Introduction
Retinoblastoma (RB) is the most common intraocular
malignant tumor in infants, and RB is also common among children
and adolescents (1). Though the
RB patient survival rate is excellent with the application of
current therapies, significant ocular damage often occurs that may
have life-long impact on patient vision (2,3).
Over the past decades, treatment options for children with
intraocular RB have dramatically changed. Attempts to avoid eye
enucleation and beam radiotherapy have lead to increased use of
globe-preserving techniques, including systemic chemotherapy,
photocoagulation, brachytherapy, cryotherapy, and thermotherapy
(4). Gene therapy provides
valuable new options for the development of new RB treatments.
Human RB occurs primarily in either the familial or
sporadic form. These two forms are both rooted in bi-allelic
mutation of the RB gene (5),
wherein local importation of the exogenous RB gene leads to
irreversible growth arrest of tumor cell selectively (6). Previously, Jia et al
(7) utilized small interference
RNA (siRNA) for vascular endothelial growth factor (VEGF) to
inhibit the formation of new blood vessels in RB models.
Additionally, a phase I study has shown that delivery of the
suicide gene (thymidine kinase) into RB tumors had effective
oncolytic effects in vivo (8).
In contemporary RB research, oncolytic adenovirus
treatment, alternatively referred to as conditionally replicating
adenovirus (CRAd) treatment, has emerged as an innovative and
promising platform for the treatment of many forms of cancer due to
the ability of these vectors to replicate selectively in tumor
cells, ultimately resulting in cell death by lysis of tumor cells
(9,10). Because viral replication is
specific to tumor cells, the natural increase in local
concentration of viral particles leads to the propagation of virus
particles throughout the tumor. This may allow relatively low,
non-toxic doses to be highly effective in elimination of entire
tumors. A facilitation strategy that increases the selectivity of
the oncolytic adenovirus is the use of tumor-specific promoters to
control the expression of adenoviral genes essential for
replication, such as E1A (11,12). These promoters give priority to
viral gene expression in tumor cells, limiting or entirely
preventing damage to healthy tissues.
During tumorigenesis, the loss of the tumor
suppressing retinoblastoma protein (Rb) that binds to E2F leads to
an apparent increase in free E2F, or increasing E2f that exists
independently of the Rb-E2F complex. The resultant abundance of
free E2F, in turn, induces high-level expression of the
E2F-responsive genes associated with retinoblastoma. Previously,
E2F-1 overexpression has been observed in many other tumor cell
lines, including specific lung and liver cancer lines. This
occurrence is likely due to frequent disruptions in the
pRB/p16INK4α/cyclin D pathway. The P16 protein specifically binds
to CDK4, which inhibits the kinase activity of CDK4. Conversely, Rb
protein is phosphorylated by CDK4. In the absence of the P16 gene,
the Rb protein is instead phosphorylated, and E2F-1 is released to
prompt the transcription of its downstream target genes. Because
these genes are essential to the transition to the S-phase, this
disrupts the life cycle of normal cells (13–15). Thus, the human E2F-1 gene promoter
is responsible for controlling the expression of key viral genes
essential for replication, making it an excellent candidate for
achieving specific tumor selectivity. E1 regulation in oncolytic
adenoviruses by the E2F-1 promoter demonstrated high selectivity,
indicating the precise level of control attainable by using the
E2F-1 promoter (16). These
findings led to the design of the study to explore an oncolytic
adenovirus with E1A regulation controlled by the E2F-1 promoter.
This innovative design allowed for the investigation of the virus
effectiveness in generating RB tumor cell death both in
vitro and in vivo in mouse models in the current study
for the first time.
Both the E1A and E1B genes are essential for
adenovirus replication. E1A acts as a cue to initiate virus
replication by activating the early adenovirus promoters, and it is
also required to drive the host cell into the S-phase of the cell
cycle for viral DNA replication (17). An adverse effect of E1A is that it
stabilizes p53, which leads to apoptosis and is unfavorable for
viral replication (18). To
prevent this, E1B55K and the Ad E4 or f6 proteins form a complex
with p53, causing its degradation through ubiquitin mediated
proteolysis (19,20). E1B19K additionally prevents
E1A-induced apoptosis by interfering with the actions of the
pro-apoptotic proteins Bak and Bax (21,22). It has been reported that the
oncolytic adenovirus dl1520 with E1B19K, as well as other
E1B55K-deleted viruses, replicated efficiently in a variety of
tumor cell lines independent of their p53 status (23–25). E1B19K deletion, however, has been
indicated to generate more rapid viral release from apoptotic
cells, resulting in enhancement to viral delivery across tumor
tissues. Based on the high activity of telomerase reverse
transcriptase (hTERT) in the human tumor cells, Doloff et al
(26) constructed an oncolytic
adenovirus, Ad-hTERT-E1A, with deletions of the viral E1B and E3
regions and an hTERT promoter-driven E1A cassette. This design
possessed strong therapeutic potential as well as an improved
safety profile compared to the previous dl1520. Ji et al
(27) constructed an oncolytic
adenovirus with an E1A controlled using the hTERT promoter armed
with the suicide gene thymidine kinase. This design demonstrated a
good killing effect in RB tumor models in situ in the eyes
of nude mice. The success of these designs provided the foundation
for the current design, containing an oncolytic adenovirus
inclusive of E1A and E1B19K controlled by hTERT promoter. The
current design was evaluated for the anti-cancer effects of the
viruses and the overall role of E1B in RB treatment.
Over the course of the current study, the targeted
oncolytic adenovirus Ad-E2F1 p-E1A was constructed for RB
treatment. In this design, E1A was controlled by the E2F1 promoter
due to the notable fact that E2F-1 activity usually increases in RB
cells. Additionally, the targeted oncolytic adenovirus Ad-TERT p-E1
was constructed, in which E1 was controlled by the TERT promoter.
The replication capacity of these recombinant viruses and the
effect to induce tumor cell death was studied in cancer cell lines
HXO-RB44, Y79, Hep3B and NCIH460. Furthermore, simultaneous
evaluation of the replication-defective adenovirus Ad-endostatin,
carrying the human endostatin gene, revealed the anti-cancer
efficacy of Ad-endostatin both in isolated treatments and when
combined with Ad-E2F1 p-E1A or Ad-E1 TERT p-E1 treatments in
situ for the treatment of RB tumor model nude mice. The results
suggest that the gene-viral therapeutic system developed herein
demonstrates the synergistic effects of viral oncolytic therapy and
anti-angiogenesis therapy, generating a novel therapeutic strategy
for human RB.
Materials and methods
Cell lines and cell culture
Cancer cell lines with Rb pathway defects used in
the current study included the human lung cancer cell line NCIH460
(ATCC, Manassas, VA, USA) that is P16 negative, human hepatocyte
cancer cell line Hep3B (ATCC), two human RB cell lines HXO-RB44
(Cancer Research Institute, Xiangya Medical College, Central South
University of China) (28), and
Y79 (Institute of Biochemistry and Cell Biology, Shanghai Institute
of Biological Science, Chinese Academy of Science, Shanghai, China)
that are Rb negative. Additionally selected were the human breast
adenocarcinoma cell line MCF-7 that is Rb positive and the HLF
human lung fibroblast cell line, both purchased from the Institute
of Biochemistry and Cell Biology, Chinese Academy of Sciences. The
human embryonic kidney cell line AD-293 used for packaging
adenoviral vectors was purchased from Stratagene (La Jolla, CA,
USA). NCIH460, Hep3B, and AD-293 cells were cultured in Dulbecco’s
modified Eagle’s medium (Gibco-BRL, Carlsbad, CA, USA) with 10%
fetal bovine serum. HXO-RB44 cells, HLF, and Y79 cells were
maintained in ready mix RPMI-1640 (Gibco-BRL) medium supplemented
with 10% fetal bovine serum. MCF-7 cells were cultured in RPMI-1640
with 10% fetal bovine serum and insulin (0.01 mg/ml).
Generation of HXO-RB44-GFP-Luc cells
HXO-RB44 cells were transfected with a plasmid
carrying an enhanced green fluorescent protein (EGFP)-luciferase
fusion gene expression cassette, kindly provided by Dr C.Y. Li
(University of Colorado Health Sciences Center, USA) using
Lipofectamine 2000 (Invitrogen, Carlsbad, USA). Positive
transfectants were selected with 0.5 mg/ml Geneticin (Invitrogen).
Single clones of positive transfectants were obtained by limited
dilution. The stably transfected HXO-RB44-EGFP-Luc cells were
maintained in RPMI-1640 medium containing 10% fetal bovine serum
(FBS) and 0.1 mg/ml Geneticin. Clones expressing high levels of
EGFP as well as luciferase were selected for further
experiments.
Construction of recombinant oncolytic
adenoviruses
A 269-bp fragment of human E2F-1 promoter (GenBank
no. S74230) was amplified from human genomic DNA, and a 1011-bp
fragment of the human adenoviral E1A gene (GenBank no. AC_000008)
was amplified by polymerase chain reaction (PCR) from AD-293
cellular genomic DNA. The E1A gene and E2F-1 promoter were
subcloned into pDC311 (Microbix Biosystems, Toronto, Canada),
resulting in an adenoviral shuttle vector pDC311-E2F1 p-E1A that
was subsequently co-transfected with the adenoviral backbone
plasmid pBHGLoxΔE1E3 (Microbix Biosystems) into AD-293 cells to
obtain oncolytic adenovirus Ad-E2F1 p-E1A.
A 1812-bp fragment of the human adenoviral E1B gene
was amplified by PCR from the pAdE1-3 plasmid (kindly provided by
Dr C.Y. Li), digested by SmaI/XbaI, and cloned into
the pIRES-neo (Clontech) to obtain pIRES-neo-E1B, in which E1B55 kD
was not expressed due to the deletion mutation of G 663. A 475-bp
fragment containing the promoter of the human telomerase reverse
transcriptase gene (GenBank no. NG_009265) was excised by
EcoRI/BamHI from pTERT p-EGFP (kindly provided by Dr
C.Y. Li) and inserted into pIRES-neo-E1B, resulting in pTERT
p-IRES-E1B. The E1A gene was digested with BamHI and
inserted into pTERT p-IRES-E1B to obtain pTERT p-E1A-IRES-E1B.
Finally, an XhoI/NotI restriction fragment of pTERT
p-E1A-IRES-E1B containing the hTERT promoter, E1A gene, E1B gene,
and downstream poly A sequences was inserted into pENTR1A
(Invitrogen). The resulting plasmid pENTR1A-TERT p-E1A-IRES-E1B and
adenoviral backbone plasmid pAd/PL-DEST (Invitrogen) were LR
recombination reacted. The homologously recombinant
pAd/PL-DEST-TERTp-E1AIRES-E1B construct was then digested with
PacI to expose the left and right viral ITRs and transfected
into AD-293 cells to generate Ad-TERT p-E1. All primers used in
adenovirus construction are listed in Table I, and the recombinant adenoviruses
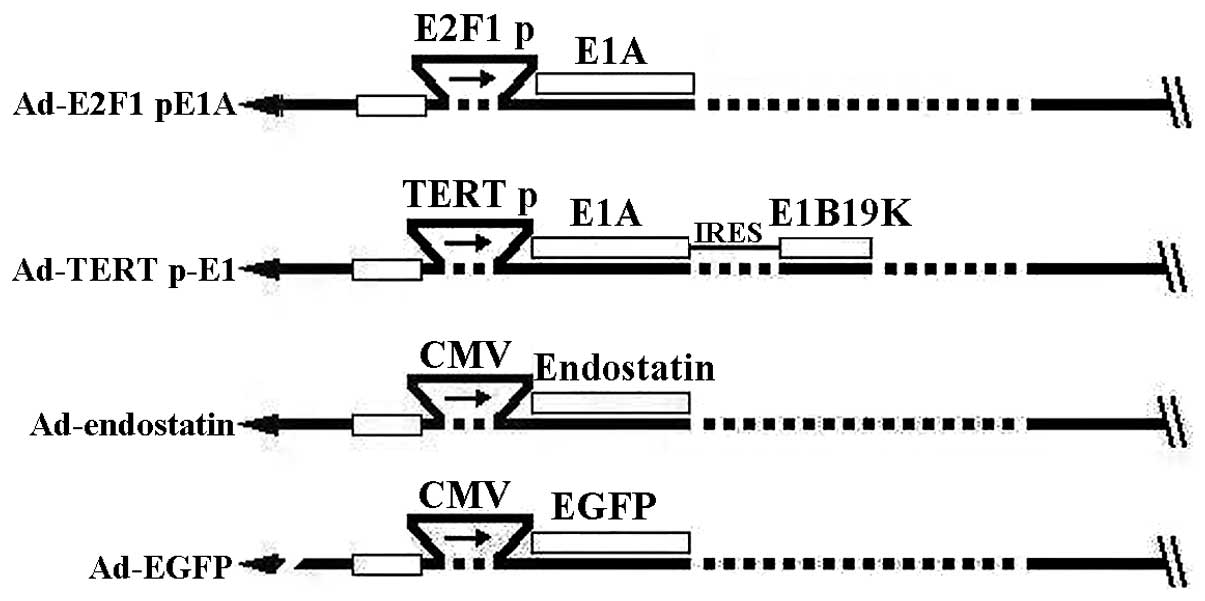
are illustrated in Fig. 1. The
wild-type adenovirus dl309 containing a partial deletion of the E3
gene not affecting replication was prepared using an identical
procedure to that described previously. This design was utilized as
an oncolytic control. Ad-endostatin was a replication-deficient
adenovirus containing endostatin (provided by Dr C.Y. Li). The
replication-deficient adenoviruses Ad-null (with the deletion of
both E1 and E3) and Ad-EGFP were preserved. Functional particle
titers for all adenoviruses were determined by plaque assays in
AD-293 cells and shown as plaque forming units/milliliter
(pfu/ml).
 | Table I.Primers used in oncolytic adenovirus
construction. |
Table I.
Primers used in oncolytic adenovirus
construction.
| Primers
sequence |
|---|
| E2F-1 promoter | F: 5′-CCG GAA TTC
CGG GGT ACC ATC CGG ACA AAG CCT GCG-3′ |
| R: 5′-CGC GGA TCC
GCG CGA GGG CTC GAT CCC GCT CCG C-3′ |
| E1A gene (pE2F1
p-EGFP-1) | F: 5′-CGG GAT CCA
TGA GAC ATA TTA TCT GCC ACG-3′ |
| R: 5′-ATA AGA ATG
CGG CCG CTT ATG GCC TGG GGC GTT TA-3′ |
| E1B gene | F: 5′-TCC CCC GGG
ATG GAG GCT TGG GAG TGT TT-3′ |
| R: 5′-GCT CTA GAT
CAA TCT GTA TCT TCA TCG CT-3′ |
| E1A gene (pTERT
p-IRES-E1B) | F: 5′-CGG GAT CCG
GGC CCA TGA GAC ATA TTA TCT GCC ACG-3′ |
| R: 5′-CGG GAT CCT
TAT GGC CTG GGG CGT TTA-3′ |
Fluorescence microscopy observation and
FACS analysis
Cells were seeded in 24-well plates one day before
adenoviral infection. The following day, cells of 60–70% confluence
were infected with Ad-EGFP either with or without oncolytic
adenovirus at the indicated multiplicity of infection (MOI). After
72 h, visualization of EGFP expression was carried out on a Zeiss
Axio Uret S100 (Carl Zeiss Microscopy, LLC, USA) equipped with a
Zeiss AxioCam color camera. In fluorescence activated cell sorting
(FACS) analysis, cell fluorescence was measured using the FACSort
with an excitation of 448 nm wavelength. Cell populations of
interest were gated and analyzed using CellQuest™ software
(Becton-Dickinson, USA).
Viral replication assays
HLF cells and log-phase tumor cells plated at 60–70%
confluence were infected with Ad-E2F1 p-E1A or Ad-TERT p-E1 at an
MOI of 10 pfu/cell. Virus inocula were removed after a 4 h
incubation period. The cells were then washed 3 times with sodium
perborate (PBS) and incubated at 37°C for 48 h. Cells were scraped
into a 1 ml medium, subjected to 3 freeze-thaw cycles, and
centrifuged. The supernatant was subsequently collected. Serial
dilutions of the supernatant were assayed for live virus particles
by standard plaque forming assays using AD-293 cells. The wild-type
adenovirus dl309 was used as a control.
Western blot analysis
Tumor cells at 60–70% confluence were infected with
different viruses at various MOIs. After 48 h, cell extracts were
prepared by lysis buffer containing 2% sodium dodecyl sulfate (SDS)
and 0.125 M Tris-HCl (pH 6.8). The total protein of the cell
extracts was measured using the Bio-Rad Protein Assay kit (Bio-Rad,
Hercules, USA). Proteins (40 μg) were separated on 10%
SDS-polyacrylamide gel and blotted onto a polyvinylidene difluoride
membrane (Roche, Mannheim, Germany). The membrane was incubated
with antibodies to E1A, and GAPDH (Santa Cruz Biotechnology Inc.,
USA) for 1 h at room temperature. After washing, the membrane was
probed with the appropriate secondary peroxidase-conjugated
antibodies and subsequently visualized using a chemiluminescence
method (ECL, Roche). Images of the bands were captured using an
image acquisition software system (ChemiDoc™ XRS+;
Bio-Rad).
In vitro cell viability assay
HXO-RB44-GFP-Luc cells and Y79 cells were plated at
50–60% confluence in 96-well dishes and 24 h later infected with
different adenoviruses at various MOIs. After 6 days, the cell
viability was measured using the cell counting kit-8 (CCK-8)
(Dojindo, Tokyo, Japan). Background refers to the absorbance of
medium alone. The percentage of cell survival was calculated using
the formula: % cell survival = (A490 nm of infected
cells − A490 nm of background)/(A490 nm
uninfected cells − A490 nm of background) × 100%.
In vivo antitumor effect
HXO-RB44-GFP-Luc cells were infected with various
adenoviruses as follows: Ad-null 50 MOI, Ad-E2F1 p-E1A 12.5 MOI,
Ad-TERT p-E1 12.5 MOI, Ad-endostatin 50 MOI, Ad-E2F1 p-E1A 12.5
MOI+Ad-endostatin 50 MOI, and Ad-TERT p-E1 12.5 MOI+Ad-endostatin
50 MOI. Virus inocula were removed after a 16 h incubation period.
The cells were then prepared at 1×105 cells/μl in
PBS. RB tumors were established by injecting HXO-RB44-GFP-Luc cells
into the vitreum of BALB/c (nu/nu) mice (Shanghai Laboratory Animal
Center, Shanghai, China). A total of 10 mice were allocated to each
group. Mice were anesthetized with 50 mg/kg pentobarbital
intraperitoneally and a topical application of 0.4% oxybuprocaine.
The pupil was dilated using an eye drop solution containing
0.5/0.5% tropicamide/phenylephrine hydrochloride (Santen
Pharmaceutical). A plastic ring filled with 2.5% cellulose was
placed on the cornea to assist in visualizing the fundus. The
injection was performed under a binocular surgical microscope.
A total of 2×105 cells prepared in 2
μl PBS were injected slowly into the midvitreous of the left
eye with a 32G needle attached to a 10 μl microsyringe. The
bioluminescent image of the HXO-RB44-GFP-Luc tumor in the vitreous
was acquired by in vivo bio-layer interferometry (BLI)
technology using a NightOwl LB 981 Molecular Imaging System
(Berthold Technologies, Bad Wildbad, Germany) on Day 15 after
injection. Each subject was injected i.p. with 100 mg/kg
D-luciferin (Molecular Imaging Products, Ann Arbor, MI, USA) in 100
μl PBS, anesthetized with pentobarbital, and images were
acquired after 5 min. The exposure time of the bioluminescent
images was set to 10 min. Images were processed and pseudocolored
using WinLight software (Berthold Technologies). Alternatively,
tumor growth was determined by fluorescence signal on Day 20 after
injection by direct stereomicroscopy (Stemi SV11; ZEISS, Jena,
German).
The onset of EGFP gene expression and its
distribution in the vitreous region was investigated. Interesting
regional optical density (IOD) = eye area (μm2) ×
mean fluorescence intensity (grey)/2(16) bit. All animal
subjects were sacrificed 42 days post-injection, and eyes were
enucleated for pathological examination. All mice were maintained
and handled in accordance with the guidelines approved by national
and local institutions.
Histopathology
Mouse eyes were fixed in 10% formalin and embedded
in paraffin. The sections were routinely stained with hematoxylin
and eosin (H&E) and blindly evaluated by two individual
pathologists.
Statistical analysis
All in vitro experiments were completed three
times under separate conditions, and the in vitro and in
vivo experimental data are presented as the mean plus or minus
standard deviation (mean ± SD). Comparisons were made using ANOVA
with appropriate post-hoc tests (Fisher’s PLSD). Survival was
analyzed by the Kaplan-Meier method, and results were compared for
statistical significance using the generalized Wilcoxon test. All
statistical analyses were performed using SPSS10.0 software.
P-values <0.05 were considered statistically significant.
Results
Construction of recombinant oncolytic
viruses
Two types of conditionally replicating adenoviruses
(CRAds), Ad-E2F1 p-E1A and Ad-TERT p-E1, were developed. In these
designs, the E1A of Ad-E2F1 p-E1A was under the control of E2F1
promoter and the E1A and E1B19K of Ad-TERT p-E1 were under the
control of human telomerase reverse transcriptase (hTERT) promoter
(Fig. 1). Absence of the
wild-type virus was confirmed by PCR for the E3 gene (data not
shown).
Selective replication of the two
recombinant oncolytic adenoviruses
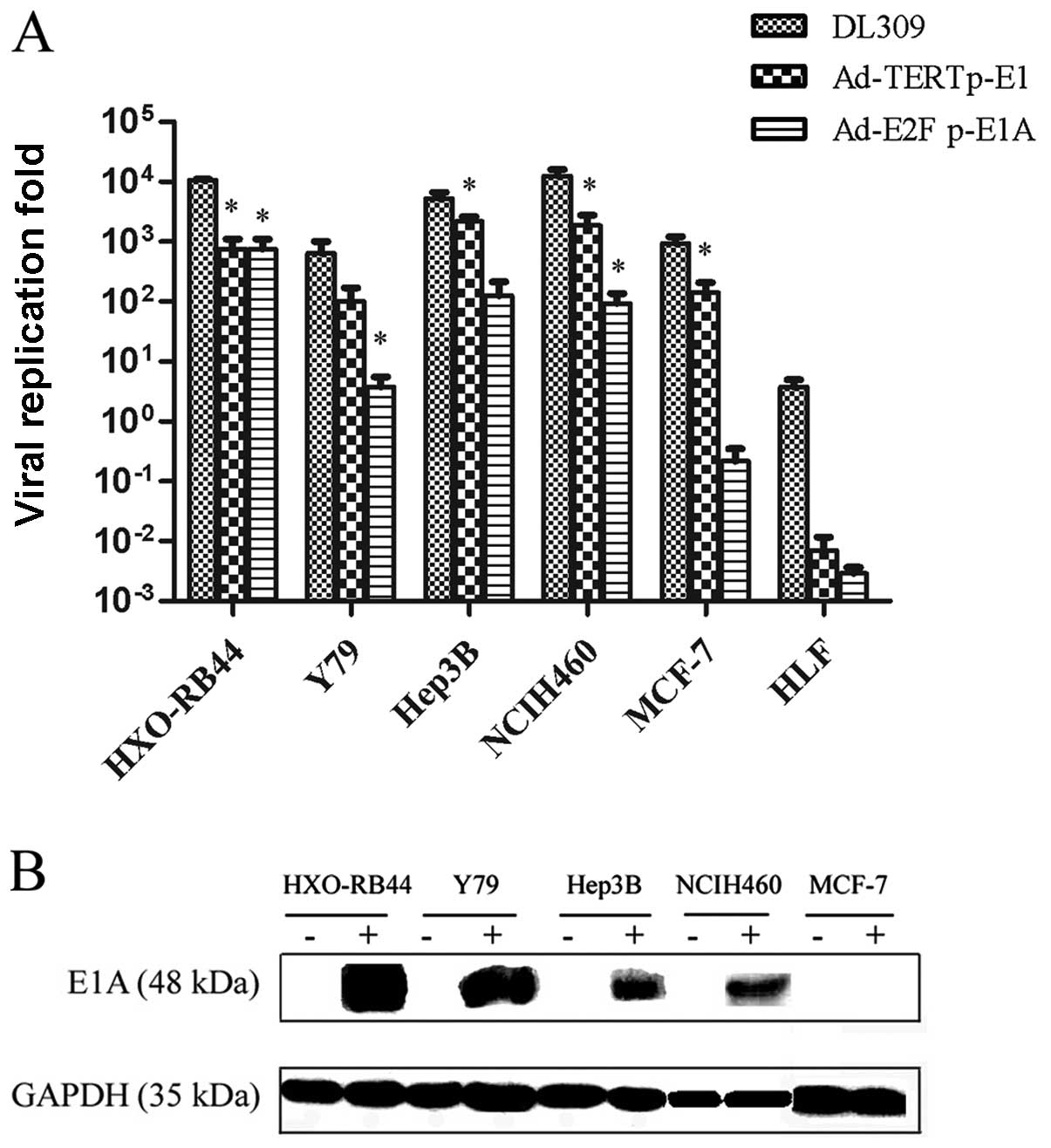
In order to demonstrate the selective replication of
the Ad-E2F1 p-E1A vector, cancer cell lines HXO-RB44, Y79, Hep3B,
NCIH460, MCF-7, and HLF cells were infected using Ad-E2F1 p-E1A.
The resultant production of viral particles was quantified with the
plaque forming assay method (Fig.
2A). The result showed that Ad-E2F1 p-E1A replicated well in
HXO-RB44, Y79, Hep3B and NCIH460 tumor cells in which the Rb
pathway was dysregulated, but not in MCF-7 and HLF cells that are
Rb positive. Ad-TERT p-E1 replicated remarkably well in tumor cells
HXO-RB44, Y79, Hep3B, NCIH460 and MCF-7, but not in HLF cells.
Moreover, Ad-TERT p-E1 replicated more efficiently in Hep3B,
NCIH460, and MCF-7 cells than Ad-E2F1 p-E1A (P<0.05) (Fig. 2A). Selective replication of the
Ad-E2F1 p-E1A was further confirmed by western blot analysis, as
the E1A protein was only expressed in HXO-RB44, Y79, Hep3B and
NCIH460 tumor cells (Fig.
2B).
CRAds for activating expression of the
GFP transgene in cancer cells
In order to assess the feasibility and effectiveness
of the strategy involving non-replicating adenovirus replication
driven by a replicative adenovirus, the fluorescence intensity was
observed when an oncolytic adenovirus was administered along with
Ad-EGFP. Both Ad-E2F1 p-E1A and Ad-TERT p-E1 showed significantly
improved EGFP-positive rates and mean fluorescence intensity in
HXO-RB44 cells and Y79 cells. Furthermore, the average fluorescence
intensity of EGFP in Hep3B cells increased, but Ad-E2F1 p-E1A
reduced EGFP expression in the tumor cell line MCF-7 with normal Rb
status (P<0.001) (Fig. 3A).
The percentage of EGFP-positive HXO-RB44 cells increased from
25.7±2.30% (25 MOI of Ad-EGFP) to 44.40±6.20% (25 MOI of Ad-E2F1
p-E1A and 25 MOI of Ad-EGFP) (P<0.05), a value close to the
level of 500 MOI Ad-EGFP (45.0±4.90%) (Fig. 3B). The EGFP-positive rate was
raised to 84.75±0.65% and 91.10±0.10%, respectively, when Ad-EGFP
was co-administered with 100 MOI and 250 MOI of Ad-E2F1 p-E1A. This
was significantly higher than that of the same amount of Ad-TERT
p-E1 (P<0.001). Moreover, Ad-E2F1 p-E1A (100, 250 and 500 MOI)
elevated the mean fluorescence intensity of EGFP to a significantly
higher level than the same MOI of Ad-TERT p-E1 (P<0.01).
Similarly, a combination of Ad-E2F1 p-E1A with Ad-EGFP resulted in
a higher EGFP-positive rate than that of Ad-TERT p-E1 plus Ad-EGFP
in Y79 cells (P<0.001) (Fig.
3C). These results suggested that the oncolytic adenovirus was
efficient in improving the expression of the exogenous gene carried
by non-replicating adenovirus.
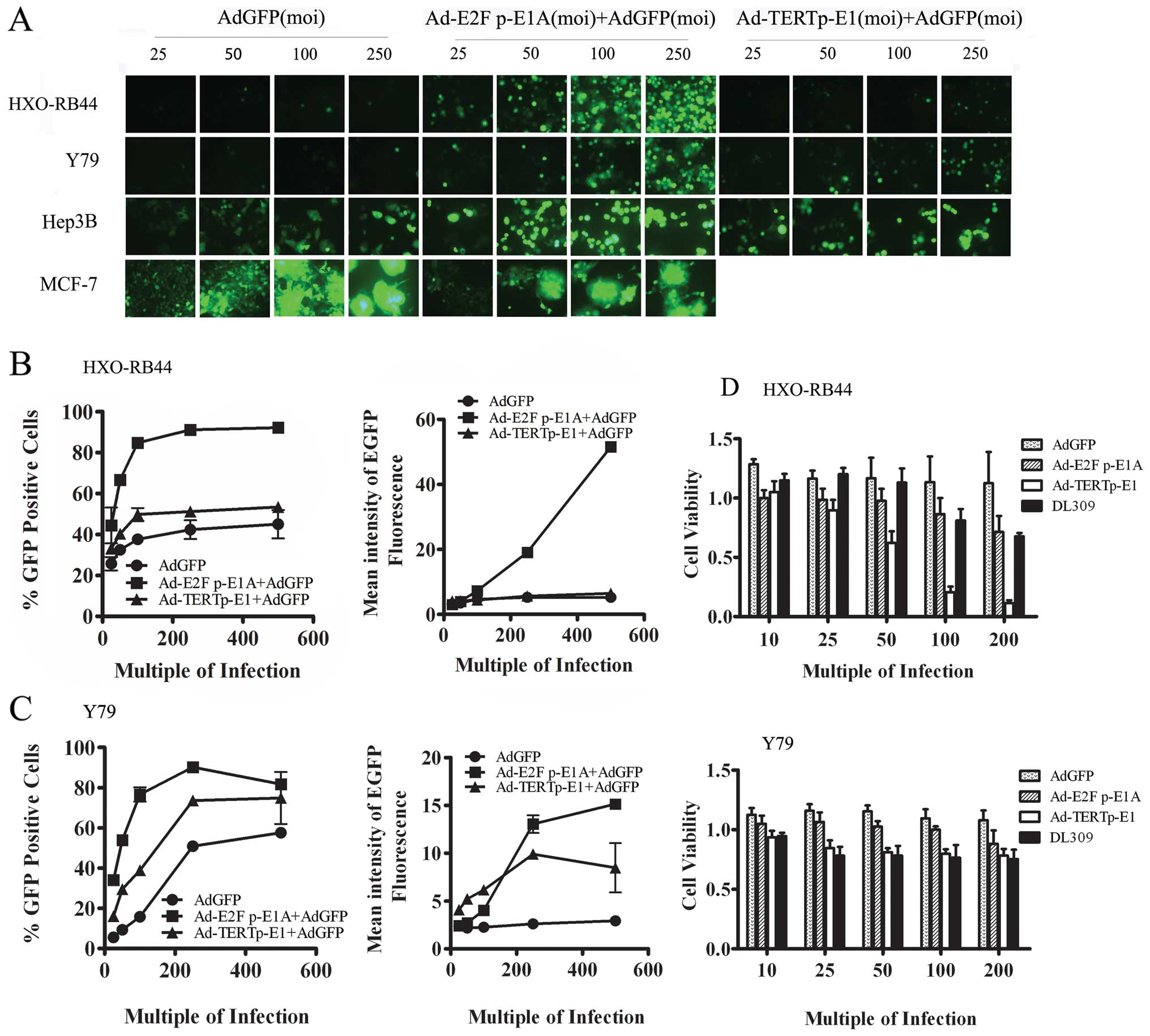 | Figure 3.The ability of oncolytic adenovirus
to activate expression of the GFP transgene in cancer cells. (A)
HXO-RB-44,Y79, Hep3B and MCF-7 cells were infected with Ad-GFP at a
MOI of 25, 50, 100, 250 pfu/cell, combined with a same amount of
Ad-E2F p-E1A or Ad-TERT p-E1 or not. At 3 days post-infection,
cells were observed and photographed by fluorescent microscope
(Zeiss, Axio 100, original magnification, ×400). (B) FACS analysis
of average percentage and mean fluorescent intensity in HXO-RB44
cells at 3 days post-infection by viral vectors. (C) FACS analysis
of average percentage and mean fluorescent intensity in Y79 cells
at 3 days post-infection by viral vectors. The average percentage
and mean fluorescent intensity were calculated from 3 individual
wells for each viral vector infection and at least 3 repeated
infection. (D) HXO-RB44 cells and Y79 cells were infected with
Ad-GFP or Ad-E2F p-E1A or Ad-TERTp-E1 or the wild-type control
virus dl309 at an MOI of 10, 25, 50, 100, 200 pfu/cell
respectively. Cell viability was measured using CCK-8 assay 7 days
post-infection. All experiments were performed in triplicates and
repeated 3 times. Data are shown as the mean ± SD.
*P<0.05, **P<0.01 and
***P<0.001. |
Cell-killing effect in vitro of Ad-E2F1
p-E1A and Ad-TERT p-E1 on RB cells
The results of CKK-8 assay showed that when HXO-RB44
cells were infected with Ad-TERT p-E1 at 50, 100 and 200 MOI, the
cell survival rate was 62.08±9.82% (P<0.05), 20.59±4.75%
(P<0.001) and 11.32±2.54% (P<0.001), respectively. The
oncolytic effect of Ad-TERT p-E1 at 100 and 200 MOI were
significantly better than those observed in dl309 and Ad-E2F1 p-E1
(P<0.001, respectively). In addition, the oncolytic effect of
dl309, Ad-E2F1 p-E1A and Ad-TERT p-E1 on Y79 cells were not
obvious. Ad-TERT p-E1 and Ad-E2F1 p-E1A combined did exhibit a
significant treatment difference (P<0.05) (Fig. 3D).
Antitumor efficacy of adenovirus for in
situ RB in nude mice
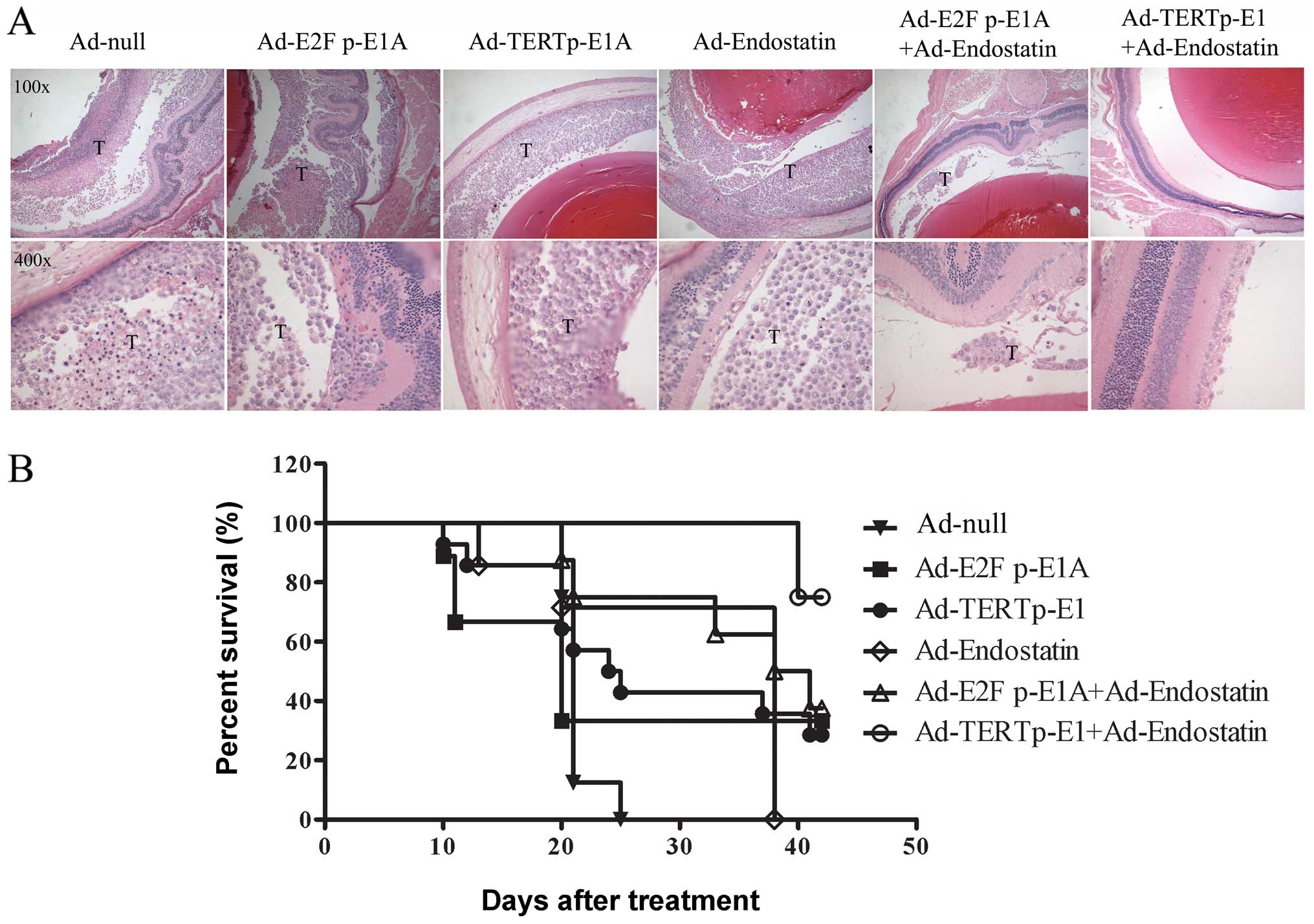
The vitreous bodies of nude mice were inoculated
with 2×105 HXO-RB44-GFP-Luc cells that were infected
with the above adenoviruses, respectively. The anti-RB effect of
oncolytic adenovirus on in vivo cells were measured. On Day
15, in vivo imaging of the fundus was performed by
intraperitoneal injection of a luciferase substrate. Compared with
Ad-E2F1 p-E1A, Ad-TERT p-E1 was shown to inhibit tumor growth more
effectively, and Ad-TERT p-E1+Ad-endostatin almost eliminated the
tumor completely (Fig. 4C).
Fluorescence signals in the nude mouse retina were investigated by
fluorescence stereoscope on Day 20 (Fig. 4A). Compared with Ad-null, Ad-E2F1
p-E1A showed no significant difference in RB tumor cell death
(P>0.05), while stronger effects were observed in the Ad-TERT
p-E1, Ad-endostatin, Ad-E2F1 p-E1A+Ad-endostatin, and Ad-TERT
p-E1+Adendostatin groups (all P<0.05) (Fig. 4B). Co-administration of
Ad-endostatin and Ad-TERT p-E1 generated a stronger oncolytic
effect than Ad-endostatin (P=0.037), comparable to that of Ad-TERT
p-E1 (P=0.097) (data not shown). Notably, only one of the 10 mice
in the Ad-endostatin+Ad-TERT p-E1 group subsequently developed RB.
These results suggest that the oncolytic adenovirus and
Ad-endostatin combination synergistically cause cell death in
cancerous RB cells in nude mice.
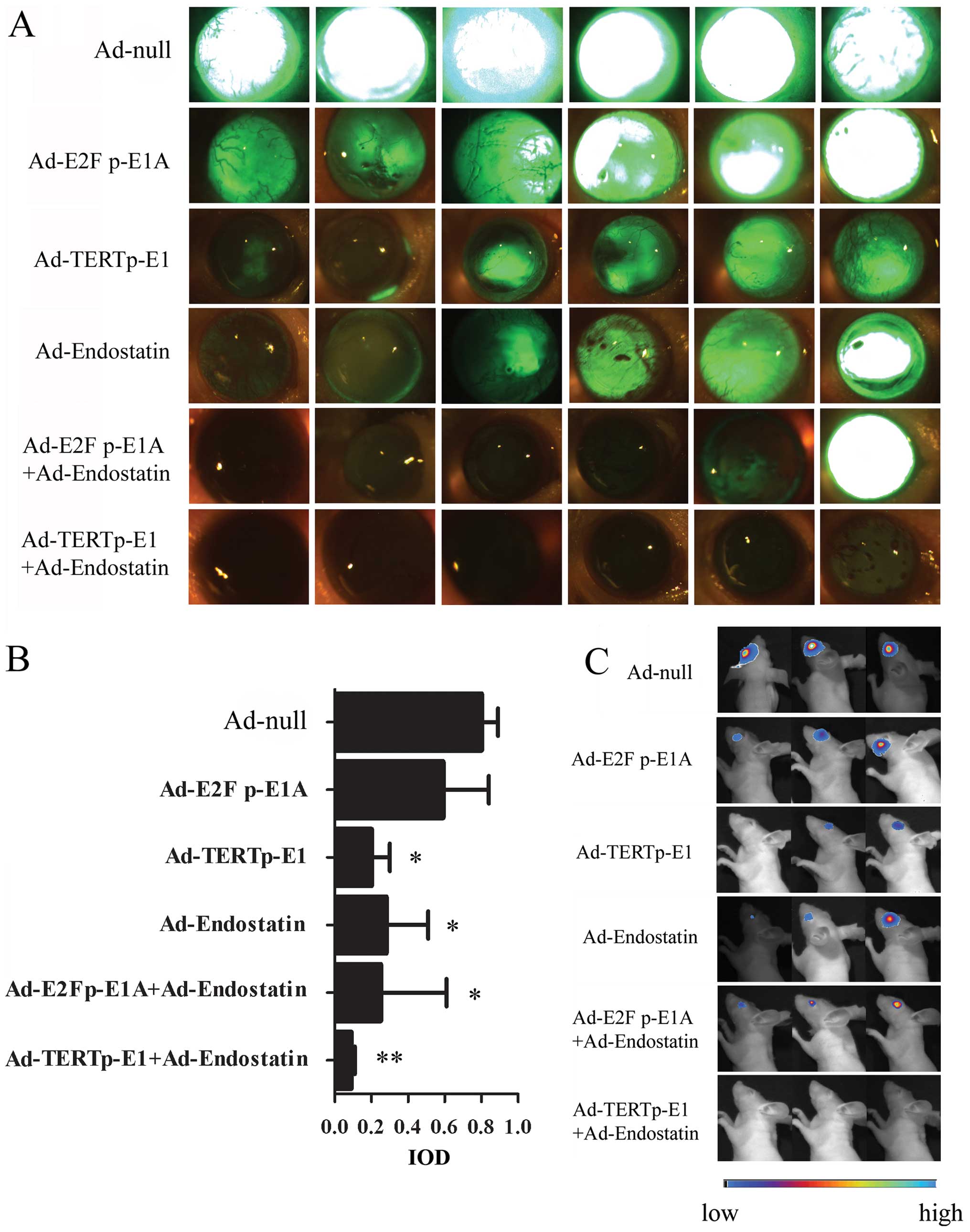 | Figure 4.Antitumor efficacy in
HXO-RB44-GFP-Luc in a mouse tumor model. HXO-RB44-GFP-Luc cells
(1×107) were infected with Ad-null (50 MOI), Ad-E2F
p-E1A (12.5 MOI), Ad-TERTp-E1 (12.5 MOI), Ad-endostatin (50 MOI),
Ad-E2Fp-E1A (12.5 MOI) + Ad-endostatin (50 MOI), Ad-TERTp-E1 (12.5
MOI) + Ad-endostatin (50 MOI) respectively. After 16 h infection,
2×105 HXO-RB44-GFP-Luc cells in 2 μl PBS were
injected into the vitreous body of BALB/c (nu/nu) mice, with 10
mice in each of the groups. (A) Fluorescence images of
HXO-RB44-GFP-Luc tumors in vivo 20 days later. (Zeiss,
StemiSV11, original magnification, ×32) (B) Comparison of
fluorescence signaling between groups. The tumor growth is
determined with IOD (interesting regional optical density) using
Axiovision 3.1 software quantitative methods. The data are shown as
the mean ± SD. *P<0.05, **P<0.01. (C)
Representative BLI images of RB tumors in each group at day 15.
(NightOwl LB 981 Molecular Imaging System; Berthold
Technologies). |
Effect of oncolytic adenovirus on the
survival time of nude mice with in situ RB
H&E staining revealed that tumor cell number in
the vitreous body was consistent with the results of green
fluorescence previously identified by IOD signal quantitative
analysis (Fig. 5A). The average
survival times of the Ad-null group, Ad-E2F1 p-E1A group, Ad-TERT
p-E1 group, Ad-endostatin group, Ad-E2F1 p-E1A+Ad-endostatin group,
and Ad-TERT p-E1+Ad-endostatin group were 21±0.19, 20±4.24,
24±3.74, 38±0, 38±5.66 and 42±0 days, respectively. The survival
rate in Ad-TERT p-E1+Ad-endostatin group was higher than that of
Ad-TERT p-E1 group (P=0.022) and Ad-endostatin group (P=0.006),
indicating that the Ad-TERT p-E1 and Ad-endostatin worked
synergistically. These results revealed that oncolytic viruses with
Ad-endostatin did significantly extend the survival time of mice
with RB tumors, though oncolytic virus treatment alone failed to
improve survival (Fig. 5B).
Discussion
A feasible solution to overcome the limitations in
capacity of recombinant adenovirus treatments, which have
previously limited the effectiveness of these treatments, is
described. This solution utilizes oncolytic adenovirus-driven
expression of therapeutic genes armed with non-replicating
adenoviruses, a novel combination approach. In this method, E1A and
E1B provided by oncolytic adenoviruses in tumor cells enable the
replication of a non-replicating adenovirus, greatly increasing the
quantity of copies of the therapeutic gene without adverse affects
to living tissues. The advantage of this strategy is that one kind
of oncolytic adenovirus can be combined with any other
non-replicating viruses carrying therapeutic genes, or even
multiple non-replicating viruses, despite the capacity limit of
adenovirus packaging. This strategy is especially useful for
individual gene therapy. Successful combination therapy using an
oncolytic virus along with an anti-angiogenesis gene carried by the
Ad-endostatin for the treatment of RB was reported for the first
time in the current study. The treatment achieved potent
synergistic antitumor effects and extended the survival time of
nude mice exhibiting RB tumors, demonstrating promising future
prospects for RB treatment in human patients.
The oncolytic virus Ad-E2F1 p-E1A selectively
replicated in HXO-RB44 cells with Rb gene deletion as well as in
Hep3B and NCIH460 cells with inactivation of the P16 pathway.
Similar results were not observed, however, in MCF-7 or HLF cells
that possess a much lower E2F1 activity. Similarly, Ad-TERT p-E1
replicated efficiently in each of the tumor cell lines described
above, with the exception of normal human cell HLF. Despite the
observed activity from each virus treatment, notable differences
were observed between Ad-E2F1 p-E1A and Ad-TERT p-E1 in replication
and oncolytic efficiency. The number of Ad-TERT p-E1 progeny virus
in tumor cells of Hep3B, NCIH460, and MCF-7 were 17-, 20-, and
642-fold-higher than those observed in Ad-E2F1 p-E1A, respectively
(all P<0.05). E1A protein extended the half-life of p53 protein,
in turn promoting premature apoptosis of the tumor cell and
limiting the viral replication ability of Ad-E2F1 p-E1A (18). The E1B19K expressed by Ad-TERT
p-E1, a functional Bcl-2 homologue, directly binds Bax,
Bak-inhibiting oligomerization, and mitochondrial pore-formation
resulting in apoptosis blockage (29,30). The biological function of E1B19K
is to inhibit receptor-induced signaling at the time of cell death
by preventing the Bax-Bak association, thus intrinsically
inhibiting induced apoptosis through the p53-dependent and
p53-independent mechanisms. The effect can be observed, for
example, in the cellular response to viral E1A proteins (31–34). The anti-apoptotic E1B19K protein
promotes viral replication, allowing it to propagate throughout the
tumor. Matsushita et al (35) also found that adeno-associated
virus production would be reduced by at least 100-fold when
adenovirus-bearing mutated E1B19K was used as a helper virus.
Polster et al (36) found
that the number of progeny virus produced by the E1B19K mutated
oncolytic virus was 10-fold lower than that produced by intact
E1B19K (36). Our results have an
important common similarity with the previous results.
The in vitro ability of Ad-TERT p-E1 to
induce targeted RB cell death was significantly higher than that of
Ad-E2F1 p-E1A (P<0.01). There are notable differences in the
structure of Ad-TERT p-E1 and Ad-E2F1 p-E1A that may play a role in
this activity difference. In each, different promoters were used to
regulate E1 or E1A and the E1 region of the wild-type adenovirus
consists of E1A, E1B19K and E1B55K, while the Ad-TERT p-E1 includes
only E1A and E1B19K and Ad-E2F1 p-E1A includes only E1A. The
ability of the Ad-hTERTE1A-CMV-HSVtk oncolytic virus to induce RB
cell death was previously reported by the authors in the absence of
the prodrug ganciclovir (GCV) on HXO-RB44 cells in vitro.
Additionally, an in vivo RB model showed comparable results
to that of Ad-E2F1 p-E1A (27),
though results were significantly weaker than those of Ad-TERT
p-E1. Tsukuda et al (37)
and Jakubczak et al (16)
also observed potent oncolytic effects of an oncolytic adenovirus
containing the whole intact adenoviral E1 region controlled by the
E2F1 promoter in tumor cell lines A549, HeLa, and SKOV-3 with
elevated E2F1 activity. Based on these observations, the
composition of the adenovirus E1 region is likely to play a key
role in the adenovirus oncolytic effect, while tumor-specific
promoters of adenoviral genes may merely correlate with the
selectivity and safety of the virus. Ultimately, it is the E1B19K
protein, not the promoters, that results in the difference in
oncolytic efficiency observed in Ad-TERT p-E1 and Ad-E2F1
p-E1A.
When combined with replication-defective virus
Ad-EGFP, both oncolytic virus Ad-TERT p-E1 and Ad-E2F1 p-E1A
produced elevation in average fluorescence intensity of EGFP and in
the EGFP-positive rate. This effect was most significant in
HXO-RB44 and Y79 cell lines, though the fluorescence intensity was
also increased in Hep3B cells. Moreover, Ad-E2F1 p-E1A was superior
to Ad-TERT p-E1 in promoting expression of the exogenous gene,
consistent with previous reports (38). In HXO-RB44 cells, Ad-TERT p-E1 was
likely to have induced greater RB tumor cell death when
co-administered with Ad-EGFP. These results explain the observation
that Ad-TERT p-E1 produced inferior results to those observed in
Ad-E2F1 p-E1A in promoting the expression of EGFP.
Compared with Ad-E2F1 p-E1A, Ad-TERT p-E1
demonstrated better tumor-targeting and ability to induce Rb tumor
cell death. The combination of Ad-TERT p-E1 and Ad-endostatin led
to an even more potent anti-cancer effect on RB mouse models in
situ, resulting in a longer survival time than either the
oncolytic virus or Ad-endostatin administered in isolation. The
strategy of replicative adenovirus driven replication of
non-replicating adenoviruses has been achieved in the combination
of the oncolytic virus and anti-angiogenesis gene. This
demonstrates great advantages as well as promising prospects for
future treatment of RB tumors in humans. In order to achieve the
goal of more effective clinical RB treatments, the appropriate
tumor-specific or tissue-specific promoter to control the
replication of oncolytic adenovirus must be identified.
Additionally, assessment and improvement in the safety of gene
therapy must be made. The choice to retain E1B19K in the oncolytic
adenovirus proved to be essential for ideal RB oncolysis, and it
provides a basis for future development of combination gene
treatments using adenoviruses for cancer treatment.
Acknowledgements
This study was supported by the
National Science Foundation of China (nos. 30500553, 30672440). We
would like to thank Professor Qian Huang for her contribution to
this study and technical assistance. Additionally, we would like to
gratefully acknowledge Yufei Wang and the entire staff at the
Central Experimental Laboratory of Shanghai Jiaotong University
Affiliated First People’s Hospital.
References
|
1.
|
Lohmann DR and Gallie BL: Retinoblastoma:
revisiting the model prototype of inherited cancer. Am J Med Genet
C Semin Med Genet. 129C:23–28. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Gatta G, Capocaccia R, Stiller C, Kaatsch
P, Berrino F and Terenziani M; EUROCARE Working Group: Childhood
cancer survival trends in Europe: a EUROCARE Working Group study. J
Clin Oncol. 23:3742–3751. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Abramson DH, Beaverson K, Sangani P, et
al: Screening for retinoblastoma: presenting signs as
prognosticators of patient and ocular survival. Pediatrics.
112:1248–1255. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Balmer A, Zografos L and Munier F:
Diagnosis and current management of retinoblastoma. Oncogene.
25:5341–5349. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Sun A, Bagella L, Tutton S, Romano G and
Giordano A: From G0 to S phase: a view of the roles played by the
retinoblastoma (Rb) family members in the Rb-E2F pathway. J Cell
Biochem. 102:1400–1404. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Sabado Alvarez C: Molecular biology of
retinoblastoma. Clin Transl Oncol. 10:389–394. 2008.
|
|
7.
|
Jia RB, Zhang P, Zhou YX, et al:
VEGF-targeted RNA interference suppresses angiogenesis and tumor
growth of retinoblastoma. Ophthalmic Res. 39:108–115. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Chevez-Barrios P, Chintagumpala M, Mieler
W, et al: Response of retinoblastoma with vitreous tumor seeding to
adenovirus-mediated delivery of thymidine kinase followed by
ganciclovir. J Clin Oncol. 23:7927–7935. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Kirn D, Martuza RL and Zwiebel J:
Replication-selective virotherapy for cancer: Biological
principles, risk management and future directions. Nat Med.
7:781–787. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Kruyt FA and Curiel DT: Toward a new
generation of conditionally replicating adenoviruses: pairing tumor
selectivity with maximal oncolysis. Hum Gene Ther. 13:485–495.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Ramachandra M, Rahman A, Zou A, et al:
Re-engineering adenovirus regulatory pathways to enhance oncolytic
specificity and efficacy. Nat Biotechnol. 19:1035–1041. 2001.
View Article : Google Scholar
|
|
12.
|
Fuerer C and Iggo R: Adenoviruses with Tcf
binding sites in multiple early promoters show enhanced selectivity
for tumour cells with constitutive activation of the wnt signalling
pathway. Gene Ther. 9:270–281. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Sherr CJ: Cancer cell cycles. Science.
274:1672–1677. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Strauss M, Lukas J and Bartek J:
Unrestricted cell cycling and cancer. Nat Med. 1:1245–1246. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Lu K, Shih C and Teicher BA: Expression of
pRB, cyclin/cyclin-dependent kinases and E2F1/DP-1 in human tumor
lines in cell culture and in xenograft tissues and response to cell
cycle agents. Cancer Chemother Pharmacol. 46:293–304. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Jakubczak JL, Ryan P, Gorziglia M, et al:
An oncolytic adenovirus selective for retinoblastoma tumor
suppressor protein pathway-defective tumors: dependence on E1A, the
E2F-1 promoter, and viral replication for selectivity and efficacy.
Cancer Res. 63:1490–1499. 2003.
|
|
17.
|
Ries SJ and Brandts CH: Oncolytic viruses
for the treatment of cancer: current strategies and clinical
trials. Drug Discov Today. 9:759–768. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Lowe SW, Ruley HE, Jacks T and Housman DE:
p53-dependent apoptosis modulates the cytotoxicity of anticancer
agents. Cell. 74:957–967. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Querido E, Marcellus RC, Lai A, et al:
Regulation of p53 levels by the E1B 55-kilodalton protein and
E4orf6 in adenovirus-infected cells. J Virol. 71:3788–3798.
1997.PubMed/NCBI
|
|
20.
|
Steegenga WT, Riteco N, Jochemsen AG,
Fallaux FJ and Bos JL: The large E1B protein together with the
E4orf6 protein target p53 for active degradation in adenovirus
infected cells. Oncogene. 16:349–357. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Cuconati A, Degenhardt K, Sundararajan R,
Anschel A and White E: Bak and Bax function to limit adenovirus
replication through apoptosis induction. J Virol. 76:4547–4558.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Farrow SN, White JH, Martinou I, et al:
Cloning of a bcl-2 homologue by interaction with adenovirus E1B
19K. Nature. 374:731–733. 1995. View
Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Hobom U and Dobbelstein M:
E1B-55-kilodalton protein is not required to block p53-induced
transcription during adenovirus infection. J Virol. 78:7685–7697.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Rao XM, Tseng MT, Zheng X, et al:
E1A-induced apoptosis does not prevent replication of adenoviruses
with deletion of E1b in majority of infected cancer cells. Cancer
Gene Ther. 11:585–593. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Rao XM, Zheng X, Waigel S, Zacharias W,
McMasters KM and Zhou HS: Gene expression profiles of normal human
lung cells affected by adenoviral E1B. Virology. 350:418–428. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Doloff JC, Waxman DJ and Jounaidi Y: Human
telomerase reverse transcriptase promoter-driven oncolytic
adenovirus with E1B-19 kDa and E1B-55 kDa gene deletions. Hum Gene
Ther. 19:1383–1400. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Ji X, Zhang J, Cheng L, et al: Oncolytic
adenovirus delivering herpes simplex virus thymidine kinase suicide
gene reduces the growth of human retinoblastoma in an in vivo mouse
model. Exp Eye Res. 89:193–199. 2009. View Article : Google Scholar
|
|
28.
|
Xu H, Wang C, Zhu H, Liu S, Xu X and Jiang
Y: Characteristics of an established retinoblastoma cell line
HXO-Rb44. Yan Ke Xue Bao. 11:16–21. 1995.
|
|
29.
|
White E: Regulation of the cell cycle and
apoptosis by the oncogenes of adenovirus. Oncogene. 20:7836–7846.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Subramanian T, Vijayalingam S and
Chinnadurai G: Genetic identification of adenovirus type 5 genes
that influence viral spread. J Virol. 80:2000–2012. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
White E: Mechanisms of apoptosis
regulation by viral oncogenes in infection and tumorigenesis. Cell
Death Differ. 13:1371–1377. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Cross JR, Postigo A, Blight K and Downward
J: Viral pro-survival proteins block separate stages in Bax
activation but changes in mitochondrial ultrastructure still occur.
Cell Death Differ. 15:997–1008. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Yoon AR, Kim JH, Lee YS, et al: Markedly
enhanced cytolysis by E1B-19kD-deleted oncolytic adenovirus in
combination with cisplatin. Hum Gene Ther. 17:379–390. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Lomonosova E, Subramanian T and
Chinnadurai G: Mitochondrial localization of p53 during adenovirus
infection and regulation of its activity by E1B-19K. Oncogene.
24:6796–6808. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Matsushita T, Okada T, Inaba T, Mizukami
H, Ozawa K and Colosi P: The adenovirus E1A and E1B19K genes
provide a helper function for transfection-based adeno-associated
virus vector production. J Gen Virol. 85:2209–2214. 2004.
View Article : Google Scholar
|
|
36.
|
Polster BM, Pevsner J and Hardwick JM:
Viral Bcl-2 homologs and their role in virus replication and
associated diseases. Biochim Biophys Acta. 1644:211–227. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Tsukuda K, Wiewrodt R, Molnar-Kimber K,
Jovanovic VP and Amin KM: An E2F-responsive replication-selective
adenovirus targeted to the defective cell cycle in cancer cells:
potent anti-tumoral efficacy but no toxicity to normal cell. Cancer
Res. 62:3438–3447. 2002.PubMed/NCBI
|
|
38.
|
Rohmer S, Quirin C, Hesse A, et al:
Transgene expression by oncolytic adenoviruses is modulated by
E1B19K deletion in a cell type-dependent manner. Virology.
395:243–254. 2009. View Article : Google Scholar : PubMed/NCBI
|