Introduction
Reactive oxygen species (ROS) cause oxidative stress
which has been implicated in the pathogenesis of various diseases.
However, ROS have recently been recognized to play a role as a
second messenger in various receptor signaling pathways (1–3).
ROS were reported to regulate the survival or proliferation of
monocyte/macrophages induced by macrophage colony-stimulating
factor (M-CSF) (4–6). The role of ROS was also reported in
osteoclast differentiation induced by receptor for activation of
nuclear factor-κB ligand (RANKL) (7,8).
However, little attention has been given to the role of ROS in
M-CSF signaling at the early stage of osteoclastic
differentiation.
Osteoclastic differentiation of hematopoietic
progenitor cells requires M-CSF and RANKL (9) which act through their receptors
c-Fms and receptor for activation of NF-κB (RANK), respectively. At
the early stages of osteoclastic differentiation, M-CSF stimulates
RANK expression in osteoclast precursor cells
(c-fms+RANK−) and induces late-stage
precursor cells (c-Fms+RANK+) which are
common progenitors for macrophage/monocytes and osteoclasts
(10). At later stages, the
binding of RANKL to RANK induces commitment of
c-Fms+RANK+ into mononuclear osteoclasts.
We previously reported that M-CSF generated ROS at
the early stages of osteoclast differentiation in osteoclast
precursor cells and that M-CSF induced RANK expression associated
with the activation of extracellular signal-regulated kinase (ERK),
but not p38 mitogen-activated protein kinase (MAPK) (11). In peripheral blood monocytes or
bone marrow-derived monocyte/macrophages, M-CSF-generated ROS
mediated the activation of ERK, p38 MAPK and Akt and the activation
of Akt and p38 MAPK or Akt led to cell survival (5,6).
Although these studies have reported the role of ROS in cell
survival or proliferation in M-CSF signaling in
monocyte/macrophages, the mechanisms by which M-CSF signaling leads
to RANK expression remain poorly understood in early-stage
osteoclast precursors.
The plasma membrane NADPH oxidase (Nox) is
recognized as one of the major players in the generation of ROS
(12). Aside from the neutrophil
Nox2 isoform (13), other Nox
isoforms have been discovered and the ROS they generate are
suggested to act as messengers in the activation of specific
signaling pathways (14). In bone
marrow-derived hematopoietic stem cells, Nox1, Nox2 and Nox4 were
reported to be expressed (15).
The expression of Nox2 and lower levels of Nox1 and Nox1-mediated
ROS generation by RANKL were reported in bone marrow-derived
monocyte/macrophages (7). It was
also suggested that a flexible compensatory mechanism existed
between Nox1 and Nox2 for RANKL-stimulated ROS generation to
facilitate osteoclast differentiation (16). However, the source of oxidant
generated by M-CSF remained to be defined in early-stage osteoclast
precursors.
In this study, we investigated the molecular basis
for M-CSF-induced ROS generation and RANK expression in the early
stages of osteoclastic differentiation. We found that the
generation of ROS by M-CSF was mediated by Nox2 and was required
for the expression of RANK associated with ERK activation and the
expression of PU.1 and MITF.
Materials and methods
Preparation of the bone marrow osteoclast
precursor cells
Bone marrow cells from the femur and tibia of
8-week-old Wistar/ST female rats were cultured for 16–24 h in MEM
with 10% fetal calf serum (FCS) in the presence of M-CSF (5 ng/ml),
and nonadherent cells were collected. The monocyte fraction at the
interface after Ficoll-Paque gradient centrifugation of the
nonadherent cells was used as bone marrow osteoclast precursor
cells (17). Animals were treated
in accordance with the protocols approved by the Animal Care
Research Committee of Nara Women's University.
Determination of intracellular reactive
oxygen species
Precursor cells were pre-cultured in the absence of
M-CSF with or without diphenylene iodonium (DPI) or PD98059 for 30
min, and then stimulated with M-CSF (20 ng/ml). After the indicated
time, cells were washed three times in MEM and incubated for 10 min
with 2′,7′-dichlorofluorescein diacetate (DCFH-DA) (10 μM). The
fluorescence of DCF was detected by fluorescence microscopy as
previously described (11).
Fluorescence intensity was measured with WinRoof software (Mitani
Co., Tokyo, Japan).
Determination of the activation of MAP
kinase and Akt, and expression of c-fms, MITF, PU.1 and RANK
Precursor cells were pre-cultured in the absence of
M-CSF with or without DPI or PD98059 for 30 min, and stimulated
with M-CSF (20 ng/ml). Cells were harvested after stimulation for 5
min, and the phosphorylated protein levels of ERK, JNK, p38 and Akt
were determined by western blot analysis. Following stimulation for
24 h, cells were used to determine the mRNA and protein levels of
c-fms, PU.1, MITF and RANK by real-time RT-PCR and western blot
analysis, respectively.
Quantitative real-time RT-PCR
Total RNA from the cell lysate was prepared using a
commercial kit (Sepasol-RNA I Super G; Nacalai Tesque, Kyoto,
Japan). The total RNA was reverse-transcribed with a first-strand
cDNA synthesis kit (Toyobo, Osaka, Japan). Real-time PCR was
performed using the cDNA, or total RNA for the negative control,
with Thunderbird SYBR qPCR Mix (Toyobo) and specific primers
(Table I) as previously described
(18). Levels of gene expression
were determined relative to an internal standard (actin) and
expressed relative to the control values.
 | Table IPrimers used for quantitative
real-time PCR. |
Table I
Primers used for quantitative
real-time PCR.
| Target | Forward primer
sequence | Reverse primer
sequence |
|---|
| Actin |
AGCCATGTACGTAGCCATCCA |
TCTCCGGAGTCCATCACAATG |
| c-fms |
TAGAGCCAGGTGCAACAGTG |
CGCATAGGGTCTTCAAGCTC |
| MITF |
TTGGAAGACATCCTGATGGAC |
GCTGCTTGTTTTCGAAGCTC |
| Nox1 |
CCCTTTGCTTCCTTCTTGAAATC |
GCACCCGTCTCTCTACAAATCC |
| Nox2 |
TGATCATCACATCCTCCACCAA |
GATGGCAAGGCCGATGAA |
| Nox3 |
GCAGCATTGGCGTGTTCTT |
GAAATGAACGCCCCTAGGATCT |
| Nox4 |
CTGCATCTGTCCTGAACCTCAA |
TCTCCTGCTAGGGACCTTCTGT |
| PU.1 |
TGGAGAAGCTGATGGCTTG |
CCTTGTGCTTGGACGAGAA |
| RANK |
ATATGCCTGCATCCCCTGAA |
TAGCCATCCGTTGAGTTGGA |
Western blot analysis
Equal amounts of protein of the cell lysates were
separated by sodium dodecyl sulfate-polyacrylamide gel
electrophoresis and transferred to membranes. Western blotting and
reprobing were performed and the chemiluminescent signals were
quantified by a densitometer as reported (19). Antibodies recognizing actin
(H-300), RANK (H-300), PU.1 (H-135), MITF (H-50) and p-ERK (E-4)
were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA,
USA). The antibodies to ERK, p38, pp38 (Thr180/Tyr182), JNK, pJNK
(Thr183/Tyr185), Akt and pAkt (Ser473) were obtained from Cell
Signaling Technology (Hitchin, UK). Protein concentrations were
measured using the BCA protein assay kit (Pierce/Thermo Fisher
Scientific Inc., Rockford, IL, USA).
Osteoclastic differentiation of bone
marrow osteoclast precursor cells
Precursor cells (1×104 cells/well of a
96-well plate or 1.5×105 cells/35-mm plate) were
cultured in MEM with 10% FCS containing M-CSF (20 ng/ml) and RANKL
(10 ng/ml) with or without DPI or PD98059. Cultures were maintained
with a change of medium every 3 days. After 5 days, cells were used
for counting the tartrate-resistant acid phosphatase
(TRAP)-positive multinucleated cells (MNCs) after TRAP staining and
the assessment of cell viability with a leukocyte acid phosphatase
kit 387-A (Sigma) and WST-8 (Cell Counting kit-8; Dojin, Japan),
respectively, as previously described (20).
RNA interference
The duplexed Stealth™ siRNA designed against Nox1
and Nox2 (Cybb), and the negative control were purchased from
Invitrogen. The sequences were: Nox1 siRNA,
5′-CCAAGGUUGUCAUGCACCCAUGUAA-3′ and
5′-UUACAUGGGUGCAUGACAACCUUGG-3′; Nox2 (Cybb) siRNA,
5′-GAUUCAGGAUGGAGGUGGGACAAUA-3′ and
5′-UAUUGUCCCACCUCCAUCCUGAAUC-3′. Precursor cells were seeded at a
density of 4×105 cells/dish in 35-mm-diameter culture
dishes or 2×105 cells/well in 96-well culture plates and
transfected with 25 nM of negative control siRNA (Stealth RNAi™
Negative Control; Invitrogen), Nox1 siRNA, or Nox2 siRNA using
X-tremeGENE HP DNA Transfection Reagent (Roche Applied Science,
Penzberg, Germany) for 24 h, according to the manufacturer's
instructions. After a change to fresh medium, the expression of
Nox1 and Nox2 was determined by RT-PCR. ROS production and RANK
expression were determined at 5 min and 24 h after stimulation with
M-CSF (20 ng/ml), respectively.
Statistical analysis
All statistical analyses were performed using
Welch's method with the Microsoft Excel data analysis program. The
differences were considered statistically significant at P<0.05.
All data are expressed as the mean ± SEM.
Results
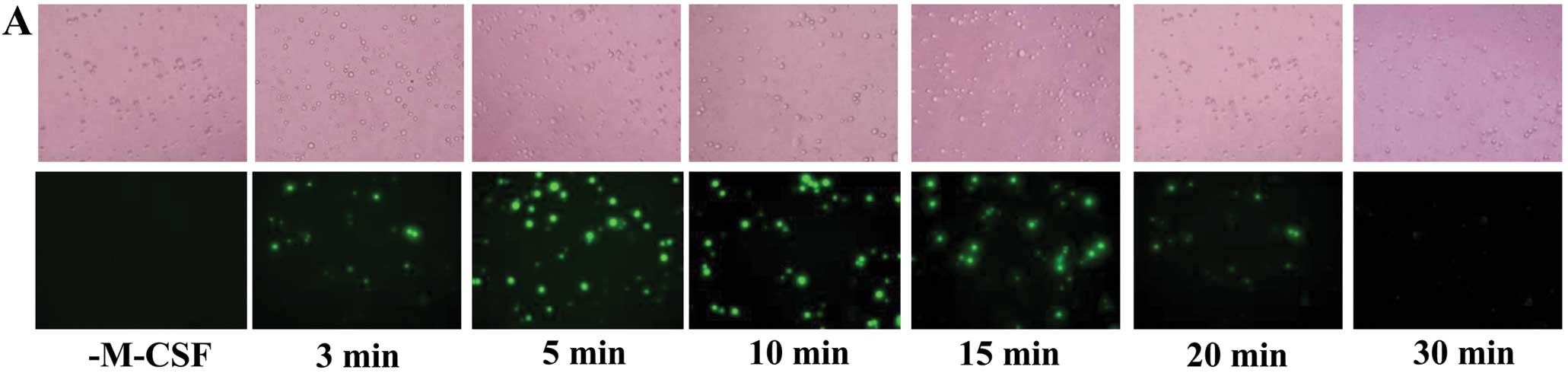
M-CSF generates ROS in osteoclast
precursor cells
Stimulation of osteoclast precursor cells with M-CSF
resulted in an increase in the intensity of DCF fluorescence
(Fig. 1A), indicating that M-CSF
induced intracellular ROS production. The production of ROS rapidly
increased to a maximum level at approximately 5 min after the M-CSF
treatment and thereafter decreased toward the basal level (Fig. 1).
Effects of NADPH oxidase inhibitor and
ERK inhibitor on M-CSF-induced ROS production, MAP kinase and Akt
activation, mRNA and protein levels of PU.1, MITF and RANK, and
osteoclast formation
Treatment of osteoclast precursor cells with DPI, a
specific inhibitor for flavoprotein that is a constituent of the
Nox complex, eliminated the rise in DCF fluorescence induced by
M-CSF (Fig. 2A). The ERK
inhibitor, PD98059, did not affect the production of ROS. ERK and
JNK were activated by M-CSF, but the phosphorylation of p38 was not
detected (Fig. 2B). DPI treatment
blocked the activation of ERK but not JNK. The phosphorylated Akt
was also increased by M-CSF, but this activation was not inhibited
by DPI or PD98059 (Fig. 2B).
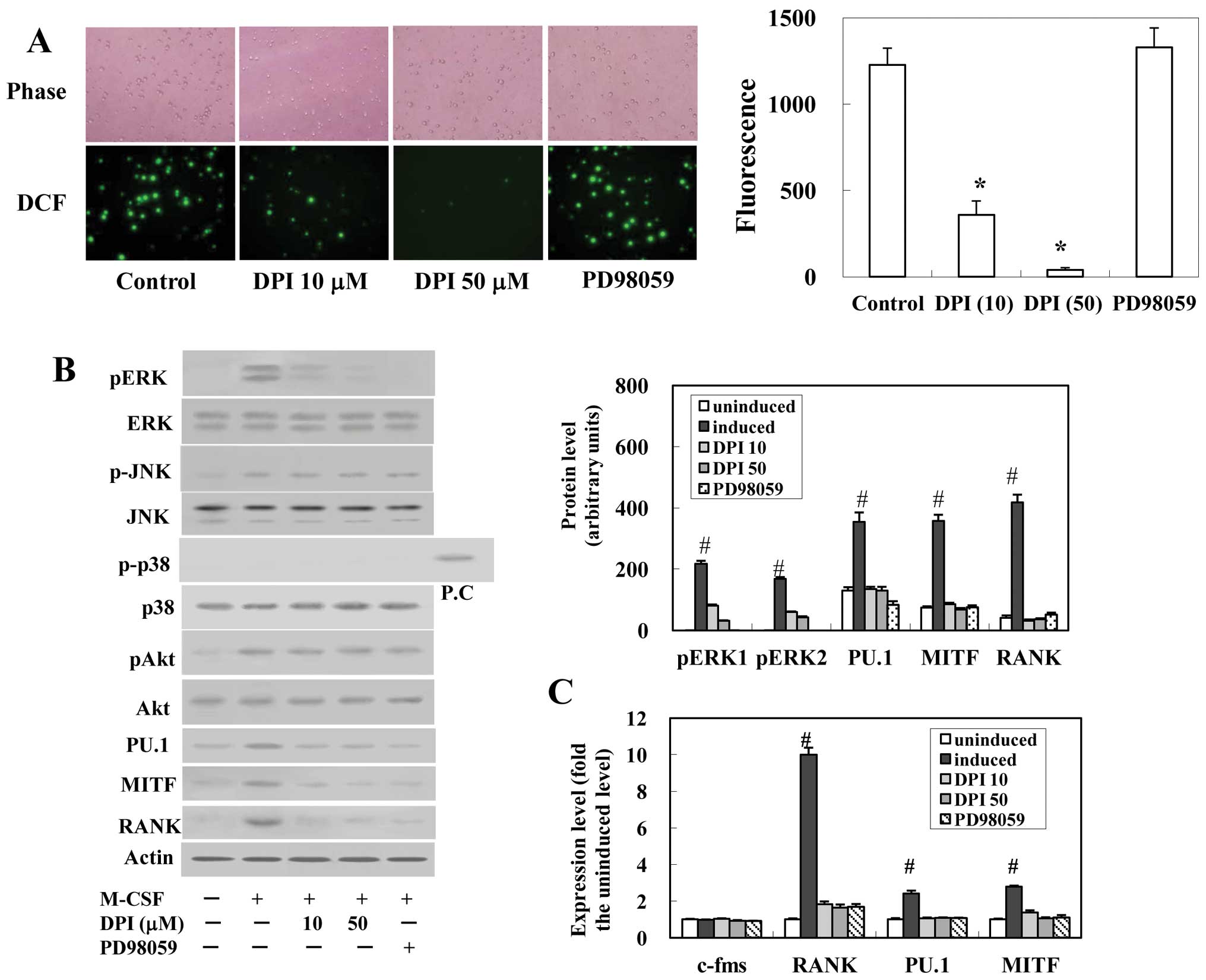 | Figure 2Effects of DPI and PD98059 on
M-CSF-induced ROS production, the activation of MAP kinase and Akt,
the mRNA and protein levels of c-fms, MITF, PU.1 and RANK, and
osteoclastogenesis. Precursor cells were pre-cultured in the
absence of M-CSF with or without DPI or PD98059 for 30 min and then
stimulated with M-CSF (20 ng/ml). (A) After stimulation for 5 min,
DCFH-DA was added and the fluorescence of DCF was detected.
Representative microscopic fields (x400 magnification; left panel)
and quantitative calculation data (right panel) are shown. (B) The
activation of ERK, JNK, p38 MAPK and Akt of the cells after
stimulation for 5 min, and the protein levels of PU.1, MITF and
RANK after the stimulation for 24 h were determined by western
blotting. The protein levels of phosphorylated ERK1 and 2 (pERK1,
pERK2), PU.1, MITF and RANK were quantified by densitometry and are
graphically represented (right panel). (C) After stimulation for 24
h, cells were harvested and the expression levels of c-fms, MITF,
PU.1 and RANK were determined by real-time RT-PCR. Levels are
expressed relative to the uninduced level. (D) Precursor cells were
cultured with M-CSF (20 ng/ml) and RANKL (10 ng/ml) for 5 days.
Cell viability and the number of TRAP-positive MNCs were
determined. Values are the mean ± SEM of four experiments.
Significantly different from the control value
(*P<0.05). Significantly different from the uninduced
value (#P<0.05). |
M-CSF increased the mRNA levels of RANK, PU.1 and
MITF to ~10-, 2.4- and 2.8-fold the uninduced (before the
stimulation of M-CSF) value, respectively, although the c-fms mRNA
levels were unchanged (Fig. 2C).
DPI or PD98059 significantly suppressed these increases. The
protein levels of RANK, PU.1 and MITF were also increased by M-CSF
and these increases were suppressed by DPI or PD98059 (Fig. 2B). The osteoclastic
differentiation of precursor cells was significantly inhibited by
the presence of DPI or PD98059 without affecting cell viability
(Fig. 2D).
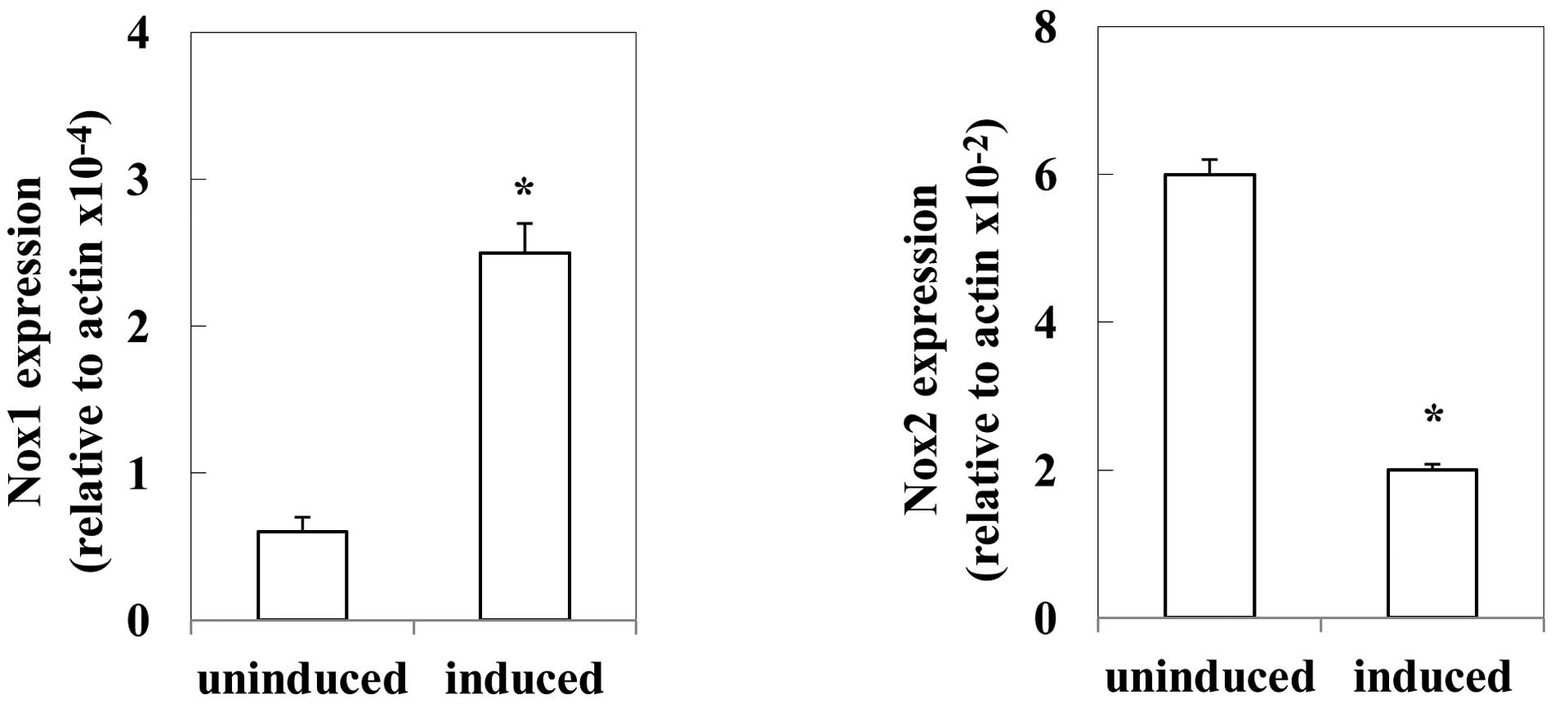
Expression of Nox isozyme
Precursor cells expressed the mRNA of Nox2, a
smaller amount of Nox1, and undetectable levels of Nox3 and Nox4.
The mRNA levels of Nox1 were ~0.1% of these of Nox2. M-CSF
decreased Nox2 expression to ~30% of the uninduced level, but
increased the Nox1 expression to 4-fold the uninduced level
(Fig. 3). The levels of Nox3 and
Nox4 were undetectable after the induction by M-CSF.
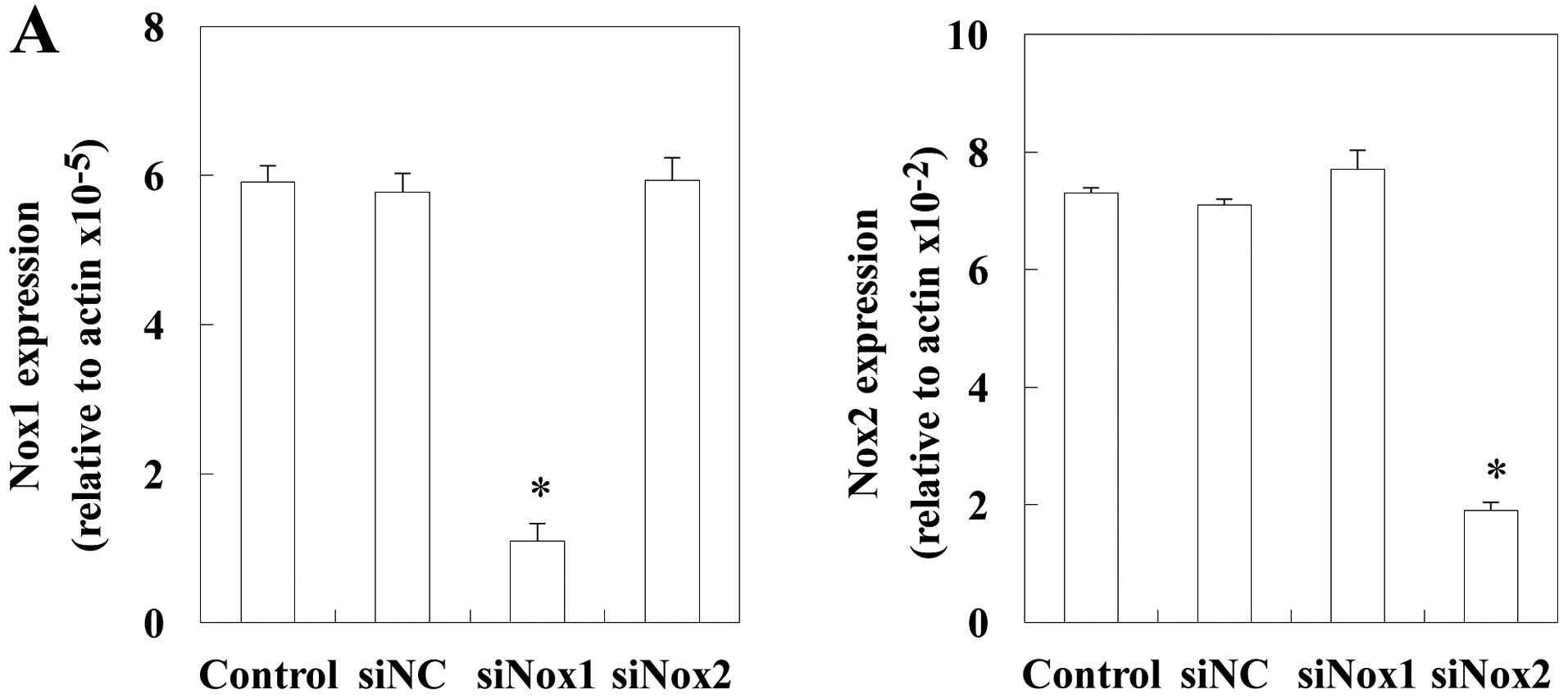
Effects of Nox1 and Nox2 siRNA on
M-CSF-induced ROS generation and RANK expression
To investigate which Nox isozyme is responsible for
the responses to M-CSF, precursor cells were treated with negative
control, Nox1 or Nox2 siRNA, and ROS production and RANK expression
were examined (Fig. 4). Nox1 or
Nox2 was effectively knocked down by the specific siRNA, as shown
by the real-time RT-PCR analysis (Fig. 4A). The silencing of Nox2 in
precursor cells resulted in a significant decrease in ROS
production in response to M-CSF (Fig.
4B). However, Nox1 knockdown had no effect on ROS production.
Nox2, but not Nox1, siRNA inhibited the expression of RANK
(Fig. 4C). These results suggest
that Nox2 is a critical mediator of M-CSF-induced RANK expression
in precursor cells (c-fms+RANK−).
Discussion
There are two types of osteoclast precursor cells,
the early-stage precursor cells expressing c-Fms, but not RANK
(c-fms+RANK−), and the late-stage precursor
cells expressing c-Fms and RANK (c-Fms+RANK+)
(10). M-CSF stimulated RANK
expression in early-stage osteoclast precursors
(c-fms+RANK−) and the binding of RANKL to
RANK triggers the differentiation of late-stage precursors into
osteoclasts. The expression of RANK caused by the binding of M-CSF
is a key step in the early stages of osteoclastogenesis.
This study clearly demonstrated a critical role for
ROS in the differentiation of early-stage osteoclast precursor
cells into late-stage precursors. M-CSF generated ROS in the
early-stage of osteoclast precursor cells. The production of ROS
was inhibited by a Nox inhibitor, DPI, indicating Nox-mediated ROS
generation. The inhibition of ROS production resulted in the
suppression of RANK expression. This study, for the first time,
revealed that Nox-mediated production of ROS was required for the
expression of RANK in early-stage osteoclast precursor cells. In
agreement with our previous finding (20), M-CSF activated ERK and JNK, but
not p38. The M-CSF-activation of ERK, but not JNK, was inhibited by
DPI treatment. Consistent with these results, the expression of
RANK was inhibited by DPI or a specific inhibitor of ERK, PD98059.
The mRNA and protein levels of PU.1 and MITF, which transactivate
RANK expression (21), were also
reduced by DPI or PD98059. Furthermore, PD98059 did not inhibit ROS
production, but suppressed RANK expression. These results suggested
that ERK activation functioned downstream of ROS-generation and
upstream of RANK expression in M-CSF signaling in early-stage
osteoclast precursors. The pathway, M-CSF/ROS generation/ERK
activation/RANK expression, was suggested to occur in the early
stages of osteoclastogenesis. In peripheral blood monocytes, the
activation of ERK by M-CSF was suggested to play a role in cellular
survival (4). Macrophages from
p47phox-/− mice, lacking a key component of the Nox
complex required for ROS generation, had no effect on
M-CSF-stimulated ERK expression, but reduced cell survival and Akt1
and p38 phosphorylation (5).
Application of DPI was reported to inhibit the responses of
monocyte/macrophages to M-CSF, including ROS production, cell
proliferation, and phosphorylation of c-Fms and Akt kinase, but not
MAP kinases such as ERK, p38 and JNK (6). These studies reported that
Nox-mediated ROS generation by M-CSF led to the activation of p38
and Akt and survival in monocyte/macrophages. However, in our
experiments using early-stage osteoclast precursor cells, neither
the activation of p38 by M-CSF nor the inhibition of Akt activation
was observed with the Nox inhibitor, DPI. DPI did not reduce the
viability of cultured precursor cells, although it decreased
osteoclast formation. These results suggested that the activation
of Akt by M-CSF was not mediated by Nox-generated ROS in
early-stage osteoclast precursor cells different from the
differentiated monocyte/macrophages and was not involved in the
signaling pathway for M-CSF-induced RANK expression. The M-CSF
signaling may differ depending on the stage of cell
differentiation, although osteoclast precursors can differentiate
into monocyte/macrophages.
Nox is recognized as a major intracellular source of
ROS. As observed in monocyte/macrophages (7,16),
Nox2 was found to be the main isotype expressed in early-stage
osteoclast precursor cells. The expression of Nox1 was also
detectable at a low level, whereas that of other members such as
Nox3 and Nox4 was undetectable. M-CSF decreased the expression of
Nox2 and increased that of Nox1. The induced level of Nox1
expression was still 1% of the induced level of Nox2. The
downregulation of Nox2 and the upregulation of Nox1 were also
observed on RANKL-stimulation (8,16).
Although the upregulation of Nox4 by RANKL-stimulation was also
reported (16), the increase in
its expression by M-CSF was not observed in this study. The siRNA
targeting Nox2, but not Nox1, inhibited the M-CSF-stimulated ROS
production and RANK expression. These results clearly indicated the
generation of ROS by M-CSF to be mediated through Nox2 in
early-stage osteoclast precursors, although a flexible compensatory
mechanism between Nox1 and Nox2 for RANKL-stimulated ROS production
was suggested in the osteoclast differentiation of marrow-derived
monocyte/macrophages (16).
In conclusion, this study provides evidence that ROS
produced in response to M-CSF via a process mediated by Nox2 act as
an intracellular signaling mediator for RANK expression through the
activation of ERK and the expression of PU.1 and MITF in
early-stage osteoclast precursor cells
(c-fms+RANK−).
Abbreviations:
|
DCFH-DA
|
2′,7′-dichlorofluorescein
diacetate
|
|
DPI
|
diphenyleneiodonium
|
|
ERK
|
extracellular signal-regulated
kinase
|
|
JNK
|
Jun amino-terminal kinase
|
|
MAPK
|
mitogen-activated protein kinase
|
|
M-CSF
|
macrophage colony-stimulating
factor
|
|
MNCs
|
multi-nucleated cells
|
|
Nox
|
NADPH (nicotinamide adenine
dinucleotide phosphate) oxidase
|
|
RANK
|
receptor for activation of nuclear
factor-κB
|
|
RANKL
|
receptor for activation of nuclear
factor-κB ligand
|
|
ROS
|
reactive oxygen species
|
|
TRAP
|
tartrate-resistant acid
phosphatase
|
References
|
1
|
Suh YA, Arnold RS, Lassegue B, et al: Cell
transformation by the superoxide-generating oxidase Mox1. Nature.
401:79–82. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lambeth JD: Nox enzymes, ROS, and chronic
disease: an example of antagonistic pleiotropy. Free Radic Biol
Med. 43:332–347. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ray PD, Huang BW and Tsuji Y: Reactive
oxygen species (ROS) homeostasis and redox regulation in cellular
signaling. Cell Signal. 24:981–990. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bhatt NY, Kelley TW, Khramtsov VV, et al:
Macrophage-colony-stimulating factor-induced activation of
extracellular-regulated kinase involves phosphatidylinositol
3-kinase and reactive oxygen species in human monocytes. J Immunol.
169:6427–6434. 2002. View Article : Google Scholar
|
|
5
|
Wang Y, Zeigler MM, Lam GK, et al: The
role of the NADPH oxidase complex, p38 MAPK, and Akt in regulating
human monocyte/macrophage survival. Am J Respir Cell Mol Biol.
36:68–77. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Choi HK, Kim TH, Jhon GJ and Lee SY:
Reactive oxygen species regulate M-CSF-induced monocyte/macrophage
proliferation through SHP1 oxidation. Cell Signal. 23:1633–1639.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lee NK, Choi YG, Baik JY, et al: A crucial
role for reactive oxygen species in RANKL-induced osteoclast
differentiation. Blood. 106:852–859. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sasaki H, Yamamoto H, Tominaga K, et al:
NADPH oxidase-derived reactive oxygen species are essential for
differentiation of a mouse macrophage cell line (RAW264.7) into
osteoclasts. J Med Invest. 56:33–41. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Asagiri M and Takayanagi H: The molecular
understanding of osteoclast differentiation. Bone. 40:251–264.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Arai F, Miyamoto T, Ohneda O, et al:
Commitment and differentiation of osteoclast precursor cells by the
sequential expression of c-Fms and receptor activator of nuclear
factor kappaB (RANK) receptors. J Exp Med. 190:1741–1754. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hie M and Tsukamoto I: Administration of
zinc inhibits osteoclastogenesis through the suppression of RANK
expression in bone. Eur J Pharmacol. 668:140–146. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Finkel T: Oxidant signals and oxidative
stress. Curr Opin Cell Biol. 15:247–254. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cross AR and Segal AW: The NADPH oxidase
of professional phagocytes - prototype of the NOX electron
transport chain systems. Biochim Biophys Acta. 1657:1–22. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bokoch GM and Knaus UG: NADPH oxidases:
not just for leukocytes anymore! Trends Biochem Sci. 28:502–508.
2003.PubMed/NCBI
|
|
15
|
Piccoli C, D'Aprile A, Ripoli M, et al:
Bone-marrow derived hematopoietic stem/progenitor cells express
multiple isoforms of NADPH oxidase and produce constitutively
reactive oxygen species. Biochem Biophys Res Commun. 353:965–972.
2007. View Article : Google Scholar
|
|
16
|
Sasaki H, Yamamoto H, Tominaga K, et al:
Receptor activator of nuclear factor-kappaB ligand-induced mouse
osteoclast differentiation is associated with switching between
NADPH oxidase homologues. Free Radic Biol Med. 47:189–199. 2009.
View Article : Google Scholar
|
|
17
|
Takarada T, Hinoi E, Kambe Y, et al:
Osteoblast protects osteoclast devoid of sodium-dependent vitamin C
transporters from oxidative cytotoxicity of ascorbic acid. Eur J
Pharmacol. 575:1–11. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hie M, Shimono M, Fujii K and Tsukamoto I:
Increased cathepsin K and tartrate-resistant acid phosphatase
expression in bone of streptozotocin-induced diabetic rats. Bone.
41:1045–1050. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hie M, Yamazaki M and Tsukamoto I:
Curcumin suppresses increased bone resorption by inhibiting
osteoclastogenesis in rats with streptozotocin-induced diabetes.
Eur J Pharmacol. 621:1–9. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hie M and Tsukamoto I: Vitamin
C-deficiency stimulates osteoclastogenesis with an increase in RANK
expression. J Nutr Biochem. 22:164–171. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ishii J, Kitazawa R, Mori K, et al:
Lipopolysaccharide suppresses RANK gene expression in macrophages
by down-regulating PU. 1 and MITF. J Cell Biochem. 105:896–904.
2008. View Article : Google Scholar : PubMed/NCBI
|