Introduction
Infertility affects approximately 15% of couples
worldwide, and the male factor is at least partly responsible in
approximately 50% of infertile couples (1). Genetic abnormalities, i.e. obstacles
to spermatogenesis, are thought to account for 15–30% of male
factor infertility (2).
Spermatogenesis is the process by which male germ cells undergo a
complex differentiation where morphological alterations lead to the
formation of differentiated sperm. The unique differentiation
mechanisms of spermatogenesis suggest the existence of cell type-
and stage-specific molecules. With the advent of genetics and
molecular biology, a variety of genes involved in spermatogenesis
have been identified during the last decade (3–9).
Recently, we identified a novel protein referred to
as spermatogenesis associated gene 12 (SPATA12) (10,11). In the testis, SPATA12 is
specifically expressed in spermatocytes, spermatids and
spermatozoa, and may be involved in the development of testicular
maturation. In contrast, the SPATA12 gene is located on
chromosome 3p14. Chromosomal abnormalities including homozygous
deletions, loss of heterozygosity and expressional deficiencies in
genes located at 3p14 have been frequently reported in many tumor
types, suggesting that this locus is likely to contain
tumour-suppressor genes (12,13). SPATA12 was also found to be absent
in testicular germ cell tumors such as seminoma, yolk sac, teratoma
and embryonal carcinoma. Flow cytometric analysis of SPATA12 in
both mouse GC-1 spg germ cells and human HeLa cells indicates that
the expression of the SPATA12 gene may delay G1 to S phase
progression in the cell cycle. In addition, SPATA12 was shown to
inhibit tumor cell colony formation. These findings suggest that
SPATA12 could be an inhibitor suppressing cell proliferation in the
process of germ cell development and in tumorigenesis. However, the
transcriptional regulations of SPATA12 in spermatogenesis remain
unclear.
The emerging technology of cDNA microarray
hybridization offers the possibility of providing a rapid,
high-throughput method for the efficient and accurate simultaneous
expression measurement of thousands of genes (14–17). Studies based on microarray or
expressed sequence tag analyses have been successfully used during
spermatogenesis, and have aided in the understanding of the
molecular mechanisms and genetic determinants of male infertility
(18–20). Based on this background, we
hypothesized that analysis of the gene expression profile induced
by SPATA12 in GC-1 spg germ cells may contribute to an
understanding of the function of SPATA12 and the possible pathways
in which SPATA12 is involved during spermatogenesis.
Materials and methods
Cell line
The mouse GC-1 spg germ cell line (ATCC CRL-2053)
was cultured in Dulbecco’s modified Eagle’s medium supplemented
with 10% FBS, 100 μg/ml penicillin-streptomycin, and was
maintained in 5% CO2 and a 95% humidified air atmosphere
at 37°C.
Transient transfection
Transfections were performed with Lipofectamine 2000
(Invitrogen). Cells were plated to 50–70% confluent culture in each
well of a 6-well plate or a 60-mm dish 24 h before transfection.
According to the manufacturer’s instructions, the GC-1 spg cells
were transfected with 4 μg pRevTRE plasmid or
pRevTRE-SPATA12 plasmid, respectively.
RNA isolation
Total RNA was extracted with the TRIzol reagent
(Invitrogen) according to the manufacturer’s protocol, digested by
RNase-free DNase (Fermentas), dissolved in diethyl
pyrocarbonate-treated water, and stored at −80°C prior to use. For
quality control, RNA purity and integrity were evaluated by agrose
gel electrophoresis and the OD260/OD280 ratio.
Microarray assay and data analysis
32K Mouse Genome Array (CapitolBio Corporation,
Beijing, China), covering 99% of the current assembly of the mouse
genome and including 32,256 Oligo DNA with 70 mer length, was
applied to investigate the possible changes in the mRNA level in
GC-1 spg cells following SPATA12 gene transfection by comparison to
control GC-1 spg cells. Total RNA was extracted using TRIzol
reagent (Invitrogen) and then applied to synthesize Cy3- or
Cy5-conjugated dUTP-labeled cDNA probe using the RNA Fluorescence
Labeling Core kit (M-MLV version 2.0; Takara, Dalian, China) and
following the manufacturer’s instructions. Hybridization, scanning,
and data extraction were conducted at CapitalBio Corporation. The
number of genes affected by SPATA12 was determined using the
scatter plots of control GC-1 spg cells vs. GC-1 spg cells
transfected with SPATA12 (named GC-1 spg-SPATA12 cells). In
addition, identified genes were also categorized specifically using
biological process ontology terms and Kyoto Encyclopedia of Genes
and Genomes (KEGG) biological pathways using the software Molecule
Annotation System 2.0 (http://:bioinfo.capitalbio.com/MAS/) (CapitolBio
Corporation).
RT-PCR and SYBR-Green real-time PCR
analysis
For cDNA synthesis, 2 μg of total RNA was
reverse transcribed using M-MLV reverse transcriptase (Promega).
Then PCR was performed in a 10 μl reaction volume containing
5.7 μl of nuclease-free water, 0.1 μl of Takara Taq
(5 U/μl), 1 μl of 10X PCR buffer, 0.8 μl of
dNTP mixture (2.5 mM), 2 μl of cDNA and 0.2 μl each
of the 20 μM gene-specific primers. After initial
denaturation for 10 min at 94°C, 27–30 cycles of PCR were
performed. Each cycle consisted of a denaturing period (30 sec at
94°C), an annealing phase (55–60°C), and an extension period (60
sec at 72°C). After the last cycle, all samples were incubated for
an additional 10 min at 72°C. PCR products were separated by 1.5%
agarose gel electrophoresis, and the DNA bands were stained with
ethidium bromide. RT-PCR signals were normalized to the signals of
the murine glyceraldehyde-3-phosphate dehydrogenase (gapdh)
gene.
For quantitative RT-PCR, a SYBR-Green real-time
RT-PCR protocol (Invitrogen) was applied using an MX3000
(Stratagene, USA) instrument. PCR was performed in a 10 μl
reaction volume containing 2.2 μl of nuclease-free water, 5
μl of Mix, 2 μl of cDNA and 0.4 μl each of the
2.5 μM gene-specific primers. The PCR profile was 95°C for 5
min followed by 94°C for 30 sec, 58°C for 20 sec, and 72°C for 20
sec for 40 cycles, with a final extension at 72°C for 10 min and
storage at 4°C. The level of gene mRNA was evaluated in automated
analysis by MxPro. All the primers for RT-PCR are shown in Table I.
 | Table I.Primers used for real-time RT-PCR and
RT-PCR experiments. |
Table I.
Primers used for real-time RT-PCR and
RT-PCR experiments.
| Gene name | Forward primer
(5′→3′) | Reverse primer
(5′→3′) | Product size
(bp) |
|---|
| Gapdh1 |
GAAGGGTGGAGCCAAAAGG |
TTGCTGACAATCTTGAGTGAGTTG | 111 |
| Ccl5 |
GCCCACGTCAAGGAGTATTTCT |
TCTCTGGGTTGGCACACACTT | 100 |
| Fbxo39 |
AGCCTGAGGAGTTGCTATTTCAGT |
ACGTTAACTTCTGCAGGGTGTTC | 100 |
| Wnt10a |
CTTCAGCCGAGGTTTTCGAGA |
CCGCAAGCCTTCAGTTTACC | 108 |
| Rtp4 |
CATCTTTGGGTGAGAAGGTGACT |
GAGATCTGGGTGGTTTTACTTTGTG | 120 |
| Sp100 |
AGCTACAACCACAGTCCCCT |
TCCTGTCCTTTTCCGTCTTCTAA | 111 |
| Zbp1 |
GACGGACAGACGTGGAAGATC |
TTGACCGGATTGTGCTGACA | 110 |
| Gys1 |
CGCTGGAAGGGTGAGCTTT |
GAAGTGGGCAACCACATACG | 156 |
| Mtss1 |
ATGGAGGCTGTGATCGAGAAG |
TCCGGCTTTGTTTATGAAGTCTT | 114 |
| Selenbp1 |
AGCCAGGTCATCCACAGGTT |
ACTTCGTGCTGTCCCCAAAG | 100 |
| Ndrg1 |
CACACAACATTTTGCTGTCTGC |
GCCAACTGATCCATTGAGGGG | 100 |
| Cyclin B1 |
TGGCCTCACAAAGCACATGA |
GCTGTGCCAGCGTGCTAATC | 77 |
| Cyclin D1 |
TAGGCCCTCAGCCTCACTC |
CCACCCCTGGGATAAAGCAC | 80 |
| Cyclin E1 |
AATTGGGGCAATAGAGAAGAGGT |
TGGAGCTTATAGACTTCGCACA | 161 |
| β-catenin |
GGCAACCCTGAGGAAGAAGA |
CACTGGTGACCCAAGCATTTT | 597 |
| Gapdh2 |
TTCAACGGCACAGTCAAGG |
TGAAGTCGCAGGAGACAACC | 694 |
| SPATA12 |
CGCGGATCCATGTCCAGTTCTGCTCTGACT |
CCCAAGCTTGCAGGATTATTATTGATTACAG | 607 |
Statistical analysis
Statistical analysis was carried out using the
Student’s t-test. P-values ≤0.05 were considered to indicate
statistically significant results.
Results
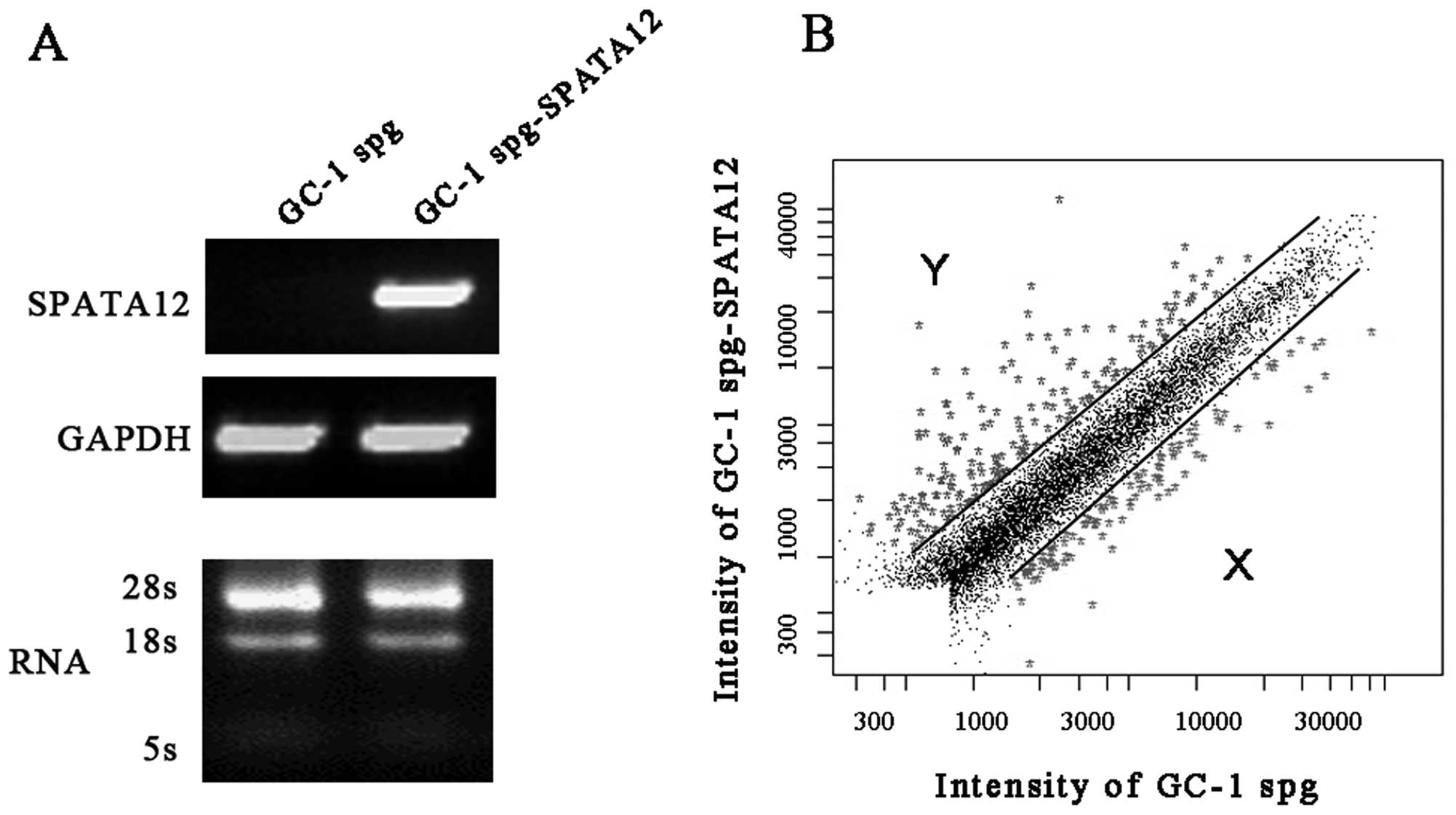
Global gene expression induced by SPATA12
in GC-1 spg cells
Global gene expression levels induced by SPATA12 in
GC-1 spg cells from the microarray analysis are represented in
Fig. 1. The differentially
expressed genes with fold changes of ≥2 or ≤0.5 (P≤0.05) were
analyzed using t-test and P-value and clustered with the software
package Cluster 3.0. Our data showed that the expression of 286 out
of 32,256 genes was altered after SPATA12 was expressed. Of these,
182 genes were upregulated and 104 genes were downregulated
specifically.
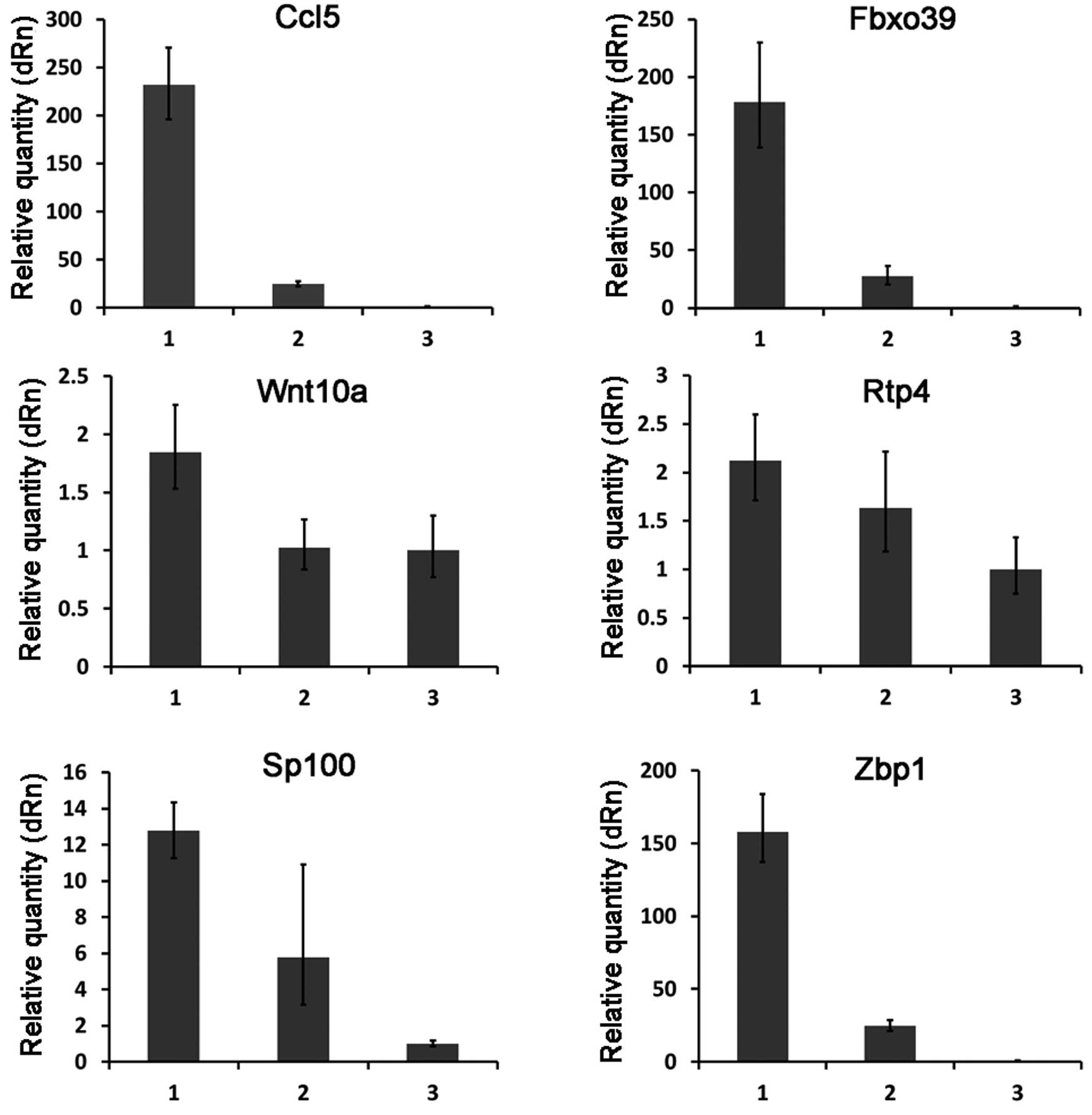
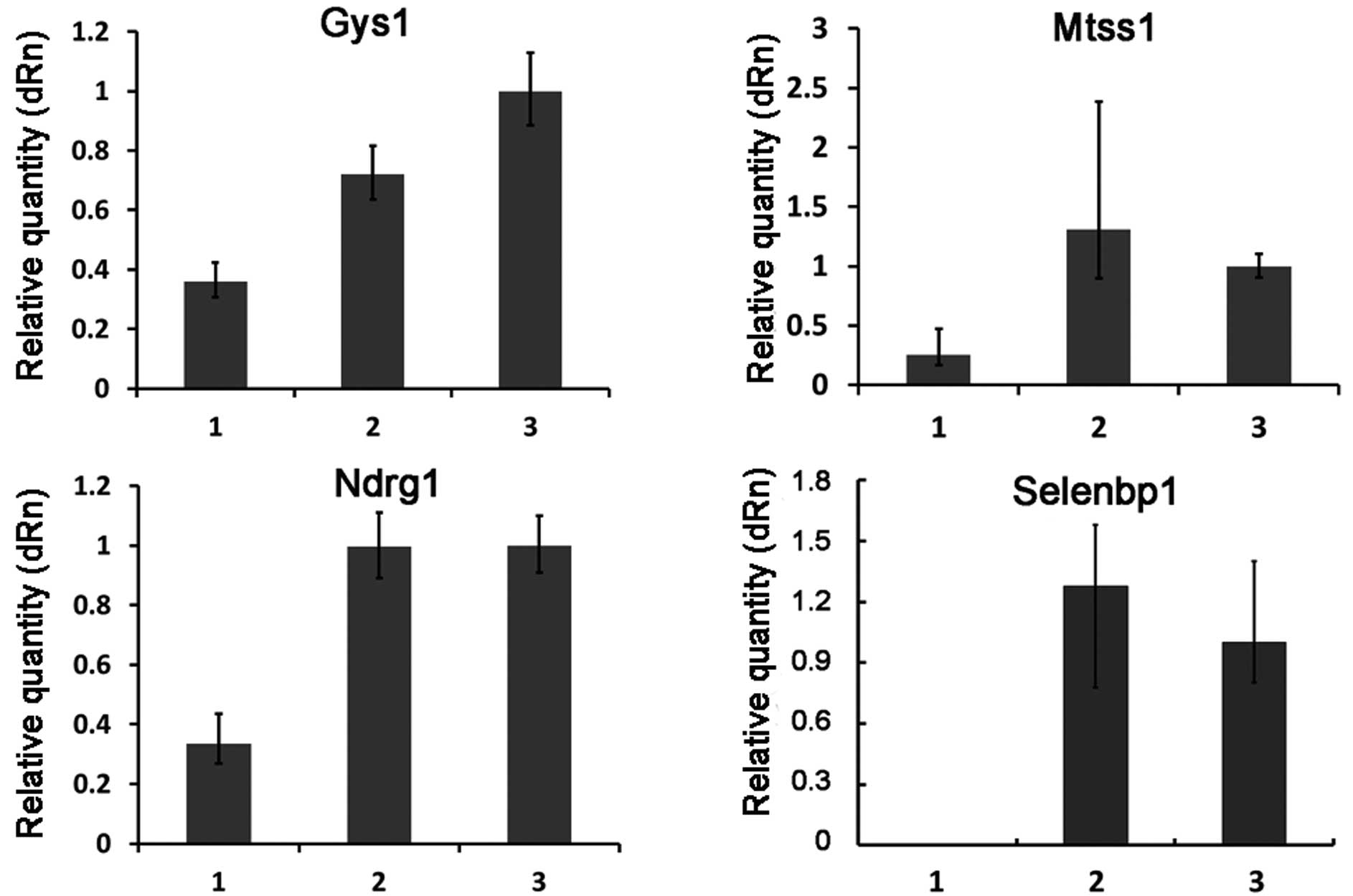
Verification of the differentially
expressed genes from the microarray data
To test the reliability of the microarray data, the
expression patterns of 10 selected genes (including 6 upregulated
genes and 4 downregulated genes) (Table II) were examined by quantitative
real-time RT-PCR. The overall profile of gene expression by
real-time RT-PCR analysis was similar to that revealed by the
microarray data for all the selected genes (Figs. 2 and 3).
 | Table II.Selected genes with differential
expression between GC-1 spg and GC-1 spg-SPATA12 cells. |
Table II.
Selected genes with differential
expression between GC-1 spg and GC-1 spg-SPATA12 cells.
| Term
description | Symbol | Ratio | Gene name | Gene function |
|---|
| Upregulated
genes | Ccl5 | 32.0033 | Chemokine (C-C
motif) ligand 5 | Cytokine activity;
chemokine activity; chemoattractant activity; immune
regulation |
| Rtp4 | 11.7258 | Receptor
transporter protein 4 | Unknown |
| Zbp1 | 8.7427 | Z-DNA binding
protein 1 | Left-handed Z-DNA
binding; RNA binding; double-stranded RNA adenosine deaminase
activity; innate immune response |
| Fbxo39 | 6.7244 | F-box protein
39 | Cancer/testis
antigen |
| Sp100 | 4.1610 | SP100 nuclear
antigen | Tumorigenesis;
immunity; gene regulation |
| Wnt10a | 2.1549 | Wingless related
MMTV integration site 10a | Signal transducer
activity; receptor binding; embryogenesis; carcinogenesis |
| Downregulated
genes | Gys1 | 0.4613 | Glycogen synthase 1
(muscle) | Catalytic activity;
glycogen (starch) synthase activity; protein binding; transferase
activity; transferase activity, transferring glycosyl groups |
| Mtss1 | 0.3599 | Metastasis
suppressor 1 | Unknown |
| Ndrg1 | 0.2371 | N-myc downstream
regulated gene 1 | Stress responses;
hormone responses; cell growth; cell differentiation; p53-mediated
caspase activation and apoptosis |
| Selenbp1 | 0.1536 | Selenium binding
protein 1 | Selenium binding;
selenium-dependent role in ubiquitination/deubiquitination-mediated
protein degradation |
Gene ontology (GO) analysis of the
microarray data
To gain insight into the potential functional
consequences of the SPATA12-induced expression in GC-1 spg cells,
GO analysis was applied to distribute genes into groups according
to biological process, molecular function and cellular component,
respectively.
Functional analysis using GO of the biological
process group revealed that genes related to immune response,
antigen processing and presentation, positive regulation of T
cell-mediated cytotoxicity, transcription regulation, and defense
response to bacterium were upregulated in the SPATA12-transfected
GC-1 spg cells (Fig. 4A and B).
This suggests that based on the GO terms these upregulated genes
combined with SPATA12 may play important roles in the physiology of
the immune response. In contrast, genes related to carbohydrate
metabolism, lipid metabolism, cholesterol biosynthesis and
glycolysis were downregulated in the SPATA12-transfected cells,
which indicates that based on these GO terms these genes affected
by SPATA12 may be involved in metabolic processes.
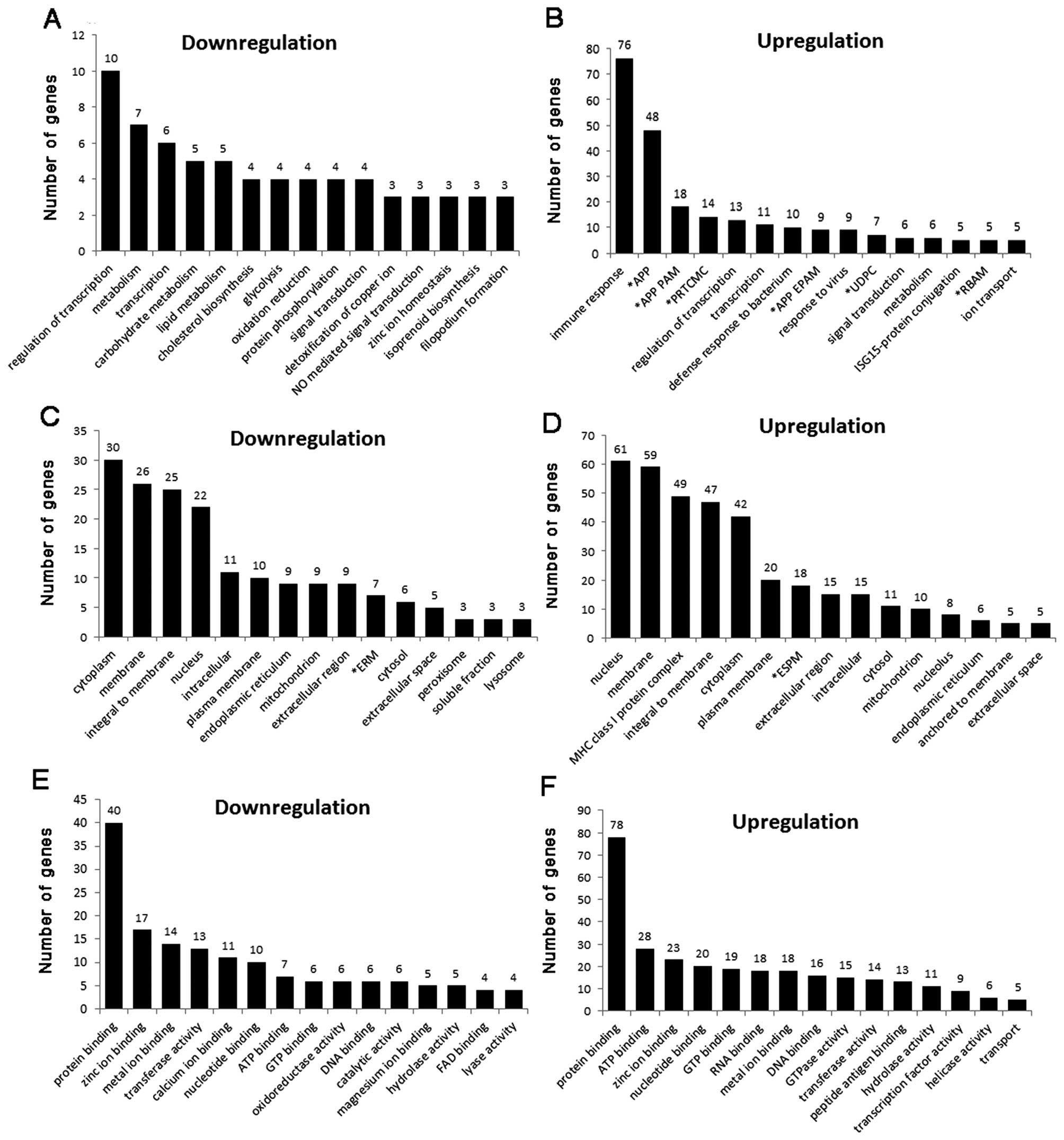 | Figure 4.The number of SPATA12-induced genes in
each of the (A and B) biological process, (C and D) cellular
component and (E and F) molecular functional categories described
in the Gene Ontology website. (A, C and E) Downregulated genes; (B,
D and F) upregulated genes. *APP, antigen processing and
presentation; *APP PAM, antigen processing and
presentation of peptide antigen via MHC class I;
*PRTCMC, positive regulation of T cell mediated
cytotoxicity; *APP EPAM, antigen processing and
presentation of exogenous peptide antigen via MHC class I;
*UDPC, ubiquitin-dependent protein catabolism;
*RBAM, response to bacterium associated molecule;
*ERM, endoplasmic reticulum membrane; *ESPM,
external side of plasma membrane. |
In the cellular component group (Fig. 4C and D), GO analysis showed that
the major differentially expressed genes, including 61 upregulated
and 22 downregulated genes, were located in the nucleus. These data
indicate that SPATA12 may play roles in the nucleus through
interacting with genes associated in these GO categories. This
observation was consistent with our previous bioinformatics
analysis report, which predicted that SPATA12 probably functions as
a testis-specific nuclear protein involved in spermatogenesis
(21).
We also noted that these genes (molecular function
group) (Fig. 4E and F), either
upregulated or downregulated in SPATA12-transfected cells, were
mostly involved in protein binding, ATP/GTP binding, zinc ion
binding or DNA/RNA binding, indicating that the function of SPATA12
may be related with the activity of ‘binding’.
Pathway analysis and the expression
change in β-catenin signaling induced by SPATA12
The analysis of the differential expression of genes
led us to wonder whether these genes may represent related pathways
in which SPATA12 is involved. Thus, biological pathway analysis was
applied to characterize the unique gene networks associated with
SPATA12 in germ cells. Most genes associated with immune-related
pathways such as antigen processing and presentation, cell
adhesion, T cell receptor signaling pathway, and
development-related pathways such as MAPK, Jak-STAT and Wnt, were
significantly overexpressed which provides evidence that both
immune responses and developmental processes may be associated with
various functions of SPATA12 (Table
III). Changes in the expression of Wnt signaling-related genes
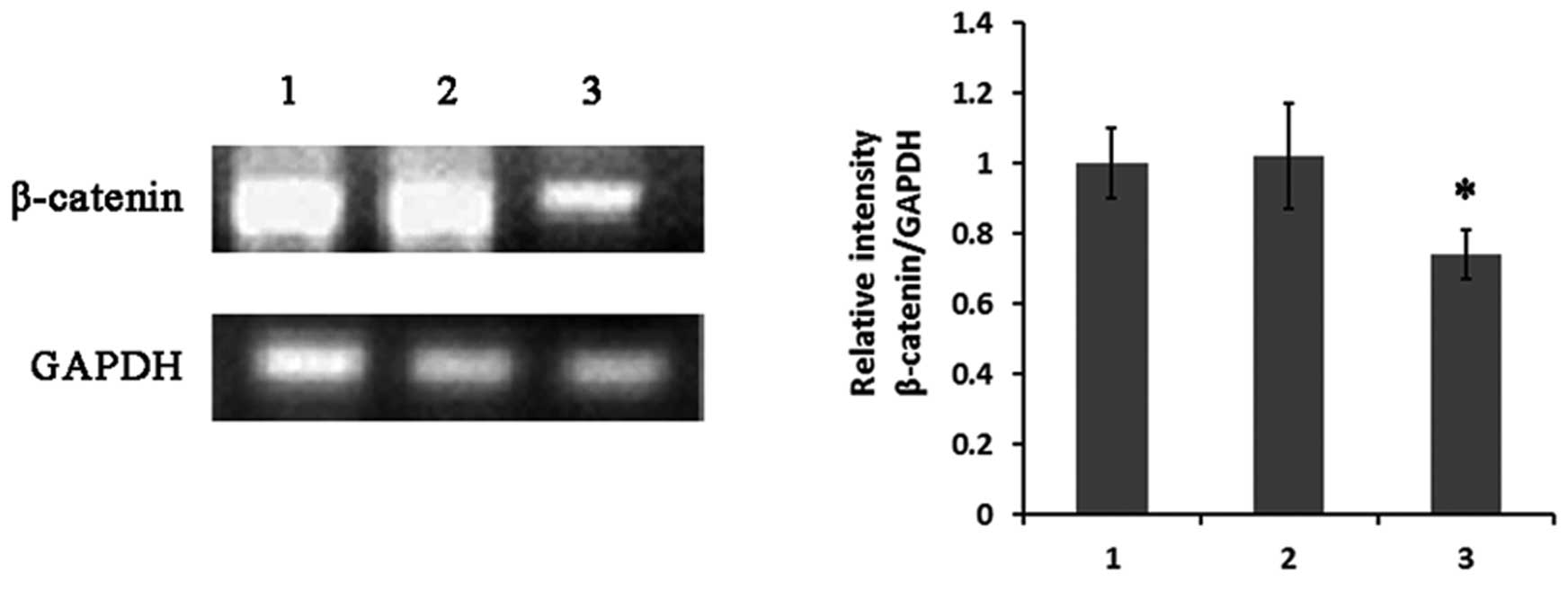
such as β-catenin supported this possibility. Semi-quantitative
RT-PCR results showed that the expression of β-catenin was
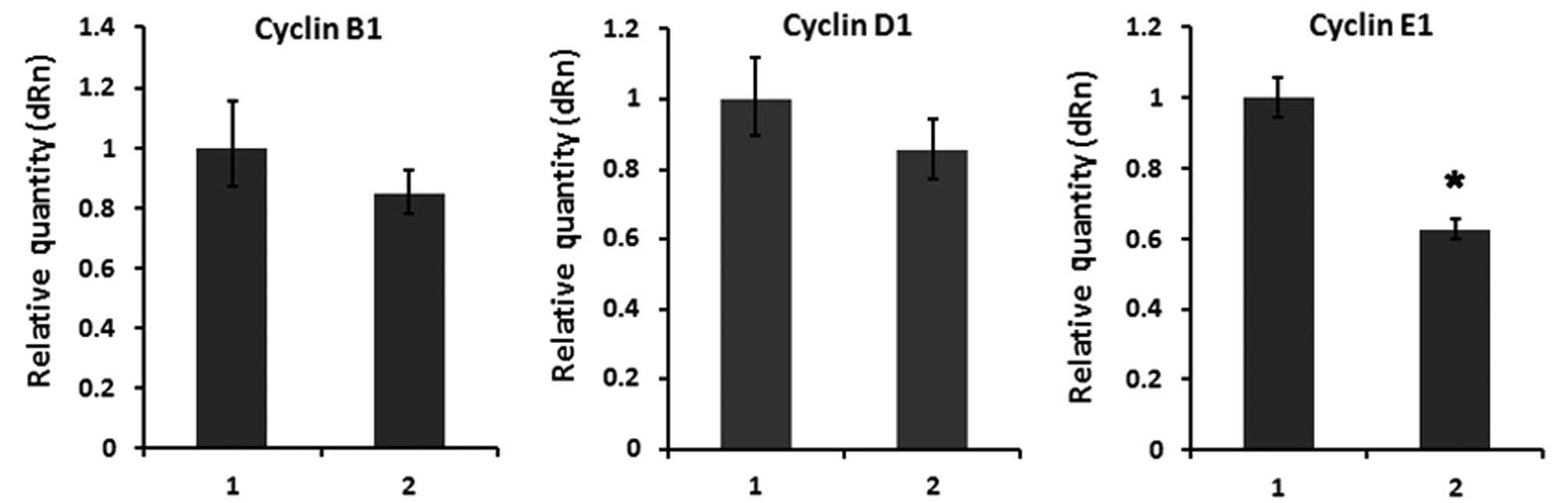
obviously downregulated (Fig. 5)
and its downstream target cell cycle gene cyclin E1 was
strongly decreased (Fig. 6) in
GC-1 spg cells transfected with SPATA12.
 | Table III.Selected pathways related with
SPATA12 identified by biological pathway analysis. |
Table III.
Selected pathways related with
SPATA12 identified by biological pathway analysis.
| Pathway name | Total | Gene (ratio) |
|---|
| Antigen processing
and presentation | 12 | H2-Q10 (2.36),
H2-Q7 (2.79), H2-T9 (2.49), H2-T3 (2.01), H2-Q6 (2.02), Tap1
(2.3442), Psme2 (2.4576), H2-K1 (2.28), H2-Q1 (2.92), Psme2b-ps
(2.4576), B2m (2.5449), H2-T23 (2.24) |
| Cell adhesion
molecules (CAMs) | 9 | H2-Q10 (2.36),
H2-Q7 (2.79), H2-T9 (2.49), H2-T3 (2.01), H2-Q6 (2.02), Itgb7
(2.73), H2-K1 (2.28), H2-Q1 (2.92), H2-T23 (2.24) |
| Type I diabetes
mellitus | 8 | H2-T23 (2.24),
H2-Q1 (2.92), H2-K1 (2.28), H2-Q6 (2.02), H2-T3 (2.01), H2-T9
(2.49), H2-Q7 (2.79), H2-Q10 (2.36) |
| Toll-like receptor
signaling pathway | 5 | Myd88 (2.0637),
Cxcl10 (5.5093), Stat1 (5.04), Cd14 (2.0947), Ccl5 (32.0033) |
| MAPK signaling
pathway | 4 | Ddit3 (2.0538),
Cd14 (2.0947), Dusp6 (2.9646), Map4k2 (0.4768) |
| Biosynthesis of
steroids | 3 | Sqle (0.4314), Pmvk
(0.4707), Fdft1 (0.4985) |
| ECM-receptor
interaction | 3 | Col6a1 (0.4919),
Col5a3 (0.4825), Itgb7 (2.7347) |
| Jak-STAT signaling
pathway | 3 | Isgf3g (3.57),
Stat1 (5.04), Stat2 (2.55) |
| Cytokine-cytokine
receptor interaction | 3 | Ccl5 (32.0033),
Vegfa (0.4651), Cxcl10 (5.5093) |
| Natural killer cell
mediated cytotoxicity | 2 | H2-T23 (2.24),
H2-K1 (2.28) |
| T cell receptor
signaling pathway | 1 | Pdk1 (0.4713) |
| Wnt signaling
pathway | 1 | Wnt10a (2.15) |
Discussion
High-density cDNA microarrays provide an important
tool to study the global patterns of gene expression. For example,
it has been used to understand interactions in a given cell, tissue
and organism under normal and diseased states (22,23), or it has been used for gene
discovery and function (24,25). Spermatogenesis is a complex
process of cell development and differentiation that requires the
highly regulated expression of multiple genes (26). Characterization and functional
analysis of new testis-specific genes related to spermatogenesis is
of momentous physiological and pathological significance in order
to understand the molecular mechanisms of spermatogenesis. Herein,
cDNA microarray was used to identify the upregulated or
downregulated genes affected by SPATA12 and obtain a global
overview on the expression patterns of these genes, aimed at
acquiring a further understanding of the function of SPATA12 and
the possible pathways in which SPATA12 is involved.
We identified 182 upregulated genes and 104
downregulated genes with a fold change of ≥2 or ≤0.5 (P≤0.05) in
expression. Through quantitative real-time RT-PCR, we confirmed the
expression of 10 (Ccl5, Fbxo39, Wnt10a, Rtp4, Sp100, Zbp1, Gys1,
Mtss1, Selenbp1 and Ndrg1) of the differentially expressed genes.
These genes along with their GO categories identified as being
differentially expressed following induction by SPATA12 may be of
significant biological interest.
Gene ontology studies provide biologically
meaningful information regarding genes including the cellular
location, molecular function and biological process. Fig. 4 lists each GO category and the
specific number of genes in their respective category that were
differentially expressed following induction by SPATA12. Biological
pathway analysis identified several related pathways such as
antigen processing and presentation, cell adhesion, MAPK, Jak-STAT
and Wnt. The differential expression of these signaling
pathway-related genes may also show that SPATA12 is associated with
immune responses in germ cell development.
Alterations in the expression of Wnt/β-catenin
signaling-related genes support this possibility. Wnt/β-catenin
signaling is one of the most important developmental signaling
pathways that control cell fate decisions and tissue patterning
during early and late embryonic development. β-catenin is a key
component of the Wnt/β-catenin signaling pathway (27–30) and was previously reported to be
expressed in the plasma membrane and cytoplasm of germ cells during
testis development. Suppression of Wnt/β-catenin signaling is
necessary for the normal development of primordial germ cells since
stabilization of β-catenin in germ cells was found to delay cell
cycle progression resulting in germ cell deficiency (31). Here, we showed that expression of
SPATA12 by transfection downregulated β-catenin in GC-1 spg
cells. Studies in the literature reported that the reduction in
β-catenin level was accompanied by inhibition of its
transactivation potential and downregulation of downstream target
genes, such as Myc, cyclin D1 and cyclin E1
(28,32,33). In the present study, our data
suggest that SPATA12 negatively regulates cell cycle-related gene
cyclin E1, which indicates that SPATA12 inhibits cell
proliferation via downregulation of β-catenin in GC-1 spg
cells. These results were shown to be of particular relevance
between SPATA12 and the β-catenin signaling pathway.
Taken together, the present study demonstrated
alterations in the gene expression profile of GC-1 spg cells
transfected with SPATA12. The functional classification of these
genes and their expression profiles provide useful information to
understand the transcriptional regulation of SPATA12. A number of
genes, GO categories and biological pathways such as immune
responses may be associated with the function of SPATA12. Moreover,
our study showed that SPATA12 may interact with the β-catenin
signaling pathway and SPATA12 could negatively regulate β-catenin
signaling during spermatogenesis.
Acknowledgements
This study was supported by the
National Natural Science Foundation of China (nos. 30872763,
81270735) and the Fundamental Research Funds for the Central
Universities of China (no. 531107040314).
References
|
1.
|
O’Flynn O’Brien KL, Varghese AC and
Agarwal A: The genetic causes of male factor infertility: a review.
Fertil Steril. 93:1–12. 2010.
|
|
2.
|
Ferlin A, Raicu F, Gatta V, Zuccarello D,
Palka G and Foresta C: Male infertility: role of genetic
background. Reprod Biomed Online. 14:734–745. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Coussens M, Maresh JG, Yanagimachi R,
Maeda G and Allsopp R: Sirt1 deficiency attenuates spermatogenesis
and germ cell function. PLoS One. 3:e15712008. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Takeuchi A, Mishina Y, Miyaishi O, Kojima
E, Hasegawa T and Isobe K: Heterozygosity with respect to Zfp148
causes complete loss of fetal germ cells during mouse
embryogenesis. Nat Genet. 33:172–176. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Zhou CX, Zhang YL, Xiao L, et al: An
epididymis-specific beta-defensin is important for the initiation
of sperm maturation. Nat Cell Biol. 6:458–464. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Roy A, Yan W, Burns KH and Matzuk MM:
Tektin3 encodes an evolutionarily conserved putative testicular
microtubules-related protein expressed preferentially in male germ
cells. Mol Reprod Dev. 67:295–302. 2004. View Article : Google Scholar
|
|
7.
|
Takahashi T, Tanaka H, Iguchi N, et al:
Rosbin: a novel homeobox-like protein gene expressed exclusively in
round spermatids. Biol Reprod. 70:1485–1492. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Kissel H, Georgescu MM, Larisch S, Manova
K, Hunnicutt GR and Steller H: The Sept4 septin locus is required
for sperm terminal differentiation in mice. Dev Cell. 8:353–364.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Zhu H, Zhu JX, Lo PS, et al: Rescue of
defective pancreatic secretion in cystic-fibrosis cells by
suppression of a novel isoform of phospholipase C. Lancet.
362:2059–2065. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Dan L, Lifang Y and Guangxiu L: Expression
and possible functions of a novel gene SPATA12 in human testis. J
Androl. 28:502–512. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Liu ZW, Lin YT, Liu XM, Yu WW, Zhang YS
and Li D: Experimental study of inhibition of tumor cell
proliferation by a novel gene SPATA12. Zhong Nan Da Xue Xue Bao Yi
Xue Ban. 37:222–227. 2012.(In Chinese).
|
|
12.
|
Ji L, Minna JD and Roth JA: 3p21.3 tumor
suppressor cluster: prospects for translational applications.
Future Oncol. 1:79–92. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Riquelme E, Tang M, Baez S, Diaz A, Pruyas
M, Wistuba II and Corvalan A: Frequent epigenetic inactivation of
chromosome 3p candidate tumor suppressor genes in gallbladder
carcinoma. Cancer Lett. 250:100–106. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Kim HJ, Joo HJ, Kim YH, et al: Systemic
analysis of heat shock response induced by heat shock and a
proteasome inhibitor MG132. PLoS One. 6:e202522011. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Korkor MT, Meng FB, Xing SY, Zhang MC, Guo
JR, Zhu XX and Yang P: Microarray analysis of differential gene
expression profile in peripheral blood cells of patients with human
essential hypertension. Int J Med Sci. 8:168–179. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Roy Choudhury D, Small C, Wang Y, Mueller
PR, Rebel VI, Griswold MD and McCarrey JR: Microarray-based
analysis of cell-cycle gene expression during spermatogenesis in
the mouse. Biol Reprod. 83:663–675. 2010.PubMed/NCBI
|
|
17.
|
King HC and Sinha AA: Gene expression
profile analysis by DNA microarrays: promise and pitfalls. JAMA.
286:2280–2288. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Zeng S and Gong Z: Expressed sequence tag
analysis of expression profiles of zebrafish testis and ovary.
Gene. 294:45–53. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Xiao P, Tang A, Yu Z, Gui Y and Cai Z:
Gene expression profile of 2058 spermatogenesis-related genes in
mice. Biol Pharm Bull. 31:201–206. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Lo LJ, Zhang ZH, Hong N, Peng JR and Hong
YH: 3640 unique EST clusters from the medaka testis and their
potential use for identifying conserved testicular gene expression
in fish and mammals. PLoS One. 3:e39152008. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
LI D and LU GX: Identification and
expression of a novel human testis-specific gene by digital
differential display. Chin Med J. 117:1791–1796. 2004.PubMed/NCBI
|
|
22.
|
Sridhar K, Ross DT, Tibshirani R, Butte AJ
and Greenberg PL: Relationship of differential gene expression
profiles in CD34+ myelodysplastic syndrome marrow cells
to disease subtype and progression. Blood. 114:4847–4858. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Creekmore AL, Silkworth WT, Cimini D,
Jensen RV, Roberts PC and Schmelz EM: Changes in gene expression
and cellular architecture in an ovarian cancer progression model.
PLoS One. 6:e176762011. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Mattison J, Weyden L, Hubbard T and Adams
DJ: Cancer gene discovery in mouse and man. Biochim Biophys Acta.
1796:140–161. 2009.PubMed/NCBI
|
|
25.
|
Chen F, Zhu HH, Zhou LF, et al: Genes
related to the very early stage of ConA-induced fulminant
hepatitis: a gene-chip-based study in a mouse model. BMC Genomics.
11:2402010. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Eddy EM: Male germ cell gene expression.
Recent Prog Horm Res. 57:103–128. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Davidson G: The cell cycle and Wnt. Cell
Cycle. 9:1667–1668. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Chang H, Gao F, Guillou F, Taketo MM, Huff
V and Behringer RR: Wt1 negatively regulates β-catenin signaling
during testis development. Development. 135:1875–1885. 2008.
|
|
29.
|
Olmeda D, Castel S, Vilaró S and Cano A:
Beta-catenin regulation during the cell cycle: implications in G2/M
and apoptosis. Mol Biol Cell. 14:2844–2860. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Peifer M and Polakis P: Wnt signalling in
oncogenesis and embryogenesis: a look outside the nucleus. Science.
287:1606–1609. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Kimura T, Nakamura T, Murayama K, et al:
The stabilization of beta-catenin leads to impaired primordial germ
cell development via aberrant cell cycle progression. Dev Biol.
300:545–553. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Tetsu O and McCormick F: Beta-catenin
regulates expression of cyclin D1 in colon carcinoma cells. Nature.
398:422–426. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Botrugno OA, Fayard E, Annicotte JS, et
al: Synergy between LRH-1 and beta-catenin induces G1
cyclin-mediated cell proliferation. Mol Cell. 15:499–509. 2004.
View Article : Google Scholar : PubMed/NCBI
|