Introduction
Guggulsterone [4,17(20)-pregnadiene-3,16-dione], is
a plant sterol derived from the gum resin (guggulu) of the
tree Commiphora mukul. This gum resin has been used for
centuries in Ayurvedic medicine to treat obesity, arthritis and
hyperlipidemia (1,2). The anti-arthritic, anti-inflammatory
and anti-lipid activities of guggulsterone have also been
demonstrated (3). Recently,
guggulsterone has been shown to exhibit antitumor activity in a
variety of human tumor cell types (4). The active compound in the resin is
cis and trans isomers of guggulsterone (3,5).
Of note, cis-guggulesterone is more potent than the
trans-isomer in inducing apoptosis in mature adipocytes
(3). Furthermore, guggulesterone
isomers have been shown to exhibit isomer-specific antitumor
activity (6–8). However, the molecular mechanism of
guggulesterone-mediated anticancer activity remains elusive.
Invasion processes require the degradation of the
extra-cellular matrix (ECM), which provides biochemical and
mechanical barriers to cell movement in cancer (9). Studies have reported that in the
matrix metalloproteinase (MMP) family, gelatinases A (72 kDa
gelatinase, type IV collagenase and MMP-2) and B (92 kDa
gelatinase, type IV collagenase and MMP-9) play a critical role in
ECM degradation and cell migration leading to tumor cell invasion
in breast cancer (10,11). Elevated MMP-9 levels have been
functionally linked to elevated metastasis in a number of tumor
types, such as brain (12),
prostate (13), bladder (14) and breast tumors (15,16). Consequently, inhibiting the
expression of MMP-9 and/or its upstream regulatory pathways may
prove to be effective in treating malignant tumors, including
breast cancer.
Protein kinase C (PKC) is involved predominantly in
the production of MMP-9 (17–21). A PKC activator,
12-O-tetradecanoylpho-bol-13-acetate (TPA), induces MMP-9
synthesis and secretion during various pathological processes, such
as tumor invasion (16,22). TPA-mediated MMP-9 expression is
regulated by activating transcription factors, such as nuclear
factor-κB (NF-κB) and activator protein-1 (AP-1) in cancer cells
(23–25). The mitogen-activated protein
kinase (MAPK) signaling pathway is important for AP-1 activation
and NF-κB activation requires IκB kinase (IKK), phosphoinositide 3
kinase-Akt, or p38 MAPK, depending on the cell type (17–21). Consequently, these findings
suggest that the main pathways involved in MMP-9 expression
following PKC activation are the MAPK, NF-κB and AP-1 pathways.
In this study, we investigated the roles of
guggulsterone isomers in regulating TPA-induced MMP-9 expression,
and thus suppressing cell invasion, as well as the mechanisms
involved.
Materials and methods
Cells and materials
MCF-7 cells were obtained from the American Type
Culture Collection (Manassas, VA, USA). Cells were cultured in DMEM
supplemented with 10% fetal bovine serum (FBS) and 1% antibiotics
at 37°C in a 5% CO2 incubator. Isomers of guggulsterone
were obtained from Steraloids Inc. (Newport, RI, USA) and dissolved
in dimethyl sulfoxide (DMSO). TPA,
3-(4,5-dimethyl-thiazol-2-yl)-2,5-diphenyltetrazoliumbromide (MTT),
and anti-β-actin antibodies were from Sigma (St. Louis, MO, USA).
Antibodies against p-IκBα, p-IKKαβ, p38, p-p38, JNK, p-JNK, ERK and
p-ERK were from Cell Signaling Technology (Beverly, MA, USA).
MMP-9, p50, p65, proliferating cell nuclear antigen (PCNA) and
horseradish peroxidase (HRP)-conjugated IgG antibodies were from
Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA). High
glucose-containing DMEM, FBS and phosphate-buffered saline (PBS)
were purchased from Gibco-BRL (Gaithersburg, MD, USA).
Determination of cell viability
The effect of guggulsterone on MCF-7 cell viability
was determined using an MTT assay (26). Briefly, cells were seeded at
3×104 cells/well and allowed to attach. After 24 h,
cells were treated with guggulsterone at 5, 10 or 20 μM.
After incubation for 24 h, cells were washed with PBS. MTT (0.5
mg/ml PBS) was then added to each well, and the plates were
incubated at 37°C for 30 min. Formazan crystals were dissolved with
DMSO (100 μl/well), and an intensity of color was detected
at 570 nm using a microplate reader (Model 3550; Bio-Rad, Richmond,
CA, USA).
Western blot analysis
MCF-7 cells (5×105) were pre-treated with
guggulsterone (10 and 20 μM) for 1 h and then incubated with
TPA for 24 h. Cells were lysed with an ice-cold lysis buffer (50 mM
Tris-HCl, pH 7.4, 1% NP-40, 0.5% sodium deoxycholate, 150 mM NaCl,
1 mM EGTA, 0.1% SDS). Protein concentration was determined using
the Bradford method (27).
Samples (20 μg) were separated by SDS-PAGE with 10%
acrylamide, and the resolved proteins were transferred onto a
hybond-PVDF membrane using a western blotting apparatus. The PVDF
membrane was incubated with 2% bovine serum albumin or 5% skim milk
to block non-specific sites, and then incubated overnight with 1
μg/ml primary antibody for MMP-9, p38, p-p38, JNK, p-JNK,
ERK, p-ERK, β-actin, p50, p65, or PCNA. HRP-conjugated IgG was used
as the secondary antibody. Protein expression levels were
determined by signal analysis using an image analyzer (Fujifilm,
Tokyo, Japan).
Gelatin zymography assay
The conditioned medium was collected after 24 h of
stimulation with TPA, mixed with non-reducing sample buffer, and
separated on a polyacrylamide gel containing 0.1% (w/v) gelatin
(25). Gels were washed at room
temperature for 30 min with 2.5% Triton X-100 solution, and
incubated at 37°C for 16 h in 5 mM CaCl2, 0.02% Brij and
50 mM Tris-HCl (pH 7.5). The gel was stained for 30 min with 0.25%
(w/v) Coomassie brilliant blue in 40% (v/v) methanol/7% (v/v)
acetic acid and photographed with an image analyzer (Fujifilm).
Proteolysis was imaged as a white zone in a dark blue field.
Densitometric analysis was performed using Multi Gauge Image
Analysis software (Fujifilm).
Quantitative real-time PCR assay
Total RNA was extracted from the cells using a
FastPure RNA kit (Takara Bio Inc., Shiga, Japan). The RNA
concentration and purity were determined by absorbance at 260/280
nm. cDNA was then synthesized from 1 μg total RNA using the
PrimeScript RT reagent kit (Takara Bio Inc.). MMP-9 and GAPDH mRNA
expression were determined by real-time PCR using the ABI PRISM
7900 sequence detection system and SYBR®-Green (Applied
Biosystems, Foster City, CA, USA). Primers used were: MMP-9
(NM-004994) sense, CCTGGAGACCTGAGAACC AATCT and antisense,
CCACCCGAGTGTAACCATAGC; and GAPDH (NM-002046) sense, ATGGAAATCCCATC
ACCATCTT and antisense, CGCCCCACTTGATTTTGG. To control for
variation in mRNA concentration, all the results were normalized to
the housekeeping gene, GAPDH. Relative quantification was performed
using the comparative ΔΔCt method according to the manufacturer’s
instructions.
Preparation of nuclear extract
The MCF-7 cells (2×106) were treated with
guggulsterone in the presence or absence of TPA for 4 h. Cells were
immediately washed twice with PBS (pH 7.5), scraped into 1.5 ml of
ice-cold PBS, and pelleted at 1,500 × g for 3 min. Cytoplasmic and
nuclear extracts were prepared from cells using the
NE-PER® Nuclear and Cytoplasmic Extraction Reagents
(Pierce Biotechnology, Rockford, IL, USA).
Electrophoretic mobility shift assay
(EMSA)
The activation of NF-κB and AP-1 was assayed with a
gel mobility shift assay using nuclear extracts. An oligonucleotide
containing the κ-chain (κB, 5′-CCGGTTAACAGAGGGGGCTTTCCGAG-3′) or
the AP-1 (5′-CGCTTGATGAGTCAGCCGGAA-3′) binding site was synthesized
and used as a probe for gel retardation assay. Two complementary
strands were annealed and labeled with [α-32P]dCTP.
Labeled oligonucleotides (10,000 cpm), 10 μg of nuclear
extracts, and a binding buffer [10 mM Tris-HCl, pH 7.6, 500 mM KCl,
10 mM EDTA, 50% glycerol, 100 ng poly(dI•dC), 1 mM DTT] were
incubated for 30 min at room temperature in a final volume of 20
μl. Reaction mixtures were analyzed by electrophoresis on 4%
polyacrylamide gels in 0.5X TBE buffer (final concentrations: 22.5
mM Tris-borate, pH 7.6, 0.5 mM EDTA). Gels were dried and examined
by autoradiography. Specific binding was controlled by competition
with a 50-fold excess of cold κB or AP-1 oligonucleotide.
Invasion assay
Invasion assays were carried out in 24-well chambers
(8-μm pore size) coated with 20 μl Matrigel diluted
in DMEM. The Matrigel coating was re-hydrated in 0.5 ml DMEM for 30
min immediately before the experiments. Cells (2×105
cells/well) were added to the upper chamber with chemoattractant in
the bottom well. Conditioned medium (0.5 ml) was added to the lower
compartment of the invasion chamber and chambers were incubated for
24 h. Following incubation, cells on the upper side of the chamber
were removed using cotton swabs, and cells that had migrated were
fixed and stained with toluidine blue solution. Invaded cells were
counted in five random areas of the membrane under a light
microscope. Data are presented as the means ± SE from three
individual experiments performed in triplicate.
Statistical analysis
Statistical analysis was performed using ANOVA and
Duncan’s test. A value of P<0.05 was considered to indicate a
statistically significant difference.
Results
Effect of guggulsterone isomers on MCF-7
cell viability
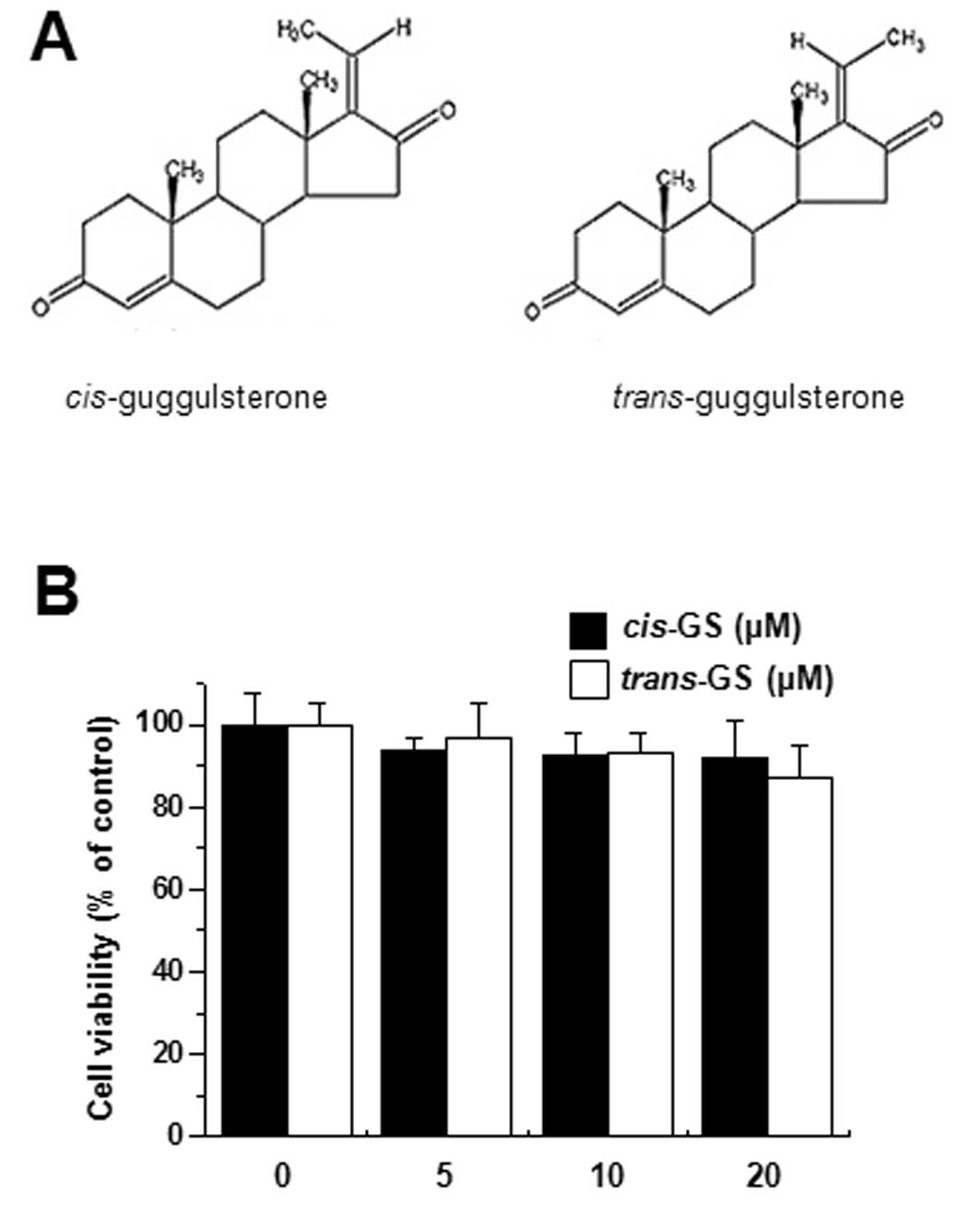
Since the cytotoxicity of guggulsterone cis-
and trans-isomers (Fig.
1A) on MCF-7 cells has not been previously reported, we
examined the cytotoxicity to avoid interference from the reagent.
The treatment of MCF-7 cells with guggulsterone isomers (5, 10 and
20 μM) for 24 h caused no significant change in cell
viability (Fig. 1B).
Effect of guggulsterone isomers on
TPA-induced MMP-9 expression in MCF-7 cells
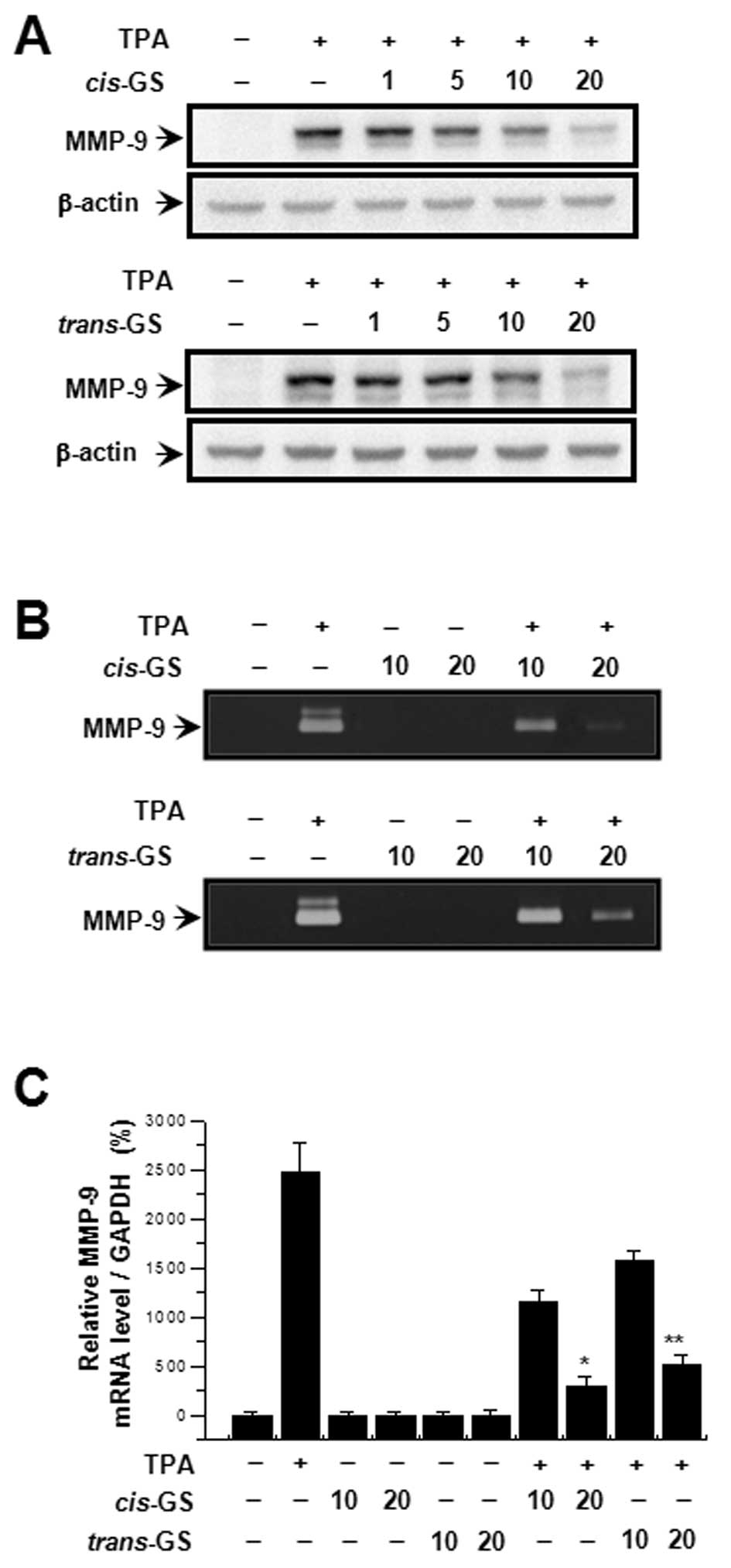
In order to investigate the effect of guggulsterone
on TPA-induced MMP-9 expression, we performed western blot
analysis, real-time PCR, and zymography with MCF-7 cells. MCF-7
cells were treated with guggulsterone isomers (1, 5, 10 and 20
μM) in the presence of 100 nM TPA for 24 h. Western blot
analysis revealed that the treatment of MCF cells with
guggulsterone isomers blocked the upregulation of TPA-induced MMP-9
expression in a concentration-dependent manner (Fig. 2A). Determination of the effects of
guggulsterone isomers on TPA-induced MMP-9 secretion revealed that
both cis- and trans-isomers substantially inhibited
TPA-induced MMP-9 secretion (Fig.
2B). Real-time PCR also showed that TPA increased the MMP-9
mRNA levels in MCF-7 cells, which was blocked by guggulsterone
isomers in a dose-dependent manner (Fig. 2C). Of note,
cis-gugulsterone decreased MMP-9 expression and secretion to
a greater extent at 20 μM than trans-guggulsterone
(Fig. 2A and B). These results
indicate that the cis-isomer is more potent than the
trans-isomer in the inhibition of TPA-induced MMP-9
expression and secretion in MCF-7 cells.
Effect of guggulsterone isomers on
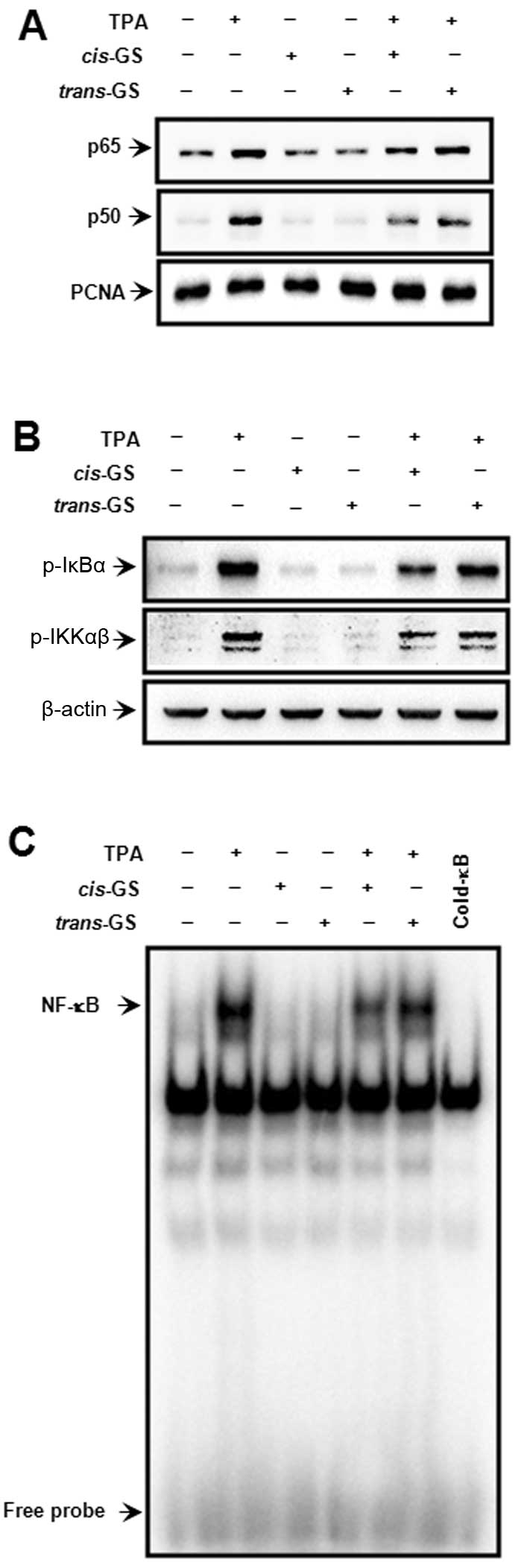
TPA-induced NF-κB activation
The MMP-9 promoter contains NF-κB and AP-1 binding
sites, both of which are centrally involved in MMP-9 gene induction
(23,24,28,29). To investigate the transcription
factor involved in inhibiting MMP-9 transcription by guggulsterone,
we first examined the effect of guggulsterone isomers on the
TPA-stimulated NF-κB signaling pathway. The treatment of MCF-7
cells with guggulsterone isomers revealed that cis-isomer,
but not trans-isomer, inhibited the TPA-stimulated nuclear
translocation of p65/p50, an NF-κB subunit and NF-κB DNA binding
activity (Fig. 3A and C), as well
as the TPA-induced phosphorylation of IκBα (Ser-32) and IKKαβ
(Ser-176/180) (Fig. 3B). These
data indicate that inhibition of the TPA-stimulated activation of
the IKK/IκB/NF-κB axis by guggulsterone is stereoisomer-specific
and that cis-guggulsterone is an inhibitor of IKK.
Effect of guggulsterone isomers on
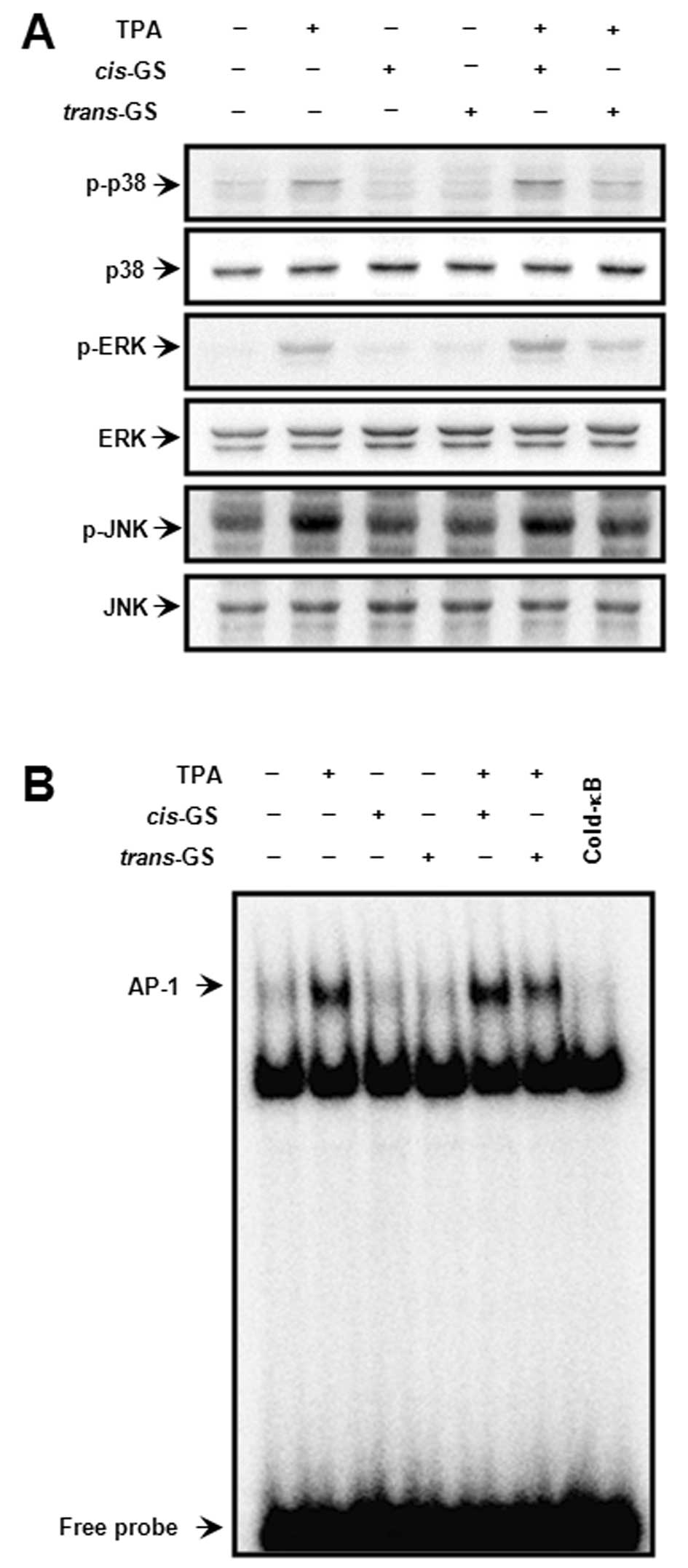
TPA-induced AP-1 activation
MAPK signaling pathways are involved in the AP-1
transcriptional activity (29).
We investigated the effect of guggulsterone on the TPA-induced
activation of MAPK and AP-1 by western blot analysis and EMSA. The
TPA-induced ERK, JNK and p38 phosphorylation, and the activation of
AP-1 binding activity were detected (Fig. 4). The activation of the signaling
molecules by TPA was significantly blocked by
trans-guggulsterone, but not cis-guggulsterone,
indicating that the inhibition of TPA-stimulated AP-1 activation by
guggulsterone is also stereoisomer-specific; that is,
trans-guggulsterone regulates TPA-mediated MAPK/AP-1
activation in MCF-7 cells.
Effect of guggulsterone isomers on
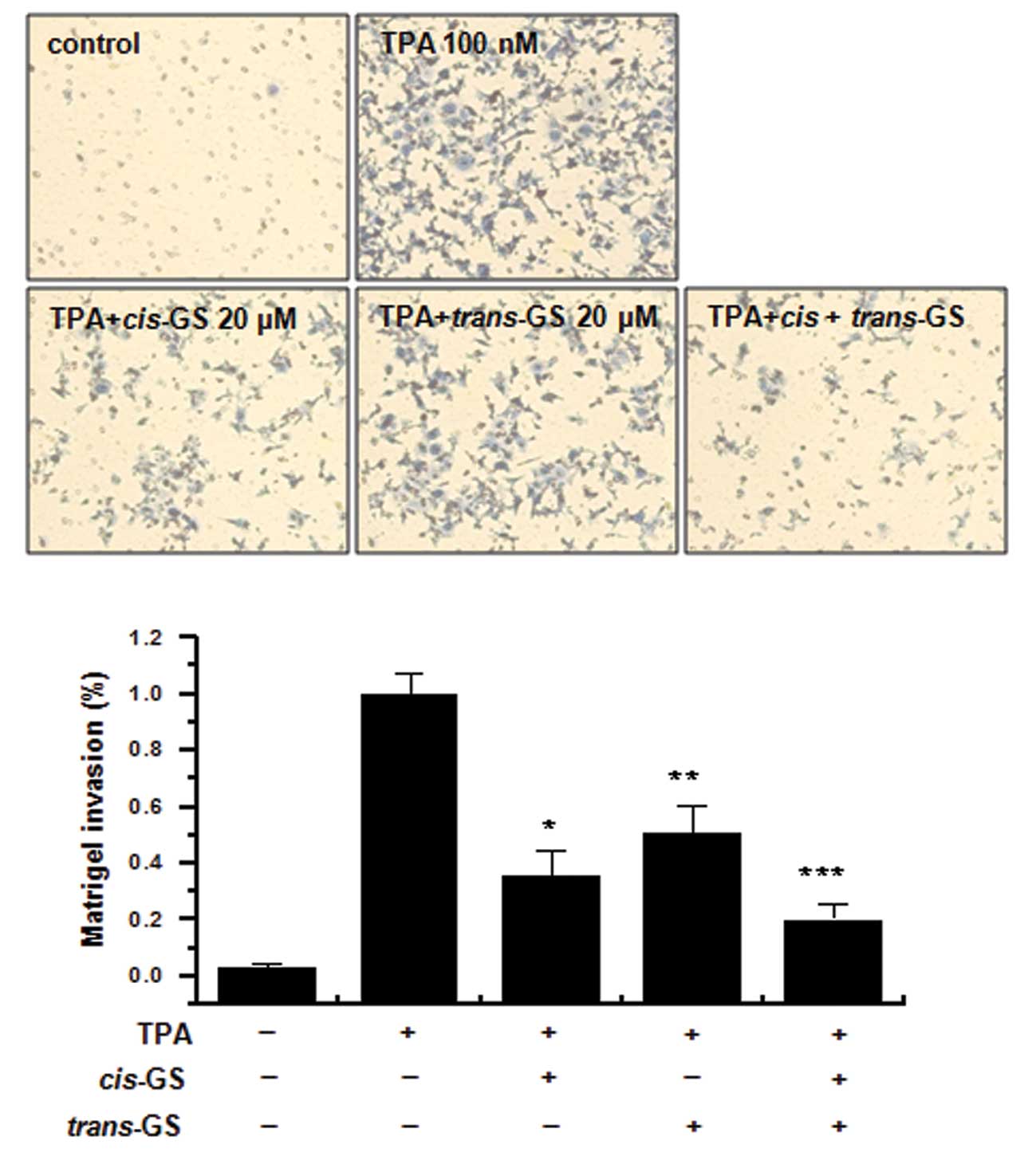
TPA-induced MCF-7 cell invasion in vitro
The effects of guggulsterone isomers on the invasive
potential of MCF-7 breast cancer cells were evaluated using a
Matrigel invasion assay. Treatment with TPA increased MCF-7 cell
invasion compared to the control MCF-7 cells. The incubation of
MCF-7 cells with TPA resulted in a 10-fold increase in the invasion
of MCF-7 cells. Treatment with cisguggulesterone decreased
the TPA-induced cell invasion by 67%, and the trans-isomer
reduced the invasion by 51% (Fig.
5). The combination of these isomers exerted an additive effect
on the inhibition of MCF-7 cell invasion, showing a 79% inhibition
of invasion.
Discussion
This study examined the effects of guggulsterone
isomers (cis and trans) on TPA-induced cell invasion,
MMP-9 expression and related molecular mechanisms in MCF-7 cells.
We found that cis-guggulsterone inhibited TPA-induced MMP
expression by blocking IKK/NF-κB signaling, whereas
trans-guggulsterone by blocking MAPK/AP-1 signaling,
suggesting that guggulsterone isomers differ in the modulation of
TPA-induced MMP-9 expressions in MCF-7 cells. The combination of
isomers exerted additive effects on the inhibition of MCF-7 cell
invasion. This study is the first to show that the combination of
guggulsterone isomers may be a potential therapeutic strategy for
breast cancer.
NF-κB is a transcription factor that plays an
important role in the induction of MMP-9 gene expression (30,31). NF-κB comprises a family of
inducible transcription factors which regulate host inflammatory
and immune responses (32).
Diverse signal transduction cascades mediate NF-κB pathway
stimulation (32). NF-κB is an
inducible dimeric transcription factor that belongs to the
Rel/NF-κB family of transcription factors and consists of two major
polypeptides, p65 and p50 (33).
NF-κB is initially located in the cytoplasm in an inactive form
complexed with IκB, an inhibitory factor of NF-κB. Various inducers
such as TPA, cytokines and stress can dissociate this complex,
presumably by IκB phosphorylation, resulting in NF-κB being
released from the complex. IKKαβ phosphorylates serine residues in
the NH2-terminus of IκB, resulting in NF-κB release and
translocation to the nucleus (30). NF-κB then translocates to the
nucleus, where it interacts with specific DNA recognition sites to
mediate gene transcription. The NF-κB elements are centrally
involved in MMP-9 gene induction by TPA (23,34). Shishodia and Aggarwal (6) showed that guggulsterone suppressed
NF-κB activation by inhibiting IKK and IκBα degradation in the
majority of tumor cells. In support of these observations, we found
that the TPA-stimulated phosphorylation of IKKαβ and IκBα and the
nuclear translocation of NF-κB were inhibited by treatment with
cis-guggulsterone, but not with trans-guggulsterone.
These findings suggest that cis-guggulsterone is a specific
inhibitor of the IKK/NF-κB pathway.
AP-1 is a sequence-specific transcriptional factor
composed of Jun, Fos and ATF family proteins, which are induced by
multiple stimuli such as TPA, cytokines, growth factors and stress
(35). The MAPK signaling pathway
plays a pivotal role in AP-1 activation (17–21). The activation of ERK results in an
increase in AP-1 activity via c-Fos induction, whereas JNK
activation leads to the c-Jun phosphorylation (17–21). In this study, the results showed
that TPA-induced ERK, JNK and p38 phosphorylation, and AP-1 binding
activity via c-Fos induction were blocked by
trans-guggulsterone, but not by cisguggulsterone.
These findings suggest that trans-guggulsterone is a
specific inhibitor of the MAPK/AP-1 pathway.
MMP-9 activation has been shown to be associated
with the progression and invasion of tumors, including mammary
tumors (36). Thus, the discovery
and development of an agent that inhibits MMP-9 expression are
important for the treatment of cancer/tumors. Guggulesterone
significantly diminished the TPA-induced cell invasion, and the
combination of these isomers exerted additive effects on the
inhibition of MCF-7 cell invasion. These results indicate that
MMP-9 may be one of the critical molecules involved in processing
tumor invasion and metastasis of breast cancer cells.
In conclusion, guggulsterone isomers downregulate
MMP-9 expression and tumor cell invasion through the
stereoisomer-specific suppression of IKK/NF-κB and MAPK/AP-1
activation. That is, cis-guggulsterone regulates the
IKK/NF-κB pathway and trans-guggulsterone regulates
MAPK/AP-1 activation. Therefore, guggulsterone isomers modulate
TPA-induced MMP-9 expression in MCF-7 cells in an isomer-specific
manner. Moreover, the combination of these isomers exerts an
additive effect on inhibition of MCF-7 cell invasion. Based on
these observations, we suggest that the combination of cis-
and trans-guggulsterone isomers may be a strong candidate
for the prevention of breast tumor invasion and metastasis.
Abbreviations:
|
TPA
|
12-O-tetradecanoylpho-bol-13-acetate;
|
|
MMP
|
matrix metalloproteinase;
|
|
IKK
|
I-κB kinase;
|
|
AP-1
|
activator protein-1;
|
|
MAPK
|
mitogen-activated protein kinase;
|
|
ECM
|
extracellular matrix
|
Acknowledgements
This study was supported by the Korea
Science and Engineering Foundation (KOSEF) (nos.
R11-2002-100-04001-0 and M10528010003-05N2801-00310), and the Korea
Research Foundation Grant (nos. KRF-2009-0076698 and
KRF-2010-0012716), Republic of Korea.
References
|
1.
|
Sinal CJ and Gonzalez FJ: Guggulsterone:
an old approach to a new problem. Trends Endocrinol Metab.
13:275–276. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Urizar NL and Moore DD: GUGULIPID: a
natural cholesterol-lowering agent. Annu Rev Nutr. 23:303–313.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Yang JY, Della-Fera MA and Baile CA:
Guggulsterone inhibits adipocyte differentiation and induces
apoptosis in 3T3-L1 cells. Obesity (Silver Spring). 16:16–22. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Shishodia S, Sethi G, Ahn KS and Aggarwal
BB: Guggulsterone inhibits tumor cell proliferation, induces
S-phase arrest, and promotes apoptosis through activation of c-Jun
N-terminal kinase, suppression of Akt pathway, and downregulation
of antiapoptotic gene products. Biochem Pharmacol. 74:118–130.
2007. View Article : Google Scholar
|
|
5.
|
Satyavati GV: Gum guggul (Commiphora
mukul) - the success story of an ancient insight leading to a
modern discovery. Indian J Med Res. 87:327–335. 1988.PubMed/NCBI
|
|
6.
|
Shishodia S and Aggarwal BB: Guggulsterone
inhibits NF-kappaB and IkappaBalpha kinase activation, suppresses
expression of anti-apoptotic gene products, and enhances apoptosis.
J Biol Chem. 279:47148–47158. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Cheon JH, Kim JS, Kim JM, Kim N, Jung HC
and Song IS: Plant sterol guggulsterone inhibits nuclear
factor-kappaB signaling in intestinal epithelial cells by blocking
IkappaB kinase and ameliorates acute murine colitis. Inflamm Bowel
Dis. 12:1152–1161. 2006. View Article : Google Scholar
|
|
8.
|
Ichikawa H and Aggarwal BB: Guggulsterone
inhibits osteoclastogenesis induced by receptor activator of
nuclear factor-kappaB ligand and by tumor cells by suppressing
nuclear factor-kappaB activation. Clin Cancer Res. 12:662–668.
2006. View Article : Google Scholar
|
|
9.
|
Woessner JF Jr: Matrix metalloproteinases
and their inhibitors in connective tissue remodeling. FASEB J.
5:2145–2154. 1991.PubMed/NCBI
|
|
10.
|
Nakajima M, Welch DR, Belloni PN and
Nicolson GL: Degradation of basement membrane type IV collagen and
lung subendothelial matrix by rat mammary adenocarcinoma cell
clones of differing metastatic potentials. Cancer Res.
47:4869–4876. 1987.PubMed/NCBI
|
|
11.
|
Egeblad M and Werb Z: New functions for
the matrix metalloproteinases in cancer progression. Nat Rev
Cancer. 2:161–174. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Saito N, Hatori T, Murata N, et al: A
double three-step theory of brain metastasis in mice: the role of
the pia mater and matrix metalloproteinases. Neuropathol Appl
Neurobiol. 33:288–298. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Castellano G, Malaponte G, Mazzarino MC,
et al: Activation of the osteopontin/matrix metalloproteinase-9
pathway correlates with prostate cancer progression. Clin Cancer
Res. 14:7470–7480. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Kanayama H: Matrix metalloproteinases and
bladder cancer. J Med Invest. 48:31–43. 2001.PubMed/NCBI
|
|
15.
|
Lin CW, Hou WC, Shen SC, et al: Quercetin
inhibition of tumor invasion via suppressing PKC
delta/ERK/AP-1-dependent matrix metalloproteinase-9 activation in
breast carcinoma cells. Carcinogenesis. 29:1807–1815. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Lee SO, Jeong YJ, Kim M, Kim CH and Lee
IS: Suppression of PMA-induced tumor cell invasion by capillarisin
via the inhibition of NF-kappaB-dependent MMP-9 expression. Biochem
Biophys Res Commun. 366:1019–1024. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Yao J, Xiong S, Klos K, et al: Multiple
signaling pathways involved in activation of matrix
metalloproteinase-9 (MMP-9) by heregulin-beta1 in human breast
cancer cells. Oncogene. 20:8066–8074. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Ruhul Amin AR, Senga T, Oo ML, Thant AA
and Hamaguchi M: Secretion of matrix metalloproteinase-9 by the
proinflammatory cytokine, IL-1beta: a role for the dual signalling
pathways, Akt and Erk. Genes Cells. 8:515–523. 2003.PubMed/NCBI
|
|
19.
|
Karin M: The regulation of AP-1 activity
by mitogen-activated protein kinases. J Biol Chem. 270:16483–16486.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Madrid LV, Mayo MW, Reuther JY and Baldwin
AS Jr: Akt stimulates the transactivation potential of the RelA/p65
Subunit of NF-kappa B through utilization of the Ikappa B kinase
and activation of the mitogen-activated protein kinase p38. J Biol
Chem. 276:18934–18940. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Weng CJ, Chau CF, Hsieh YS, Yang SF and
Yen GC: Lucidenic acid inhibits PMA-induced invasion of human
hepatoma cells through inactivating MAPK/ERK signal transduction
pathway and reducing binding activities of NF-kappaB and AP-1.
Carcinogenesis. 29:147–156. 2008. View Article : Google Scholar
|
|
22.
|
Nabeshima K, Inoue T, Shimao Y and
Sameshima T: Matrix metalloproteinases in tumor invasion: role for
cell migration. Pathol Int. 52:255–264. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Hong S, Park KK, Magae J, et al:
Ascochlorin inhibits matrix metalloproteinase-9 expression by
suppressing activator protein-1-mediated gene expression through
the ERK1/2 signaling pathway: inhibitory effects of ascochlorin on
the invasion of renal carcinoma cells. J Biol Chem.
280:25202–25209. 2005. View Article : Google Scholar
|
|
24.
|
Woo MS, Jung SH, Kim SY, et al: Curcumin
suppresses phorbol ester-induced matrix metalloproteinase-9
expression by inhibiting the PKC to MAPK signaling pathways in
human astroglioma cells. Biochem Biophys Res Commun. 335:1017–1025.
2005. View Article : Google Scholar
|
|
25.
|
Kim KH, Park BH, Tu Y, et al:
Polarization-sensitive optical frequency domain imaging based on
unpolarized light. Opt Express. 19:552–561. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Plumb JA, Milroy R and Kaye SB: Effects of
the pH dependence of
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium
bromide-formazan absorption on chemosensitivity determined by a
novel tetrazolium-based assay. Cancer Res. 49:4435–4440. 1989.
|
|
27.
|
Bradford MM: A rapid and sensitive method
for the quantitation of microgram quantities of protein utilizing
the principle of protein-dye binding. Anal Biochem. 72:248–254.
1976. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Azuma M, Aota K, Tamatani T, et al:
Suppression of tumor necrosis factor alpha-induced matrix
metalloproteinase 9 production in human salivary gland acinar cells
by cepharanthine occurs via down-regulation of nuclear factor
kappaB: a possible therapeutic agent for preventing the destruction
of the acinar structure in the salivary glands of Sjogren’s
syndrome patients. Arthritis Rheum. 46:1585–1594. 2002.
|
|
29.
|
Hwang YP, Yun HJ, Kim HG, Han EH, Lee GW
and Jeong HG: Suppression of PMA-induced tumor cell invasion by
dihydroartemisinin via inhibition of PKCalpha/Raf/MAPKs and
NF-kappaB/AP-1-dependent mechanisms. Biochem Pharmacol.
79:1714–1726. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Eberhardt W, Huwiler A, Beck KF, Walpen S
and Pfeilschifter J: Amplification of IL-1 beta-induced matrix
metalloproteinase-9 expression by superoxide in rat glomerular
mesangial cells is mediated by increased activities of NF-kappaB
and activating protein-1 and involves activation of the
mitogen-activated protein kinase pathways. J Immunol.
165:5788–5797. 2000.
|
|
31.
|
Lungu G, Covaleda L, Mendes O,
Martini-Stoica H and Stoica G: FGF-1-induced matrix
metalloproteinase-9 expression in breast cancer cells is mediated
by increased activities of NF-kappaB and activating protein-1. Mol
Carcinog. 47:424–435. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Yamamoto Y and Gaynor RB: Therapeutic
potential of inhibition of the NF-kappaB pathway in the treatment
of inflammation and cancer. J Clin Invest. 107:135–142. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Thanos D and Maniatis T: NF-kappa B: a
lesson in family values. Cell. 80:529–532. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Chung TW, Moon SK, Chang YC, et al: Novel
and therapeutic effect of caffeic acid and caffeic acid phenyl
ester on hepatocarcinoma cells: complete regression of hepatoma
growth and metastasis by dual mechanism. FASEB J. 18:1670–1681.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Bakiri L, Matsuo K, Wisniewska M, Wagner
EF and Yaniv M: Promoter specificity and biological activity of
tethered AP-1 dimers. Mol Cell Biol. 22:4952–4964. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Scorilas A, Karameris A, Arnogiannaki N,
et al: Overexpression of matrix-metalloproteinase-9 in human breast
cancer: a potential favourable indicator in node-negative patients.
Br J Cancer. 84:1488–1496. 2001. View Article : Google Scholar : PubMed/NCBI
|