-1 rev transcripts in human cells. Nat
Biotech. 19:500–505. 2002.PubMed/NCBI
|
|
8
|
Novina CD, Murray MF, Dykxhoorn DM,
Beresford PJ, Riess J, Lee SK, Collman RG, Lieberman J, Shankar P
and Sharp PA: siRNA-directed inhibition of HIV-1 infection. Nat
Med. 8:681–686. 2002.PubMed/NCBI
|
|
9
|
Yamamoto T, Omoto S, Mizuguchi M, Mizukami
H, Okuyama H, Okada N, Saksena NK, Brisibe EA, Otake K and Fuji YR:
Double-stranded nef RNA interferes with human immunodeficiency
virus type 1 replication. Microbiol Immunol. 46:809–817. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Song E, Lee SK, Dykxhoorn DM, Novina C,
Zhang D, Crawford K, Cerny J, Sharp PA, Lieberman J, Manjunath N
and Shankar P: Sustained small interfering RNA-mediated human
immunodeficiency virus type 1 inhibition in primary macrophages. J
Virol. 77:7174–7181. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Park WS, Hayafune M, Miyano-Kurosaki N and
Takaku H: Specific HIV-1 env gene silencing by small interfering
RNAs in human peripheral blood mononuclear cells. Gene Ther.
10:2046–2050. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Martínez MA, Gutiérrez A, Armand-Ugón M,
Blanco J, Parera M, Gómez J, Clotet B and Esté JA: Suppression of
chemokine receptor expression by RNA interference allows for
inhibition of HIV-1 replication. AIDS. 16:2385–2390. 2002.
|
|
13
|
Qin XF, An DS, Chen IS and Baltimore D:
Inhibiting HIV-1 infection in human T cells by lentiviral-mediated
delivery of small interfering RNA against CCR5. Proc Natl Acad Sci
USA. 100:183–188. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Anderson J, Banerjea A and Akkina R:
Suppression of HIV-1 infection by a stem-loop structured anti-CXCR4
siRNA. AIDS Res Hum Retroviruses. 19:699–706. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee RC, Feinbaum RL and Ambros V: The
C. elegans heterochronic gene lin-4 encodes small RNAs with
antisense complementarity to lin-14. Cell. 75:843–854. 1993.
|
|
16
|
Lagos-Quintana M, Rauhut R, Lendeckel W
and Tuschl T: Identification of novel genes coding for small
expressed RNAs. Science. 294:853–858. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lau NC, Lim LP, Weinstein EG and Bartel
DP: An abundant class of tiny RNAs with probable regulatory roles
in Caenorhabditis elegans. Science. 294:858–862. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bartel DP: MicroRNAs: genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hammond SM: Dicing and slicing the core
machinery of the RNA interference pathway. FEBS Lett.
579:5822–5829. 2005.PubMed/NCBI
|
|
20
|
Tang G: siRNA and miRNA: an insight into
RISCs. Trends Biochem Sci. 30:106–114. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tomari Y and Zamore PD: Perspective:
machines for RNAi. Genes Dev. 19:517–529. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kim VN: MicroRNA biogenesis: coordinated
cropping and dicing. Nat Rev Mol Cell Biol. 6:376–385. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shiomi M: Functions and regulatory
mechanisms of small RNAs expressed in living organisms. Exp Med.
25:794–799. 2007.
|
|
24
|
Aravin AA, Lagos-Quintana M, Yalcin A,
Zavolan M, Marks D, Snyder B, Gaasterland T, Meyer J and Tuschl T:
The small RNA profile during Drosophila melanogaster
development. Dev Cell. 5:337–350. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Aravin A, Gaidatzis D, Pfeffer S,
Lagos-Quintana M, Landgraf P, Iovino N, Morris P, Brownstein MJ,
Kuramochi-Miyagawa S, Nakano T, Chien M, Russo JJ, Ju J, Sheridan
R, Sander C, Zavolan M and Tuschl T: A novel class of small RNAs
bind to MILI protein in mouse testes. Nature. 442:203–207.
2006.PubMed/NCBI
|
|
26
|
Girard A, Sachidanandam R, Hannon GJ and
Carmell MA: A germline-specific class of small RNAs binds mammalian
Piwi proteins. Nature. 442:199–202. 2006.PubMed/NCBI
|
|
27
|
Grivna ST, Beyret E, Wang Z and Lin H: A
novel class of small RNAs in mouse spermatogenic cells. Genes Dev.
20:1709–1714. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lau NC, Seto AG, Kim J, Kuramochi-Miyagawa
S, Nakano T, Bartel DP and Kingston RE: Characterization of the
piRNA complex from rat testes. Science. 313:363–367. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Saito K, Nishida KM, Mori T, Kawamura Y,
Miyoshi K, Nagami T, Siomi H and Siomi MC: Specific association of
Piwi with rasiRNAs derived from retrotransposon and heterochromatic
regions in the Drosophila genome. Genes Dev. 20:2214–2222.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Vagin VV, Sigova A, Li C, Seitz H, Gvozdev
V and Zamore PD: A distinct small RNA pathway silences selfish
genetic elements in the germline. Science. 313:320–324. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Watanabe T, Takeda A, Tsukiyama T, Mise K,
Okuno T, Sasaki H, Minami N and Imai H: Identification and
characterization of two novel classes of small RNAs in the mouse
germline: retrotransposon-derived siRNAs in oocytes and germline
small RNAs in testes. Genes Dev. 20:1732–1743. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nishida KM, Saito K, Mori T, Kawamura Y,
Nagami-Okada T, Inagaki S, Siomi H and Siomi MC: Gene silencing
mechanisms mediated by Aubergine piRNA complexes in
Drosophila male gonad. RNA. 13:1911–1922. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Klattenhoff C and Theurkauf W: Biogenesis
and germline functions of piRNAs. Development. 135:3–9. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kuramochi-Miyagawa S, Watanabe T, Gotoh K,
Totoki Y, Toyoda A, Ikawa M, Asada N, Kojima K, Yamaguchi Y, Ijiri
TW, Hata K, Li E, Matsuda Y, Kimura T, Okabe M, Sakaki Y, Sasaki H
and Nakano T: DNA methylation of retrotransposon genes is regulated
by Piwi family members MILI and MIWI2 in murine fetal testes. Genes
Dev. 22:908–917. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Watanabe T, Totoki Y, Toyoda A, Kaneda M,
Kuramochi-Miyagawa S, Obata Y, Chiba H, Kohara Y, Kono T, Nakano T,
Surani MA, Sakaki Y and Sasaki H: Endogenous siRNAs from naturally
formed dsRNAs regulate transcripts in mouse oocytes. Nature.
453:539–543. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Clever JL, Miranda D Jr and Parslow TG:
RNA structure and packaging signals in the 5 leader region of the
human immunodeficiency virus type 1 genome. J Virol.
76:12381–12387. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Russell RS, Hu J, Beriault V, Mouland AJ,
Laughrea M, Kleiman L, Wainberg MA and Liang C: Sequences
downstream of the 5 splice donor site are required for both
packaging and dimerization of human immunodeficiency virus type 1
RNA. J Virol. 77:84–96. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Habu Y, Miyano-Kurosaki N, Kitano M, Endo
Y, Yukita M, Ohira S and Takaku H, Nashimoto M and Takaku H:
Inhibition of HIV-1 gene expression by retroviral vector-mediated
small-guide RNAs that direct specific RNA cleavage by tRNase ZL.
Nucleic Acids Res. 33:235–243. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Nashimoto M: Conversion of mammalian tRNA
3′ processing endoribonuclease to four-base-recognizing RNA
cutters. Nucleic Acids Res. 23:3642–3647. 1995.
|
|
40
|
Nashimoto M: Specific cleavage of target
RNAs from HIV-1 with 5′ half tRNA by mammalian tRNA 3′ processing
endoribonuclease. RNA. 2:523–524. 1996.
|
|
41
|
Takaku H, Minagawa A, Takagi M and
Nashimoto M: A candidate prostate cancer susceptibility gene
encodes tRNA 3′ processing endoribonuclease. Nucleic Acids Res.
31:2272–2278. 2003.
|
|
42
|
Takaku H, Minagawa A, Takagi M and
Nashimoto M: The N-terminal half-domain of the long form of tRNase
Z is required for the RNase 65 activity. Nucleic Acids Res.
32:4429–4438. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kato K, Habu Y and Takaku H: Micro RNA
like inhibition of HIV-1 replication. Nucleic Acids Symp Ser (Oxf).
52:509–510. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Sugiyama R, Hayafune M, Habu Y, Yamamoto N
and Takaku H: HIV-1 RT-dependent DNAzyme expression inhibits HIV-1
replication without the emergence of escape viruses. Nucleic Acids
Res. 39:589–598. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Keith G, Heitzler J, el Adlouni C, Glasser
AL, Fix C, Desgres J and Dirheimer G: The primary structure of
cytoplasmic initiator tRNA(Met) from Schizosaccharomyces pombe.
Nucleic Acids Res. 21:29491993. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Planelles V, Bachelerie F, Jowett JB,
Haislip A, Xie Y, Banooni P, Masuda T and Chen IS: Fate of the
human immunodeficiency virus type 1 provirus in infected cells: a
role for vpr. J Virol. 69:5883–5889. 1995.PubMed/NCBI
|
|
47
|
Sakai A, Hirabayashi Y, Aizawa S, Tanaka
M, Ida S and Oka S: Investigation of a new p24 antigen detection
system by the chemiluminescence-enzyme-immuno-assay. Kansenshogaku
Zasshi. 73:205–212. 1999.(In Japanese).
|
|
48
|
Zuker M: Mfold web server for nucleic acid
folding and hybridization prediction. Nucleic Acids Res.
31:3406–3415. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Enright AJ, John B, Tushl T, Sander C and
Marks DS: Micro RNA targets in Drosophila. Genom Biol.
5:R12003. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Myers JW, Jones JT, Meyer T and Ferrell JE
Jr: Recombinant Dicer efficiently converts large dsRNAs into siRNAs
suitable for gene silencing. Nat Biotechnol. 3:324–328. 2003.
View Article : Google Scholar : PubMed/NCBI
|