Introduction
The p53 gene is a well-defined tumor-suppressor
gene. The gene produces a 53-kDa phosphoprotein that was first
characterized as the major cellular protein associated with the T
antigen encoded by simian virus 40 (SV40), a small DNA virus (1–3).
SV40 and other oncogenic viruses target p53 in order to induce cell
proliferation, thus increasing the number of cells carrying their
genomes. p53 plays a role in many abnormal types of cell
proliferation, in apoptosis in response to DNA injury, in the
prevention of replication of genomes that have suffered DNA damage
(2,4–9),
in the inhibition of tumor angiogenesis (10), and in embryogenesis (11). In general, the target genes of p53
can be grouped into categories of biological activities that
include apoptosis, growth arrest, DNA repair, checkpoint responses
and balancing of aerobic respiration and glycolysis (12).
Apart from the transcription-dependent induction of
apoptosis, p53 also induces apoptosis through a mitochondrial
pathway. In this pathway, p53 binds to the outer mitochondrial
membrane, induces its permeabilization, and forms complexes with
the protective Bcl XL and Bcl-2 proteins. The binding of p53 to
these factors triggers cytochrome c release and caspase activation
(13–16).
Pifithrin-α is a reversible inhibitor of
p53-mediated apoptosis and p53-dependent gene transcription.
Notably, transcriptional-dependent apoptosis is repressed by
pifithrin, while mitochondrial-dependent apoptosis is not repressed
(17).
We previously demonstrated and characterized p53
expression in the rodent corneal epithelium (18–20). Others have found that UV
irradiation was able to induce the p53 protein (8) and p53-dependent apoptosis in the
corneal epithelium (21–23). However, its causative contribution
to apoptosis has yet to be determined. In the present study we
examined changes in apoptosis and p53 expression and functional
activity in rodent corneal epithelium in response to UV
irradiation.
Materials and methods
Animals
C57BL/6 mice were obtained from the Animal Facility
of Haifa Technion. Eyes of animals were enucleated after sacrifice
by CO2 narcosis. The use of animals adhered to the
ethical guidelines of and was supervised by the Technion Animal
Welfare Committee.
Cell culture
In order to investigate corneal epithelial cells
derived from the ocular surface, we used a corneal epithelial cell
culture system, as previously described (24). Outgrowing cells from corneal
explants formed in the suspension epithelial cell culture. These
cells were grown in RPMI-1640 medium supplemented with 10% fetal
bovine serum, 2 mmol/l of L-glutamine, MEM-Eagle vitamin solution
concentrate (100×), 50 μg/ml penicillin and 50 μg/ml
streptomycin (Beit Haemek, Israel).
Immunohistochemistry
p53 immunostaining was performed using the
monoclonal antibodies: Mab-248, which binds to an N-terminal or
central epitope of the p53 molecule (25–27); and Mab-421, which recognizes a
C-terminal epitope of the p53 protein (26,28) (both a kind gift from Professor V.
Rotter, Weizmann Institute of Science, Rehovot, Israel) and a
monoclonal antibody against phosphorylated Ser15 of the p53 protein
(Cell Signaling Technology). Immunostaining with these antibodies
was performed overnight at 4°C in a humidity chamber, followed by
the avidin-biotin secondary antibody staining technique, in which a
biotinylated secondary antibody reacts with several
peroxidase-conjugated streptavidin molecules (Dako Labs 2 kit;
DakoCytomation, Glostrup, Denmark). Alternatively, the
HistoMouse™-Max kit (Zymed Laboratories, Inc., South San Francisco,
CA, USA) was used. All control samples were processed in the
absence of the primary antibody. The slides were washed, mounted
with an aqueous mounting medium and photographed within a few hours
under a digital microscope camera (Zeiss Axioscope 2 with image
processing software Image-Pro Plus version 6). Light intensity and
contrast were standardized for a respective culture with an
appropriate control.
Apoptotic index determination
On-slide in situ terminal deoxynucleotidyl
transferase-mediated dUTP-biotin nick end-labeled (TUNEL) assay was
used. The apoptotic index (AI) was determined using the ApopTag
marker (Oncor, Inc., Gaithersburg, MD, USA) and was calculated as
the percentage of TUNEL-positive cells per 1,000 cells, according
to a previously described procedure (29).
Fluorescence staining of p53
immunoreactive protein
Twenty-four hours after plating, cells were
incubated with 200 nmol/l of MitoTracker Red 580 (Molecular Probes,
Eugene, OR, USA) in culture medium for 40 min, after which the dye
was removed, and cells were treated with or without 15 μM of
pifithrin-α (Sigma, St. Louis, MO, USA) for 2 h. The cells were
then irradiated with a UV lamp (312 nm) at 150 mJ/cm2.
The Petri dish was placed 15 cm above a UV light source (4×6 W,
312-nm tube, power 50 W, TFP-10M, Vilber Lourmant, Torcy, France)
for 5 min. The UV dosimetry was performed using a UV light meter
(YK-34UV; Lutron Electronic, Taiwan). Following UV irradiation,
cells were fixed in absolute methanol for 10 min, placed on a slide
and dried. After rinsing with cold PBS (pH 7.4) cells were
permeabilized with 0.5% Triton X-100 for 10 min at room temperature
(RT). After blocking, the anti-p53 antibody (Mab-421) was added
(without dilution) and incubated at RT for 2 h followed by
incubation with anti-mouse IgG-FITC (Sigma) (1:128 dilution) for 1
h. After removal of the antibodies, the cells were rinsed with PBS
and mounted with UltraMount (Lab Vision, UK). Fluorescence was
immediately observed using either an Axioscop 2 or Leica laser
scanning confocal microscope (Bensheim, Germany).
Protein determination
Protein was quantitated by the method of Bradford
(30) using bovine serum albumin
as a standard. In brief, unknown protein concentrations were
determined spectrophotometically (595 nm), following the binding of
the dye, Coomassie Brilliant Blue, to both unknown protein
preparations and predetermined standard concentrations of bovine
serum albumin, and their optical densities were compared.
Western blot analysis
Ocular cell protein extracts were prepared in lysis
buffer (1% Triton X-100 and 0.1% SDS in PBS), as previously
described (20). Equal amounts of
protein derived from the epithelial cell cultures were compared to
each other by western blot analysis, following resolution of
protein samples (50 μg/well) by standard denaturing SDS 7.5%
poly-acrylamide gel electrophoresis, using standard conditions
(31). Proteins were transferred
to nitrocellulose membranes (Schleicher and Schuell Bioscience
GmbH, Germany), as previously described (20). Bovine serum albumin blocked (BSA,
2% in TBS-T) and blotted nitrocellulose membranes were subjected to
western blot analysis using the Mab-248 antibody. This was followed
by incubation with rabbit anti-mouse IgG (whole-molecule)
conjugated to horseradish peroxidase (HPR) (Sigma) at a dilution of
1:1,000 for 1 h at RT. Between incubations, the blots were washed
three times for 10 min/wash with 1X PBS containing 0.05% Tween-20.
Enhanced chemilumi-nescence (ECL) substrates to develop the results
(Amersham, Buckinghamshire, UK) and Sea-Blue protein molecular
weight markers (Novartis, San Diego, Ca, USA) were used on each
gel. Fifty micrograms of p53-M clone 314 cell extract (a kind gift
from Professor V. Rotter, Weizman Institute) (32) was used as a p53-positive control
(p53-M), while 50 μg of BSA was used as a negative control
(NC).
Quantitative p53 mRNA determination by
real-time PCR
Total RNA from mouse corneal epithelium cells was
extracted with the MasterPure RNA Purification kit (Epicentre
Biotechnologies, Madison, WI, USA). Total RNA concentrations were
determined spectrophotometrically by measuring the absorbance at
260 nm. cDNA was generated from 2 μg of total RNA using
Verso™ reverse transcriptase-RT and random primers (Verso™ cDNA
kit; Thermo Scientific, Surrey, UK), according to the
manufacturer’s instructions. Primers and probes for β-actin and
mouse p53 genes were designed by Primer Design Ltd., Southampton,
UK (Table I). p53 mRNA production
was measured by quantitative real-time PCR by means of Rotor-Gene
6000 (Corbett Life Science/Qiagen) amplification with ABsolute Blue
Mix (Thermo Scientific).
 | Table IQuantitative real-time PCR
conditions. |
Table I
Quantitative real-time PCR
conditions.
| mRNA | Denaturation
parameters (temperature/time) | Data collection
(temperature/time) | Extension
(temperature/time) | No. of cycles |
|---|
| Mus musculus
p53 Accession: M13873 (sequences after alignments) | 95°C/15 sec | 50°C/30 sec | 72°C/15 sec | 30 |
Sense
primer
Antisense primer
Probe |
GAACCGCCGACCTATCCTTA
GCACAAACACGAACCTCAAA
cagtggCTGTCCCGTCCCAGAAGGTTCCCACTG |
Statistical analysis
Data are reported as means ± SEM and P<0.05 was
considered to indicate a statistically significant result. Groups
were compared by ANOVA with Student-Newman-Keuls post hoc analysis
or Kruskal-Wallis nonparametric ANOVA, with Dunn’s post hoc
analysis, as appropriate.
Results
p53 immunostaining and subcellular
localization in the corneal epithelium after UV exposure (in
vitro)
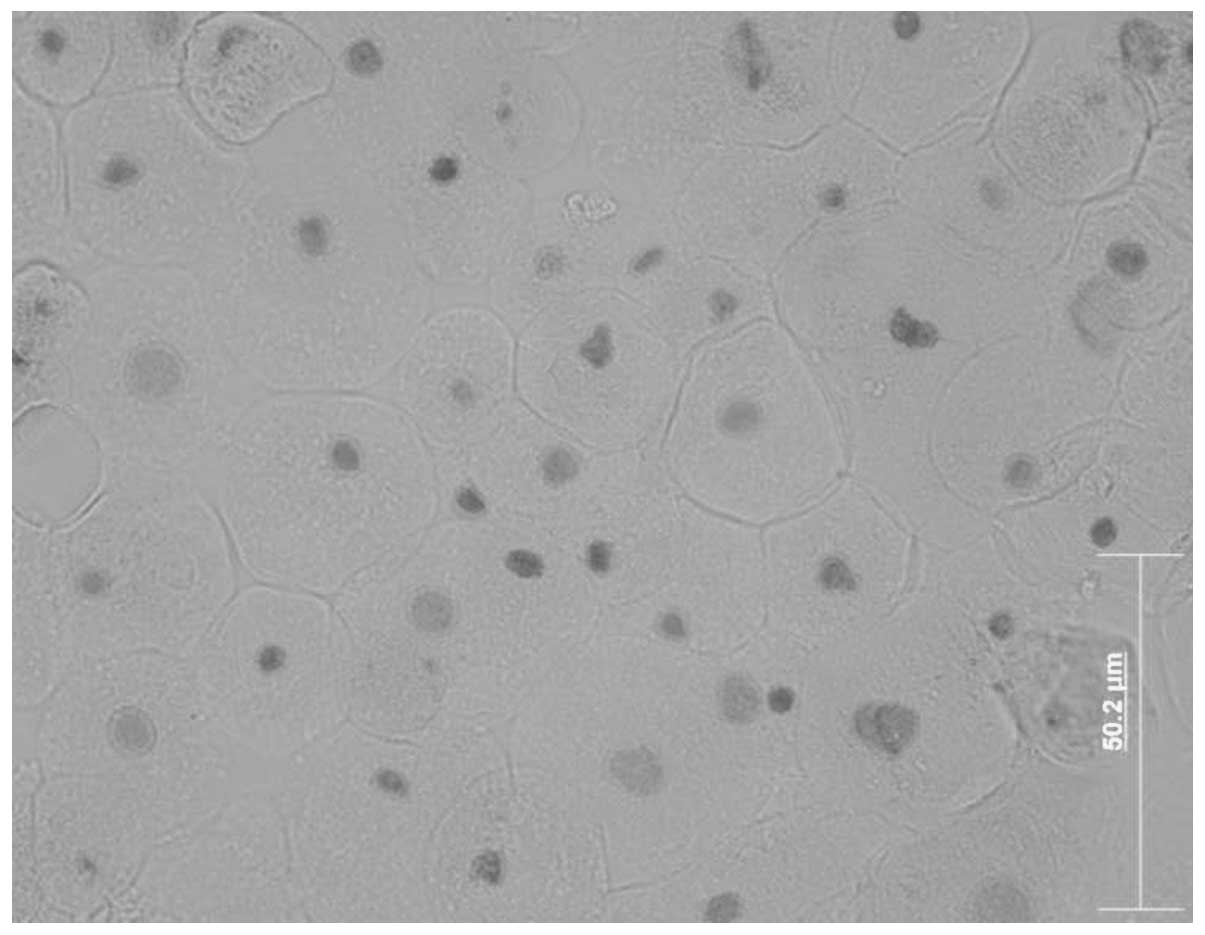
In order to investigate corneal epithelial cells on
the ocular surface, we used a corneal epithelial cell culture
system from corneal explant outgrowths (24). The migrating cells formed in the
epithelial cell culture resembled native corneal epithelial cells
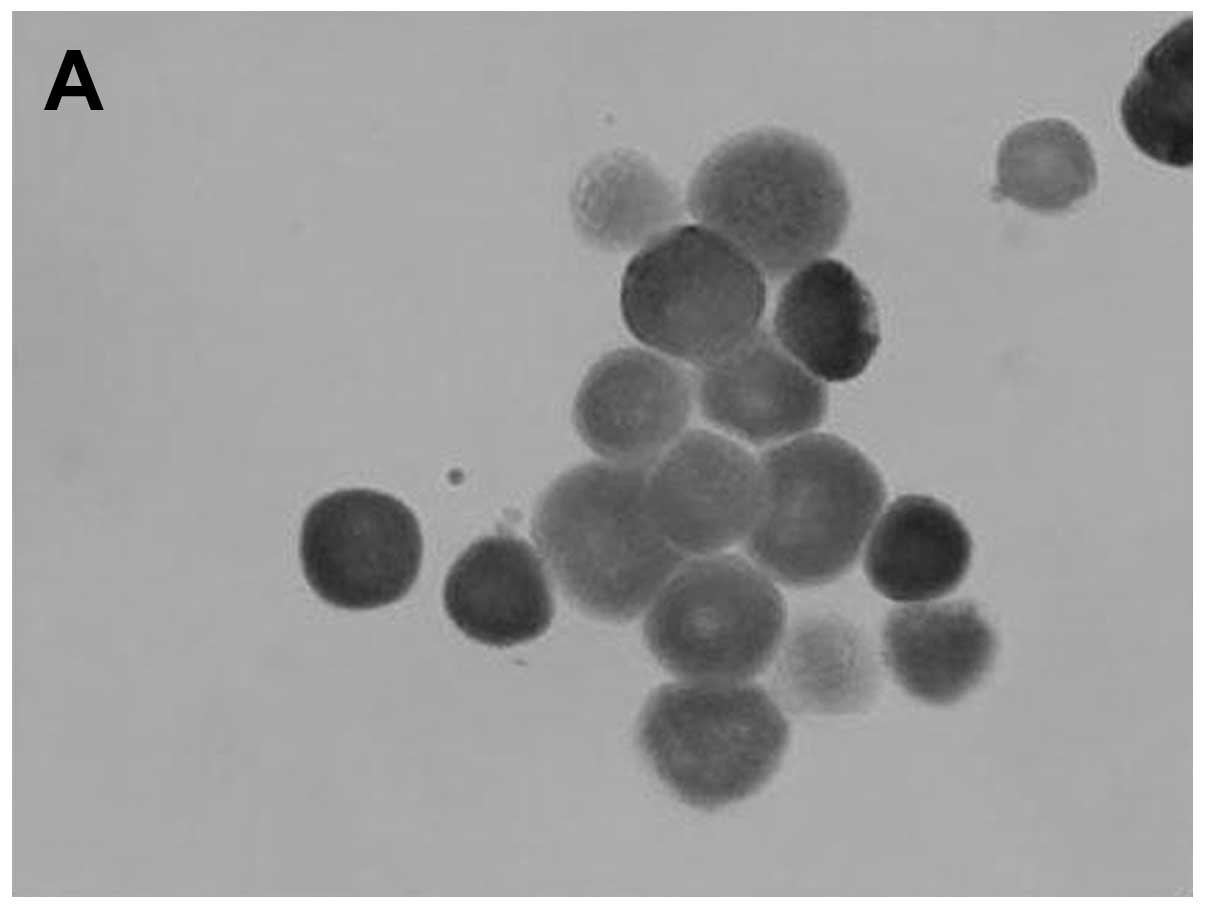
(Fig. 1). Cells migrating from
the explants expressed p53 protein in their cytoplasm and
demonstrated positive staining with Mab-421, negative staining with
Mab-248 and negative p53 staining with a monoclonal antibody
against phosphory-lated Ser15 (Fig.
2 and Table II). Corneal
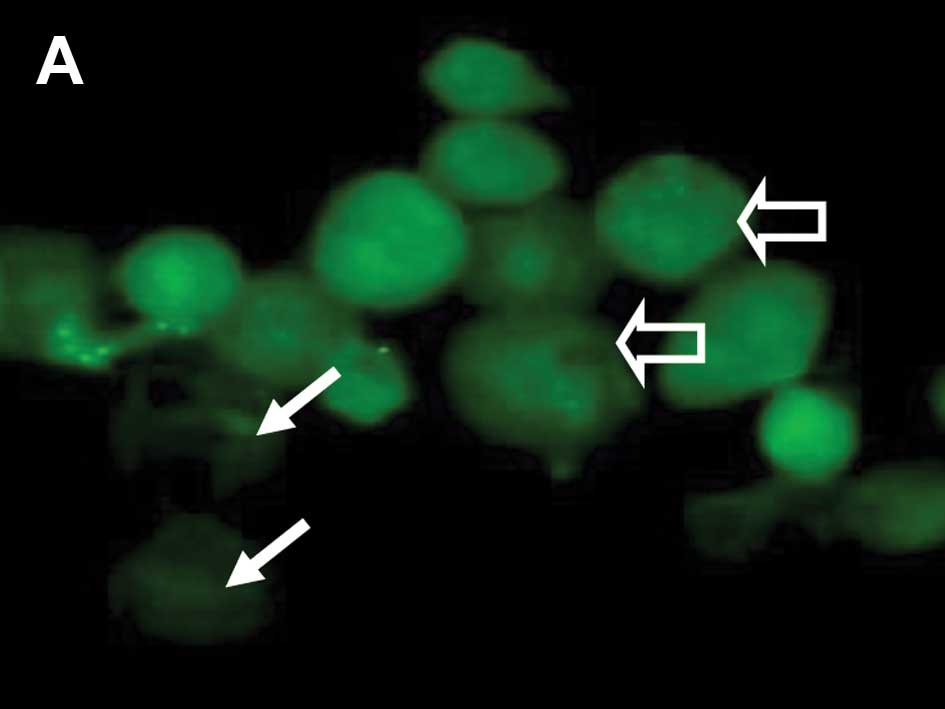
epithelial cells positive for cytoplasmic p53 staining were noted
to be with and without MitoTracker uptake and mitochondrial
staining (Fig. 3A and B). Cells
with negative Mab-421 staining also demonstrated punctuate labeling
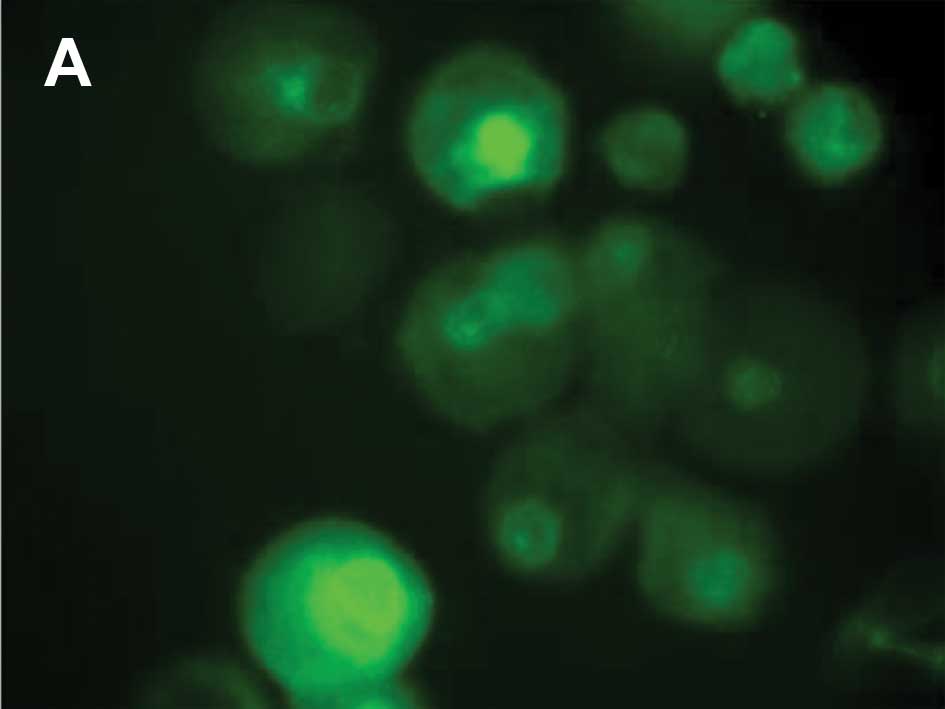
corresponding to MitoTracker uptake by mitochondria (Fig. 3C). From 30 min until 24 h
following UV irradiation, p53 translocated to the nucleus when
compared to the control cells without p53 staining in the nucleus
(Fig. 4A).
 | Table IIp53 expression and apoptosis
following UV-irradiation in corneal epithelial cell cultures. |
Table II
p53 expression and apoptosis
following UV-irradiation in corneal epithelial cell cultures.
| Epithelial cell
culture | Pifithrin | Staining with
Mab-421 | Staining with Mab
phospho-p53, Ser15 | Apoptotic index
(mean ± SE) |
|---|
| Control | | ++ | - | 5.82±0.3 |
| + | ++ | - | 5.21±0.25 |
| 30 min following
UV | | +++ | ++ | 12.12±0.63 |
| + | +++ | ++ | 11.28±0.55 |
| 2 h following
UV | | ++++ | ++ | 28.0±1.3 |
| + | ++++ | ++ | 19.10±0.96a |
| 6 h following
UV | | +++ | ++ | 31.02±1.53 |
| + | +++ | ++ | 20.09±1.1a |
| 24 h following
UV | | ++ | - | 25.63±1.05 |
| + | ++ | - | 8.48±0.47a |
p53 staining in the corneal epithelial cells was
rapidly altered following UV illumination. Positive cytoplasmic p53
staining with Mab-248 and the Mab against phosphorylated Ser15 was
noted within 30 min following UV exposure (Table II). p53 staining with Mab-421 was
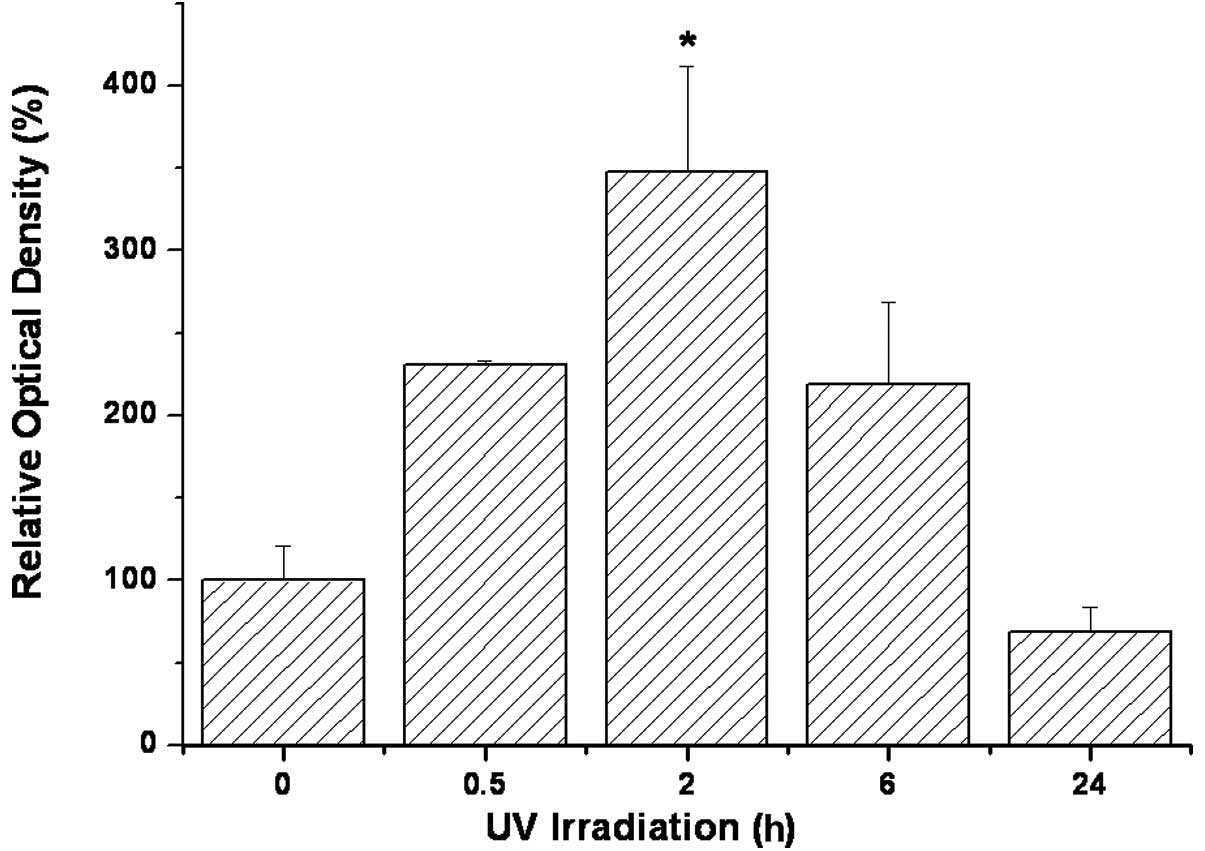
intensified following UV illumination, peaking at 2 h and returning
to a level lower than that of the control within 24 h after UV
treatment (Fig. 5). Apoptosis, as
measured by TUNEL staining, was intensified after UV illumination
and peaked within 6 h following UV treatment (Fig. 6). Addition of pifithrin-α (a
reversible inhibitor of p53-mediated apoptosis and p53-dependent
gene transcription) did not alter apoptosis in the control cells,
but did decrease apoptosis of the UV-irradiated cells. The
apoptotic index (AI) was decreased by pifithrin; the maximal
decrease occurred 24 h after UV irradiation (Table II).
Western blot analysis
To confirm our in situ staining data, western
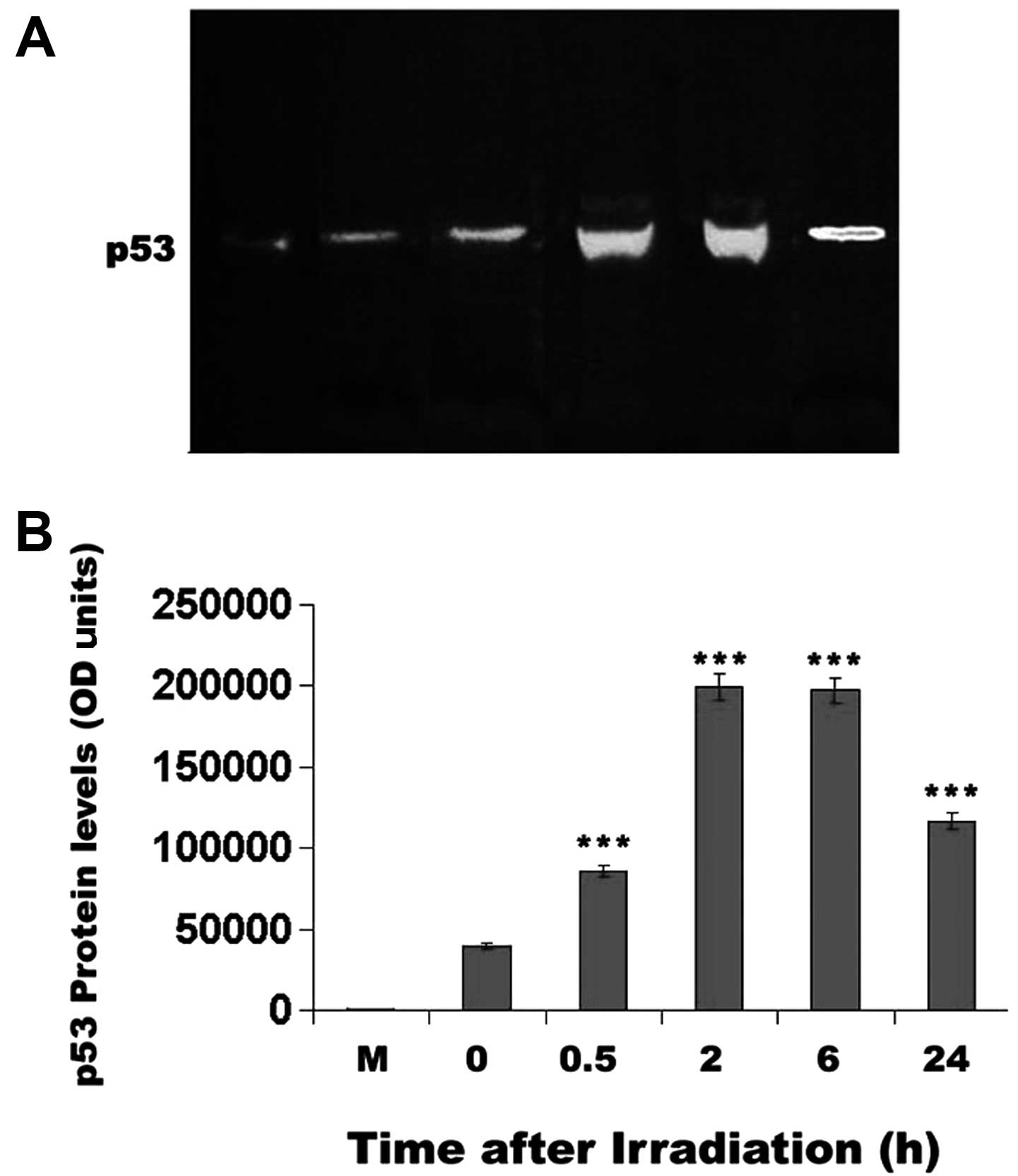
blot analysis was performed. The western blot analysis (Fig. 7) corroborated the results of the
p53 staining of the mouse corneal epithelial cells. Specifically,
p53 protein levels reached a maximum 2 h after UV irradiation
(P<0.001) and decreased only after 24 h, without being reduced
below unirradiated control levels (Fig. 7).
Quantitative p53 mRNA determination
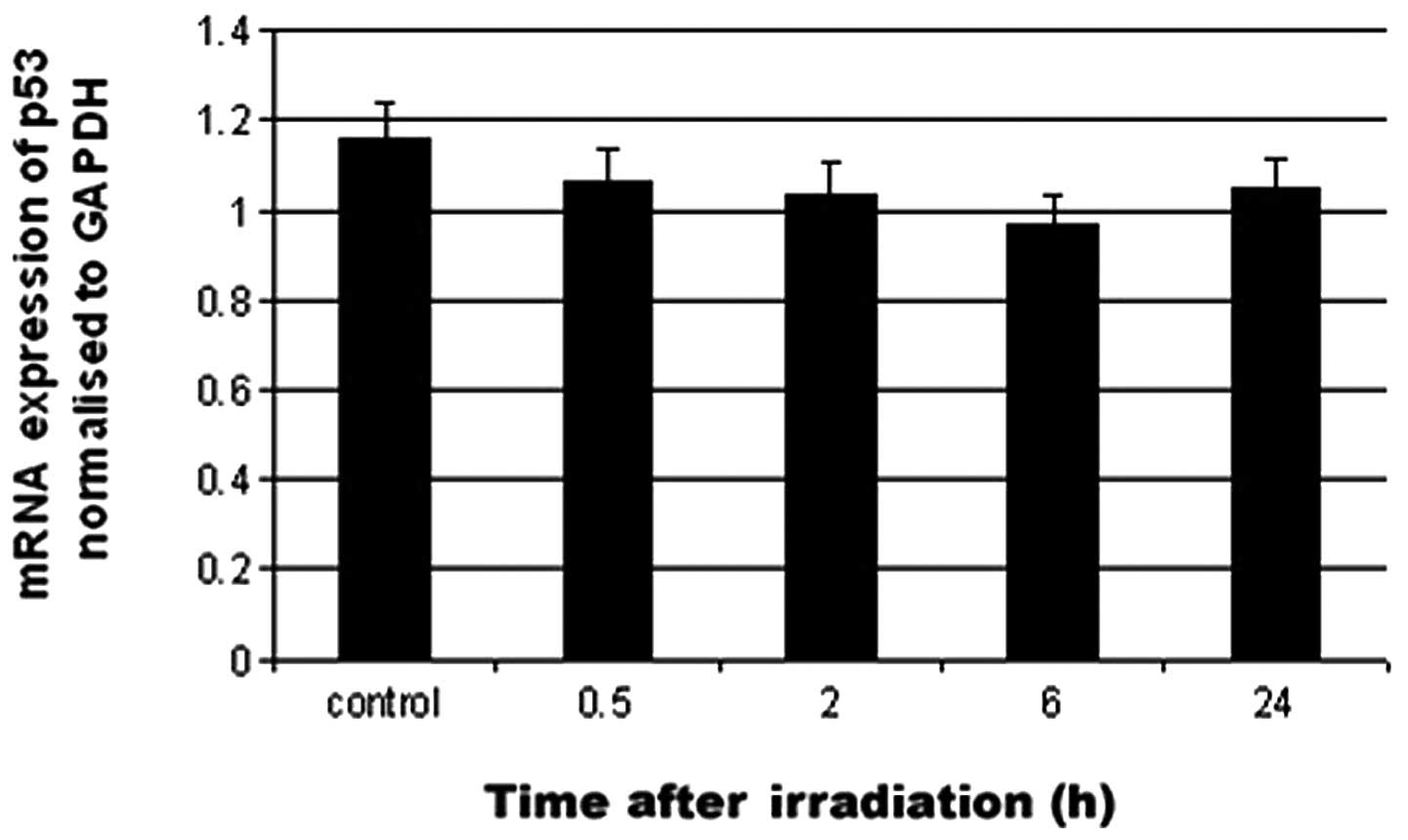
To assess whether the UV-induced p53 regulation
occurred at the RNA level, real-time PCR was carried out on
UV-irradiated tissue. p53 mRNA levels following UV irradiation of
the mouse corneal epithelium were not significantly changed as
compared with control (Fig.
8).
Discussion
In the present study, we demonstrated the presence
of p53 in the cytoplasm of cultured primary corneal epithelial
cells. This result was identical to previous results noted in
intact corneal epithelia in rats (18), mice (20) as well as in other species
(19). Hence, the in vitro
tissue culture model for p53 expression as described in the present
study may truly represent what occurs in vivo for p53
expression in the cornea. We demonstrated, in the corneal
epithelial cells, that a nuclear translocation of cytoplasmic p53,
occurred following UV irradiation. Moreover, the UV irradiation
under the same condition induced epithelial cell apoptosis as
measured by TUNEL staining. UV-induced p53 nuclear translocation
did not appear to be regulated by transcriptional mechanisms.
We previously reported that the p53 protein is
strongly expressed in normal vertebrate adult corneal epithelium
(19). In that previous study,
immunostaining of frozen eye sections, similar to the cultured
corneal epithelium in the present study, did not show p53
immunostaining for all of the p53 Mabs used, while western blot
analysis was positive for all antibodies used. We suggested that
several p53-binding proteins may compete for antibody epitope sites
and hence block certain ‘non-staining’ p53 Mabs.
We found differential staining of Mab-421 and
Mab-248 on the immunohistochemical preparations and identical
positive staining of these same antisera upon western blot analysis
of corneal epithelial cell protein extracts. Similar data were
obtained in our previous studies in vivo (19,20). The antibody Mab-248 binds to an
N-terminal (3,25,26) or central (27) epitope of p53, while Mab-421 binds
to a C-terminal epitope (26,28). The non-reactivity of Mab-248 in
native corneal and conjunctival epithelium, as noted using
immunohistochemical techniques, may indicate the presence of an
N-terminal or central region p53 binding protein, such as
αB-crystallin. αB-crystallin interacts with p53 in the cytoplasm of
cells (33), hence covering a
putative antibody epitope. The limbo-corneal epithelium
constitutively expresses αB-crystallin with higher levels in the
proliferative compartment (34).
Upon denaturation, as occurs in western blotting, any protein
binding noncovalently to p53 would thus be removed from its binding
site, so that all the internal p53 epitopes become exposed
(20). This would explain the
differential Mab reactivity noted by immunohistochemistry and the
apparent equal reactivity of these same monoclonal antibodies upon
western blot analysis.
The regulation of p53 activity is mainly
post-translational. Stabilization is an essential step for p53 to
function efficiently in response to UV irradiation. This study
demonstrated that UV irradiation of corneal epithelium cultures
induced cytoplasmic p53 phosphorylation within 30 min. Until p53
appeared in the nucleus, we noted an increase in apoptosis that may
have been caused by the original cytoplasmic p53 protein. The
cytoplasmic p53 protein of the corneal epithelium may participate
in transcriptional- and mitochondrial-dependent apoptotic pathways,
as it is always present in the corneal epithelium cytoplasm
(20).
There have been reports of an ‘inactive’ p53 isoform
found in the cytoplasm (35). In
the present study we also found large amounts of cytoplasmic p53 in
cultured rodent corneal epithelium. This may represent examples of
transitory ‘inactive’ p53. Upon UV irradiation, rapid
phosphorylation of cytoplasmic p53 may clear damaged cells by
inducing apoptosis (36). This is
consistent with the rapid transit of cytoplasmic p53 protein into
the nucleus upon UV irradiation and suggests the presence of an
active p53 form in the cytoplasm. We found that p53 transcriptional
activity was not significantly altered by UV irradiation. We,
therefore, suggest that the increase in the p53 protein after UV
irradiation is the result of post-translational stabilization. A
similar observation was noted by others in the human epidermis
(8,37).
In order to clearly understand how p53 functions in
regulating the cell cycle, it is important to identify all of its
possible functions and all of the proteins interacting with it. The
present study demonstrated the existence of functionally active
cytoplasmic p53 protein in normal cultured rodent corneal
epithelium cells. Additional studies are warranted to fully
characterize the role of the p53 protein and its regulation as part
of its physiological function in normal corneal epithelium.
Acknowledgements
We thank Professor Varda Rotter and Dr
Naomy Goldfinger (Weizman Institute of Science, Rehovot, Israel)
for kindly providing the Mabs-421 and -248 as well as the
p53-positive control, p53-M clone 314 cell extract. We also would
like to thank Drs Elvira Bormusov and Aviva Lazarovich (Technion,
Haifa, Israel) for their technical assistance and Ms. Judith Rondel
for the critical reading of our manuscript. We would also like to
acknowledge grant support for this study to Y.T. and A.P. from the
Center for Immigrant Scientist Absorption of the Ministry of
Absorption, State of Israel as well as to Y.T. and G.W. from the
Edward S. Mueller Eye Research Fund, Israel.
References
|
1
|
Linzer DI and Levine AJ: Characterization
of a 54K Dalton cellular SV40 tumor antigen present in
SV40-transformed cells and unifected embryonal carcinoma cells.
Cell. 17:43–52. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lane DP: Cancer. p53, guardian of the
genome. Nature. 358:15–16. 1992. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lane DP, Stephen CW, Midgley CA, Sparks A,
Hupp TR, Daniels DA, Greaves R, Reid A, Vojtesek B and Picksley SM:
Epitope analysis of the murine p53 tumour suppressor protein.
Oncogene. 12:2461–2466. 1996.PubMed/NCBI
|
|
4
|
Kastan MB, Onyekwere O, Sidransky D,
Vogelstein B and Craig RW: Participation of p53 protein in the
cellular response to DNA damage. Cancer Res. 51:6304–6311.
1991.PubMed/NCBI
|
|
5
|
Hartwell L: Defects in a cell cycle
checkpoint may be responsible for the genomic instability of cancer
cells. Cell. 71:543–546. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Unger T, Nau MN, Segal S and Minna JD:
p53: a transdominant regulator of transcription whose function is
ablated by mutations occurring in human cancer. EMBO J.
11:1383–1390. 1992.PubMed/NCBI
|
|
7
|
Clarke AR, Purdie CA, Harrison DJ, Morris
RG, Bird CC, Hooper ML and Wyllie AH: Thymocyte apoptosis induced
by p53-dependent and -independent pathways. Nature. 362:849–852.
1993. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hall PA, Mckee PH, Du P, Manage H, Dover R
and Lane DP: High levels of p53 protein in UV-irradiated normal
human skin. Oncogene. 8:203–207. 1993.PubMed/NCBI
|
|
9
|
Hall PA and Lane DP: Tumour supressors: a
developing role for p53? Curr Biol. 7:R144–R147. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Teodoro JG, Evans SK and Green MR:
Inhibition of tumor angio-genesis by p53: a new role for the
guardian of the genome. J Mol Med. 85:1175–1186. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sah VP, Attardi LD, Mulligan GJ, Williams
BO, Bronson RT and Jacks T: A subset of p53-deficient embryos
exhibit exencephaly. Nat Genet. 10:175–180. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Matoba S, Kang JG, Patino WD, Wragg A,
Boehm M, Gavrilova O, Hurley PJ, Bunz F and Hwang PM: p53 regulates
mitochondrial respiration. Science. 312:1650–1653. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Erster S and Moll UM: Stree-induced p53
runs a transcription-independent death program. Biochem Biophys Res
Commun. 331:843–850. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mihara M, Erster S, Zaika A, Petrenko O,
Chittenden T, Pancoska P and Moll UM: p53 has a direct apoptogenic
role at the mitochondria. Mol Cell. 11:577–590. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chipuk JE, Kuwana T, Bouchier-Hayes L,
Droin NM, Newmeyer DD, Schuler M and Green DR: Direct activation of
Bax by p53 mediates mitochondrial membrane permeabilization and
apoptosis. Science. 303:1010–1014. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Murphy ME, Leu JI and George DL: p53 moves
to mitochondria: a turn on the path to apoptosis. Cell Cycle.
3:836–839. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bonini P, Cicconi S, Cardinale A, Vitale
C, Serafino AL, Ciotti MT and Marlier LN: Oxidative stress induces
p53-mediated apoptosis in glia: p53 transcription-independent way
to die. J Neurosci Res. 75:83–95. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tendler Y, Weisinger G, Coleman R, Diamond
E, Lischinsky S, Kerner H, Rotter V and Zinder O: Tissue-specific
p53 expression in the nervous system. Brain Res Mol Brain Res.
72:40–46. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tendler Y, Panshin A, Weisinger G and
Zinder O: Identification of cytoplasmic p53 protein in corneal
epithelium of vertebrates. Exp Eye Res. 82:674–681. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pokroy R, Tendler Y, Pollack A, Zinder O
and Weisinger G: p53 expression in the normal murine eye. Invest
Ophthalmol Vis Sci. 43:1736–1741. 2002.PubMed/NCBI
|
|
21
|
Estil S, Kravik K, Haaskjold E, Refsum SB,
Bjerknes R and Wilson G: Pilot study on the time course of
apoptosis in the regenerating corneal epithelium. Acta Ophthalmol
Scand. 80:517–523. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ren H and Wilson G: The effect of
ultraviolet-B irradiation on the cell shedding rate of the corneal
epithelium and changes of p53 expression. Acta Ophthalmol.
72:447–452. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ren H and Wilson G: Apoptosis in the
corneal epithelium. Invest Ophthalmol Vis Sci. 37:1017–1025.
1996.PubMed/NCBI
|
|
24
|
Lomako J, Lomako WM, Decker SJ, Carraway
CA and Carraway KL: Non-apoptotic desquamation of cells from
corneal epithelium: putative role for Muc4/sialomucin complex in
cell release and survival. J Cell Physiol. 202:115–124. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wade-Evans A and Jenkins JR: Precise
epitope mapping of the murine transformation-associated protein,
p53. EMBO J. 4:699–706. 1985.PubMed/NCBI
|
|
26
|
Yewdell JW, Gannon JV and Lane DP:
Monoclonal antibody analysis of p53 expression in normal and
transformed cells. J Virol. 59:444–452. 1986.PubMed/NCBI
|
|
27
|
Yin X, Fontoura B, Morimoto T, Robert B
and Carroll RB: Cytoplasmic complex of p53 and eEF2. J Cell
Physiol. 196:474–482. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Arai N, Nomura D, Yokota K, Wolf D, Brill
E, Shohat O and Rotter V: Immunologically distinct p53 molecules
generated by alternative splicing. Mol Cell Biol. 6:3232–3239.
1986.PubMed/NCBI
|
|
29
|
Gavrieli Y, Sherman Y and Ben-Sasson SA:
Identification of programmed cell death in situ via specific
labelling of nuclear DNA fragmentation. J Cell Biol. 119:493–501.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bradford MM: A rapid and sensitive method
for the quantitation of microgram quantities of protein utilizing
the principle of protein-dye binding. Anal Biochem. 72:248–254.
1976. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sambrook J, Fritsch EF and Maniatis T:
Molecular Cloning: A Laboratory Manual. 2nd edition. Cold Spring
Harbor Laboratory Press; New York: pp. 1834–1874. 1989
|
|
32
|
Almog N, Li R, Peled A, Schwartz D,
Wolkowicz R, Goldfinger N, Pei H and Rotter V: The murine
C’-terminal alternatively spliced form of p53 induces attenuated
apoptosis in myeloid cells. Mol Cell Biol. 17:713–722. 1997.
|
|
33
|
Liu S, Li J, Tao Y and Xiao X: Small heat
shock protein alphaB-crystallin binds to p53 to sequester its
translocation to mitochondria during hydrogen peroxide-induced
apoptosis. Biochem Biophys Res Commun. 354:109–114. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Grueterich M, Alge C, Fuchs A, Kampik A
and Welge-Luessen U: Alpha-B-crystallin in the limbal and corneal
epithelium. Invest Ophthalmol Vis Sci. 44E:13582003.
|
|
35
|
Shaulsky G, Goldfinger N, Tosky M, Levine
AJ and Rotter V: Nuclear localization is essential for the activity
of p53 protein. Oncogene. 6:2055–2065. 1991.PubMed/NCBI
|
|
36
|
Bean LJ and Stark GR: Phosphorylation of
serines 15 and 37 is necessary for efficient accumulation of p53
following irradiation with UV. Oncogene. 20:1076–1084. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Qin JZ, Chaturvedi V, Denning MF, Bacon P,
Panella J, Choubey D and Nickoloff BJ: Regulation of apoptosis by
p53 in UV-irradiated human epidermis, psoriatic plaques and
senescent keratinocytes. Oncogene. 21:2991–3002. 2002. View Article : Google Scholar : PubMed/NCBI
|