Introduction
Parkinson’s disease (PD) is a degenerative disease
of the central nervous system that severely endangers human health.
Recently, it has been shown that the main pathological change
associated with PD is the denaturing death of dopaminergic neurons
(DAs) in the mesencephalon substantia nigra compact of
patients.
Lin et al (1) first isolated and purified glial cell
line-derived neurotrophic factor (GDNF) in 1993 and found that GDNF
exhibited neurotrophic effects on DAs of cultured embryonic
mesencephalons, as well as stimulated the survival and
differentiation of DAs in the ventral mesencephalon of mammals
(1,2).
In order to realize the protective effect of GDNF on
DAs, the specific intercellular signaling pathway must be activated
by its membrane receptor. Driven by this signaling pathway, the
cytoplasmic transcriptional molecules and substances enter into the
nucleus, thus exerting influences on the expression of related
genes of certain substances, conferring protection and aiding in
the survival of the cell. The composition of the GDNF membrane
receptor system is relatively clear. It is mainly composed of two
parts: the ligand binding receptor GDNF family receptor α1 (GFRα1);
the known transmembrane signal transduction receptor Ret (3,4),
NCAM140 (5) and integrin β1
(6–8).
During the protection of DAs via GDNF, the main
activated intracellular signaling pathway was found to be the
PI3K/Akt pathway (9). Studies
have shown that the mechanisms involved in the activation of PI3K
by GDNF occur as follows. After GDNF binds to GFRα1, the RET kinase
is activated by phosphorylation of its intracellular portion, thus
activating the PI3K/Akt pathway (10). GDNF can phosphorylate the
intracellular ‘adaptor protein’ Fyn via NCAM, thus activating the
PI3K/Akt pathway (11); and GDNF
can activate the PI3K/Akt pathway via integrin β1 and the ‘adaptor
protein’ Shc (12). Therefore, it
is clear that GDNF can directly or indirectly activate PI3K/Akt via
a transmembrane receptor to protect DAs.
N-cadherin is a transmembrane adhesive protein. Its
cytoplasmic region can interact with intercellular proteins of
various scopes (13–15). It is well known that N-cadherin
shares a similar structure and function to NCAM and integrin β1,
which can also mediate adhesion and signal transduction (16). The extracellular portion of Ret
that binds to GDNF/GFRα1 has a cadherin-like domain as well
(17). Our previous, and
unpublished data, demonstrated that N-cadherin binds to GDNF
(18,19). Thus, there is an urgent need to
confirm that GDNF also activates the PI3K/Akt signaling pathway via
N-cadherin, in order to confer a protective effect on DAs. This
knowledge will contribute to the development of treatment
strategies for PD.
In order to verify the above concepts, in the
present study, the N-cadherin gene were knocked down. Using flow
cytometry (FCM) and Hoechst 33258 staining, we confirmed the
protective effect of GDNF on DAs. In addition, through
immunoblotting and immunohistochemical staining, we demonstrated
the PI3K/Akt signaling pathway was activated following inhibition
of N-cadherin expression, and we examined the phosphorylation
levels and the correlation of phosphorylation between N-cadherin
and Akt following treatment with GDNF.
Materials and methods
Ethics statement
Adult male Sprague-Dawley (SD) rats were provided by
the Experimental Animal Center of Xuzhou Medical College. This
research was approved by the Experimentation Committee of Xuzhou
Medical College, and all animals were treated according to the
national and international rules of animal welfare. For the present
study, there were no suitable alternative approaches, and care was
taken to minimize animal distress.
Cell lines
The MN9D cell line was kindly provided by Professor
Qunyuan Xu from Capital Medical University. The cryopreserved MN9D
cells were thawed and cultured in a flask for 3 days. The cultured
cells were digested and then re-suspended with DMEM/F12
(Sigma-Aldrich) plus 10% FBS and 1% B27 (Gibco), transferred to a
6-well cell culture plate that was pre-coated with polylysine
(Sigma-Aldrich), and cultured at the density of
2–5×105/well. The plate was kept at 37°C in 5%
CO2, in a moisture balanced standard incubator for
culture. After a 24-h culture, the serum containing DMEM/F12 was
replaced by serum-free culture medium, Neurobasal™ medium (Gibco)
containing 2% B27 and 4 mM glutamine.
N-cadherin RNAi
MN9D cells were transiently transfected with a
control vector and an N-cadherin gene-specific interfering plasmid
using Lipofectamine 2000 transfection reagent (Invitrogen)
according to the manufacturer’s protocol. Changes in N-cadherin
expression were evaluated 3–4 days post siRNA transfection. Stable
N-cadherin-knockdown MN9D cells were used for immunoblotting and
immunocytochemistry assays.
Stereotaxic injection at the substantia
nigra of the rat lateral cerebral ventricle
The stereotactic instrument was calibrated, the
weighed animal was intraperitoneally injected with 360 mg/kg of a
chloral hydrate solution, the head was immobilized and sheared, and
the skin of the head was sterilized with 2% iodine and 75% alcohol.
A 6-cm incision was cut along the sagittal suture, the fascia and
muscles were stripped and the periosteum was separated to expose
the sutura. The skull was cleaned with 30% hydrogen peroxide, and
the point of bleeding on the skull was immediately stanched with
bone wax. The stereoscopic position was determined according to the
rat brain atlas (Paxinos G and Watson C: The Rat Brain in
Stereotaxic Coordinates, 2nd edition, Academic Press, Inc.). The
insertion of the needle was marked on the skull with locating pins
referring to the median line, anterior and posterior fontanels. The
injection needle was fixed and inserted into the brain according to
the stereoscopic location coordinate. The injection was then
conducted.
Immunocytochemistry
MN9D cells were inoculated in a 24-well plate (to
allow cells to creep onto a coverslip), and then cultured in serum-
and phenol red-free DMEM/F12 medium for 24 h. The cells were
injured by addition of 200 μM 6-OHDA for 30 min, followed by
4% paraformaldehyde for fixation. After washing cells with PBS,
sections were blocked with 5% goat serum-PBS and incubated
overnight at 4°C. The control group did not receive the primary
antibody. Then the other wells received 150 μl of the
primary antibody (prepared with 0.3% Triton X-100 blocking
solution). The primary antibodies were monoclonal rat anti-human
N-cadherin (1:100; Cell Signaling Technology), polyclonal rabbit
anti-human phosphorylated N-cadherin (phospho-N-cadherin) (1:5;
Santa Cruz Biotechnology, Inc.), polyclonal rabbit anti-human Akt
(1:100; Cell Signaling Technology), monoclonal rat anti-human
phosphorylated Akt (1:50; Santa Cruz Biotechnology, Inc.), diluted
with PBS and incubated overnight. After washing with PBS, the
sections were incubated with the secondary antibodies: FITC-labeled
goat anti-rabbit secondary antibody (analytically pure, 1:200;
Zhongshan Co.) or Cy3-labeled goat anti-rat secondary antibody
(1:200) overnight. After washing the cells with PBS, the coverslip
was removed and sealed with a fluorescence mountant. It was mounted
reversely onto the glass slide (with the cells facing down). The
fixed cells were observed using confocal microscopy at a
magnification of ×400.
Western blot analysis
Immunoblotting was carried out using 20 μg of
the total cell extracts. The MN9D cells were collected and washed
with cold PBS, and then plasma and membrane proteins were extracted
with a eukaryotic cell membrane extraction kit (Thermo Scientific).
The protein concentration was assayed using the Lowry method. Equal
quantities of protein sample were separated by 10%
SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and then
electrotransferred to a nitrocellulose (NC) membrane
(Sigma-Aldrich) according to the methods of Gu et al
(20). The NC membrane was
blocked with 5% fat-free milk (containing 0.2% Tween-20) in PBS.
Then the primary antibodies were added: phosphorylated Akt (p-Akt)
(Ser473, 1:1,000), total Akt (1:1,000) (both from Cell Signaling
Technology), N-cadherin (1:100), phospho-N-cadherin (Tyr860)
(1:500), β-actin (1:1,000) (all were from Santa Cruz Biotechnology,
Inc.). The membranes were then incubated overnight at 4°C. The
membrane was cleaned with washing buffer and then the alkaline
phosphatase-bound goat anti-rat, goat anti-rabbit and rabbit
anti-goat antibodies were added to the respective membranes
(IgG-AP, secondary antibody). The membrane was stored at 37°C for 2
h, after which, the membrane was colored with NBT/BCIP (Amersham)
and cleaned with washing buffer 3 times (5 min each time). The
reaction was terminated by rinsing the membrane with water.
Densitometry to quantify protein expression levels was performed
using ImageJ software (NCBI).
Statistical analysis
Data were analyzed with one-way or two-way ANOVA
using SPSS16.0 software, and expressed as means ± standard
deviation. P<0.05 was considered to indicate a statistically
significant result.
Results
The phosphorylation level of N-cadherin
(Tyr860) is related to the concentration of GDNF
Rats were stereotaxically injected in the right
substantia nigra with GDNF at different doses (25 ng/4 μl,
50 ng/4 μl, 75 ng/4 μl and 100 ng/4 μl) and
PBS (4 μl) for 15 min. The untreated rats served as the
control group. The rats were then decapitated and the substantia
nigra was extracted. phospho-N-cadherin (Tyr860) levels were
detected by western blot analysis using β-actin as an internal
control. According to the statistical results, we found that the
level of phospho-N-cadherin (Tyr860) in the G 50 ng group was the
highest (Fig. 1A).
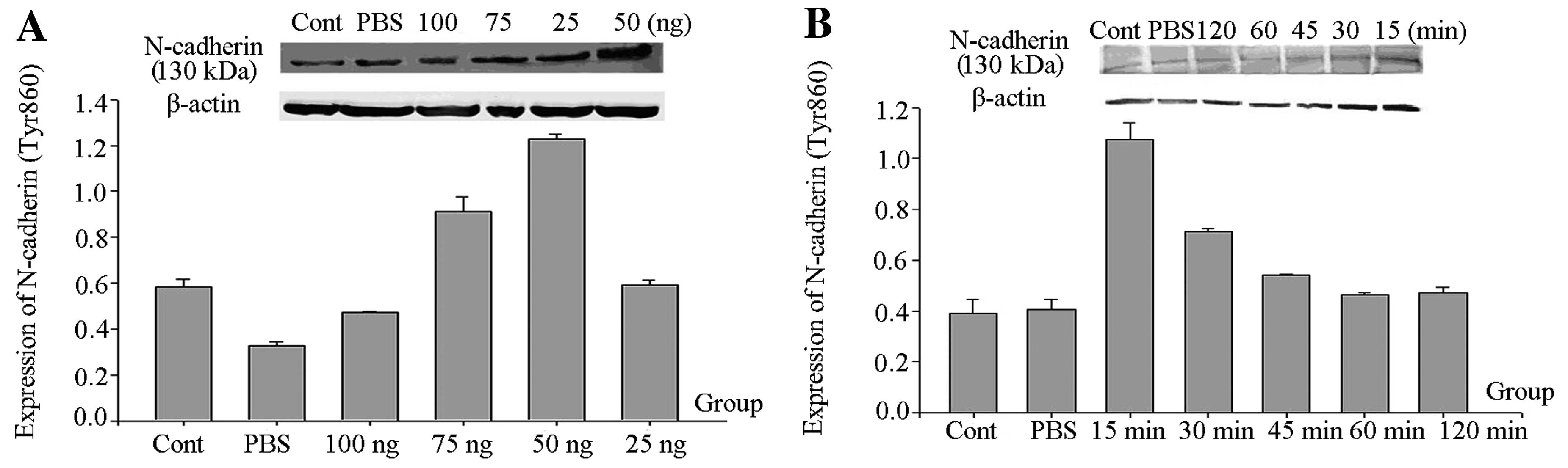 | Figure 1(A) Western blot analysis of the
amounts of phospho-N-cadherin (Tyr860) at different GDNF
concentrations. Rats were stereotaxically injected in the right
substantia nigra with GDNF at different doses (25 ng/4 μl,
50 ng/4 μl, 75 ng/4 μl and 100 ng/4 μl) and
PBS (4 μl) for 15 min. The untreated rats served as the
control group. The rats were then decapitated and the substantia
nigra was extracted. Finally, phospho-N-cadherin (Tyr860) and
β-actin levels were detected by western blot analysis. From left to
right, the bands are: control, PBS, GDNF (G) 100 ng, G 75 ng, G 25
ng and G 50 ng. According to statistical results, we found that the
level of phospho-N-cadherin (Tyr860) in the G 50 ng group was the
fhighest. (B) Western blot analysis for the amount of
phospho-N-cadherin (Tyr860) at different times following GDNF
treatment. Rats were stereotaxically injected in the right
substantia nigra with 50 ng/4 μl of GDNF for 15, 30, 45 min,
1 and 2 h; and 4 μl PBS for 15 min. The untreated rats
served as the control group. The rats were then decapitated and the
substantia nigra was extracted. Finally, phospho-N-cadherin
(Tyr860) and β-actin levels were examined by western blot analysis.
From left to right, the bands are control, PBS, G 2 h, G 1 h, G 45
min, G 30 min and G 15 min. According to the statistical results,
we found that the level of phospho-N-cadherin (Tyr860) in the G 15
min group was the highest. |
The phosphorylation level of N-cadherin
(Tyr860) is related to exposure time
Rats were stereotaxically injected in the right
substantia nigra with 50 ng/4 μl of GDNF for 15, 30, 45 min,
1 and 2 h; and 4 μl PBS for 15 min. The untreated rats
served as the control group. The rats were then decapitated and the
substantia nigra was extracted. phospho-N-cadherin (Tyr860) levels
were examined by western blot analysis using β-actin as an internal
control. We found that the level of phospho-N-cadherin (Tyr860) in
the G 15 min group was the highest (Fig. 1B).
Following interference of N-cadherin
expression, the protective effect of GDNF on DAs was
attenuated
Based on the above results, in order to further
determine whether N-cadherin mediates the protective effects of
GDNF on DAs, the N-cadherin gene was knocked down using a small
interfering RNA plasmid. MN9D cells were transfected with Si-cad 3
plasmids or Si-control plasmids, or treated with Lipofectin alone.
Forty-eight hours later, GDNF (50 ng/ml) was administered for 2 h.
Afterwards, cells were treated with 6-OHDA (200 μM) for 30
min and then cultured with serum-free medium for 24 h. The
protective effect of GDNF on DAs was examined by Hoechst 33258
assay. The rate of cell apoptosis was analyzed by flow cytometry
(FCM). FCM and Hoechst 33258 results indicated that the
6-OHDA-treated plates contained the highest number of apoptotic
cells, while the control group had the lowest number of apoptotic
cells; for the GDNF + 6-OHDA group, Lipofectin + GDNF + 6-OHDA
group and Si-control + GDNF + 6-OHDA group, the differences in the
numbers of apoptotic cells achieved no statistical significance.
These groups had lower apoptosis rates than the interference group
of si-cadherin + GDNF + 6-OHDA (Fig.
2). These results showed that 6-OHDA damaged DAs; GDNF exerted
a protective effect on DAs, and following interference of
N-cadherin expression, the protective effect of GDNF was
attenuated.
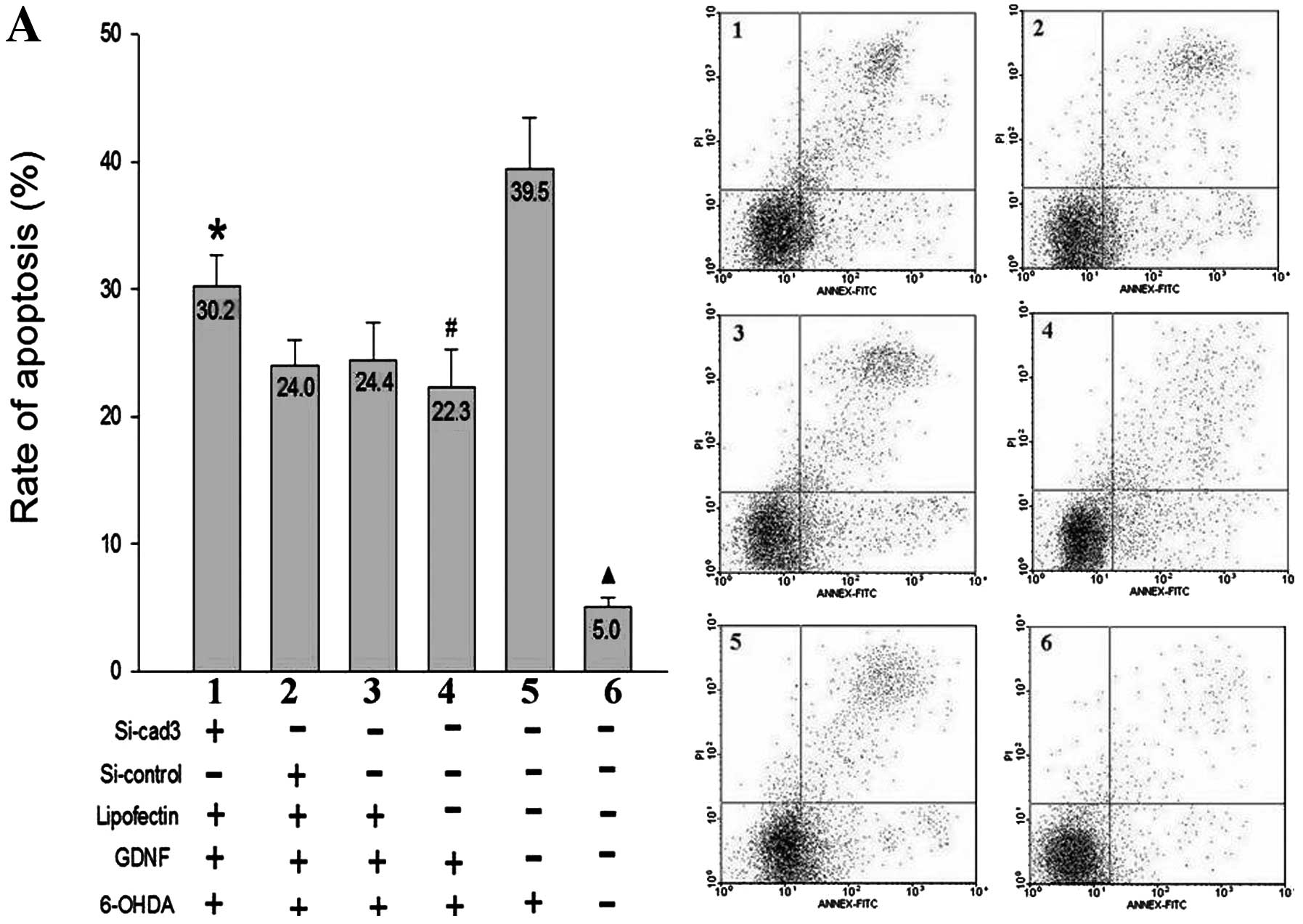 | Figure 2Protective effect of GDNF on damaged
MN9D cells following interference of the N-cadherin gene. The
values shown on the bars of the histograms indicate the apoptosis
rates. (A) Corresponding figures for the flow cytometric assay. (B)
The rate of apoptosis and the related cell morphology of MN9D cells
as determined by Hoechst 33258 staining. 1, si-cad 3 + GDNF +
6-OHDA group; 2, si-control + GDNF + 6-OHDA group; 3, Lip + GDNF +
6-OHDA group; 4, GDNF + 6-OHDA group; 5, 6-OHDA group; 6, control.
*P<0.01, significant difference between 1 and 2 or 3
or 4. #P<0.01, significant difference between 4 and
5. ▴P<0.01, significant difference between group 6
and 1,2,3,4 or 5. |
Following interference of N-cadherin
expression, activation of the PI3K/Akt signaling pathway was
affected
We aimed to ascertain the intracellular signaling
pathway activated when GDNF confers protection to DAs. Akt, also
known as protein kinase B (PKB), is the main downstream target,
which is activated and phosphorylated by many growth factors, and
it participates in many signal transduction pathways (21). Therefore, Akt phosphorylation can
be regarded as an index for measuring PI3K activity (22,23). Previous studies have shown that
when the extracellular portion of N-cadherin is bound to its
corresponding ligand, signaling pathways, such as PI3K/Akt, are
activated. Thus, in order to confirm whether GDNF also activates
the PI3K/Akt signaling pathway via N-cadherin, we used RNA
interference of N-cadherin expression, and examined N-cadherin and
Akt amounts and their phosphorylation levels.
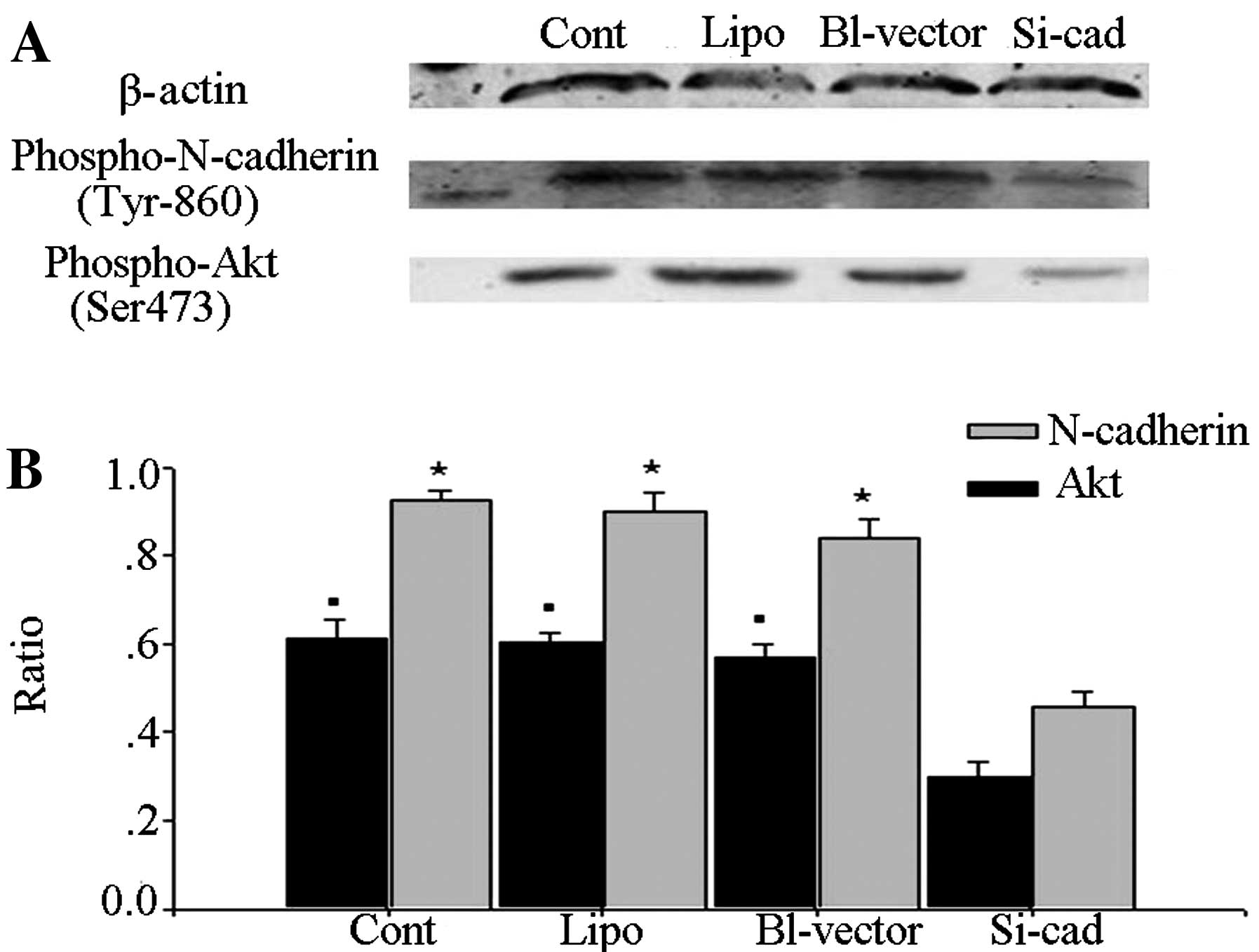
Following interference of N-cadherin expression, the
immunoblotting results showed that the quantities of
phospho-N-cadherin (Tyr860) and p-Akt were significantly decreased
in the interfering plasmid-transfected group (Fig. 3). This result indicated that the
amount of p-Akt was positively correlated to the amount of
phospho-N-cadherin (Tyr860).
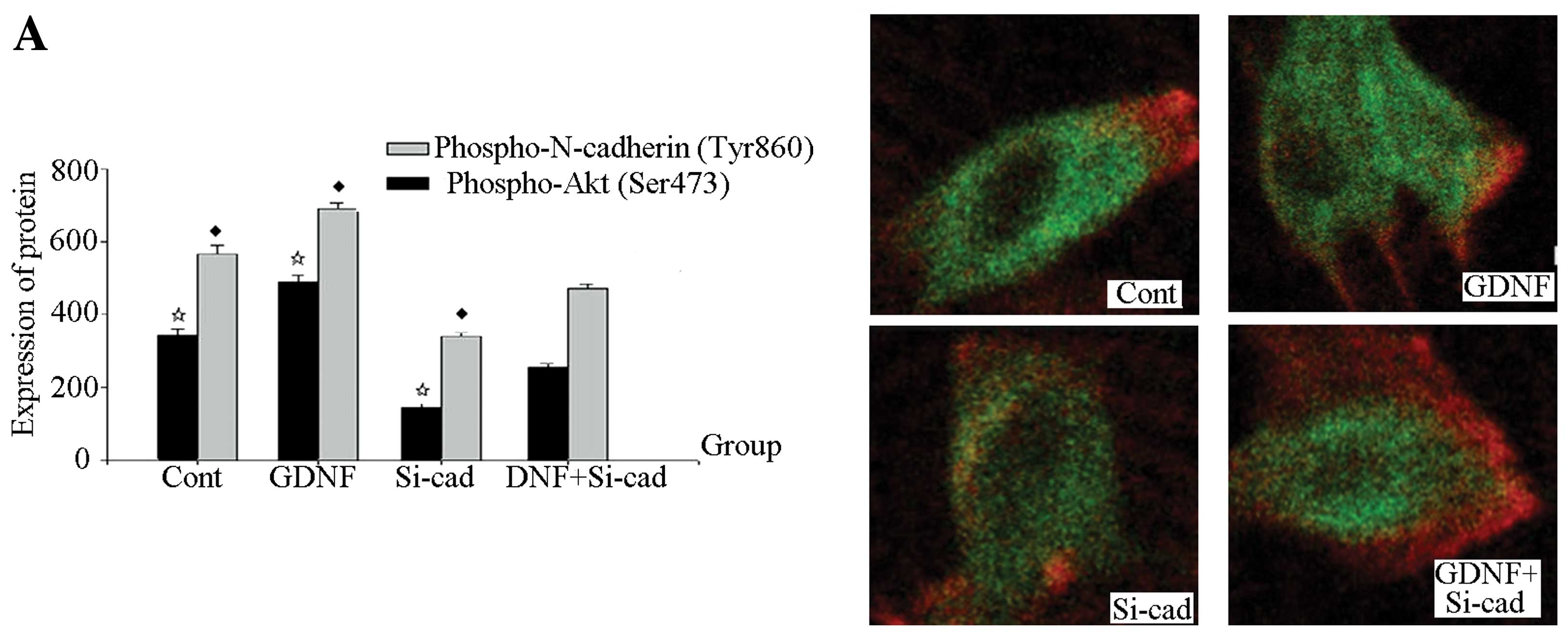
Immunohistochemistry showed that the quantities of
phospho-N-cadherin (Tyr860) and p-Akt in the interfering
plasmid-transfected group and the interference plasmid transfection
+ GDNF group were lowest, and the difference between the 2 groups
had no statistical significance (P>0.05). Additionally, the
quantities of phospho-N-cadherin (Tyr860) and Akt proteins in the
GDNF group were the highest, and that in the control group ranked
second (Fig. 4A). The interfering
plasmid-transfected group had the lowest total N-cadherin quantity,
and the intergroup differences in these quantities in the other
groups had no statistical significance (P>0.05) (Fig. 4C); the intergroup difference of
total Akt quantity also had no statistical significance (P>0.05)
(Fig. 4B).
The above results indicate that GDNF activates the
PI3K/Akt pathway via N-cadherin, and following interference of
N-cadherin expression, the activation of the PI3K/Akt pathway by
GDNF was affected.
Phospho-N-cadherin (Tyr860) and p-Akt
were decreased in a GDNF dose- and time-dependent manner
In order to further determine whether GDNF activates
the PI3K/Akt signaling pathway via N-cadherin, we carried out
experiments to determine the effects of varying GDNF times and
doses on phospho-N-cadherin (Tyr860) and p-Akt, and the correlation
between the two proteins was analyzed using statistical methods. We
performed two experiments. In the first experiments, the rats were
stereotaxically injected in the right substantia nigra with GDNF of
different doses (25 ng/4 μl, 50 ng/4 μl, 75 ng/4
μl and 100 ng/4 μl) or PBS (4 μl) for 15 min.
The untreated rats served as the control group. In the second
experiment, the rats were stereotaxically injected in the right
substantia nigra with 50 ng/4 μl of GDNF for 5, 15, 30, 45
min; and 4 μl PBS for 15 min. The rats were then decapitated
and the substantia nigra was extracted. Western blot analysis
showed that at different GDNF doses at the same treatment time,
phospho-N-cadherin (Tyr860) and p-Akt levels were highest in the 50
ng group. The phosphorylation levels declined as the dose increased
(P<0.05) (Fig. 5A). In the
experiment using the same GDNF dose but for varying treatment
times, phospho-N-cadherin (Tyr860) and p-Akt levels were the
highest in the 15 min group. The phosphorylation declined as the
time period of treatment lengthened (Fig. 5B). The changes in the
phospho-N-cadherin (Tyr860) and p-Akt shared the same trend and
showed a positive correlation in the statistical analysis.
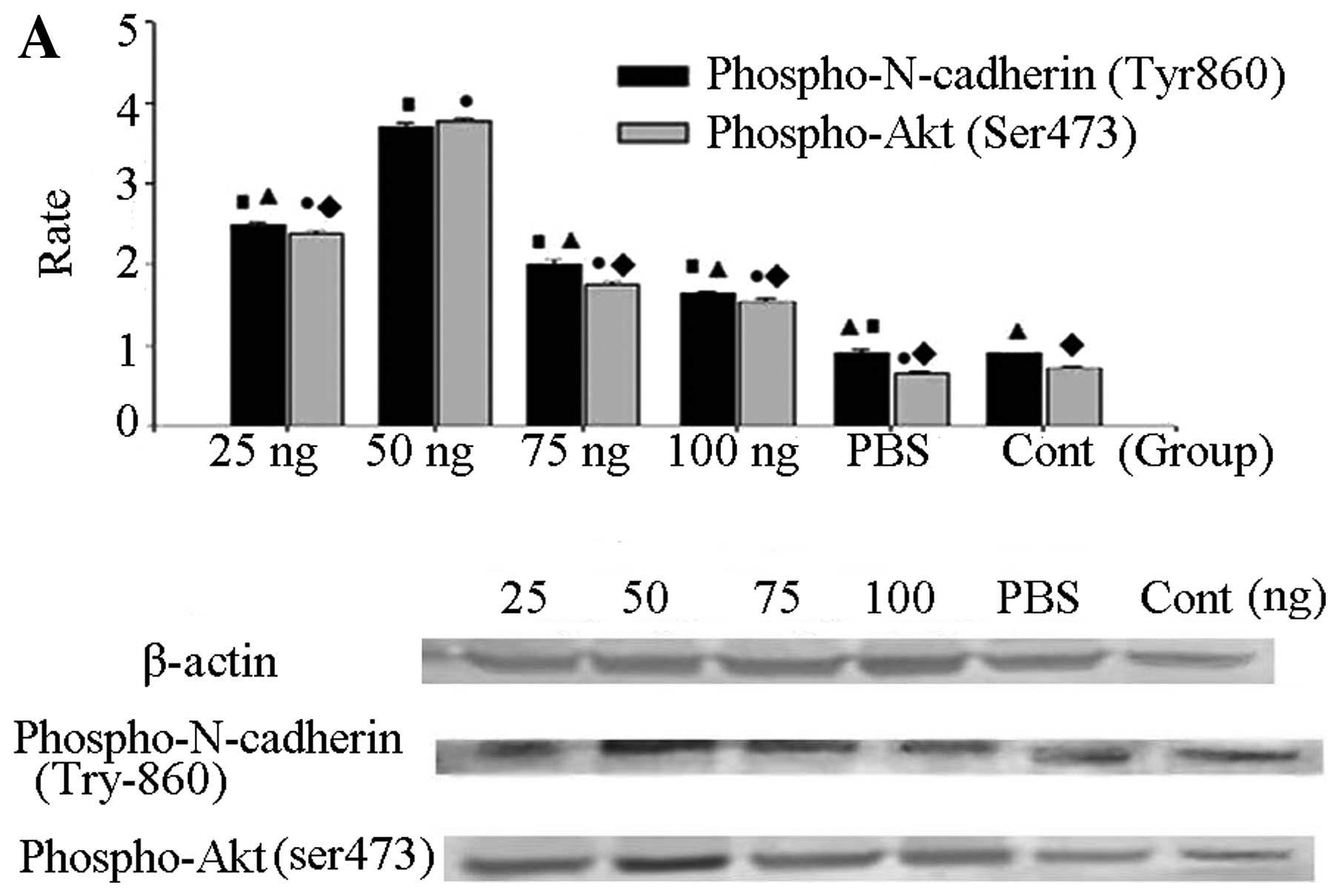 | Figure 5(A) Western blot analysis for
phospho-N-cadherin (Tyr860) and phospho-Akt (Ser473) at different
doses of GDNF. Rats were stereotaxically injected in the right
substantia nigra with GDNF at different doses (25 ng/4 μl,
50 ng/4 μl, 75 ng/4 μl and 100 ng/4 μl) or PBS
(4 μl) for 15 min. The untreated rats served as the control
group. The rats were then decapitated and the substantia nigra was
extracted. phospho-N-cadherin (Tyr860), phospho-Akt (Ser473) and
β-actin levels were examined by western blot analysis. Statistical
results indicated that the amount of phospho-N-cadherin (Tyr860)
was highest in the G 50 ng group, similar to phospho-Akt (Ser473).
Moreover, there was a positive correlation between the amounts of
phospho-N-cadherin (Tyr860) and phospho-Akt (Ser473). (B) Western
blot analysis for phospho-N-cadherin (Tyr860) and phospho-Akt
(Ser473) at different treatment times. Rats were stereotaxically
injected in the right substantia nigra with 50 ng/4 μl of
GDNF for 5, 15, 30, 45 min; and 4 μl PBS for 15 min. The
untreated rats served as the control group. The rats were then
decapitated and the substantia nigra was extracted. Finally,
phospho-N-cadherin (Tyr860) and β-actin levels were examined by
western blot analysis. From left to right, the bands are 5, 15, 30,
45 min, 4 μl PBS for 15 min and control. According to
statistical results, we found that the level of phospho-N-cadherin
(Tyr860) and phosphor-Akt (Ser473) in the G 15 min group was the
highest. ▴P<0.05 and ♦P<0.05 vs. 50 ng
group and 15 min group; ▪P<0.05 and
•P<0.05 vs. control group. |
Discussion
The present study reports a number of significant
findings. We systematically identified that, due to the effect of
GDNF, Tyr860 on the intracellular portion of N-cadherin was
phosphorylated, and the phosphorylation level of N-cadherin at
Tyr860 was correlated with the time and dose of GDNF. In order to
ascertain the appropriate protective concentration of GDNF, we
designed this assay. Regarding the most optimal concentration of
GDNF, it was found to be higher in vivo than in
vitro, which could be caused by the compensation of some other
metabolic pathways in vivo. Here, we only show the results
in vivo. GDNF protects nigra-striatal dopamine neurons in
animal models of PD, and its administration has been assessed as a
disease-modifying therapy for patients with PD (24,25). We also determined the most
effective concentration and treatment time for GDNF to protect DAs.
This finding has high significance for the development of medicines
in the clinic for PD patients.
Cadherin is a cell-cell adhesion receptor commonly
over-expressed in tumor cells that contributes to cell growth. The
PI3K/Akt pathway is also associated with different types of cancers
in most studies. It is known that neuron cells are terminating
cells, which can only be repaired, and rarely divide or
differentiate. The motor symptoms of PD result from the death of
dopamine-generating cells in the substantia nigra, a region of the
midbrain; the cause of this cell death is unknown. We assume this
pathway may be able to protect these cells from death.
Additionally, following interference of N-cadherin
gene expression, we found that the apoptotic rate of MN9D cells
increased after damage by 6-OHDA, even after the addition of GDNF.
DA damage by 6-OHDA is still a typical model of PD. Although,
sometimes, this model varies from actual PD cases, because there
are many different causes for PD. Thus, this model is useful for
the mechanistic study of PD, which is still a very popular tool in
current research.
Based on our results (Fig. 3), the N-cadherin gene was knocked
down, but this pathway was still open. Thus, N-cadherin may not be
the only mediator in this pathway, which is not contradictory to
previous findings. In order to determine whether N-cadherin is the
main mediator in this pathway, we need to systematically compare
those mediators which have been previously identified. When the
N-cadherin gene was knocked down, the protective effect of GDNF was
significantly weakened. Therefore, we speculated that N-cadherin is
involved in the biological process of GDNF’s protection of DAs.
In the event that N-cadherin participates in the
biological process of the protective effect of GDNF on DAs, then
determining the activated signaling pathway is crucial. Previous
research has demonstrated that the main intracellular signaling
pathway activated in GDNF’s protective effect on DAs is the
PI3K/Akt signaling pathway. In addition, several studies have
demonstrated that when the extracellular portion of N-cadherin is
bound to its corresponding ligand, its intra-cellular portion
activates the PI3K/Akt and Ras/Raf/MAPK signaling pathways
(26,27). Therefore, we further investigated
the PI3K/Akt signaling pathway following interference of N-cadherin
expression. The results indicated that, when N-cadherin expression
was significantly decreased, the Akt phosphorylation level
triggered by GDNF was also markedly decreased, which demonstrated
that the activation of the PI3K/Akt pathway was affected.
In order to further confirm whether GDNF activates
the PI3K/Akt signaling pathway via N-cadherin, our research focused
on the changes in phosphorylation of both N-cadherin and Akt
following different time periods and GDNF doses. The results
demonstrated that the phosphorylation levels of the two proteins
were time- and GDNF dose-dependent, and related analyses also
showed that changes in the phosphorylation changes of the two
proteins were closely correlated.
In conclusion, N-cadherin was found to mediate the
protective effect of GDNF on DAs, and the intracellular signaling
pathway activated by this effect was the PI3K/Akt pathway. The
specific mechanism by which N-cadherin activates the PI3K/Akt
signaling pathway is still unclear. This is a key concept which
requires further study. The present study offers important insights
into the mechanisms of Parkinson’s disease, and our findings may
aid in drug development for patients with Parkinson’s disease.
Acknowledgements
This study was funded by the National
Natural Science Research Funds of China (grant nos. 30570564 and
30870797) and the Priority Academic Program Development of Jiangsu
Higher Education Institutions (PAPD).
References
|
1
|
Lin LF, Doherty D, Lile J, Bektesh S and
Collins F: GDNF: a glial cell line-derived neurotrophic factor for
midbrain dopaminergic neurons. Science. 260:1130–1132. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tomac A, Lindqvist E, Lin LF, et al:
Protection and repair of the nigrostriatal dopaminergic system by
GDNF in vivo. Nature. 373:335–339. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Trupp M, Arenas E, Fainzilber M, et al:
Functional receptor for GDNF encoded by the c-ret proto-oncogene.
Nature. 381:785–789. 1996. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jing S, Wen D and Yu Y: GDNF-induced
activation of the RET protein tyrosine kinase is mediated by
GFR-alpha, a novel receptor for GDNF. Cell. 85:1113–1124. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Paratcha G, Ledda F and Ibáñez CF: The
neural cell adhesion molecule NCAM is an alternative signaling
receptor for GDNF family ligands. Cell. 113:867–879. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chao CC, Ma YL, Chu KY, Eminy H and Lee Y:
Integrin alphav and and NCAM mediate the effects of GDNF on DA
neuron survival, outgrowth, DA turnover and motor activity in rats.
Neurobiol Aging. 24:105–116. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Funahashi H, Takeyama H, Sawai H, et al:
Alteration of integrin expression by glial cell line-derived
neurotrophic factor (GDNF) in human pancreatic cancer cells.
Pancreas. 27:190–196. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cao JP, Yu JK, Li C, Sun Y, Yuan HH, Wang
HJ and Gao DS: Integrin beta1 is involved in the signaling of glial
cell line-derived neurotrophic factor. J Comp Neurol. 509:203–210.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang HJ, Cao JP, Yu JK and Gao DS: Role of
PI3-K/Akt pathway and its effect on glial cell line-derived
neurotrophic factor in midbrain dopamine cells. Acta Pharmacol Sin.
28:166–172. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Coulpier M, Anders J and Ibanez CF:
Coordinated activation of autophosphorylation sites in the RET
receptor tyrosine kinase: importance of tyrosine 1062 for GDNF
mediated neuronal differentiation and survival. J Biol Chem.
277:1991–1999. 2002. View Article : Google Scholar
|
|
11
|
Glaser T, Brose G, Franceschini I, et al:
Neural cell adhesion molecule polysialylation enhances the
sensitivity of embryonic stem cell-derived neural precursors to
migration guidance cues. Stem Cells. 25:3016–3025. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Decker L and Ffrench-Constant C: Lipid
rafts and integrin activation regulate oligodendrocyte survival. J
Neurosci. 24:3816–3825. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Grazia Lampugnani M, Zanetti A, Corada M,
et al: Contact inhibition of VEGF-induced proliferation requires
vascular endothelial cadherin, beta-catenin, and the phosphatase
DEP-1/CD148. J Cell Biol. 161:793–804. 2003.PubMed/NCBI
|
|
14
|
Drees F, Pokutta S, Yamada S, Nelson WJ
and Weis WI: Alpha-catenin is a molecular switch that binds
E-cadherin-beta-catenin and regulates actin-filament assembly.
Cell. 123:903–915. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Reynolds AB: p120-catenin: past and
present. Biochim Biophys Acta. 1773:2–7. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Neugebauer KM, Tomaselli KJ, Lilien J and
Reichardt LF: N-cadherin, NCAM, and integrins promote retinal
neurite outgrowth on astrocytes in vitro. J Cell Biol.
107:1177–1187. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kjaer S and Ibáñez CF: Identification of a
surface for binding to the GDNF-GFR alpha 1 complex in the first
cadherin-like domain of RET. J Biol Chem. 278:47898–47904. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Patil SB, Brock JH, Colman DR and Huntley
GW: Neuropathic pain- and glial derived neurotrophic
factor-associated regulation of cadherins in spinal circuits of the
dorsal horn. Pain. 152:924–935. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cao JP, Li FZ, Zhu YY, Yuan HH, Yu ZQ and
Gao DS: Expressions and possible roles of GDNF receptors in the
developing dopaminergic neurons. Brain Res Bull. 83:321–330. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gu Z, Jiang Q and Zhang G: c-Jun
N-terminal kinase activation in hippocampal CA1 region was involved
in ischemic injury. Neuroreport. 12:897–900. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cantley LC: The phosphoinositide 3-kinase
pathway. Science. 296:1655–1657. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lin CH, Yeh SH, Lu KT, et al: A role for
the PI-3 kinase signaling pathway in fear conditioning and synaptic
plasticity in the amygdala. Neuron. 31:1–20. 2001.PubMed/NCBI
|
|
23
|
Edström A and Ekström PA: Role of
phosphatidylinositol 3-kinase in neuronal survival and axonal
outgrowth of adult mouse dorsal root ganglia explants. J Neurosci
Res. 74:726–735. 2003.PubMed/NCBI
|
|
24
|
Richardson RM, Kells AP, Rosenbluth KH, et
al: Interventional MRI-guided putaminal delivery of AAV2-GDNF for a
planned clinical trial in Parkinson’s disease. Mol Ther.
19:1048–1057. 2011.PubMed/NCBI
|
|
25
|
Pascual A, Hidalgo-Figueroa M, Gómez-Díaz
R and López-Barneo J: GDNF and protection of adult central
catecholaminergic neurons. J Mol Endocrinol. 46:R83–R92. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Takeichi M: The cadherins: cell-cell
adhesion molecules controlling animal morphogenesis. Development.
102:639–655. 1988.PubMed/NCBI
|
|
27
|
Hulit J, Suyama K, Chung S, et al:
N-Cadherin signaling potentiates mammary tumor metastasis via
enhanced extracellular signal-regulated kinase activation. Cancer
Res. 67:3106–3116. 2007. View Article : Google Scholar
|