Introduction
Despite major advances in the understanding and
treatment of atherosclerosis, coronary heart disease continues to
contribute to significant morbidity and mortality in the general
population. It is increasingly recognized that inflammation plays
an important role in the development of atherosclerosis (1–3).
Lipoprotein-associated phospholipase A2
(Lp-PLA2), also termed platelet-activating factor
acetylhydrolase (PAFAH), is one of the most studied circulating
biomarkers of inflammation in the setting of atherosclerosis
(2). This enzyme is predominantly
associated with LDL in humans and HDL in mice, and increasing
evidence suggests that it plays a pivotal role in the pathogenesis
of atherosclerosis (4). For
example, it is known to be an important predictor of
atherothrombotic events and a direct participant in the formation
of atherosclerosis (5).
Biochemically, Lp-PLA2 reacts with oxidized
phospholipids to generate the pro-inflammatory by-products
lysophosphatidylcholine (LPC) and oxidized non-esterified fatty
acid (oxNEFA), both of which are implicated in the progression of
atherosclerosis (5).
Specifically, LPC is known to increase the expression of vascular
adhesion molecules, to upregulate several cytokines and the CD40
ligand, and to stimulate macrophage proliferation, all of which
play a critical role in atherosclerosis (6).
Multiple in vitro and in vivo studies
have collectively suggested a causative role of Lp-PLA2
in the development of atherosclerosis. Therefore, we hypothesized
that the inhibition of its activity may have beneficial effects
(5). The effect of RNA
interference (RNAi) of Lp-PLA2 on atherosclerosis in
mouse models has not previously been studied, as such we used a
lentiviral-mediated RNAi approach which has been proven to be
efficacious in silencing target genes in dividing and nondividing
cells (7,8). Traditional concepts of inflammation
in atherosclerosis are regarded as an ‘inside-out’ responses,
holding the central tenet that inflammatory responses are initiated
at the intima. Therefore, we proceeded with transfection using the
transluminal approach. As such, in the present study, we aimed to
delineate the effect of lentiviral-mediated RNAi of
Lp-PLA2 on the progression of atherosclerosis and
associated inflammation in apolipoprotein E-deficient mice, to
further establish the role of Lp-PLA2 in
atherosclerosis.
Materials and methods
Lentiviral vectors for Lp-PLA2
RNAi
The target sequence (5′-GCAAGCTGGAATTCTCCTTTG-3′)
within the murine Lp-PLA2 mRNA was chosen as the target
for RNAi. A scrambled shRNA sequence (5′-TTCTCCGAACGTGTCACGT-3′)
served as a negative control (NC). Vectors were constructed as
previously described (9,10). The titers averaged
1×109 transduction units (TU)/ml.
Cell culture
The RAW 264.7 mouse macrophage cell line was
routinely cultured in DMEM. When cells had grown to 90% confluence,
Lp-PLA2 interfering lentiviruses and
lenti-scrambled-shRNA were then used to transfect RAW 264.7 cells
at a multiplicity of infection (MOI) of 50. Previous studies
demonstrated that unstimulated macrophage cells failed to produce
detectable levels of Lp-PLA2, while oxidized (ox)LDL
upregulated the expression of Lp-PLA2 in a
concentration- and time-dependent manner (11). In our preliminary cell
experiments, the expression of Lp-PLA2 reached the
platform stage after 60 μg/ml of oxLDL stimulation.
Therefore, we pretreated the cells with 60 μg/ml oxLDL. Next
we investigated the effects of RNAi on the expression of
Lp-PLA2, monocyte chemotactic protein-1 (MCP-1) and
interleukin-6 (IL-6) by quantitative real-time PCR. Non-lentivirus
and lentivirus-containing NC shRNA transfection served as
controls.
Animals and experimental protocol
One hundred and four male apolipoprotein E-deficient
mice received a high-fat diet (0.25% cholesterol and 15% cocoa
butter) and underwent constrictive collar placement around the left
common carotid artery after anesthesia with an intraperitoneal
injection of pentobarbital sodium (30–50 mg/kg), using the method
of von der Thüsen et al (12). In brief, the common carotid
arteries were dissected and a constrictive silastic collar (0.30
mm) was placed on the left common carotid artery near its
bifurcation by placement of 3 circumferential silk ties. The
sham-operated group underwent cervical incision and closure without
the placement of a constricting collar or instillation of a
lentiviral suspension. Subsequently, the entry wound was closed and
the animals were returned to their cage for recovery from
anesthesia. A heating pad and a heating lamp were used to maintain
body temperature. Eight weeks following surgery, the mice were
randomly assigned to the following 5 groups: i) sham-operated group
(n=18), without collar placement or lentiviral suspension
instillation; ii) control group (n=18), the mice had their collars
removed without virus infusion; iii) NC group (n=32), had their
collars removed and received an infusion of 50 μl
(5×107 TU) NC viral suspension instilled into the left
common carotid arteries via the external carotid under anaesthesia;
the suspension was left in situ for 30 min and the skin
incision was subsequently closed with silk sutures (12); iv) RNAi group 1 (RNAi1) (n=18),
had their collars removed and received an intravenous (systemic)
injection of 50 μl RNAi viral suspension injected into the
tail vein of mice and v) RNAi group 2 (RNAi2) (n=18), had their
collars removed and received a local infusion of 50 μl RNAi
viral suspension instilled into the left common carotid arteries
via the external carotid (13).
To check the transfection efficiency of the lentivirus in
atherosclerotic plaques, mice of the NC group were sacrificed at a
rate of 2 mice every week after transfection. Cryosections were
observed with an Olympus microscope with fluorescent light to
identify GFP expression. The remaining mice were all sacrificed at
the end of week 15, and the left common carotid arteries were
collected for histopathological analysis. The animal experimental
protocol complied with the Animal Management Rules of the Chinese
Ministry of Health (document no. 55, 2001) and was approved by the
Ethics Committee of Zhengzhou University (Zhengzhou, China).
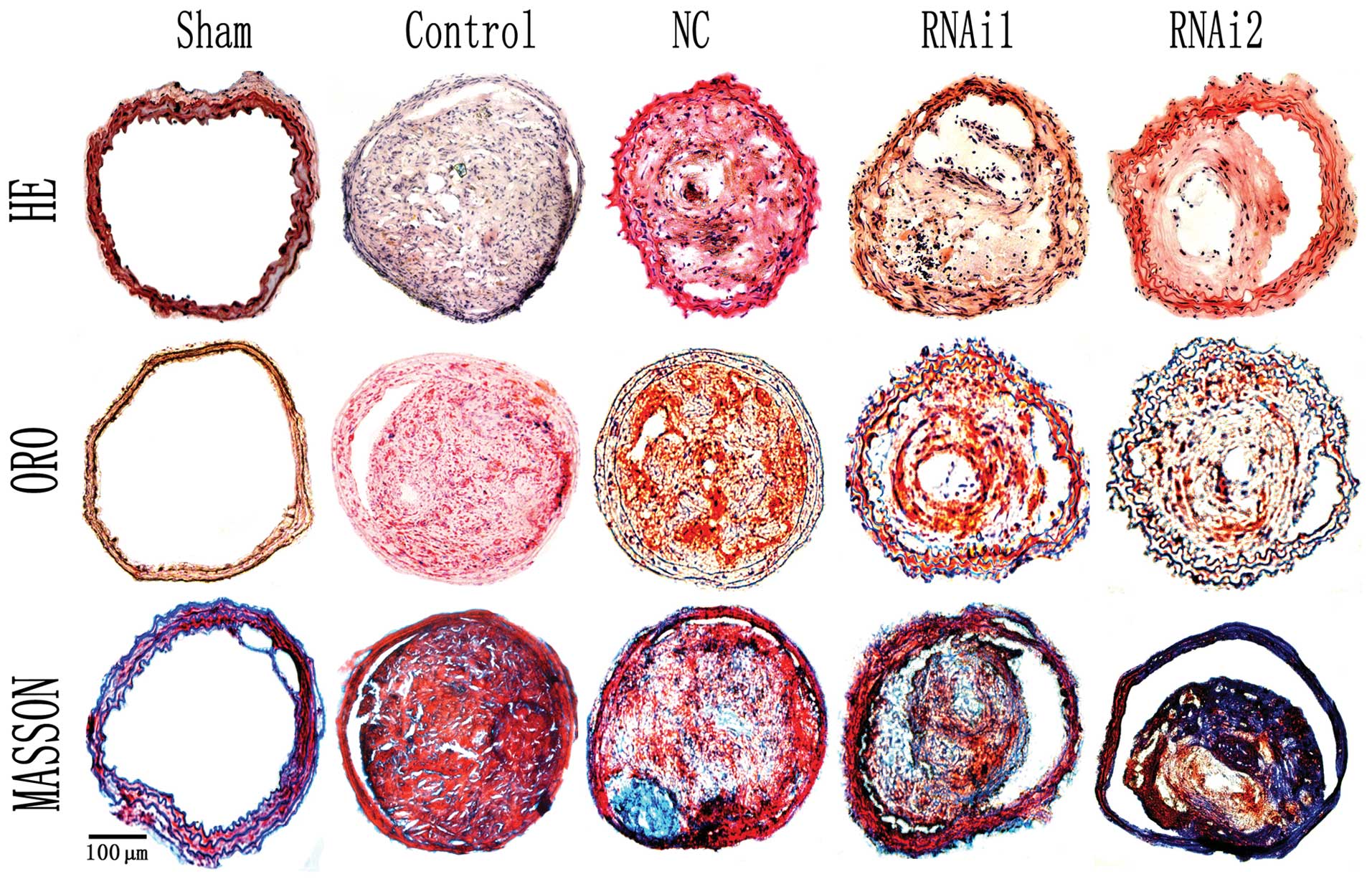
Histological analysis
The left common carotid artery was carefully
excised, embedded in OCT compound, and underwent histological
analysis for identification of the apex of the lesion, which
displays the smallest lumen. Sections were stained with hematoxylin
and eosin (H&E). Collagen and lipid deposition in plaques was
identified by Masson’s trichrome and Oil Red O (ORO) staining,
respectively.
RNA extraction and RT-PCR
Total RNA was extracted with TRIzol reagent.
Complementary DNA was synthesized using the reverse transcription
kit (CoWin Bioscience Co., Ltd., Beijing, China). PCR products were
synthesized using SYBR-Green RT-PCR Master Mix and were analyzed
with a RT-PCR cycler and detection system (ABI Prism 7300 Sequence
Detection System; PE Applied Biosystems, Foster City, CA, USA).
Quantitative values were obtained from the threshold cycle (Ct)
value. The specific primer sequences were designed by Primer
Premier 5 software. The specific primers used were as follows:
5′-ACAACCACGGCCTTCCCTACTT-3′ and 5′-TTTCTCATTTCCACGATTTCCC-3′ for
IL-6; 5′-CTG GACAACATACTGCTAACCG-3′ and 5′-TCAAATGCT
CCTTGATTTCTGG-3′ for IL-10; 5′-CCAGAGATTCAG ATGTGGAGTT-3′ and
5′-TGGCAGAGTTGATAAAGA GGAG-3′ for Lp-PLA2;
5′-GCCTGACTCTGGTGATTT CTTG-3′ and 5′-TGTTGATGTCTGCTTCTCCCTG-3′ for
MMP-8; 5′-GCTCAGCCAGATGCAGTTAACG-3′ and
5′-TCTTGGGGTCAGCACAGACCTC-3′ for MCP-1; 5′-TGT
CTACTGAACTTCGGGGTGA-3′ and 5′-TGGTTTGCTACG ACGTGGGCTA-3′ for TNF-α;
and 5′-GCTATGCTCTCC CTCACGCCAT-3′ and 5′-TCACGCACGATTTCCCTCTC AG-3′
for β-actin. The results were analyzed by the 2−ΔΔCt
method, which reflects the difference in threshold for the target
gene relative to that of β-actin.
Western blot analysis
Tissues were collected and lysed with protease
inhibitor in 1X lysis buffer on ice for 10 to 15 min. Homogenates
were centrifuged at 12,000 × g for 20 min on ice, and the
supernatants were collected and the protein content was quantified
using the BCA method, and then SDS-PAGE electrophoresis was
performed. Proteins were transferred to PVDF membranes. Membranes
were then blocked with 5% non-fat milk and incubated overnight with
primary antibodies against Lp-PLA2, MMP-8 (Abcam,
Cambridge, UK), TNF-α, IL-6 and β-actin (Zhongshan Biological
Technology Co. Ltd, Beijing, China). After washing with TBS-T, the
membranes were incubated with horseradish peroxidase
(HRP)-conjugated secondary antibodies at room temperature for 60
min. Subsequently, the appropriate HRP-conjugated secondary
antibodies were used, and the blots were probed and then exposed to
X-ray film.
Plasma lipid and biological analysis
Plasma concentration of Lp-PLA2, IL-10,
MMP-8, TNF-α, total cholesterol (TC), and triglyceride (TG) were
measured using quantitative sandwich enzyme immunoassay (commercial
ELISA kits) following the manufacturer’s recommendation (CoWin
Bioscience Co., Ltd.).
Statistical analysis
Data are presented as mean values ± standard
deviation (SD). Data were compared among groups using one-way
analysis of variance (ANOVA) followed by the Student-Newman-Keuls
(SNK) test for post-hoc comparisons. All statistical analyses were
performed using SPSS version 16.0 software (SPSS, Chicago, IL,
USA). P<0.05 was considered to indicate a statistically
significant result.
Results
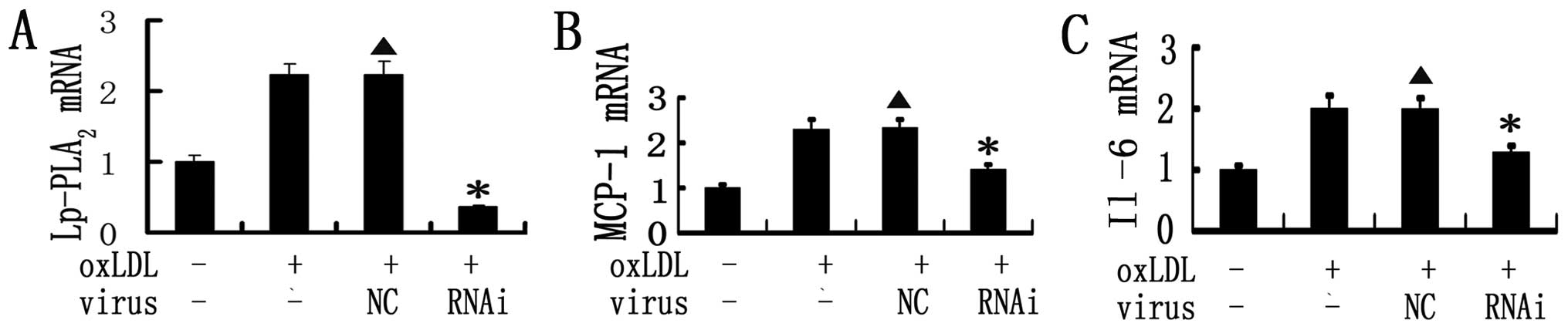
Effects of RNAi on the expression of
Lp-PLA2 and pro-inflammatory cytokines in vitro
RAW 264.7 cells showed very low expression of
Lp-PLA2 before oxLDL stimulation. After 60 μg/ml
of oxLDL pretreatment, the expression of Lp-PLA2
increased sharply. Mouse RAW 264.7 cells were then transduced with
MOI 50 of each vector to determine their efficiency. Our results
demonstrated that Lp-PLA2 RNAi led to an 83.8%
(P<0.001) decrease in Lp-PLA2 mRNA expression in RAW
264.7 cells. As expected, no effect was observed following
scrambled shRNA infection. In addition, our study demonstrated that
Lp-PLA2 RNAi inhibited the augmentation of MCP-1 and
IL-6 induced by oxLDL (Fig.
1).
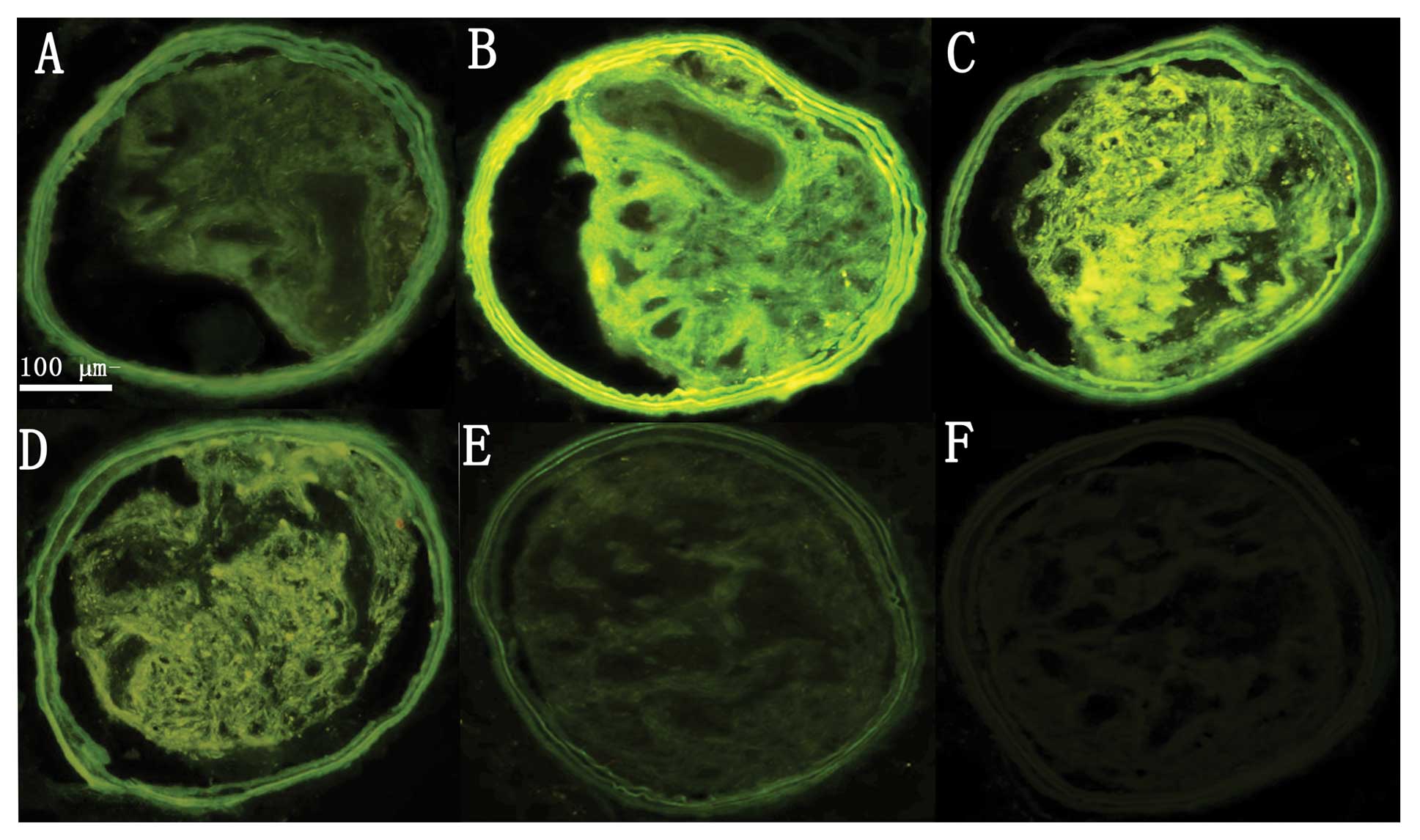
Safety and efficiency of RNAi in
vivo
Following surgery and lentiviral infection, all mice
were apparently healthy, and no animals died before the day of
sacrifice. Previous studies have indicated that GFP expression
provides an efficient and convenient way to detect the transfection
efficiency of lentiviruses (7,13).
Therefore, GFP fluorescence in carotid artery plaques was examined
once a week after transfection (Fig.
2). Slight GFP fluorescence in the carotid plaques was
displayed 1 week after transfection. The strongest GFP fluorescence
was manifested 2 and 3 weeks after transfection. Modest GFP
fluorescence was visualized 4–6 weeks after transfection. Faint
fluorescence was still visible 7 weeks after transfection. These
results demonstrated efficient in vivo transfection of
Lp-PLA2 shRNA in the carotid plaques of the mice.
Effective silencing of Lp-PLA2
expression by RNAi in vivo
At the end of the study, the plaques in the
sham-operated group showed extremely low mRNA and protein
expression of Lp-PLA2 as well as the plasma
concentration of Lp-PLA2. The mRNA and protein
expression of Lp-PLA2, as well as the plasma
concentration Lp-PLA2 were significantly higher in the
control and NC group compared with that in the sham-operated group.
In the RNAi1 and RNAi2 groups, Lp-PLA2 mRNA expression
was reduced by 42.5 and 58.3% (both P<0.01), the
Lp-PLA2 protein level was decreased by 15.6 and 46.2%
(both P<0.01) and the plasma concentration of Lp-PLA2
was lowered by 28.2 and 40.8% (both P<0.05), respectively,
compared to those in the control and NC groups (Figs. 3A and 5). In contrast, the control group did
not differ from the NC groups in Lp-PLA2 expression.
These results indicate that transluminal local administration of
the lentivirus was more effective in silencing Lp-PLA2
expression.
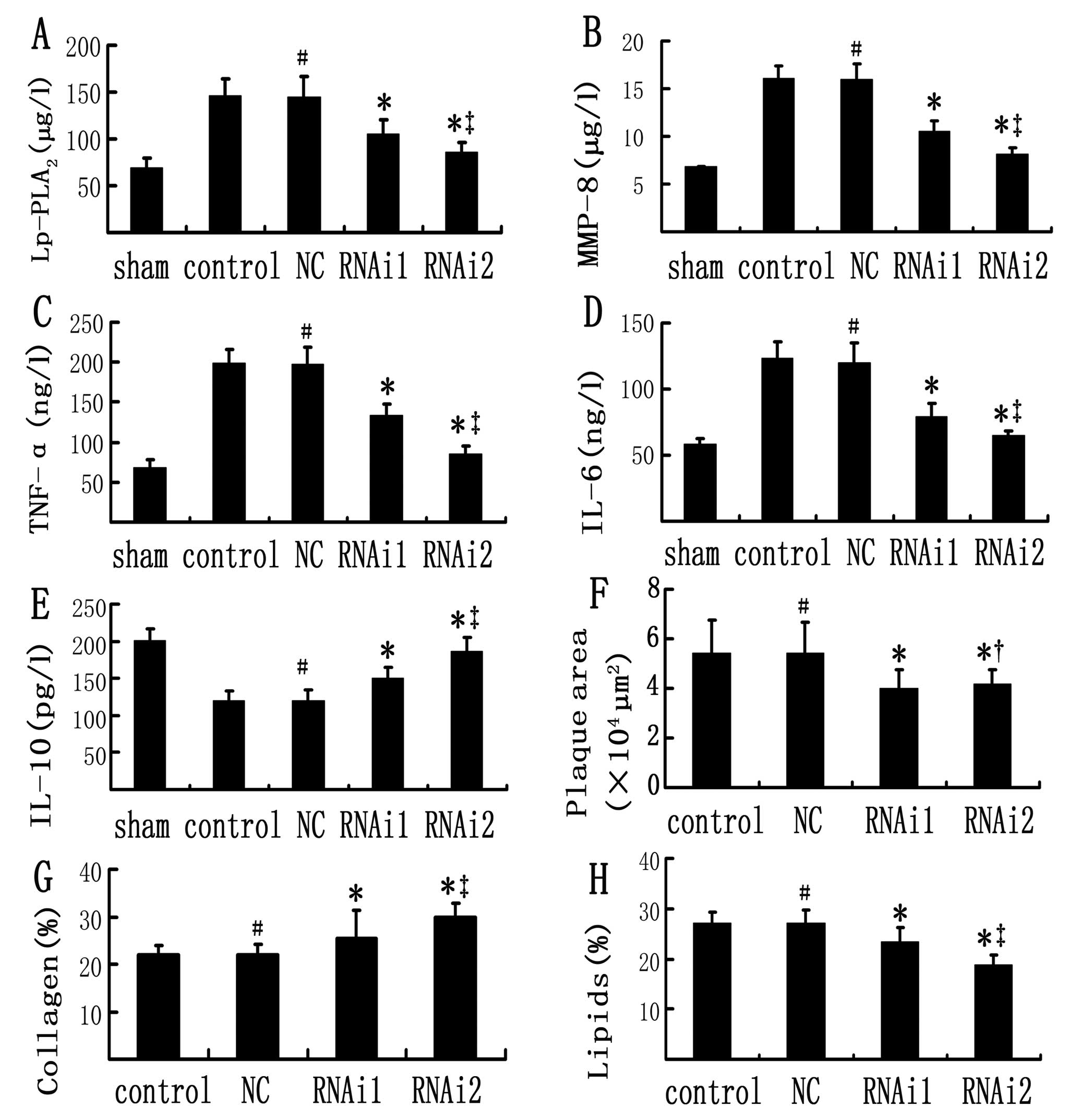 | Figure 3(A–E) Comparison of plasma
inflammatory markers in the sham-operated, control, NC, RNAi1 and
RNAi2 groups; (F–H) Comparison of plaque morphology in the
different groups. Concentrations of (A) Lp-PLA2, (B)
MMP-8, (C) TNF-α, (D) IL-6 and (E) IL-10 were measured by ELISA at
week 15. (F) Plaque area, (G) collagen content and (H) lipid
content are shown for the control, NC, RNAi1 and RNAi2 groups. Data
are expressed as the mean ± SD (n=18). No significant differences
were found between control and NC groups. *P<0.05 vs.
control and NC groups; ‡P<0.05 vs. RNAi1 group;
†P>0.05 vs. RNAi1 group; #P>0.05 vs.
control group (one-way ANOVA). Lp-PLA2,
lipoprotein-associated phospholipase A2; MMP-8, matrix
metalloproteinase-8; TNF-α, tumor necrosis factor-α; RNAi, RNA
interference; IL, interleukin. |
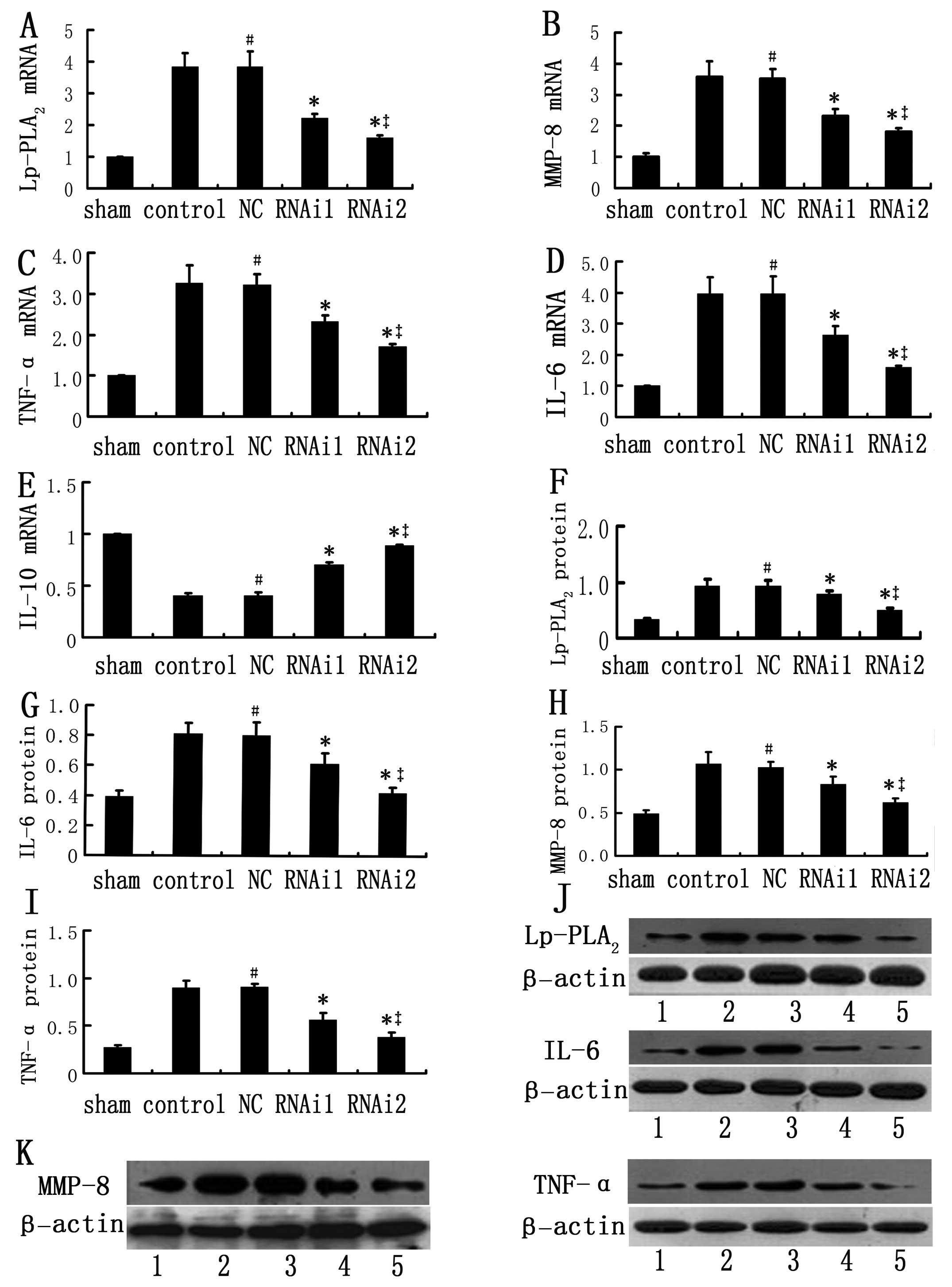 | Figure 5Expression of inflammatory genes in
atherosclerotic plaques in the sham-operated, control, NC, RNAi1
and RNAi2 groups after harvesting of tissues at week 15. (A–E) mRNA
expression of inflammatory genes. Relative mRNA expression of
Lp-PLA2 and inflammatory genes was measured by real
time-PCR in the sham-operated, control, NC, RNAi1 and RNAi2 groups
for 3 independent experiments. The expression levels of the target
gene in the sham-operated group were considered as 1, its relative
expression levels in other groups were presented as a ratio with
that of the sham-operated group. (F–K) Densitometric analysis of
protein expression of inflammatory genes and representative
immunoblots. Accumulation of target protein was normalized against
β-actin protein levels, determined as an internal control. Data are
expressed as the mean ± SD (n=6). No significant differences were
found between the control and NC groups. #P>0.05 vs. control
group; *P<0.05 vs. control and NC groups;
‡P<0.05 vs. RNAi1 group (one-way ANOVA). Lane 1,
sham-operated group; lane 2, control group; lane 3, negative
control (NC) group; lane 4, RNAi1 group; lane 5, RNAi2 group.
Lp-PLA2, lipoprotein-associated phospholipase
A2; MMP-8, matrix metalloproteinase-8; TNF-α, tumor
necrosis factor-α; RNAi, RNA interference; IL, interleukin. |
No effect of Lp-PLA2 RNAi on
body weight and plasma lipid profile
As expected, we observed no significant differences
in the TC and TG levels among the 5 groups of mice. Additionally,
the body weights of mice in all groups were not significantly
different (Table I).
 | Table IBody weight, plasma TC and TG levels
among all groups. |
Table I
Body weight, plasma TC and TG levels
among all groups.
| Sham | Control | NC | RNAi1 | RNAi2 |
|---|
| BW (g) | 27.4±3.7 | 27.8±3.6 | 26.7±3.2 | 27.5±3.9 | 27.8±3.5 |
| TC (mmol/l) | 29.5±3.5 | 30.3±2.3 | 29.9±3.0 | 29.8±3.3 | 30.1±2.3 |
| TG (mmol/l) | 3.0±0.9 | 3.0±1.0 | 3.0±0.9 | 3.0±0.8 | 2.9±0.9 |
Lp-PLA2 RNAi normalizes plasma
inflammatory markers
Control and NC groups demonstrated a significant
increase in the plasma concentration of pro-inflammatory cytokines
MMP-8, TNF-α and IL-6 together with a reduction in the
anti-inflammatory cytokine, IL-10, when compared with the
sham-operated group (Fig. 3).
These changes were partially reversed after RNAi. This was
particularly evident in the RNAi2 group, which showed significantly
lower mRNA expression of pro-inflammatory cytokines than the
control and NC groups did (P<0.01), reaching values similar to
those observed in the sham-operated group.
Lp-PLA2 RNAi attenuates the
formation of atherosclerotic plaques
The cross-sectional plaque areas for the 2 RNAi
groups were found to be significantly lower than those values in
the control and NC groups at week 15 (P<0.01) (Fig. 4). As expected, no significant
difference in plaque area was found between the control and NC
groups. Interestingly, we also observed that the plaque area for
the RNAi2 group was only moderately lower than that of the RNAi1
group, which was not statistically significant (P>0.05)
(Figs. 3 and 4). This result suggested that delivery
of Lp-PLA2 shRNA either locally or systemically did not
differentially affect plaque size.
The relative content of lipids and collagen in
plaques was determined by histological staining (Figs. 4 and 5). The relative content of collagen in
plaques of the control, NC, RNAi1, and RNAi2 groups was 22.1, 22.0,
25.3 and 29.8%, respectively, and was significantly higher in the
RNAi1 and RNAi2 groups (P<0.01). In comparison with the control
and NC group, the relative increase in the collagen content in
plaques of the RNAi1 and RNAi2 groups was 14.7 and 35.1%,
respectively.
The relative content of lipids in plaques of the 4
groups was 27.1, 27.0, 23.5 and 18.9%, respectively, and was
significantly lower in the RNAi1 and RNAi2 groups than that in the
control and NC groups (P<0.01). The relative reduction in lipid
content in plaques of the RNAi1 and RNAi2 groups was 26.2 and
30.1%, respectively, compared with the control and NC groups. In
contrast, no significant difference in the content of lipids and
collagen was found between the control and NC group. Note, that at
the end of the study period, no atherosclerotic lesions were found
in the left common carotid artery of the sham-operated group. Taken
together, these data indicate that the 2 RNAi groups showed less
lipid content and higher collagen content than the control and NC
groups did. Although the 2 RNAi groups were both effective in
attenuating atherosclerotic plaque formation and decreased plaque
vulnerability, transluminal local delivery of Lp-PLA2
shRNA exhibited enhanced improvement of plaque stability than
systemic administration. Plaque area and composition among all
groups were not statistically significant at the time of
transfection.
Effects of RNAi on the expression of
inflammatory genes within the plaque
The plaques in the sham-operated group showed very
low expression of MMP-8, TNF-α and IL-6 at the end of the study, in
comparison, the expression of pro-inflammatory cytokines was
sharply increased in the control and NC groups. Silencing of
Lp-PLA2 RNAi was able to reverse these increases; in the
RNAi2 group there was significantly lower mRNA and protein
expression of pro-inflammatory cytokines, as compared to the
control and NC groups (P<0.05), reaching values similar to those
observed in sham-operated mice. In contrast to these factors which
were increased in atherosclerotic plaques, mRNA expression of IL-10
was diminished in control and NC groups compared with the
sham-operated group, and this effect was attenuated after RNAi. As
expected, we observed no significant differences in the expression
of inflammatory genes between the control and NC groups (Fig. 5).
Discussion
The major finding of the present study was that the
mRNA and protein expression of Lp-PLA2 can be
effectively knocked down in carotid plaques of apolipoprotein
E-deficient mice using lentiviral-mediated RNAi. This led to
reduced local inflammatory cytokine expression, decreased lipid
content in plaques, increased collagen content in plaques and
reduced atherosclerotic plaque areas and vulnerability.
Importantly, transluminal local delivery of Lp-PLA2 was
found to be more effective than systemic administration, thus
providing a potential therapeutic approach for the treatment of
atherosclerosis.
Atherosclerosis is a chronic inflammatory disease of
the vascular wall (7). It is
known that the inflammatory process contributes significantly to
the initiation, progression and rupture of atherosclerotic plaques
(1,14,15). Lp-PLA2 produces 2 types
of inflammatory mediators, LPC and oxNEFA, which trigger
significant inflammatory responses, such as cell adhesion,
inflammatory gene expression, and cell death (16,17). Both experimental and
epidemiological studies have presented evidence that the
circulating concentration of Lp-PLA2 is associated with
progression of atherosclerosis after adjusting for established risk
factors (2,19,20). Given what is known about the
actions of Lp-PLA2 and the epidemiological data,
Lp-PLA2 provides an attractive target for assessing
cardiovascular disease risk and a therapeutic target for
interventions to reduce atherosclerosis. Previous studies have
demonstrated that darapladib, a selective Lp-PLA2
inhibitor, attenuated inflammation and necrotic core formation in
animal models of atherosclerosis (21,22). However, darapladib did not reduce
the primary end point of coronary plaque deformability, nor alter
the plasma hs-CRP concentration in a phase II clinical study
(23,24). In summary, experimental and
epidemiological evidence remains equivocal concerning the
potentially pro-atherogenic and anti-atherogenic effects of
Lp-PLA2 inhibition by darapladib. RNAi is a clinically
feasible method with which to downregulate the expression of target
genes efficiently and selectively (18). In the present study,
lentiviral-mediated Lp-PLA2 RNAi was used as a
therapeutic approach for atherosclerosis. Traditional concepts of
inflammation in atherosclerosis are regarded as an ‘inside-out’
responses, holding the central tenet that inflammatory responses
are initiated at the luminal surface, and later propagate outward
toward the adventitia. Therefore, we proceeded with transfection
using a transluminal approach. The efficacy of lentiviral-mediated
RNAi was confirmed by an observed decrease in the mRNA and protein
expression of Lp-PLA2 and the observation of GFP
fluorescence in the plaques. This effect was associated with a
reversal of the observed increase in the expression of
pro-inflammatory cytokines (MMP-8, TNF-α and IL-6), and an
attenuation of the decrease in expression of anti-inflammatory
cytokines (IL-10), as well as, decreased plaque content of lipids,
increased plaque content of collagen, and finally lowered
atherosclerotic plaque area and vulnerability of the plaques. We
denied the possibility that the beneficial effects observed in the
RNAi group were caused by nonspecific immune stimulation induced by
transfection since no significant effect was found between the
control and NC groups.
Several lines of evidence suggest that the precise
role of Lp-PLA2 in atherosclerosis in mice, with a
lipoprotein profile different to humans, is controversial, with
previous studies proposing seemingly contradictory anti- and
pro-atherogenic functions. Lp-PLA2 is an enzyme mainly
associated with HDL in mice and LDL in humans (4). A study by Tellis and Tselepis
(25) suggested that the role of
Lp-PLA2 in atherosclerosis may depend on its lipoprotein
carrier in plasma, and that HDL-associated Lp-PLA2
contributes to the reduction of atherosclerosis, whereas
LDL-associated Lp-PLA2 stimulates this process.
Nevertheless, other research has demonstrated that increased plasma
Lp-PLA2 is associated with susceptibility to
atherosclerosis in mice (26),
and that patients with coronary heart disease exhibit reduced
LDL-Lp-PLA2 mass and catalytic efficiency, suggesting a
diminished ability to degrade pro-inflammatory phospholipids.
Moreover, considerable evidence has been obtained for the
pro-atherogenic roles of Lp-PLA2in vitro and
in vivo (26–28).
It was initially thought that Lp-PLA2
exerts an anti-atherogenic and anti-inflammatory effect by
hydrolyzing and inactivating platelet activating factor (PAF), a
well-known pro-inflammatory factor that contributes to inflammation
and atherosclerosis (29).
Despite this, individuals with reduced levels of Lp-PLA2
activity do not display rampant inflammatory responses anticipated
from uncontrolled PAF accumulation, and acute bronchoconstriction
to inhaled PAF does not vary in these individuals (24,30,31). Notably, responsiveness to PAF is
not altered in Japanese subjects with a genetic variant in
Lp-PLA2 (Val276Phe) that results in absence of the
circulating enzyme (32,33). In addition, clinical trials failed
to show measurable benefit of recombinant human Lp-PLA2
(also termed PAFAH) in patients with asthma or septic shock
(34,35). Furthermore, a recent report by Liu
et al (24) indicated that
circulating PAF is primarily cleared by PAF receptor-independent
transport, rather than intravascular hydrolysis by PAFAH. In
summary, we find no evidence that Lp-PLA2 hydrolyzes PAF
in vivo.
In the present study, we observed a marked effect of
RNAi on circulating inflammatory markers. These results are in
agreement with previous studies showing that atherosclerosis is an
inflammatory process (5,14,19,36). LPC, the hydrolyzing product of
Lp-PLA2, has been shown to contribute to oxidative
stress in macrophages and their tissue accumulation. Macrophages
are the most significant source of Lp-PLA2 in the
vascular wall. By virtue of these processes, Lp-PLA2 is
involved in a positive-feedback loop of inflammation and
atherosclerosis. Macrophages are the main source of
pro-inflammatory cytokines, such as MMP-8, IL-6 and TNF-α. High
levels of Lp-PLA2 and pro-inflammatory cytokines may
therefore favor the development of vulnerable plaques. Our study
suggests that Lp-PLA2 RNAi decreased the expression of
pro-inflammatory cytokines, thereby playing an anti-atherogenic and
anti-inflammatory role. A possible explanation for this beneficial
effect may be that Lp-PLA2 RNAi attenuated the
accumulation of macrophages in atherosclerotic plaques, as
indicated by our cell experiments revealing that RNAi attenuated
the expression of MCP-1, IL-6 and Lp-PLA2 evoked by
oxLDL. MMP-8 possesses proteolytic activity on several matrix
proteins particularly type I collagen and on various non-matrix
proteins (37). The RNAi groups
manifested diminished MMP-8 expression and vulnerability of the
plaques, which was in agreement with a previous study indicating
that atherosclerotic lesions in MMP-8-deficient mice had increased
collagen content (37). Recent
studies suggest that IL-10 may be a key mediator of vascular
protection in atherosclerosis (38). A major role for IL-10 is to
inhibit expression of pro-inflammatory cytokines including MMP-8,
IL-6 and TNF-α (37,39,40). These pro-inflammatory cytokines
are also known to contribute to vascular inflammation, plaque
destabilization and thrombosis (37). In the present study, higher levels
of MMP-8, TNF-α and IL-6 were found in the carotid arteries of
control and NC mice, and this effect was almost completely
abolished by RNAi. Additionally, we observed a marked increase in
the collagen content of plaques in the RNAi groups, suggesting
increased plaque stability. This effect was more pronounced in the
group receiving transluminal local transfection of the lentivirus,
supporting the idea that transluminal local delivery of
Lp-PLA2 shRNA was superior to systemic administration in
stabilizing atherosclerotic plaques. Collectively, our results
suggest that Lp-PLA2 RNAi decreased the expression of
pro-inflammatory cytokines, as well as it increased the expression
of anti-inflammatory cytokines, thereby playing an anti-atherogenic
and anti-inflammatory role in the stabilization of vulnerable
plaques.
Lp-PLA2 is upregulated in atherosclerotic
plaques and macrophages undergoing apoptosis within the necrotic
core and fibrous cap of vulnerable and ruptured plaques, but not
within stable lesions. As Lp-PLA2 predominantly existed
in advanced plaques, it may play an important role in advanced
lesions and the determination of plaque instability, but not at
earlier stages of atherogenesis. Therefore, the duration of the
present investigation was 7 weeks, which was longer than the 3-week
duration used in the study of Quarck et al (29).
The present study had several limitations. Firstly,
we only measured the plasma concentration of Lp-PLA2 at
the end of the investigation, which may not reflect the true
activity of Lp-PLA2 over time. Secondly, our data
revealed no difference in plaque area between the 2 RNAi groups.
This may be due to the relatively small number of animals
undergoing histological analysis, thus we cannot exclude the
possibility that the lack of difference was due to low statistical
power. Further research will be needed to clarify these
details.
In summary, our study demonstrated that
lentiviral-mediated RNAi was effective in knocking down
Lp-PLA2 expression in apolipoprotein E-deficient mice,
which resulted in reduced inflammatory gene expression, diminished
plaque area, decreased lipid content, increased collagen content
and reduced plaque vulnerability, independent of the plasma
lipoprotein profile. In addition, transluminal local delivery of
Lp-PLA2 shRNA was superior to systemic administration in
stabilizing atherosclerotic plaques.
Acknowledgements
This study was supported by grants
from the Key Scientific and Technological Project of Henan Province
(no. 112102310174), the Scientific Fund for Distinguished Young
Scholars in Henan Province (no. 094100510017), the Research Team
Project of the First Affiliated Hospital of Zhengzhou University,
and the Science and Technology Fund for Innovation Leading Talents
of Health in Henan Province (no. 3027).
References
|
1
|
Ross R: Atherosclerosis - an inflammatory
disease. N Engl J Med. 340:115–126. 1999. View Article : Google Scholar
|
|
2
|
Suchindran S, Rivedal D, Guyton JR, et al:
Genome-wide association study of Lp-PLA(2) activity and mass in the
Framingham Heart Study. PLoS Genet. 6:e10009282010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Manrique C, Lastra G, Gardner M and Sowers
JR: The renin angiotensin aldosterone system in hypertension: roles
of insulin resistance and oxidative stress. Med Clin North Am.
93:569–582. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chait A, Han CY, Oram JF and Heinecke JW:
Thematic review series: the immune system and atherogenesis.
Lipoprotein-associated inflammatory proteins: markers or mediators
of cardiovascular disease? J Lipid Res. 46:389–403. 2005.
View Article : Google Scholar
|
|
5
|
Sudhir K: Clinical review:
lipoprotein-associated phospholipase A2, a novel
inflammatory biomarker and independent risk predictor for
cardiovascular disease. J Clin Endocrinol Metab. 90:3100–3105.
2005.PubMed/NCBI
|
|
6
|
Elkind MS, Tai W, Coates K, Paik MC and
Sacco RL: High-sensitivity C-reactive protein,
lipoprotein-associated phospholipase A2, and outcome after ischemic
stroke. Arch Intern Med. 166:2073–2080. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Qi LH, Wang Y, Gao F, et al: Enhanced
stabilization of atherosclerotic plaques in apolipoprotein
E-knockout mice by combinatorial Toll-like receptor-1 and -2 gene
silencing. Hum Gene Ther. 20:739–750. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Morris KV and Rossi JJ:
Lentiviral-mediated delivery of siRNAs for antiviral therapy. Gene
Ther. 13:553–558. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mäkinen PI, Koponen JK, Kärkkäinen AM, et
al: Stable RNA interference: comparison of U6 and H1 promoters in
endothelial cells and in mouse brain. J Gene Med. 8:433–441.
2006.PubMed/NCBI
|
|
10
|
Follenzi A and Naldini L: HIV-based
vectors. Preparation and use. Methods Mol Med. 69:259–274.
2002.PubMed/NCBI
|
|
11
|
Wang WY, Li J, Yang D, Xu W, Zha RP and
Wang YP: OxLDL stimulates lipoprotein-associated phospholipase
A2 expression in THP-1 monocytes via PI3K and p38 MAPK
pathways. Cardiovasc Res. 85:845–852. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
von der Thüsen JH, van Berkel TJ and
Biessen EA: Induction of rapid atherogenesis by perivascular
carotid collar placement in apolipoprotein E-deficient and
low-density lipoprotein receptor-deficient mice. Circulation.
103:1164–1170. 2001.PubMed/NCBI
|
|
13
|
von der Thüsen JH, van Vlijmen BJ, Hoeben
RC, et al: Induction of atherosclerotic plaque rupture in
apolipoprotein E−/− mice after adenovirus-mediated
transfer of p53. Circulation. 105:2064–2070. 2002.PubMed/NCBI
|
|
14
|
De Castro SH, Faria Neto HC and Gomes MB:
Platelet-activating factor acetylhydrolase (PAF-AH) activity in
patients with type 1 diabetes mellitus. Arq Bras Cardiol.
88:179–184. 2007.
|
|
15
|
Libby P: Changing concepts of
atherogenesis. J Intern Med. 247:349–358. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Miller RG, Costacou T and Orchard TJ:
Lipoprotein-associated phospho lipase A2, C-reactive
protein, and coronary artery disease in individuals with type 1
diabetes and macroalbuminuria. Diab Vasc Dis Res. 7:47–55.
2010.
|
|
17
|
Ballantyne CM, Hoogeveen RC, Bang H, et
al: Lipoprotein-associated phospholipase A2, high-sensitivity
C-reactive protein, and risk for incident coronary heart disease in
middle-aged men and women in the Atherosclerosis Risk in
Communities (ARIC) study. Circulation. 109:837–842. 2004.
View Article : Google Scholar
|
|
18
|
Mäkinen PI, Lappalainen JP, Heinonen SE,
et al: Silencing of either SR-A or CD36 reduces atherosclerosis in
hyperlipidaemic mice and reveals reciprocal upregulation of these
receptors. Cardiovasc Res. 88:530–538. 2010.PubMed/NCBI
|
|
19
|
Persson M, Nilsson JA, Nelson JJ, Hedblad
B and Berglund G: The epidemiology of Lp-PLA(2): distribution and
correlation with cardiovascular risk factors in a population-based
cohort. Atherosclerosis. 190:388–396. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Koenig W and Khuseyinova N:
Lipoprotein-associated and secretory phospholipase A2 in
cardiovascular disease: the epidemiological evidence. Cardiovasc
Drugs Ther. 23:85–92. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wilensky RL, Shi Y, Mohler ER III, et al:
Inhibition of lipoprotein-associated phospholipase A2
reduces complex coronary atherosclerotic plaque development. Nat
Med. 14:1059–1066. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hu MM, Zhang J, Wang WY, et al: The
inhibition of lipoprotein-associated phospholipase A2
exerts benefcial effects against atherosclerosis in LDLR-defcient
mice. Acta Pharmacol Sin. 32:1253–1258. 2011.PubMed/NCBI
|
|
23
|
Serruys PW, García-García HM, Buszman P,
et al: Effects of the direct lipoprotein-associated phospholipase
A(2) inhibitor darapladib on human coronary atherosclerotic plaque.
Circulation. 118:1172–1182. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu J, Chen R, Marathe GK, Febbraio M, Zou
W and McIntyre TM: Circulating platelet-activating factor is
primarily cleared by transport, not intravascular hydrolysis by
lipoprotein-associated phospholipase A2/PAF acetylhydrolase. Circ
Res. 108:469–477. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tellis CC and Tselepis AD: The role of
lipoprotein-associated phospholipase A2 in
atherosclerosis may depend on its lipoprotein carrier in plasma.
Biochim Biophys Acta. 1791:327–338. 2009.PubMed/NCBI
|
|
26
|
Singh U, Zhong S, Xiong M, Li TB,
Sniderman A and Teng BB: Increased plasma non-esterified fatty
acids and platelet-activating factor acetylhydrolase are associated
with susceptibility to atherosclerosis in mice. Clin Sci.
106:421–432. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kuniyasu A, Tokunaga M, Yamamoto T, et al:
Oxidized LDL and lysophosphatidylcholine stimulate plasminogen
activator inhibitor-1 expression through reactive oxygen species
generation and ERK1/2 activation in 3T3-L1 adipocytes. Biochim
Biophys Acta. 1811:153–162. 2011. View Article : Google Scholar
|
|
28
|
Tsironis LD, Katsouras CS, Lourida ES, et
al: Reduced PAF-acetylhydrolase activity associated with Lp(a) in
patients with coronary artery disease. Atherosclerosis.
177:193–201. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Quarck R, De Geest B, Stengel D, et al:
Adenovirus-mediated gene transfer of human platelet-activating
factor-acetylhydrolase prevents injury-induced neointima formation
and reduces spontaneous atherosclerosis in apolipoprotein
E-deficient mice. Circulation. 103:2495–2500. 2001. View Article : Google Scholar
|
|
30
|
Karasawa K: Clinical aspects of plasma
platelet-activating factor acetylhydrolase. Biochim Biophys Acta.
1761:1359–1372. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Karasawa K, Harada A, Satoh N, Inoue K and
Setaka M: Plasma platelet activating factor-acetylhydrolase
(PAF-AH). Prog Lipid Res. 42:93–114. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Naoki K, Asano K, Satoh N, et al: PAF
responsiveness in Japanese subjects with plasma PAF acetylhydrolase
deficiency. Biochem Biophys Res Commun. 317:205–210. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mohler ER III, Ballantyne CM, Davidson MH,
et al: The effect of darapladib on plasma lipoprotein-associated
phospholipase A2 activity and cardiovascular biomarkers
in patients with stable coronary heart disease or coronary heart
disease risk equivalent: the results of a multicenter, randomized,
double-blind, placebo-controlled study. J Am Coll Cardiol.
51:1632–1641. 2008.PubMed/NCBI
|
|
34
|
Opal S, Laterre PF, Abraham E, et al:
Recombinant human platelet-activating factor acetylhydrolase for
treatment of severe sepsis: results of a phase III, multicenter,
randomized, double-blind, placebo-controlled, clinical trial. Crit
Care Med. 32:332–341. 2004. View Article : Google Scholar
|
|
35
|
Henig NR, Aitken ML, Liu MC, Yu AS and
Henderson WR Jr: Effect of recombinant human platelet-activating
factor-acetylhydrolase on allergen-induced asthmatic responses. Am
J Respir Crit Care Med. 162:523–527. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lavi S, McConnell JP, Rihal CS, et al:
Local production of lipoprotein-associated phospholipase
A2 and lysophosphatidylcholine in the coronary
circulation: association with early coronary atherosclerosis and
endothelial dysfunction in humans. Circulation. 115:2715–2721.
2007.PubMed/NCBI
|
|
37
|
Laxton RC, Hu Y, Duchene J, et al: A role
of matrix metalloproteinase-8 in atherosclerosis. Circ Res.
105:921–929. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Didion SP, Kinzenbaw DA, Schrader LI, Chu
Y and Faraci FM: Endogenous interleukin-10 inhibits angiotensin
II-induced vascular dysfunction. Hypertension. 54:619–624. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Brasier AR, Recinos A III and Eledrisi MS:
Vascular inflammation and the renin-angiotensin system.
Arterioscler Thromb Vasc Biol. 22:1257–1266. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yanai H, Tomono Y, Ito K, Furutani N,
Yoshida H and Tada N: The underlying mechanisms for development of
hypertension in the metabolic syndrome. Nutr J. 7:102008.
View Article : Google Scholar : PubMed/NCBI
|