Introduction
Taurine, a β-aminosulfonic acid, is the most
abundant free amino acid in excitable mammalian tissues, with
intracellular concentrations of 20–70 mmol/kg in the heart
(1,2). Accumulating evidence indicates that
taurine may play a cytoprotective role in the heart. Indeed, the
oral supplementation of taurine has been shown to be effective in
animal models and human patients with congestive heart failure and
cardiomyopathy (3–6). The main mechanisms behind the
cytoprotective effects of taurine include the maintenance of
calcium (Ca2+) homeostasis, the regulation of osmotic
balance and antioxidant and anti-apoptotic activity (6–10).
Physiologically, high intracellular taurine levels are maintained
by the combination of membrane taurine transporter (TAUT) activity
and endogenous biosynthesis. Since the capacity to synthesize
taurine in most tissues is limited (including the heart),
maintenance of the large intracellular taurine pool mainly depends
upon the activity of TAUT (11,12).
Inducing arrhythmias and even heart failure, acute
myocardial ischemia (AMI) is one of the most serious cardiovascular
events. In this setting, the supplementation of exogenous taurine
has been demonstrated to produce preventive and therapeutic effects
on AMI (13,14). However, it seems unlikely that
taurine treatment is simply a replacement therapy. Therefore, in
this study, we investigated changes in taurine content and TAUT
expression in hypoxic cardiomyocytes and ischemic myocardial
tissues treated or not with taurine.
Materials and methods
Animals, cell lines, protocol and
procedure
Experimental procedures were performed using
pathogen-free, adult male Sprague-Dawley (SD) rats, weighing
250–300 g (Shanghai Institute of Materia Medica, Chinese Academy of
Sciences, Shanghai, China). The experimental protocol was approved
by the Shanghai Medical Experimental Animal Care and Use Committee.
SD rats were anesthetized by intraperitoneal injections of ketamine
(100 mg/kg). The rats were placed in a supine position after being
shaved on the chest and then intubated with positive-pressure
ventilation (180 ml/min) with room air using a SAR-830/A Small
Animal Ventilator (CWE, Inc., Weston, WI, USA). Under sterile
conditions, the heart was exposed via a left thoracotomy at the
level of the 5th intercostal space. AMI was created by left
coronary artery ligation 2 mm below the left atrium with a 6-0
prolene suture. Regional myocardial ischemia was confirmed through
the observation of a rapid discoloration over the anterior surface
of the left ventrical together with the development of akinesia and
dilatation over the area at risk. The sham-operated control rats
only received thoracotomy without left coronary artery ligation.
All rats received an intraperitoneal injection of 1 ml
physiological saline or 100 mg/kg/day of taurine (Sigma) for 3
consecutive days before modeling. The rats were assigned into 4
groups (n=18/group): i) control group (untreated sham-operated
group); ii) taurine-treated control group (sham-operated control
group treated with taurine); iii) AMI group; and iv)
taurine-treated AMI group. In each group, 6 animals were euthanized
30 min after modeling, 6 were euthanized 2 h after modeling, and 6
were euthanized 2 weeks after modeling at the completion of an
echocardiogram examination.
H9C2 cells were purchased from the Shanghai
Institute of Cell Biology, Chinese Academy of Sciences, Shanghai,
China. The H9C2 cells were maintained at 37°C with 5% carbon
dioxide (CO2) in air atmosphere in Dulbecco’s modified
Eagle’s medium (DMEM) supplemented with 10% (volume/volume)
heat-inactivated fetal bovine serum and antibiotics (100 U/ml
penicillin and 100 mg/ml streptomycin). For hypoxic conditions, the
H9C2 cells were cultured at 37°C with 1% oxygen and 5%
CO2 in a hypoxic incubator (Ruskinn Invivo2 200; Ruskinn
Technology). In the taurine treatment groups various doses of
taurine were added to the DMEM 24 h prior to the induction of
hypoxia. To measure the taurine content in the cardiomyocytes, the
medium was removed and replaced with standard incubation buffer
[mmol/l: KCl 3, NaCl 125, KH2PO4 12,
MgSO4 1.2, CaCl2 1.3, NaHCO3 5,
HEPES 20, pH 7.4, and 0.2% bovine serum albumin (BSA)].
Measurement of taurine content by
high-performance liquid chromatography (HPLC)
Taurine content in the hypoxic cardiomyocytes and
myocardial tissues and the plasma of AMI rats was measured by
reversed-phase HPLC. In addition, the plasma of 10 AMI patients and
10 healthy volunteers along with their clinical data were collected
and taurine content in the plasma was also measured by HPLC. The
study was approved by the Zhongshan Hospital Research Ethics
Committee and the informed consent was obtained from each patient
according to the committee’s regulations. Approximately
1×106 hypoxic cardiomyocytes or 0.1 g ischemic cardiac
tissue were homogenized with normal saline. The homogenates were
centrifuged at 14,000 rpm for 15 min at 4°C. The supernatants or
the plasma were mixed with 0.2 M perchloric acid and centrifuged at
12,000 rpm for 15 min at 4°C.
HPLC was performed according to a previous study
(15). Briefly, the supernatants
were purified in a dual-bed ion-exchange column (2.5 cm of AG 1-X8
100–200 mesh in the chloride form; >2.5 cm of AG 50W-X8 200–400
mesh in the hydrogen form), eluted with 2 ml ultrapure water, and
then lyophilized. The sample or standard were dissolved in 100 μl
of water prior to HPLC analysis on a Waters system (Waters Corp.,
Milford, MA, USA) equipped with a 3.9×150 mm Nova-Pak
C18 column and a model 470 scanning fluorescence
detector. Isocratic elution was performed at a flow rate of 2
ml/min using 43% solvent A (0.05 mol/l
NaH2PO4, pH 5.3, plus 5 mol/l NaOH) mixed
with 57% solvent B (0.05 mol/l NaH2PO4 in 75%
methanol-water). Glutamine, added after ion exchange
chromatography, was used as the internal standard. The standard
curve was linear for the taurine content in the sample
concentration, and the recovery of taurine was >90%. The value
of taurine was expressed as nmol/mg of protein in cardiomyocytes,
μmol/g in cardiac tissues, and μmol/l in plasma.
Quantitative real-time PCR analysis
Total ribonucleic acid (RNA) was extracted from the
cells and frozen ischemic areas of the cardiac tissue specimens
(n=6/group) using TRIzol reagent (Invitrogen). Total RNA (2 μg) was
reverse transcribed using a PrimeScript RT reagent kit (Takara).
Reverse transcription-polymerase chain reaction (PCR) was performed
before quantitative real-time PCR. Messenger RNA expression was
determined by real-time PCR using SYBR Premix Ex Taq II (Takara).
PCR amplification cycles were programmed for 30 sec at 95°C,
followed by 40 cycles of 95°C for 30 sec, 60°C for 30 sec and 72°C
for 40 sec. Data were collected after each annealing step, and
α-tubulin was used as the endogenous control. mRNA expression was
calculated using the 2-ΔΔCt method. The primers used for
the amplification of rat genes were as follows: TAUT forward,
5′-CAACTTCACTTCGCCTGTGA-3′ and reverse, 5′-CTTGCTCTTGTGCCATGAAG-3′;
cysteine sulfinate decarboxylase (CSD) forward,
5′-TGATCCCTGAGGATCTGGAG-3′ and reverse, 5′-ACTCAAATCCTTCCCGCTTT-3′;
and α-tubulin forward, 5′-CACCCGTCTTCAGGGCTTCTTGGTTT-3′ and
reverse, 5′-CATTTCACCATCTGGTTGGCTGGCTC-3′.
Western blot analysis
Protein was extracted from the cells and the
ischemic areas of cardiac tissues. The protein concentration was
determined using the BCA protein assay (Pierce). Equal amounts of
protein were subjected to 12% sodium dodecyl sulfate-polyacrylamide
gel electrophoresis (SDS-PAGE). Following gel electrophoresis, the
proteins were transferred onto polyvinylidene difluoride (PVDF)
membranes (Immobilon-P; Millipore). The membranes were blocked for
1 h at room temperature in 5% non-fat dry milk in Tris-buffered
saline (TBS) containing 0.05% Tween-20. Following overnight
incubation at 4°C with primary antibodies, the membranes were
incubated with horseradish peroxidase-labeled secondary antibody
(Chemicon) for 1 h at room temperature. Peroxidase activity was
detected by chemiluminescence (SuperSignal West Femto Luminol
Substrate and Peroxide Buffer; Pierce). The primary antibodies used
included anti-TAUT (1:100; Santa Cruz Biotechnology, Inc.),
anti-Bax (1:1,000; Epitomics), anti-Bcl-2 (1:1,000; Cell Signaling
Technology) and anti-tubulin (1:200; Beyotime Technology)
antibodies.
Immunofluorescence and
immunohistochemical staining for TAUT
Cardiomyocytes were stained by immunofluorescence
and the ischemic areas of the cardiac tissue sections were stained
by immunohistochemistry to assess the distribution of TAUT, as
described below. First, the cultured cells were grown on slides and
then washed and fixed. Cells were then incubated with primary
antibody against TAUT (1:50; Santa Cruz Biotechnology, Inc.) and
rabbit anti-goat tetramethyl rhodamine isothiocyanate-conjugated
secondary antibody (1:200; Invitrogen) before staining with
4′,6-diamidino-2-phenylindole (DAPI). The fluorescent images were
visualized under a confocal microscope (FV-1000; Olympus). The
ischemic areas of the cardiac tissue sections were incubated with
primary antibody against TAUT (1:50; Santa Cruz Biotechnology,
Inc.), and donkey anti-goat IgG/horseradish peroxidase (1:100;
Serotec) was applied as the secondary antibody. For the negative
controls, primary antibodies were replaced by phosphate-buffered
saline (PBS). Staining was evaluated by 2 independent
observers.
Hematoxylin and eosin (H&E) and TUNEL
staining
The formalin-fixed and paraffin-embedded tissues
were cut into sections (4 μm thick) for histological studies using
H&E and terminal deoxynucleotidyl transferase-mediated
deoxyuridine triphosphate nick end-abeling (TUNEL) staining. TUNEL
staining was performed using a TUNEL kit (No. 11684817910, Roche)
according to the manufacturer’s instructions. At least 5 random
fields from each section were counted in order to quantify the
histological images.
Annexin V and propidium iodide (PI)
assays
The H9C2 cells were cultured in DMEM in 6-well
plates to produce colonies at 80–90% confluence. Various doses of
taurine were added to the culture solution, and the cells were
incubated for 24 h prior to the induction of hypoxia. In order to
detect early apoptotic activity, an Annexin V-FITC/PI Apoptosis
Detection kit (KeyGen) was used according to the manufacturer’s
instructions. H9C2 cells were washed with cold PBS and added to 200
μl of the Annexin V-binding buffer. The samples were immediately
analyzed by flow cytometry after they were stained with 2 μl of
FITC-labeled Annexin V and 2 μl of PI (BD FACSAria™).
Statistical analysis
Data were analyzed using the computer program SPSS
15.0 (SPSS, Inc.) by means of an unpaired two-tailed Student’s
t-test. Continuous data are expressed as the means ± SEM. Results
were considered statistically significant at a P-value of
<0.05.
Results
Low-dose taurine reduces the apoptosis of
cardiomyocytes and improves cardiac function
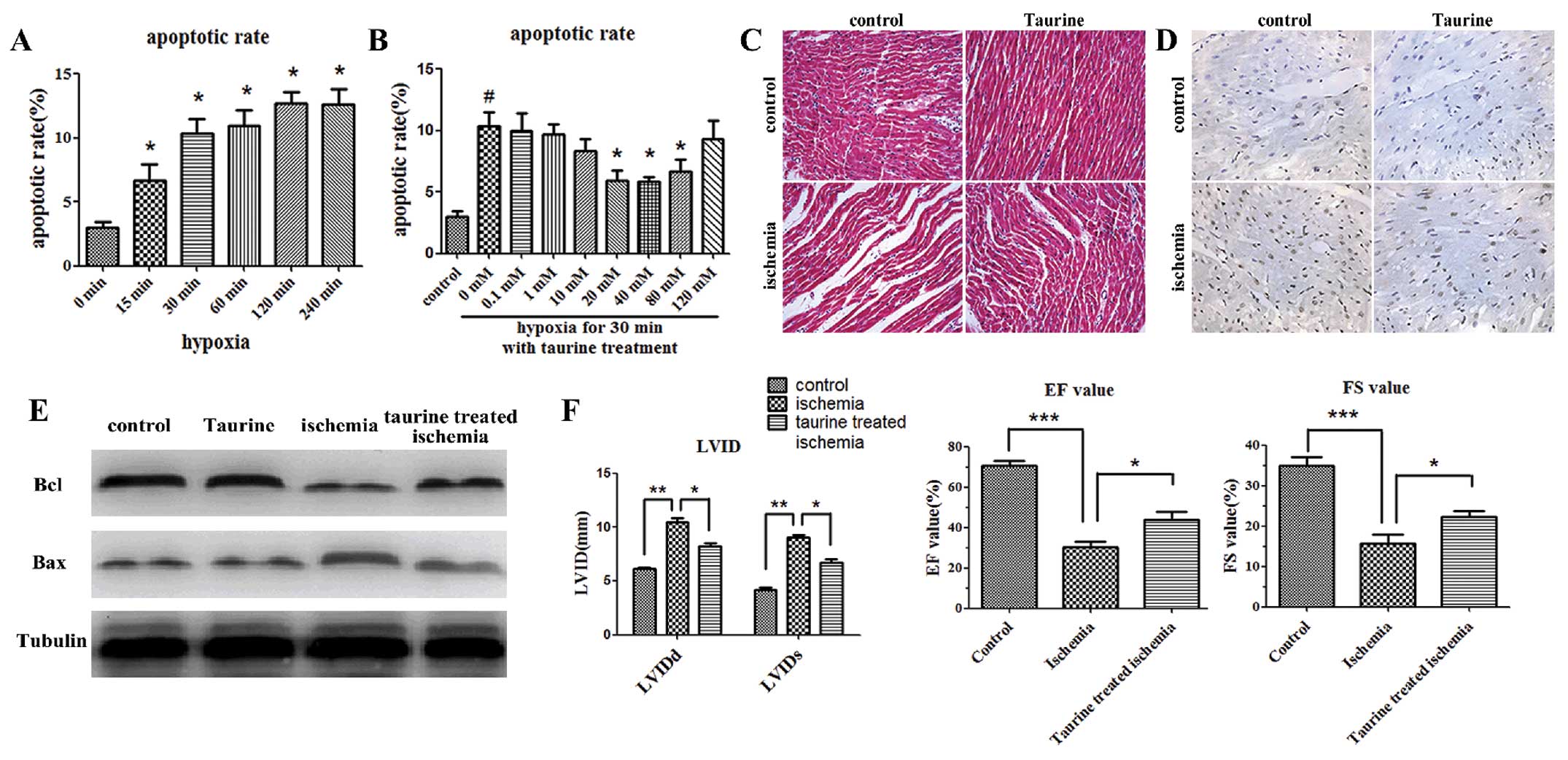
Annexin V and PI assays were conducted in
vitro to assess the apoptosis of cardiomyocytes. The early
apoptosis of cardiomyocytes was increased within 15 min after the
induction of hypoxia (Fig. 1A).
In addition, low-dose taurine treatment (40 mM) significantly
reduced the apoptosis of cardiomyocytes induced by hypoxia, whereas
high-dose taurine treatment (120 mM) was not effective (Fig. 1B).
As shown by H&E staining, the cardiomyocytes in
the ischemic myocardial tissues 2 h after AMI were stretched and
narrow in shape, and the gaps between these cardiomyocytes were
wider in vivo when compared with the control group 2 h after
modeling. The gaps between the cardiomyocytes were narrower when
the AMI rats were treated with taurine (100 mg/kg/day) (Fig. 1C). Furthermore, TUNEL staining
indicated that there were more apoptotic cardiomyocytes 2 h after
modeling in the ischemic cardiac tissues than in the control ones.
Moreover, the administration of taurine inhibited the apoptosis of
the cardiomyocytes (Fig. 1D). We
also detected the anti-apoptotic gene, Bcl-2, and the pro-apoptotic
gene, Bax, in the cardiac tissues by western blot analysis. Our
data showed that the expression of Bcl-2 was significantly
decreased 2 h after modeling in the AMI group as compared with the
control group, and that the supplementation of exogenous taurine
significantly elevated the levels of Bcl-2. On the contrary, the
expression of Bax was increased in the AMI group, which was
reversed by taurine treatment (Fig.
1E).
Echocardiogram examinations were performed 2 weeks
after modeling. Cardiac function was determined by the left
ventricular diastolic and systolic internal dimensions (LVIDd and
LVIDs, respectively), the percentage of fractional shortening (FS)
and the ejection fraction (EF). The LVIDd and LVIDs were
significantly increased in the AMI group, and were reduced
following taurine treatment. Accordingly, the FS and EF were
significantly decreased in the AMI group, and this decrease was
also reversed following taurine treatment (Fig. 1F).
Low-dose taurine increases the
intracellular taurine content which decreases under hypoxic and
ischemic stress conditions
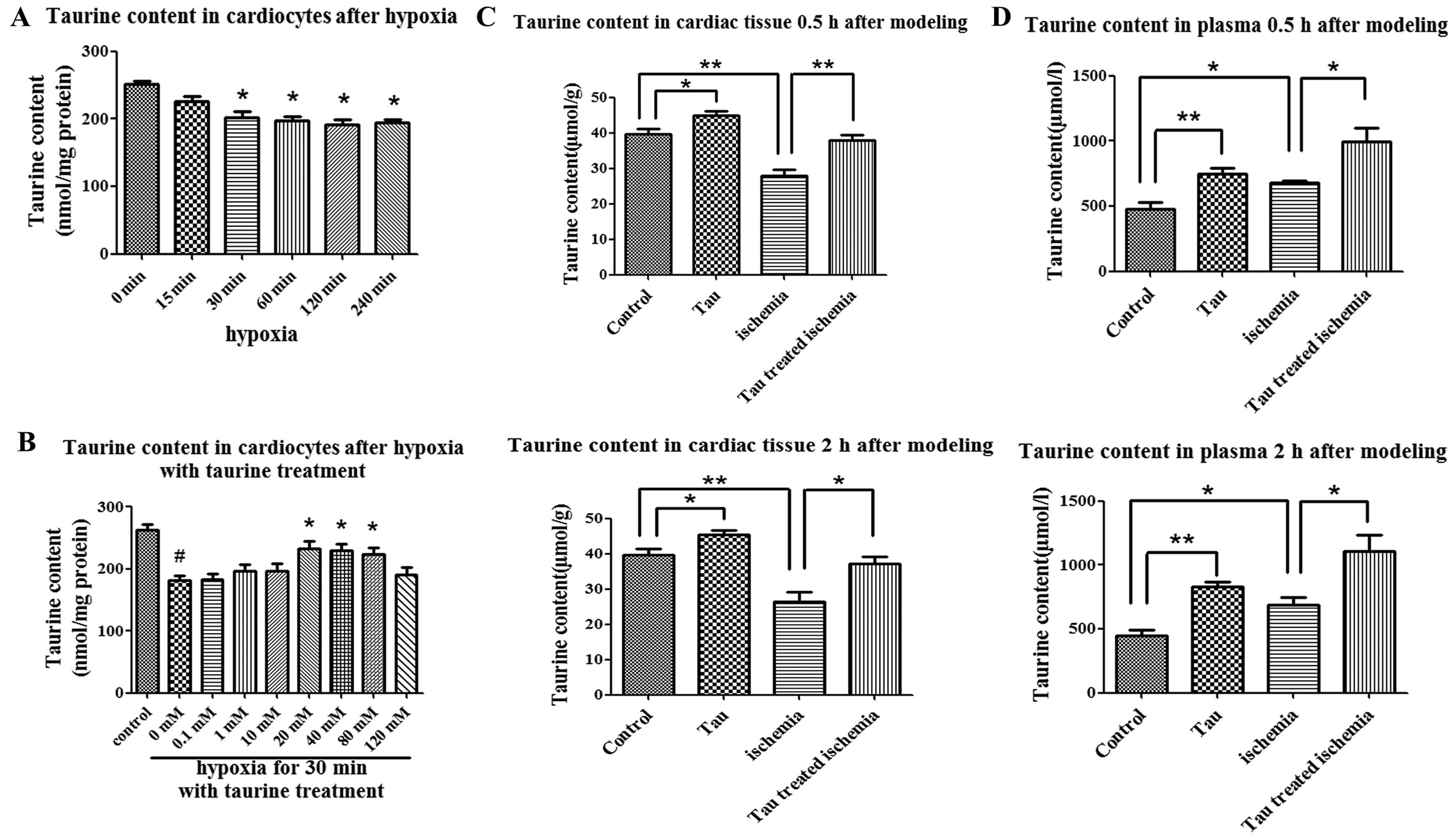
The taurine content in the cardiomyocytes was
202.5±8.2 nmol/mg protein when the cells were cultured under
hypoxic conditions for 30 min, which was significantly decreased as
compared with the cells cultured under normoxic conditions
(251.3±5.2 nmol/mg protein, P<0.01) (Fig. 2A). In contrast to high-dose
taurine (120 nM), low-dose taurine (40 mM) elevated the
intracellular taurine content, which was decreased under hypoxic
conditions (Fig. 2B).
In rats with AMI, the taurine content in the
ischemic areas of the cardiac tissues 30 min and 2 h after modeling
was 27.99±1.66 and 26.54±2.57 μmol/g, respectively. These levels
were significantly lower than those of the control group at 30 min
(39.57±1.56 μmol/g) and at 2 h (39.71±1.63 μmol/g) after modeling
(P<0.05) (Fig. 2C). On the
contrary, the plasma taurine content in the rats with AMI was
increased as compared with that in the rats of the control group at
30 min (680.0±15.67 vs. 480.6±52.14 μmol/l) and at 2 h (685.1±63.56
vs. 448.7±41.47 μmol/l) after modeling (P<0.05) (Fig. 2D). This was in accordance with the
change in plasma taurine content in AMI patients which was
increased compared with the control group (493.7±51.94 vs.
358.5±12.59 μmol/l, P<0.05) (Table
I). When the AMI rats were treated with taurine, the taurine
content in the cardiac tissues content was elevated to 37.99±1.49
and 37.13±2.08 μmol/g at 30 min and at 2 h after modeling,
respectively; these levels were significantly higher than those in
the AMI group not treated with taurine (P<0.05) (Fig. 2C). Finally, the taurine content in
the plasma was increased following taurine treatment in the control
and AMI rats due to the supplementation of exogenous taurine
(Fig. 2D).
 | Table IClinical characteristics of the
patients. |
Table I
Clinical characteristics of the
patients.
| Characteristic | AMI | Control | P-value |
|---|
| Age (years) | 68±7.5 | 58±14.5 | >0.05 |
| Male/female | 10/0 | 8/2 | >0.05 |
| Hypertension (%) | 60% | 40% | >0.05 |
| Diabetes (%) | 40% | 20% | >0.05 |
| Cholesterol
(mmol/l) | 4.17±0.17 | 3.64±0.21 | >0.05 |
| Triglyceride
(mmol/l) | 1.85±0.36 | 1.53±0.33 | >0.05 |
| Taurine content in
plasma (μmol/l) | 493.7±51.94 | 358.5±12.59 | 0.029 |
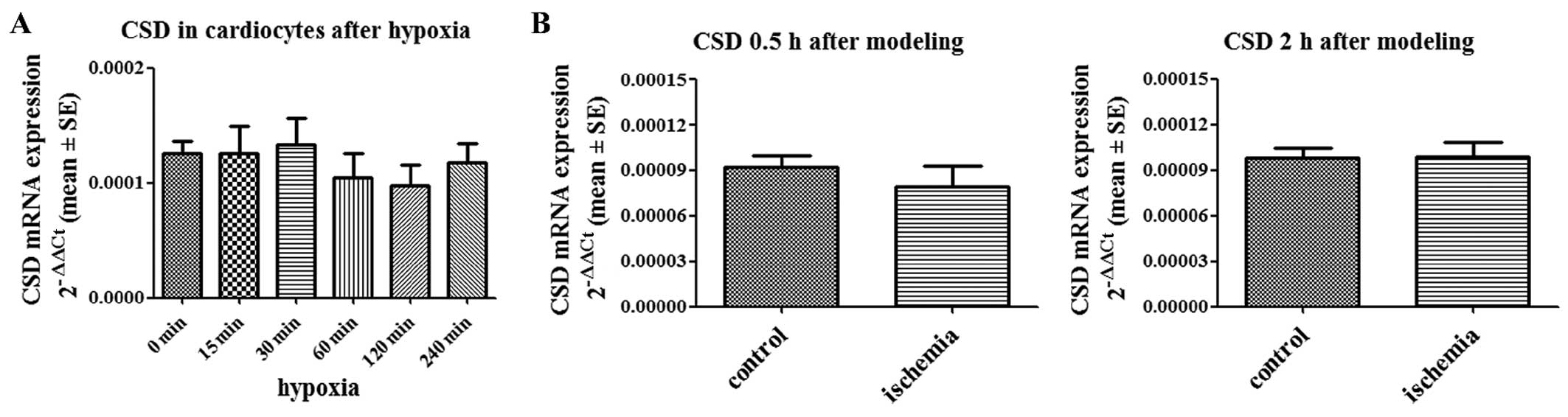
CSD expression levels are not altered in
hypoxic cardiomyocytes and ischemic cardiac tissues
CSD, which is involved in biosynthesis pathways, is
the rate-limiting enzyme in taurine biosynthesis (16,17). We quantified the expression of CSD
mRNA in hypoxic cardiomyocytes and ischemic cardiac tissues. No
difference was found between hypoxic cardiomyocytes and normoxic
cardiomyocytes (Fig. 3A). CSD
expression was also not altered in the ischemic areas of the
cardiac tissue from the rats with AMI compared with the controls
(Fig. 3B).
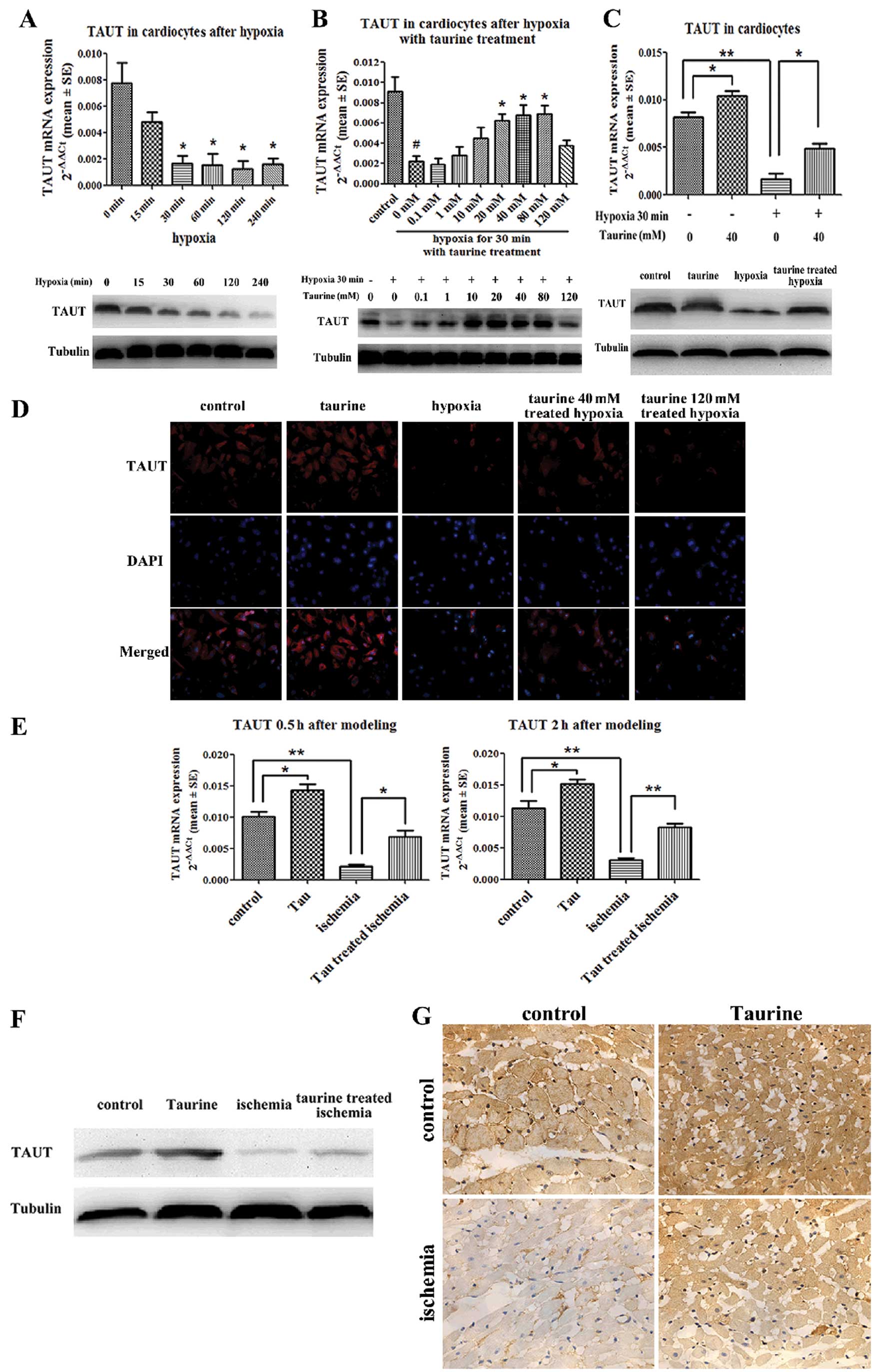
Low-dose taurine upregulates TAUT
expression, which is downregulated by hypoxia and AMI
Real-time quantitative reverse transcription PCR
revealed that the expression of TAUT mRNA in the cardiomyocytes was
downregulated at 30 min under hypoxic conditions. Western blot
analysis and immunofluorescence demonstrated that the protein
expression of TAUT was also downregulated 30 min after hypoxia,
which paralleled the changes in TAUT mRNA expression (Fig. 4A). Furthermore, low-dose taurine
(40 mM) significantly upregulated TAUT mRNA expression, which had
been downregulated by hypoxia. However, high-dose taurine (120 mM)
had no effect on the expression of TAUT mRNA. Western blot analysis
and immunofluorescence also confirmed that the protein levels of
TAUT were in accordance with the mRNA levels (Fig. 4B and D). Our results also
demonstrated that low-dose taurine upregulated the mRNA and protein
levels of TAUT in the normoxic cardiomyocytes (Fig. 4C and D).
As compared with the control group, the mRNA
expression of TAUT in the cardiac tissues in the AMI group was
reduced by 78% at 30 min after modeling and by 82% at 2 h after
modeling (Fig. 4E). In addition,
the protein levels of TAUT in the cardiac tissues obtained 2 h
after modeling measured by western blot analysis and
immunohistochemistry were in accordance with the changes in the
mRNA levels described above (Fig. 4F
and G). As compared with the AMI rats not treated with taurine,
those treated with low-dose taurine had mRNA levels of TAUT in
cardiac tissues that were elevated by 3.1-fold at 30 min after
modeling and by 2.7-fold at 2 h after modeling. (Fig. 4E). Moreover, the protein levels of
TAUT were also reversed by taurine treatment (Fig. 4F and G).
Discussion
Coronary heart disease is the most common cause of
mortality worldwide (18), and
AMI can lead to serious consequences such as arrhythmia and heart
failure. There are many preventative and therapeutic interventions
for coronary heart disease, such as statin therapy, anticoagulants
and percutaneous coronary intervention.
Populations with higher fish consumption have lower
cardiovascular death rates than populations with high meat
consumption (19), which may be
associated with high taurine concentrations in fish (20). Taurine is abundant in cardiac
tissue and exerts protective effects on many tissues and cells
(particularly cardiomyocytes) by its osmoregulatory,
anti-oxidative, Ca2+-modulating, and plasma
membrane-stabilizing effects (1).
In addition, taurine effectively prevents myocardial
ischemia-induced apoptosis by inhibiting the assembly of the
Apaf-1/caspase-9 apoptosome and the Akt/caspase-9 pathway (9,10).
Taurine prevents arsenic-induced myocardial pathophysiology by
attenuating NF-κB activation via IKK, p38 and the JNK MAPK
signaling pathways (21). In
addition, taurine prevents the apoptosis of cardiomyocytes by
inhibiting NADPH oxidase and calpain activation (22).
Endogenous taurine is synthesized from methionine
and cysteine, which are its amino acid precursors. CSD is
considered as the key rate-limiting enzyme in the biosynthesis of
taurine (23). Mammalian species
such as humans and rats are unable to synthesize sufficient taurine
and must rely on dietary sources to maintain their requirements.
Moreover, intracellular concentrations of taurine are much higher
than extracellular concentrations (24). TAUT, which is a high-affinity and
low-capacity sodium- and chloride-dependent transporter located on
the cell membrane, maintains this transmembrane gradient. The
expression of TAUT is downregulated by high concentrations of
glucose, the p53 tumor suppressor protein, endothelin and certain
diseases, such as hypertension. On the contrary, hypertonicity,
tumor necrosis factor-α and nitric oxide upregulate the expression
of TAUT (25).
The present study confirmed that taurine treatment
inhibited the apoptosis of cardiomyocytes. The echocardiogram
examination showed that the EF and FS values which were
significantly decreased in the rats with AMI, were reversed
following taurine treatment. The apoptosis of cardiomyocytes which
increased under hypoxic and ischemic conditions was also inhibited
following taurine treatment. Multiple studies have revealed the
protective effects of taurine on cardiomyocytes (4–6,
9). However, changes in
intracellular taurine content and the expression of TAUT in
cardiomyocytes have not been previously clarified following taurine
treatment.
A previous study demonstrated that the taurine
content in the myocardium and aortic wall in spontaneously
hypertensive rats was decreased, which may be a result of the
decreased TAUT activity and affinity, and the downregulation of
TAUT gene expression (26). Our
study demonstrated that taurine concentrations in hypoxic
cardiomyocytes and ischemic cardiac tissues were reduced as
compared with the controls. Inversely, the taurine concentrations
in plasma were higher in the rats with AMI than in the control
rats, which was confirmed in AMI patients. Although the expression
of CSD mRNA was similar in the hypoxic and normal cardiomyocytes
and in the cardiac tissues of rats with or without AMI, the mRNA
and protein expression of TAUT in the hypoxic cardiomyocytes and
ischemic myocardial tissues was decreased as compared with the
controls. As CSD is the key rate-limiting enzyme in the
biosynthesis of taurine, these results suggest that the
biosynthesis of taurine was not altered in the hypoxic
cardiomyocytes and ischemic cardiac tissues. The reason why taurine
concentrations were decreased in hypoxic cardiomyocytes and
ischemic cardiac tissues and increased in the plasma of AMI rats
appears secondary to the reduced expression of TAUT, ultimately
causing dysfunctional taurine transport. The downregulation of TAUT
expression and decreased concentrations of taurine occurred 30 min
after modeling, which indicates that the concentration of taurine
may be an early marker for AMI. This is significant, as the early
diagnosis of AMI can lead to early interventional therapies.
Cardiac troponin T is a classical and useful marker of AMI;
however, it is generally upregulated 2 or 3 h after ischemia.
Consequently, the concentration of taurine captures our attention
since it changes within <30 min following the induction of
ischemia. Of note, the concentration of taurine can be detected by
a non-invasive examination termed nuclear magnetic resonance
spectroscopy (NMRS) in the hippocampal formation of rats (27). Further studies are required to
detect the taurine content in ischemic cardiac tissues by NMRS.
Taurine supplementation may prevent cardiomyocytes
from undergoing apoptosis. Our results revealed that treatment with
low-dose (40 mM) but not high-dose (120 mM) taurine increased the
expression of TAUT and the taurine content in hypoxic
cardiomyocytes. Previous studies have demonstrated that
extracellular taurine can regulate the expression of TAUT in many
other cell types. For instance, it has been reported that low-dose
taurine treatment (0.1, 1 and 10 mM) reverses diabetes-induced or
high glucose-induced decreases in TAUT expression in retinal glial
cells (28). Additionally, TAUT
expression has been shown to be downregulated in HepG2 human
hepatoblastoma cells following treatment with 50 mM of
extracellular taurine (29).
Various doses of taurine may also have different effects. For
example, it has been shown in rat myocardial mitochondria that
low-dose taurine (5 and 10 mM) increases Ca2+-ATPase
activity, whereas high-dose taurine (20 mM) inhibits
Ca2+-ATPase activity (30). Our in vitro study
demonstrated that low-dose taurine upregulated TAUT expression and
increased the intracellular content of taurine, but that high-dose
taurine had no effect. In our study, in vivo taurine
supplementation by intraperitoneal injection increased the
expression of TAUT in cardiac tissues in normal rats as well as in
rats with AMI. As a result, the concentration of taurine in cardiac
tissue was also elevated in both taurine-treated normal rats and
taurine-treated AMI rats. The dose of taurine (100 mg/kg/day) used
for our in vivo study may be considered a low-dose according
to a previous study (31). In
fact, we did not administer high-dose taurine in vivo since
in clinical practice, lower drug doses are preferable. These
results suggest that the upregulation of TAUT expression by taurine
supplementation occurs through a positive feedback pathway. The
elevated intra-cardiocyte taurine content and the upregulation of
TAUT expression were protective factors for the cardiomyocytes
under ischemic conditions. Taurine therapy, even at a low-dose, is
a reliable interventional modality to protect cardiomyocytes in
rats with AMI.
In conclusion, taurine exerts a protective effect on
the ischemic myocardium. Low-dose but not high-dose taurine
upregulates TAUT expression and increases the intra-cardiocyte
taurine content in hypoxic cardiomyocytes and ischemic myocardial
tissues. Further studies are required to evaluate the mechanism
behind TAUT dysfunction in cardiovascular disease and to
investigate the clinical applications of taurine as a therapeutic
agent in AMI.
Acknowledgements
This study was supported by a grant from the
National Basic Research Program of China (no. 2011CB503905).
References
|
1
|
Huxtable RJ: Physiological actions of
taurine. Physiol Rev. 72:101–163. 1992.
|
|
2
|
Chapman RA, Suleiman MS and Earm YE:
Taurine and the heart. Cardiovasc Res. 27:358–363. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Azuma J, Hasegawa H, Sawamura A, et al:
Therapy of congestive heart failure with orally administered
taurine. Clin Ther. 5:398–408. 1983.PubMed/NCBI
|
|
4
|
Azuma J, Sawamura A, Awata N, et al:
Therapeutic effect of taurine in congestive heart failure: a
double-blind crossover trial. Clin Cardiol. 8:276–282. 1985.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Takihara K, Azuma J, Awata N, et al:
Beneficial effect of taurine in rabbits with chronic congestive
heart failure. Am Heart J. 112:1278–1284. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Oudit GY, Trivieri MG, Khaper N, et al:
Taurine supplementation reduces oxidative stress and improves
cardiovascular function in an iron-overload murine model.
Circulation. 109:1877–1885. 2004. View Article : Google Scholar
|
|
7
|
Schaffer S, Takahashi K and Azuma J: Role
of osmoregulation in the actions of taurine. Amino Acids.
19:527–546. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Satoh H and Sperelakis N: Review of some
actions of taurine on ion channels of cardiac muscle cells and
others. Gen Pharmacol. 30:451–463. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Takatani T, Takahashi K, Uozumi Y, et al:
Taurine prevents the ischemia-induced apoptosis in cultured
neonatal rat cardiomyocytes through Akt/caspase-9 pathway. Biochem
Biophys Res Commun. 316:484–489. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Takatani T, Takahashi K, Uozumi Y, et al:
Taurine inhibits apoptosis by preventing formation of the
Apaf-1/caspase-9 apoptosome. Am J Physiol Cell Physiol.
287:C949–C953. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bitoun M, Levillain O and Tappaz M: Gene
expression of the taurine transporter and taurine biosynthetic
enzymes in rat kidney after antidiuresis and salt loading. Pflugers
Arch. 442:87–95. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Han X, Budreau AM and Chesney RW: Adaptive
regulation of MDCK cell taurine transporter (pNCT) mRNA:
transcription of pNCT gene is regulated by external taurine
concentration. Biochim Biophys Acta. 1351:296–304. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Takahashi K, Ohyabu Y, Takahashi K, et al:
Taurine renders the cell resistant to ischemia-induced injury in
cultured neonatal rat cardiomyocytes. J Cardiovasc Pharmacol.
41:726–733. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Oriyanhan W, Yamazaki K, Miwa S, Takaba K,
Ikeda T and Komeda M: Taurine prevents myocardial
ischemia/reperfusion-induced oxidative stress and apoptosis in
prolonged hypothermic rat heart preservation. Heart Vessels.
20:278–285. 2005. View Article : Google Scholar
|
|
15
|
Shi YR, Gao L, Wang SH, et al: Inhibition
of taurine transport by high concentration of glucose in cultured
rat cardiomyocytes. Metabolism. 52:827–833. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Reymond I, Bitoun M, Levillain O and
Tappaz M: Regional expression and histological localization of
cysteine sulfinate decarboxylase mRNA in the rat kidney. J
Histochem Cytochem. 48:1461–1468. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Beetsch JW and Olson JE: Taurine synthesis
and cysteine metabolism in cultured rat astrocytes: effects of
hyperosmotic exposure. Am J Physiol. 274:C866–C874. 1998.PubMed/NCBI
|
|
18
|
World Health Organization. Global Burden
of Disease. WHO Press; Geneva: 2008
|
|
19
|
Mozaffarian D and Rimm EB: Fish intake,
contaminants, and human health: evaluating the risks and the
benefits. JAMA. 296:1885–1899. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nittynen L, Nurminen ML, Korpela R and
Vapaatalo H: Role of arginine, taurine and homocysteine in
cardiovascular diseases. Ann Med. 31:318–326. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ghosh J, Das J, Manna P and Sil PC:
Taurine prevents arsenic-induced cardiac oxidative stress and
apoptotic damage: role of NF-kappa B, p38 and JNK MAPK pathway.
Toxicol Appl Pharmacol. 240:73–87. 2009. View Article : Google Scholar
|
|
22
|
Li Y, Arnold JM, Pampillo M, Babwah AV and
Peng T: Taurine prevents cardiomyocyte death by inhibiting NADPH
oxidase-mediated calpain activation. Free Radic Biol Med. 46:51–61.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bitoun M and Tappaz M: Gene expression of
the transporters and biosynthetic enzymes of the osmolytes in
astrocyte primary cultures exposed to hyperosmotic conditions.
Glia. 32:165–176. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ramamoorthy S, Leibach FH, Mahesh VB, et
al: Functional characterization and chromosomal localization of a
cloned taurine transporter from human placenta. Biochem J.
300:893–900. 1994.PubMed/NCBI
|
|
25
|
Tappaz ML: Taurine biosynthetic enzymes
and taurine transporter: molecular identification and regulations.
Neurochem Res. 29:83–96. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shi YR, Qi YF, Bu DF, et al: Dysfunction
of myocardial and vascular taurine transport in spontaneously
hypertensive rats. Sheng Li Xue Bao. 54:359–364. 2002.PubMed/NCBI
|
|
27
|
Melo TM, Nehlig A and Sonnewald U:
Metabolism is normal in astrocytes in chronically epileptic rats: a
(13)C NMR study of neuronal-glial interactions in a model of
temporal lobe epilepsy. J Cereb Blood Flow Metab. 25:1254–1264.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zeng K, Xu H, Mi M, et al: Effects of
taurine on glial cells apoptosis and taurine transporter expression
in retina under diabetic conditions. Neurochem Res. 35:1566–1574.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Satsu H, Terasawa E, Hosokawa Y and
Shimizu M: Functional characterization and regulation of the
taurine transporter and cysteine dioxygenase in human
hepatoblastoma HepG2 cells. Biochem J. 375:441–447. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chang L, Zhao J, Xu J, Jiang W, Tang CS
and Qi YF: Effects of taurine and homocysteine on calcium
homeostasis and hydrogen peroxide and superoxide anions in rat
myocardial mitochondria. Clin Exp Pharmacol Physiol. 31:237–243.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sahin MA, Yucel O, Guler A, et al: Is
there any cardioprotective role of Taurine during cold ischemic
period following global myocardial ischemia? J Cardiothorac Surg.
6:312011. View Article : Google Scholar : PubMed/NCBI
|