Introduction
Skin aging can be divided into intrinsic
(chronologic) aging, which is the process of senescence that
affects all body organs and extrinsic aging (photoaging), which
occurs due to exposure to environmental factors. An important
factor that influences extrinsic aging is sunlight, particularly
exposure to ultraviolet (UV)B irradiation, which causes photoaging.
Chronic exposure of human skin to UVB radiation results in
photoaging and induces the production of matrix metalloproteinases
(MMPs) (1).
MMPs are responsible for the degradation of the
collagenous extracellular matrix (ECM) in connective tissues
(2). MMP-1 preferentially
degrades fibrillar collagens, which maintain the tensile strength
of fetal membranes. In contrast, MMP-3 degrades an extremely wide
array of ECM substrates and can activate secreted zymogenic forms
of other MMPs (3).
UV exposure is an important factor in photoaging. UV
irradiation of cultured human dermal fibroblasts (HDFs) in
vitro or human skin in vivo induces the production of
MMPs (4–6). Excessive matrix degradation by
UV-induced MMPs secreted by various types of cells (e.g.,
keratinocytes, fibroblasts and inflammatory cells) has been shown
to contribute significantly to connective tissue damage that occurs
during photoaging (7,8). UVB irradiation can induce MMP
expression by activating transcription factors, such as nuclear
factor-κB (NF-κB) and activator protein-1 (AP-1) (9,10).
The mitogen-activated protein kinase (MAPK) signaling pathway is
important for AP-1 activation; IκB kinase (IKK), phosphoinositide 3
kinase (PI3K)-Akt and p38 MAPK have been shown to activate NF-κB,
depending on the cell type (11,12). Thus, the inhibition of UVB-induced
MMP expression and/or its upstream regulatory pathways is critical
for the treatment of photoaging of the skin.
Decursin is a coumarin compound found in the roots
of Angelica gigas Nakai, which has been traditionally used
in Korean folk medicine as a tonic and for the treatment of anemia
and other diseases (13).
Decursin induces cell cycle arrest and apoptosis in human prostate,
breast, bladder and colon cancer cells (14–16). Recent reports have demonstrated
that decursin blocks MMP-9 expression through the inhibition of
NF-κB activation in macrophages and cancer cells (17–19). However, the inhibitory effects of
UVB-induced MMP expression through NF-κB activation by decursin are
not yet well defined.
In the present study, we evaluated the preventive
effects of decursin on the UVB-induced production of MMPs in HDFs.
Decursin blocked the UVB-induced NF-κB pathway, which inhibits the
expression of MMPs. These results suggest that decursin is useful
for the prevention of skin photoaging.
Materials and methods
Materials
3-(4,5-Dimethyl-thiazol-2-yl)-2,5-diphenyltetrazolium bromide
(MTT), dimethyl sulfoxide (DMSO) and anti-β-actin antibody were
purchased from Sigma (St. Louis, MO, USA). Primary antibodies for
MMP-1 and MMP-3 were obtained from R&D Systems (Minneapolis,
MN, USA). Dulbecco’s modified Eagle’s medium (DMEM) with high
glucose level, Medium 154, growth supplement, fetal bovine serum
(FBS) and phosphate-buffered saline (PBS) were obtained from
Gibco-BRL (Gaithersburg, ME, USA). Primary antibodies for p50, p65,
IκBα, proliferating cell nuclear antigen (PCNA) and horseradish
peroxidase (HRP)-conjugated IgG were obtained from Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA).
Plant extracts and purification
The roots of A. gigas Nakai (Umbelliferae
family) were extracted serially with methanol, ethylacetate and
n-butanol and fractionated. From the ethyl-acetate fraction,
decursin was isolated using silica gel column chromatography. After
column chromatography, the structure of the purified coumarin
compounds, decursin (C19H20O5) and
decursinol angelate (molecular weight, 328 g) were characterized by
gas chromatography (Shimadzu, Kyoto, Japan), nuclear magnetic
resonance (JEOL JNM-LA 400; Japan) and mass spectroscopy (JEOL-AX
505WA) at Daegu Haany University, Daegu, Korea.
Isolation and culture of HDFs
HDFs were aseptically isolated from foreskin. The
epidermis and dermis were separated by incubation in media with 0.9
U/ml dispase at 4°C for 16 h. After the epidermis and dermis were
mechanically separated, the dermis was minced, attached to the
surface of a tissue culture flask and incubated with DMEM
containing 10% FBS for 1–2 weeks (20). Dermal fibroblasts that spread as
radial outgrowths from the attached pieces of dermis were cultured
in DMEM containing 10% FBS and 1% antibiotics at 37°C in a 5%
CO2 incubator.
UV irradiation
HDFs were rinsed twice with PBS and irradiated using
a UVB cross-linker (6×8 W, 312 nm; Model CL-508M; Vilber Lourmat,
Paris, France) (20). HEKn were
irradiated using a Stratalinker UV crosslinker (Model 2400; Agilent
Technologies, Cold Spring, NY, USA). Immediately after irradiation,
fresh serum-free medium was added to the HDFs, and complete growth
medium was added to the HEKn. Responses were measured after
incubation for each experimental condition. The same schedule of
medium changes was followed for control cells.
Determination of cell viability
The protective effect of decursin against UV-induced
cytotoxicity of HDFs was determined using the MTT assay. Briefly,
HDFs were seeded at a density of 3×104 cells/plate and
allowed to attach. After 24 h, the cells were treated with various
concentrations of decursin (1, 5, 10, 30 and 50 μM). After
incubation for 24 h, the cells were washed twice with PBS and MTT
(0.5 mg/ml PBS) was added to each well. The plates were incubated
at 37°C for 30 min. Formazan crystals that had formed were
dissolved by adding DMSO (100 μl/well) and the absorbance
was measured at 570 nm using a microplate reader (Model 3550;
Bio-Rad, Richmond, CA, USA).
Trypan blue exclusion test for
cytotoxicity
Cells were seeded onto a 10-cm dish and allowed to
attach for 24 h. They were then treated with UVB at 25
mJ/cm2. After 24 h, the cells were detached from the
wells by treatment with trypsin, followed by staining with trypan
blue; non stained cells were counted under an optical microscope
with a hemocytometer.
Western blot analysis
HDFs (2×106 cells) were irradiated with
UVB (25 or 15 mJ/cm2); the cells were treated with
decursin for 24 h and lysed using 40 μl of ice cold
M-PER® Mammalian Protein Extraction Reagent (Pierce
Biotechnology, Inc., Rockford, IL, USA). Protein concentrations in
the lysates were determined using the Bradford method (21). Samples were separated using 10%
SDS-PAGE gels with 3% stacking gels; the resolved proteins were
transferred to a Hybond™-PVDF membrane using a western blot
apparatus (Bio-Rad). Polyvinylidene fluoride (PVDF) membranes were
blotted with 1 μg/ml of primary antibodies for MMP-1, MMP-3,
p50, p65, PCNA, or β-actin. HRP-conjugated IgG was used as a
secondary antibody. Protein expression levels were determined by
analyzing the signals captured on the PVDF membranes using an image
analyzer (LAS-1000; Fuji Film, Japan).
Quantitative real-time PCR assay
Total RNA was extracted from cells using a FastPure™
RNA kit (Takara Bio, Inc., Shiga, Japan). RNA concentration and
purity were determined by measuring the absorbance at both 260 and
280 nm. Then, cDNA was synthesized from 1 μg of total RNA
using a PrimeScript™ RT reagent kit (Takara Bio, Inc.). MMP-1 and
MMP-3 mRNA expression levels were analyzed using real-time PCR with
the ABI PRISM 7900 sequence detection system and the SYBR-Green
reagent (Applied Biosystems, Foster City, CA, USA). Primers used in
the reaction were:MMP-1 (NM 002424.2)
sense,5′-AGTGACTGGGAAACCGATGCTGA-3′ and antisense,
5′-CTCTTGGCAAATCTGGCCTGTAA-3′; MMP-3 (NM 002422) sense,
5′-ATTCCATGGAGCCAGGCTTTC-3′ and antisense,
5′-CATTTGGGTCAAACTCCAACTGTG-3′ and GAPDH (NM 002046) sense,
5′-ATGGAAATCCC ATCACCATCTT-3′ and antisense, 5′-CGCCCCACTTGA
TTTTGG-3′. To control for variations in the mRNA concentration, all
results were normalized to the housekeeping gene GAPDH. Relative
quantitations were performed using the comparative ΔΔCt method
according to the manufacturer’s instructions.
Determination of MMP-1 and MMP-3
secretion with ELISA
HDFs were seeded in 100-mm culture dishes at a
density of 2×106 cells/dish and then irradiated with UVB
(25 mJ/cm2). Following 24 h of incubation, the culture
supernatants were collected and centrifuged at 10,000 × g for 5 min
to remove the particulate matter and stored at −80°C in fresh
tubes. The protein concentration in the supernatants was determined
using the Bradford method (21).
The active MMP-1 in culture supernatants was quantified by
fluorescent assay, using the Fluorokine E Human Active MMP-1
Fluorescent assay kit, and MMP-3 in the cell culture supernatants
was then determined using Quantikine ELISA kits (all from R&D
Systems), according to the manufacturer’s protocol.
Preparation of nuclear extract
HDFs (2×106 cells) were irradiated with
25 mJ/cm2 UVB and then treated with decursin for 3 h.
Cells were immediately washed twice, scraped into 1.5 ml of ice
cold PBS (pH 7.9) and then pelleted at 12,000 × g for 30 sec.
Cytoplasmic and nuclear extracts were prepared from cells using
NE-PER® Nuclear and Cytoplasmic Extraction Reagents
(Pierce Biotechnology).
Electrophoretic mobility shift assay
(EMSA)
Activation of NF-κB and AP-1 was assayed with a gel
mobility shift assay using nuclear extracts. An oligonucleotide
containing the κ-chain (κB, 5′-CCGGTTAACAGAGGGGGCTTTCCGAG-3′) or
AP-1 (5′-CGCTTGATGAGTCAGCCGGAA-3′) binding site was synthesized and
used as a probe for the gel retardation assay. The two
complementary strands were annealed and labeled with [α-32P]dCTP.
Labeled oligonucleotides (10,000 cpm), 10 μg of nuclear
extracts and binding buffer [10 mM Tris-HCl (pH 7.6), 500 mM KCl,
10 mM EDTA, 50% glycerol, 100 ng poly(dI·dC), 1 mM dithiothreitol]
were then incubated for 30 min at room temperature in a final
volume of 20 μl. The reaction mixtures were analyzed by
electrophoresis on 4% polyacrylamide gels in 0.5X Tris-borate
buffer. The gels were dried and examined by autoradiography.
Specific binding was controlled by competition with a 50-fold
excess and cold AP-1 oligonucleotide.
Statistical analysis
Statistical analysis was performed using analysis of
variance (ANOVA) and Duncan’s test. A P-value <0.05 was
considered to indicate a statistically significant result.
Results
Decursin protects HDFs against UVB
irradiation
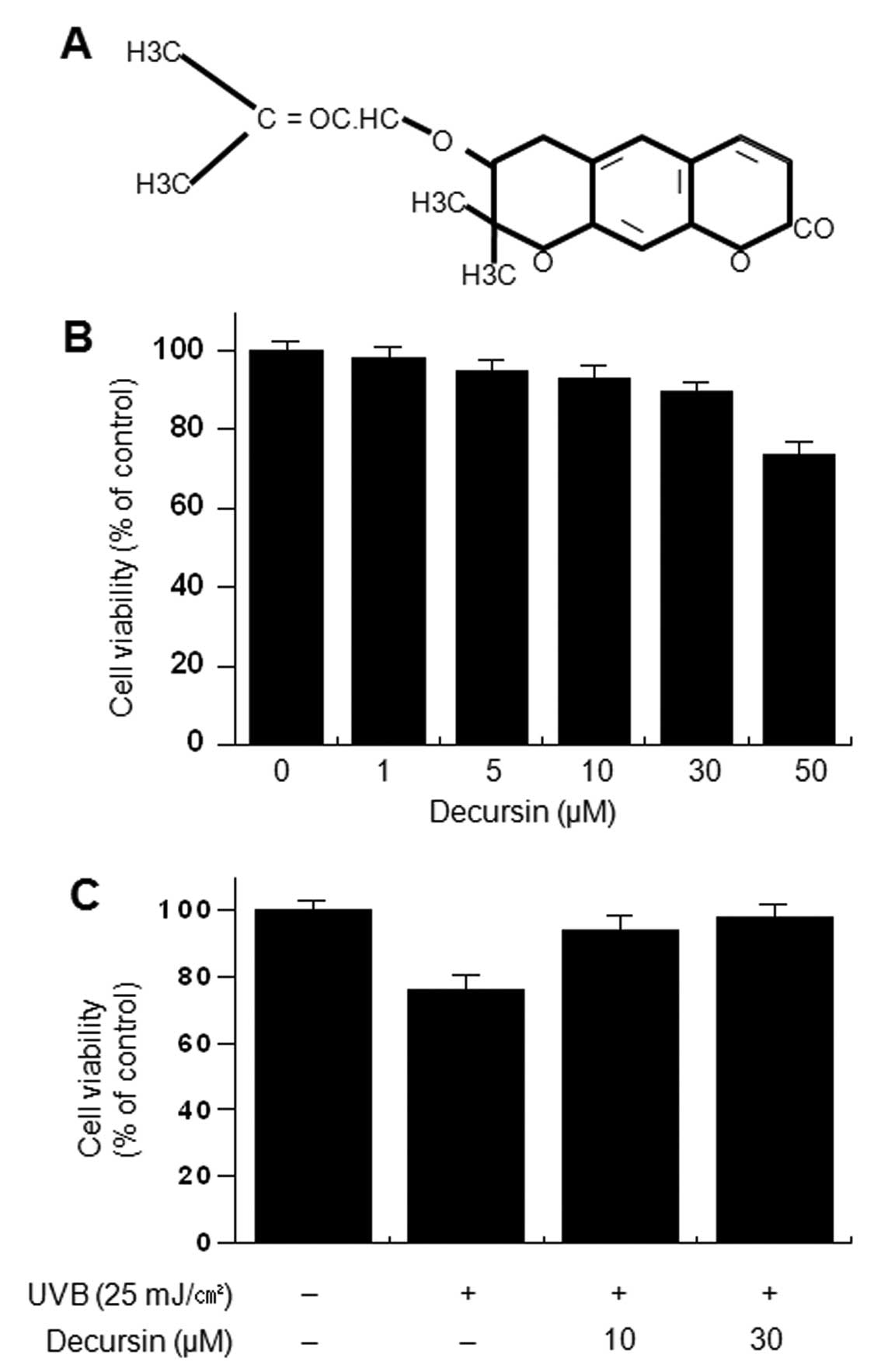
The structure of decursin is shown in Fig. 1A. To investigate the cytotoxicity
of decursin, HDFs were treated with various concentrations of
decursin for 24 h. Cell viability was determined using the MTT
assay. Decursin did not cause a significant change in the viability
of HDFs up to 50 μM (Fig.
1B). To investigate the cell protective effect of decursin on
UVB-induced cytotoxicity, cells were incubated with the indicated
concentrations of decursin for 24 h in the presence of UVB.
UVB-induced cytotoxicity was determined using the trypan blue
exclusion test. Decursin (10 and 30 μM) significantly
inhibited cell toxicity induced by UVB irradiation (Fig. 1C).
Decursin inhibits the UVB-induced
expression and secretion of MMP-1 and MMP-3 in HDFs
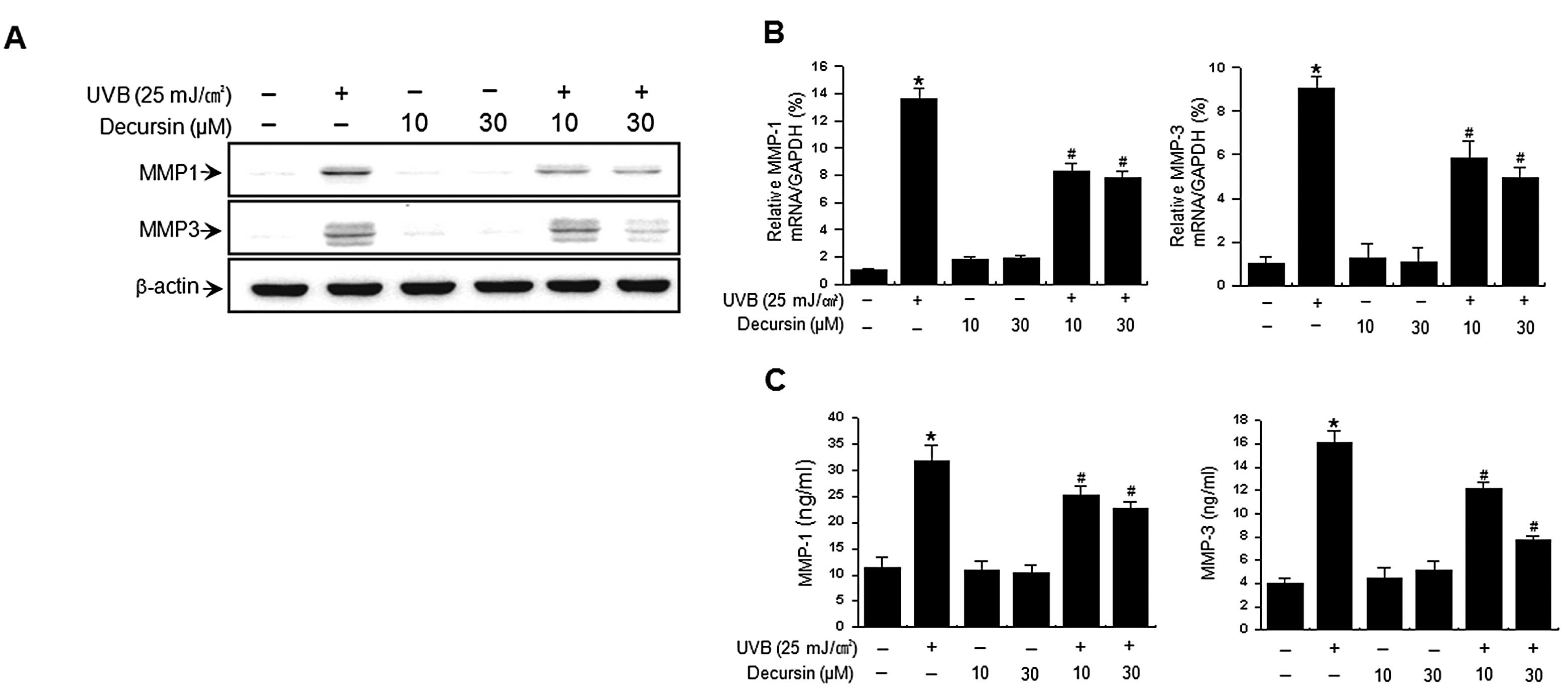
UVB activates MMP secretion, which is a hallmark of
skin aging (4,5,22).
We examined the effects of decursin on UVB-induced expression of
MMP-1 and MMP-3. Western blot analysis revealed that irradiation of
HDFs with UVB (25 mJ/cm2) markedly increased MMP-1 and
MMP-3 levels (Fig. 2A). The
UVB-induced increase in MMP levels was significantly reduced by
treatment with decursin. Consistent with these results, real-time
PCR analysis also showed an increase in expression of MMP-1 and
MMP-3 mRNA after UVB irradiation while treatment of HDFs with
decursin suppressed this UVB-induced increase in MMP-1 and MMP-3
expression (Fig. 2B). We also
determined the effect of decursin on UVB-induced MMP secretion with
ELISA. UVB irradiation of HDFs resulted in an increase in MMP-1 and
MMP-3 secretion, while decursin significantly diminished the
UVB-induced MMP-1 and MMP-3 secretion (Fig. 2C). Decursin itself had no effects
on expression and secretion of MMP-1 and MMP-3 in HDFs. These
results indicate that decursin inhibits the UVB-induced expression
and secretion of MMP-1 and MMP-3 in HDFs.
Effect of decursin on UVB-induced NF-κB
and AP-1 DNA-binding activities
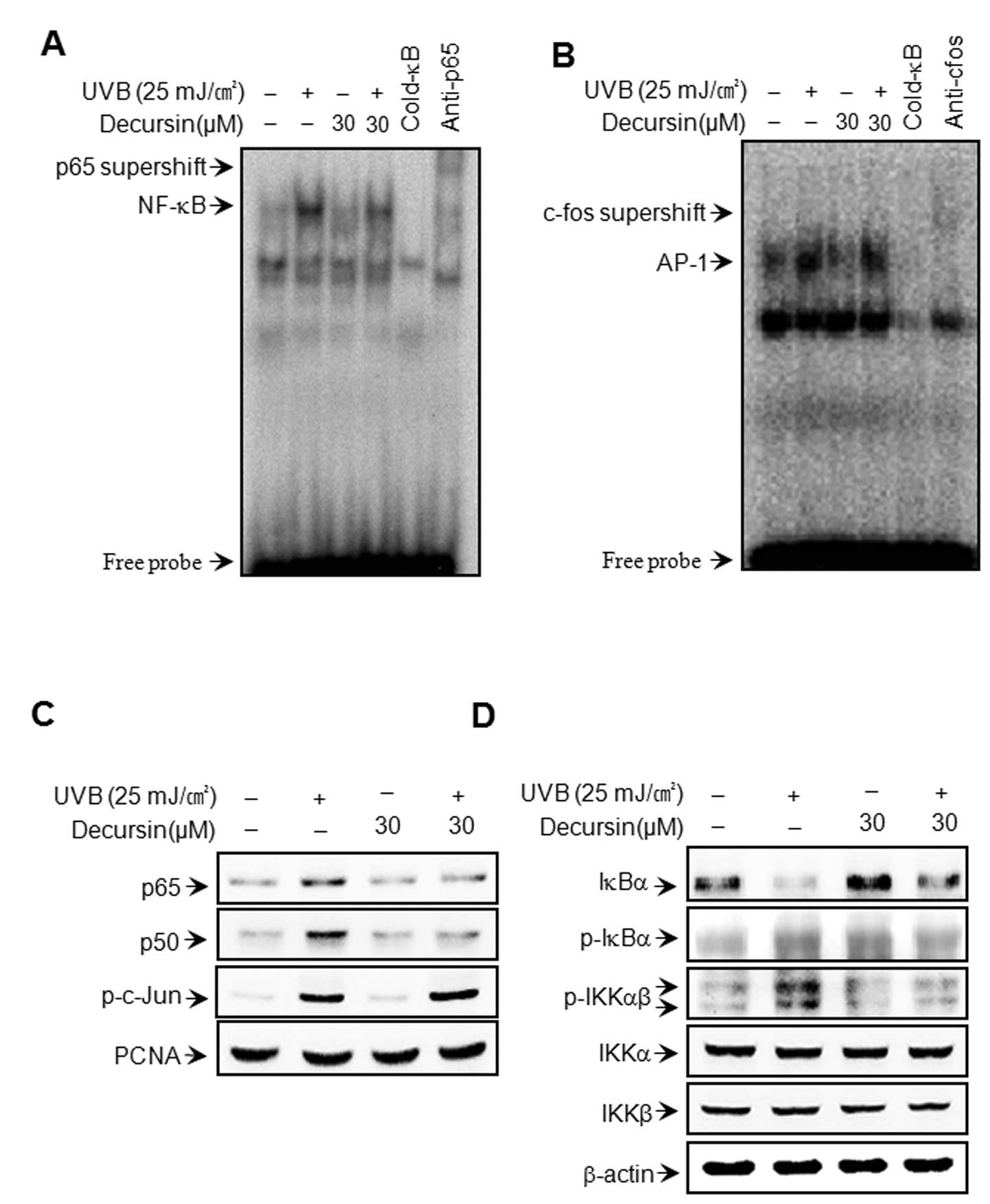
To clarify the mechanism of decursin-mediated
inhibition of MMP-1 and MMP-3 expression, the effect of decursin on
UVB-induced activation of NF-κB and AP-1 was evaluated using EMSA
and western blot analysis. As shown in Fig. 3A and B, pre-treatment with
decursin inhibited UVB-induced DNA binding activity of NF-κB, but
not AP-1. Decursin itself had no effect on the DNA binding activity
of NF-κB or AP-1. Additionally, we determined the levels of p65,
p50, p-c-Jun in the nuclear fraction. Cell treatment with UVB
resulted in increased levels of p65, p50 and p-c-Jun; however,
decursin blocked the UVB-induced translocation of p65 and p50 to
the nucleus (Fig. 3C). These
results suggest that decursin specifically blocks NF-κB activation
in HDFs. The IκB kinase (IKK) enzyme complex is part of the signal
transduction cascade upstream of NF-κB. IKK specifically
phosphorylates the inhibitory IκB protein. Under basal conditions,
the cytoplasmic protein IκB directly binds to p65 and p50 subunits
and represses their nuclear translocation. IKK phosphorylation
results in the dissociation of IκB from NF-κB and thereby activates
NF-κB (23–26). Therefore, we determined the
changes in the levels of p-IKKαβ and p-IκBα in the cytoplasmic
fraction. The cytoplasmic fraction of UVB-stimulated HDFs showed
higher levels of p-IKKαβ and p-IκBα than in unstimulated cells;
however, the UVB-induced increase in the levels of p-IKKαβ and
p-IκBα was significantly suppressed by treatment with decursin
(Fig. 3D).
Effect of decursin on the UVB-induced MAP
kinase signaling pathway
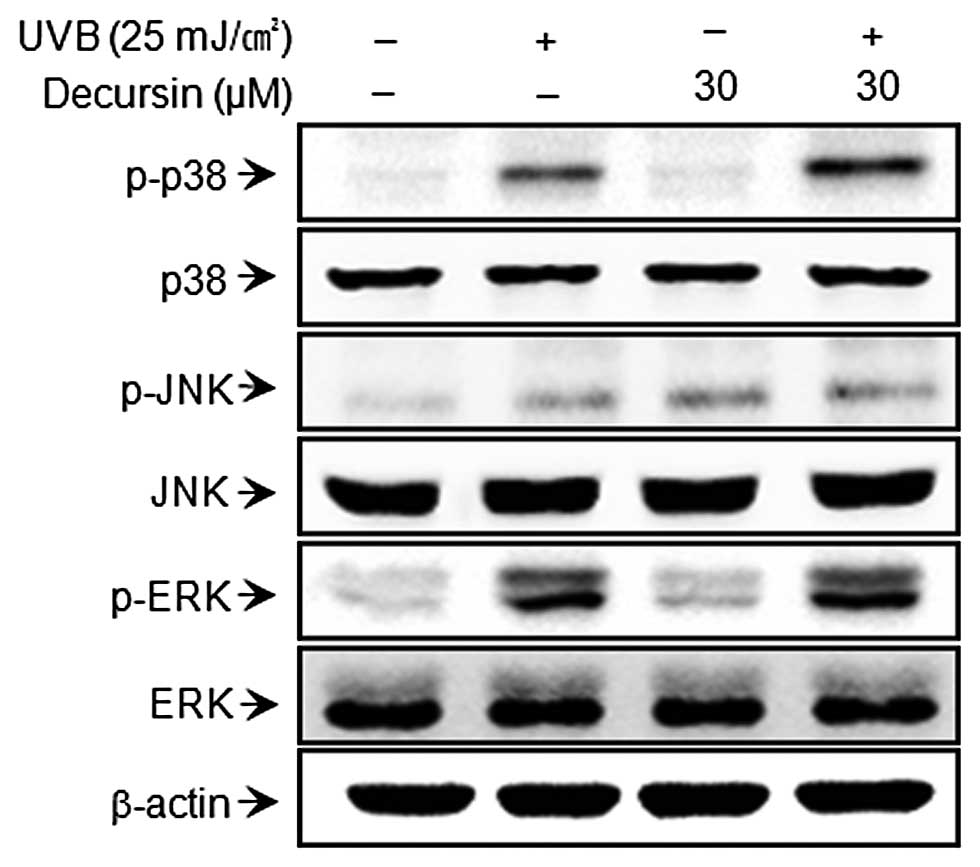
Since MAP kinase is an upstream regulator of NF-κB
and AP-1, the role of MAP kinase (ERK, p38 and JNK) in the
activation of MMP expression is fairly well understood (27,28). We investigated the effect of
decursin on UVB-induced activation of MAP kinase. Decursin showed
no effects on MAPK (Fig. 4).
These results suggest that the MAPK pathway is not involved in the
regulation of UVB-induced expression of MMP by decursin.
Discussion
In the present study, we demonstrated the preventive
effects of decursin on photoaging caused by MMP-1 and MMP-3. In
previous studies, decursin was found to prevent MMP-9 expression by
suppression of the NF-κB pathway in cancer cells and macrophages
(17–19). Our results also demonstrated that
decursin blocked UVB-induced activation of NF-κB, which has an
important role in MMP-1 and MMP-3 expression.
Skin aging can be attributed to extrinsic aging
(photoaging) and intrinsic (chronological) aging. Photoaging
involves premature skin aging caused by repeated exposure to the
sun (8,29,30). UV irradiation of cultured HDFs
in vitro or human skin in vivo was found to induce
the expression of MMP-1 and MMP-3, which play important roles in
ECM components during skin aging (5,6,31).
Varani et al (32)
reported that MMP levels increase and collagen synthesis decreases
in sun-protected human skin in vivo as age increases.
Moreover, it was suggested that excessive matrix degradation by
UV-induced MMPs secreted by various types of cells (e.g.,
keratinocytes, fibroblasts and inflammatory cells) contributes
substantially to connective tissue damage that occurs during skin
photoaging (7,8,33).
Thus, we focused on the targets of decursin’s action in signal
transduction pathways involved in the induction of the two major
MMP family members after UVB irradiation.
UV irradiation includes three types: UVA
(wavelength, 320–400 nm), UVB (280–320 nm) and UVC (200–280 nm). In
particular, studies concerning skin have focused on UVB intensity
due to stratospheric ozone depletion (34,35). It is well known that the
UVB-inducible genes involved in skin aging are primarily composed
of several MMPs involved in the degradation of the connective
tissues of the skin (22,35). Recent studies have focused on the
regulatory molecular mechanisms underlying UVB-induced upregulation
of MMPs (36,37).
The present study found that transcription factors
may be targets of decursin during UV-induced skin damage. NF-κB and
AP-1 are ubiquitous transcription factors that govern the
expression of genes encoding cytokines, chemokines, growth factors,
cell adhesion molecules and several acute phase proteins in healthy
and disease states (38,39). Therefore, the development of
strategies that target these transcription factors may provide
novel therapeutic tools for treating or preventing various
diseases. UVB-mediated photoaging is prevented by the suppression
of NF-κB and AP-1 activation (31,40,41). In fact, NF-κB and AP-1 are known
to increase MMP-1 expression in the dermis (42,43). These studies suggest that NF-κB
and AP-1 play important roles in MMP expression after UV
irradiation. Previous studies demonstrated that NF-κB and AP-1 are
molecular targets in decursin-treated cells (44,45), which suggests that targeting NF-κB
and AP-1 in UV irradiation-mediated MMP expression by using
decursin may provide a novel therapeutic tool for treating or
preventing photoaging. Our results showed that decursin strongly
blocked UVB-induced NF-κB activation (Fig. 3). Furthermore, decursin
significantly inhibited UVB-induced expression of MMPs in HDFs
(Fig. 2). Our data indicate that
decursin is a potent inhibitor of UVB-mediated NF-κB activation,
which blocks the UVB-induced expression of MMPs in HDFs.
The MAPK pathway is involved in the regulation of
cell proliferation, apoptosis, cytokine expression and MMP
production. The three major MAPK families, JNK, ERK and p38 kinase,
are expressed in HDFs, and the active phosphorylated forms can also
be detected (46,47). Previous studies have shown that
the MAPK signaling pathway is important for AP-1 activation; I-κB
kinase (IKK), phosphoinositide 3 kinase (PI3K)-Akt and p38 MAPK
have been shown to activate NF-κB, depending on the cell type
(11,12,48–50). In this study, decursin displayed no effects on
phosphorylation of p38, JNK and ERK. These data indicate that
decursin is involved in NF-κB, but not in the MAPK signaling
pathway in HDFs
In conclusion, the development of novel MMP
inhibitors may be a promising strategy for skin cancer therapy and
photoaging. Our results demonstrate that decursin is a potent
inhibitor of UVB-induced expression of MMPs that blocks the NF-κB
signaling pathway in HDFs. Therefore, decursin may be a potential
therapeutic candidate for the prevention and treatment of
photoaging.
Acknowledgements
This study was supported by the
National Research Foundation of Korea (NRF) grant funded by the
Korea government (MEST) (nos. 2011-0030716 and 2011-0023921).
References
|
1.
|
Ho JN, Lee YH, Park JS, et al: Protective
effects of aucubin isolated from Eucommia ulmoides against
UVB-induced oxidative stress in human skin fibroblasts. Biol Pharm
Bull. 28:1244–1248. 2005.PubMed/NCBI
|
|
2.
|
Scharffetter-Kochanek K, Brenneisen P,
Wenk J, et al: Photoaging of the skin from phenotype to mechanisms.
Exp Gerontol. 35:307–316. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Knauper V, Lopez-Otin C, Smith B, Knight G
and Murphy G: Biochemical characterization of human collagenase-3.
J Biol Chem. 271:1544–1550. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Pillai S, Oresajo C and Hayward J:
Ultraviolet radiation and skin aging: roles of reactive oxygen
species, inflammation and protease activation, and strategies for
prevention of inflammation-induced matrix degradation - a review.
Int J Cosmet Sci. 27:17–34. 2005. View Article : Google Scholar
|
|
5.
|
Brenneisen P, Sies H and
Scharffetter-Kochanek K: Ultraviolet-B irradiation and matrix
metalloproteinases: from induction via signaling to initial events.
Ann NY Acad Sci. 973:31–43. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Rittié L and Fisher GJ: UV-light-induced
signal cascades and skin aging. Ageing Res Rev. 1:705–720.
2002.PubMed/NCBI
|
|
7.
|
Fisher GJ, Datta SC, Talwar HS, et al:
Molecular basis of sun-induced premature skin ageing and retinoid
antagonism. Nature. 379:335–339. 1996. View
Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Chung JH, Seo JY, Lee MK, et al:
Ultraviolet modulation of human macrophage metalloelastase in human
skin in vivo. J Invest Dermatol. 119:507–512. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Cooper SJ and Bowden GT: Ultraviolet B
regulation of transcription factor families: roles of nuclear
factor-kappa B (NF-kappaB) and activator protein-1 (AP-1) in
UVB-induced skin carcinogenesis. Curr Cancer Drug Targets.
7:325–334. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Bell S, Degitz K, Quirling M, Jilg N, Page
S and Brand K: Involvement of NF-kappaB signalling in skin
physiology and disease. Cell Signal. 15:1–7. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Karin M: The regulation of AP-1 activity
by mitogen-activated protein kinases. J Biol Chem. 270:16483–16486.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Madrid LV, Mayo MW, Reuther JY and Baldwin
AS Jr: Akt stimulates the transactivation potential of the RelA/p65
subunit of NF-kappa B through utilization of the Ikappa B kinase
and activation of the mitogen-activated protein kinase p38. J Biol
Chem. 276:18934–18940. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Ahn Q, Jeong SJ, Lee HJ, et al: Inhibition
of cyclooxygenase-2-dependent survivin mediates decursin-induced
apoptosis in human KBM-5 myeloid leukemia cells. Cancer Lett.
298:212–221. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Yim D, Singh RP, Agarwal C, Lee S, Chi H
and Agarwal R: A novel anticancer agent, decursin, induces G1
arrest and apoptosis in human prostate carcinoma cells. Cancer Res.
65:1035–1044. 2005.PubMed/NCBI
|
|
15.
|
Jiang C, Guo J, Wang Z, et al: Decursin
and decursinol angelate inhibit estrogen-stimulated and
estrogen-independent growth and survival of breast cancer cells.
Breast Cancer Res. 9:R772007. View
Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Kim WJ, Lee SJ, Choi YD and Moon SK:
Decursin inhibits growth of human bladder and colon cancer cells
via apoptosis, G1-phase cell cycle arrest and extracellular
signal-regulated kinase activation. Int J Mol Med. 25:635–641.
2010.PubMed/NCBI
|
|
17.
|
Kim WJ, Lee MY, Kim JH, Suk K and Lee WH:
Decursinol angelate blocks transmigration and inflammatory
activation of cancer cells through inhibition of PI3K, ERK and
NF-κB activation. Cancer Lett. 296:35–42. 2010.PubMed/NCBI
|
|
18.
|
Kim JH, Jeong JH, Jeon ST, et al: Decursin
inhibits induction of inflammatory mediators by blocking nuclear
factor-kappaB activation in macrophages. Mol Pharmacol.
69:1783–1790. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Lee SH, Lee JH, Kim EJ, et al: A novel
derivative of decursin, CSL-32, blocks migration and production of
inflammatory mediators and modulates PI3K and NF-kappaB activities
in HT1080 cells. Cell Biol Int. 36:683–688. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Lee YR, Noh EM, Jeong EY, et al:
Cordycepin inhibits UVB-induced matrix metalloproteinase expression
by suppressing the NF-kappaB pathway in human dermal fibroblasts.
Exp Mol Med. 41:548–554. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Bradford MM: A rapid and sensitive method
for the quantitation of microgram quantities of protein utilizing
the principle of protein-dye binding. Anal Biochem. 72:248–254.
1976. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Fisher GJ, Kang S, Varani J, et al:
Mechanisms of photoaging and chronological skin aging. Arch
Dermatol. 138:1462–1470. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Jacobs MD and Harrison SC: Structure of an
IkappaBalpha/NF-kappaB complex. Cell. 95:749–758. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Regnier CH, Song HY, Gao X, Goeddel DV,
Cao Z and Rothe M: Identification and characterization of an
IkappaB kinase. Cell. 90:373–383. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Mercurio F, Zhu H, Murray BW, et al: IKK-1
and IKK-2: cytokine-activated IkappaB kinases essential for
NF-kappaB activation. Science. 278:860–866. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Karin M: How NF-kappaB is activated: the
role of the IkappaB kinase (IKK) complex. Oncogene. 18:6867–6874.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Chung TW, Moon SK, Chang YC, et al: Novel
and therapeutic effect of caffeic acid and caffeic acid phenyl
ester on hepatocarcinoma cells: complete regression of hepatoma
growth and metastasis by dual mechanism. FASEB J. 18:1670–1681.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Eberhardt W, Huwiler A, Beck KF, Walpen S
and Pfeilschifter J: Amplification of IL-1 beta-induced matrix
metalloproteinase-9 expression by superoxide in rat glomerular
mesangial cells is mediated by increased activities of NF-kappa B
and activating protein-1 and involves activation of the
mitogen-activated protein kinase pathways. J Immunol.
165:5788–5797. 2000.
|
|
29.
|
Uitto J: Connective tissue biochemistry of
the aging dermis. Age-related alterations in collagen and elastin.
Dermatol Clin. 4:433–446. 1986.PubMed/NCBI
|
|
30.
|
Gilchrest BA: A review of skin ageing and
its medical therapy. Br J Dermatol. 135:867–875. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Chung JH, Hanft VN and Kang S: Aging and
photoaging. J Am Acad Dermatol. 49:690–697. 2003. View Article : Google Scholar
|
|
32.
|
Varani J, Perone P, Fligiel SE, Fisher GJ
and Voorhees JJ: Inhibition of type I procollagen production in
photodamage: correlation between presence of high molecular weight
collagen fragments and reduced procollagen synthesis. J Invest
Dermatol. 119:122–129. 2002. View Article : Google Scholar
|
|
33.
|
Chung JH, Seo JY, Choi HR, et al:
Modulation of skin collagen metabolism in aged and photoaged human
skin in vivo. J Invest Dermatol. 117:1218–1224. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Lloyd RE, Larson RA, Adair TL and Tuveson
RW: Cu(II) sensitizes pBR322 plasmid DNA to inactivation by UV-B
(280–315 nm). Photochem Photobiol. 57:1011–1017. 1993.PubMed/NCBI
|
|
35.
|
Brenneisen P, Wenk J, Wlaschek M, Krieg T
and Scharffetter-Kochanek K: Activation of p70 ribosomal protein S6
kinase is an essential step in the DNA damage-dependent signaling
pathway responsible for the ultraviolet B-mediated increase in
interstitial collagenase (MMP-1) and stromelysin-1 (MMP-3) protein
levels in human dermal fibroblasts. J Biol Chem. 275:4336–4344.
2000.
|
|
36.
|
Kim JK, Mun S, Kim MS, Kim MB, Sa BK and
Hwang JK: 5,7-Dimethoxyflavone, an activator of PPARalpha/gamma,
inhibits UVB-induced MMP expression in human skin fibroblast cells.
Exp Dermatol. 21:211–216. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Chiang HM, Chen HC, Lin TJ, Shih IC and
Wen KC: Michelia alba extract attenuates UVB-induced
expression of matrix metalloproteinases via MAP kinase pathway in
human dermal fibroblasts. Food Chem Toxicol. 50:4260–4269. 2012.
View Article : Google Scholar
|
|
38.
|
Bakiri L, Matsuo K, Wisniewska M, Wagner
EF and Yaniv M: Promoter specificity and biological activity of
tethered AP-1 dimers. Mol Cell Biol. 22:4952–4964. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
39.
|
Chen FE and Ghosh G: Regulation of DNA
binding by Rel/NF-kappaB transcription factors: structural views.
Oncogene. 18:6845–6852. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
40.
|
Adhami VM, Afaq F and Ahmad N: Suppression
of ultraviolet B exposure-mediated activation of NF-kappaB in
normal human keratinocytes by resveratrol. Neoplasia. 5:74–82.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
41.
|
Tanaka K, Hasegawa J, Asamitsu K and
Okamoto T: Prevention of the ultraviolet B-mediated skin photoaging
by a nuclear factor kappaB inhibitor, parthenolide. J Pharmacol Exp
Ther. 315:624–630. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
42.
|
Bond M, Baker AH and Newby AC: Nuclear
factor kappaB activity is essential for matrix metalloproteinase-1
and -3 upregulation in rabbit dermal fibroblasts. Biochem Biophys
Res Commun. 264:561–567. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
43.
|
Chung JH: Photoaging in Asians.
Photodermatol Photoimmunol Photomed. 19:109–121. 2003. View Article : Google Scholar
|
|
44.
|
Liu Q, Loo WT, Sze SC and Tong Y: Curcumin
inhibits cell proliferation of MDA-MB-231 and BT-483 breast cancer
cells mediated by down-regulation of NFkappaB, cyclinD and MMP-1
transcription. Phytomedicine. 16:916–922. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
45.
|
Kim SY, Jung SH and Kim HS: Curcumin is a
potent broad spectrum inhibitor of matrix metalloproteinase gene
expression in human astroglioma cells. Biochem Biophys Res Commun.
337:510–516. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
46.
|
Shim JS, Kwon YY, Han YS and Hwang JK:
Inhibitory effect of panduratin A on UV-induced activation of
mitogen-activated protein kinases (MAPKs) in dermal fibroblast
cells. Planta Med. 74:1446–1450. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
47.
|
Bae JY, Choi JS, Choi YJ, et al:
(−)Epigallocatechin gallate hampers collagen destruction and
collagenase activation in ultraviolet-B-irradiated human dermal
fibroblasts: involvement of mitogen-activated protein kinase. Food
Chem Toxicol. 46:1298–1307. 2008.
|
|
48.
|
Yao J, Xiong S, Klos K, et al: Multiple
signaling pathways involved in activation of matrix
metalloproteinase-9 (MMP-9) by heregulin-beta1 in human breast
cancer cells. Oncogene. 20:8066–8074. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
49.
|
Ruhul Amin AR, Senga T, Oo ML, Thant AA
and Hamaguchi M: Secretion of matrix metalloproteinase-9 by the
proinflammatory cytokine, IL-1beta: a role for the dual signalling
pathways, Akt and Erk. Genes Cells. 8:515–523. 2003.PubMed/NCBI
|
|
50.
|
Weng CJ, Chau CF, Hsieh YS, Yang SF and
Yen GC: Lucidenic acid inhibits PMA-induced invasion of human
hepatoma cells through inactivating MAPK/ERK signal transduction
pathway and reducing binding activities of NF-kappaB and AP-1.
Carcinogenesis. 29:147–156. 2008. View Article : Google Scholar : PubMed/NCBI
|