Introduction
Ulcerative colitis (UC), a chronic relapsing
inflammatory bowel disease (IBD) restricted to the large bowel, is
a major public health threat worldwide, particularly in China
(1,2). UC is characterized by periods of
remission punctuated by clinical exacerbations and frequent
relapses. Moreover, 1% of UC cases with extensive disease of long
duration progress to colorectal cancer (CRC) (3). A previous study revealed that the
immune response plays an important role in the pathophysiology of
UC (4), and a cytokine profile
with dysregulation of both Th1 [producing interferon-γ (IFN-γ)] and
Th2 [producing interleukin-4 (IL-4)] is implicated in the
development of UC (5–7). However, it is still unresolved
whether a Th1- or Th2-type of immune response dominates in UC.
Research has demonstrated that UC is an atypical Th2
response, and levels of Th1 cytokines (such as IFN-γ mRNA) in
colonic lamina propria T lymphocytes (CD3+ LPL) are
reduced (7). However, an
abnormality in IL-4 is not observed in UC (7,8),
and Th2-related cytokines do not appear to be useful as predictive
markers in relation to the outcome of UC patients treated with
infliximab (9). Therefore, the
Th2 immune response has not been conclusively demonstrated in UC.
Other studies have shown that tumor necrosis factor-α (TNF-α; a
Th1-cytokine) and other cytokines, such as IFN-γ and IL-6, are
expressed at a high level in blood samples or colonic mucosa
(10–14), suggesting that a higher Th1
response may be closely associated with the development of UC. One
possible explanation for this discrepancy may be that in addition
to the local immune response, circulating T lymphocytes in
peripheral blood mononuclear cells (PBMCs) and cytokines may also
contribute to this inconsistency, since our previous studies
revealed that circulating T lymphocytes are elevated and implicated
in the pathogenesis of several autoimmune diseases, including
rheumatoid arthritis and immune thrombocytopenic purpura (15,16). However, the expression profile of
circulating T lymphocytes and related cytokines in UC remains
unclear.
Similar to CD4+ T (Th) lymphocytes,
CD8+ T (Tc) lymphocytes may also be functionally
subdivided into mutually exclusive type 1 (T1) and type 2 (T2)
subsets based on the secretion of either IFN-γ or IL-4 (15). Tc cells are able to kill target
cells directly, and are involved in several autoimmune diseases.
However, to date, no data exist concerning the Tc cell profile in
UC. In addition, recent findings of a newly identified
CD4+ Th cell subset, known as Th17 cells, may shed light
on the study of the Th17-mediated immune response in UC (17–19). Th17 cells are characterized as
preferential producers of several cytokines, such as IL-17A (also
termed IL-17), IL-17F, IL-21, IL-22 and IL-6, and may have evolved
for host protection against the microbes for which Th1 or Th2
immunity are not well suited (20). Just as T-bet controls the Th1
lineage, the activity of the transcription factor retinoic
acid-related orphan receptor-γt (RORγt) is required for Th17 cell
differentiation (21). RORγt may
be activated by IL-6 and IL-23, and consequently enhances Th17 cell
development by signal transducer and activator of transcription
3/suppressor of cytokine signaling 3 (STAT3/SOCS3) in UC and
UC-induced carcinogenesis (22–24). Moreover, the STAT3 level has been
found to be associated with aggravation of UC (25). All of these data suggest that the
RORγt-STAT3-Th17 pathway plays an important role in the progression
of UC.
The basis for new biological therapy requires new
knowledge of immunological molecules that mediate immune disorders
(26). Therefore, from a clinical
point of view, it is essential to understand the immune status of
UC. To further investigate the role of circulating Th17, Th1 and
Tc1 cells in the pathogenesis of UC, we examined the levels of
circulating Th17, Th1 and Tc1 cells, and analyzed their correlation
with clinicopathological features. Moreover, the roles of RORγt and
STAT pathways in the progression of UC were also evaluated.
Materials and methods
Patients and specimens
Fifty-five patients with active UC (24 males, age
range 31–72 years, median age 57 years and 31 females, age range
32–68 years, median age 53 years) and 21 patients with UC in
remission (9 males, age range 28–65 years, median age 51 years and
12 females, age range 31–70 years, median age 55 years) were
enrolled in this study between August 2008 and June 2010 at the
Department of Gastroenterology, Qilu Hospital, Shandong University
(Shandong, China). Patients with active UC were diagnosed according
to routine clinical, endoscopic and histopathological features
(27). Patient WBC counts ranged
from 8.3 to 14.8×109/l with a median count of
11.6×109/l. The clinical characteristics of the patients
are summarized in Table I. The
Ulcerative Colitis Disease Activity Index (UCDAI; score 0–12) was
employed to evaluate the degree of UC, as previously described
(28–30). Patients in remission were enrolled
in the study according to previously described criteria (29). Of the patients in remission, 5
patients had received no medication 6 weeks prior to sampling, 10
patients received only 5-aminosalicylic acid, and 6 patients
received a combination of prednisolone and 5-aminosalicylic acid.
In order to exclude any potential bias caused by the influence of
medicine, patients receiving 5-aminosalicylic acid or
6-mercaptopurine/azathioprine therapies were excluded if the dose
had been altered within 30 days or within 3 months, respectively.
In addition, we also recruited patients with Crohn’s disease (CD)
(7 males and 9 females, age range 25–59 years, median 43 years).
Twenty-three healthy volunteers (9 males and 14 females, age range
23–68 years, median age 49 years) with no history of IBD nor other
autoimmune diseases were recruited as healthy controls. Their WBC
counts ranged from 4.1 to 8.7×109/l with a median count
of 6.2×109/l.
 | Table IRelationship between circulating
Th17, Th1, Tc1 cells and clinicopathological variables in the
patients with active ulcerative colitis. |
Table I
Relationship between circulating
Th17, Th1, Tc1 cells and clinicopathological variables in the
patients with active ulcerative colitis.
| Parameters | n | Th17 (%) | P-value | Th1 (%) | P-value | Tc1 (%) | P-value |
|---|
| Age (years) |
| <50 | 30 | 2.97
(2.42–3.53) | 0.787 | 14.00
(11.10–16.90) | 0.837 | 15.69
(9.21–22.17) | 0.864 |
| ≥50 | 25 | 3.07
(2.71–3.43) | | 13.37
(10.84–14.90) | | 15.24
(10.18–20.30) | |
| Gender |
| Male | 24 | 3.01
(2.41–3.61) | 0.575 | 14.35
(8.03–18.77) | 0.773 | 14.91
(10.65–19.17) | 0.441 |
| Female | 31 | 3.05
(2.53–3.58) | | 16.49
(12.10–20.88) | | 15.69
(8.74–22.64) | |
| Disease
duration |
| <10 years | 38 | 2.95
(2.53–3.38) | 0.334 | 14.25
(9.65–18.85) | 0.382 | 14.29
(8.47–20.10) | 0.084 |
| ≥10 years | 17 | 3.18
(2.59–3.77) | | 16.43
(12.63–20.23) | | 15.24
(10.18–20.30) | |
| No. of
relapses |
| <5 | 27 | 3.05
(2.72–3.39) | 0.73 | 17.00
(10.57–24.29) | 0.537 | 14.29
(8.29–20.29) | 0.644 |
| ≥5 | 28 | 3.06
(2.60–3.52) | | 15.32
(11.82–18.82) | | 15.69
(11.32–20.07) | |
| Disease
activity |
| Mild +
moderate | 37 | 2.81
(2.48–3.14) | 0.007b | 14.38
(7.38–21.38) | 0.014a | 14.91
(10.36–19.46) | 0.016a |
| Severe | 18 | 3.48
(2.89–4.07) | | 17.41
(11.35–21.47) | | 17.35
(10.17–24.53) | |
| Extent of
diseases |
|
Proctosigmoiditis | 18 | 2.87
(2.07–3.67) | 0.001b | 14.70
(9.28–20.12) | 0.002b | 15.89
(12.26–19.52) | 0.117 |
| Left-sided
colitis | 25 | 2.86
(2.36–3.36) | | 18.01
(14.35–21.67) | | 14.73
(12.12–19.34) | |
| Total colitis | 12 | 3.65
(3.17–4.13) | | 21.87
(17.47–26.27) | | 16.70
(11.37–22.03) | |
| ESR (mm/h) |
| <20 | 26 | 2.24
(1.81–2.67) | 0.001b | 13.44
(11.32–15.56) | 0.010a | 17.04
(12.52–21.56) | 0.531 |
| ≥20 | 29 | 3.46
(3.02–3.90) | | 16.48
(10.08–22.16) | | 15.86
(12.35–19.37) | |
| CRP (mg/l) |
| <25 | 31 | 2.73
(2.24–3.22) | 0.005b | 12.75
(9.54–15.96) | 0.001b | 14.21
(10.01–18.21) | 0.358 |
| ≥25 | 26 | 3.13
(2.81–3.45) | | 16.12
(11.35–20.89) | | 16.06
(10.23–21.89) | |
Venous blood samples of all subjects were collected
in heparin-containing tubes. At the same time, plasma was collected
by centrifugation at 4°C (3,000 × g for 10 min), and then stored at
−80°C until use. The research protocol was approved by the Medical
Ethics Committee of Qilu Hospital, Shandong University, and written
informed consent was obtained from all subjects prior to conducting
the study.
Antibodies and reagents
Phorbol myristate acetate (PMA), ionomycin and
monensin were from eBioscience (San Diego, CA, USA). Ficoll-Paque
was from Pharmacia Diagnostics (Uppsala, Sweden). PE-Cy5-conjugated
anti-CD3, FITC-conjugated anti-CD8, PE-conjugated IL17A or IFN-γ
monoclonal antibodies were purchased from eBioscience. Anti-human
phospho-STAT3, anti-human STAT3, anti-human phospho-STAT5 and
anti-STAT5 antibodies were purchased from Cell Signaling
Technology, Inc. (Boston, MA, USA).
Flow cytometric analysis
Intracellular cytokines were assessed via flow
cytometry to reflect cytokine-producing cells, as previously
described (15,31). Briefly, heparinized peripheral
whole blood (400 μl) was incubated with RPMI-1640 medium (1:1) at
37°C in 5% CO2 for 4 h in the presence of PMA (25
ng/ml), ionomycin (1 μg/ml) and monensin (1.7 μg/ml). PMA and
ionomycin are pharmacological T cell-activating agents. They have
the advantage of stimulating T cells of any antigen specificity and
may mimic signals generated by the TCR complex. Monensin may lead
to an accumulation of cytokines in the cells since it blocks
intracellular transport mechanisms.
The cells were stained with anti-CD3 labeled with
PE-Cy5 and anti-CD8 labeled with FITC at room temperature in the
dark for 15 min. CD3+CD8− T cells were used
to delimitate CD4+ T cells as CD4 is down-modulated when
cells are activated by PMA (32).
After three washes with PBS, the cells were further stained with
anti-IL-17A labeled with PE for Th17 detection or anti-IFN-γ
labeled by PE for Th1 or Tc1 detection after fixation and
permeabilization according to the manufacturer’s instructions.
Isotype controls were used to correct compensation and confirm
antibody specificity. After another three washes, cells were
suspended in PBS and then immediately analyzed by flow cytometry
(FACScan, BD Biosciences Pharmingen). Data from 10,000 events was
acquired and analyzed by the software WinMDI 2.8.
RNA extraction and quantitative real-time
PCR assay
Ten milliliters of peripheral blood was obtained
from all subjects, and PBMCs were isolated by gradient
centrifugation on Ficoll-Paque. PBMCs were then applied to an
RNeasy mini-column (Qiagen GmbH, Hilden, Germany) and processed
according to the manufacturer’s instructions. The concentration of
RNA was determined using an Eppendorf Biophotometer (Brinkmann
Instruments, Westbury, NY, USA).
For reverse transcription-PCR, 1 μg of total RNA was
converted to cDNA using a reverse transcription kit (Fermentas Life
Science, USA) in a 20 μl volume. For quantitative real-time PCR,
cDNA was amplified in triplicate in the LightCycler 2.0 (Roche),
with SYBR-Green Real-Time PCR Master Mix (Toyobo Co., Ltd.) and
primers for T-bet or RORγt. GAPDH served as the internal standard.
The average cycle threshold (Ct) value of triplicate wells with
each primer set was calculated as the amount of gene product
present in the sample. The relative gene expression level was
determined by the ratio between the Ct value for the target genes
and GAPDH. The cycling conditions were as following:
pre-denaturation for 30 sec at 95°C followed by 40 cycles of 5 sec
at 95°C and 30 sec at 60°C. The primers are as follows: T-bet
forward, GTGCTCCAGTCCCTCCATA and reverse, TCAGCTGAGTAATCTCGGCA
(product size, 166 bp); RORγt forward, GCTGGTTAGGATGTGCCG and
reverse, GGATGCTTTGGCGATGA (product size, 310 bp); GAPDH forward,
GGTGGTCTCCTCTGACTTCAACAG and reverse, GTTGCTGTAGCCAAATTCGTTGT
(product size, 126 bp).
Western blot analysis
PBMCs were isolated using Ficoll-Paque and lysed on
ice. Total protein (30 μg) was separated by 10% sodium dodecyl
sulfate polyacrylamide gels and then transferred onto
nitrocellulose membranes. The membranes were incubated with the
primary antibody (1:1,000) overnight at 4°C. The specific
horseradish peroxidase-conjugated goat anti-rabbit or goat
anti-mouse secondary antibody was used to blot the target proteins,
and the immunoreactivity of target proteins was detected using an
enhanced chemiluminescence (ECL) detection kit.
Enzyme-linked immunosorbent assay
(ELISA)
The ELISA assay was performed on the plasma of all
subjects. The plasma levels of IL-17 and IFN-γ were determined
using commercial ELISA kits (IL-17; R&D Systems, Minneapolis,
MN, USA and IFN-γ; Jingmei, Beijing, China) according to the
manufacturer’s instructions.
Detection of erythrocyte sedimentation
rate (ESR) and C-reactive protein (CRP)
ESR was measured via the Westergren method. Plasma
CRP was detected via the rate as determined by nephelometry
according to the manufacturer’s instructions. Positive and negative
controls were used.
Statistical analysis
Data are mainly presented as means ± SD. The SPSS
software package (version 13.0; SPSS Inc., Chicago, IL, USA) was
used for all statistical analyses. The distribution of the samples
was assessed by the Kolmogorov-Smirnov test. Pearson correlation
test was applied to analyze the correlation of Th17, Th1 and Tc1
cells. Other experimental data were analyzed by Kruskal-Wallis test
or Mann-Whitney U test wherever appropriate. Tukey post-hoc
comparison was performed when statistical significance (P<0.05)
was found between observations.
Results
Alteration of the level of circulating
Th17 cells in patients with active and inactive UC
Our previous studies indicated that the level of
Th17 cells is elevated in several autoimmune diseases, including
rheumatoid arthritis and immune thrombocytopenic purpura (15,16). To investigate the alteration in
circulating Th17 cells in UC, we analyzed the expression of IL-17
in T cells by flow cytometry, based on cytokine patterns after
in vitro stimulation by PMA/ionomycin in short-term culture.
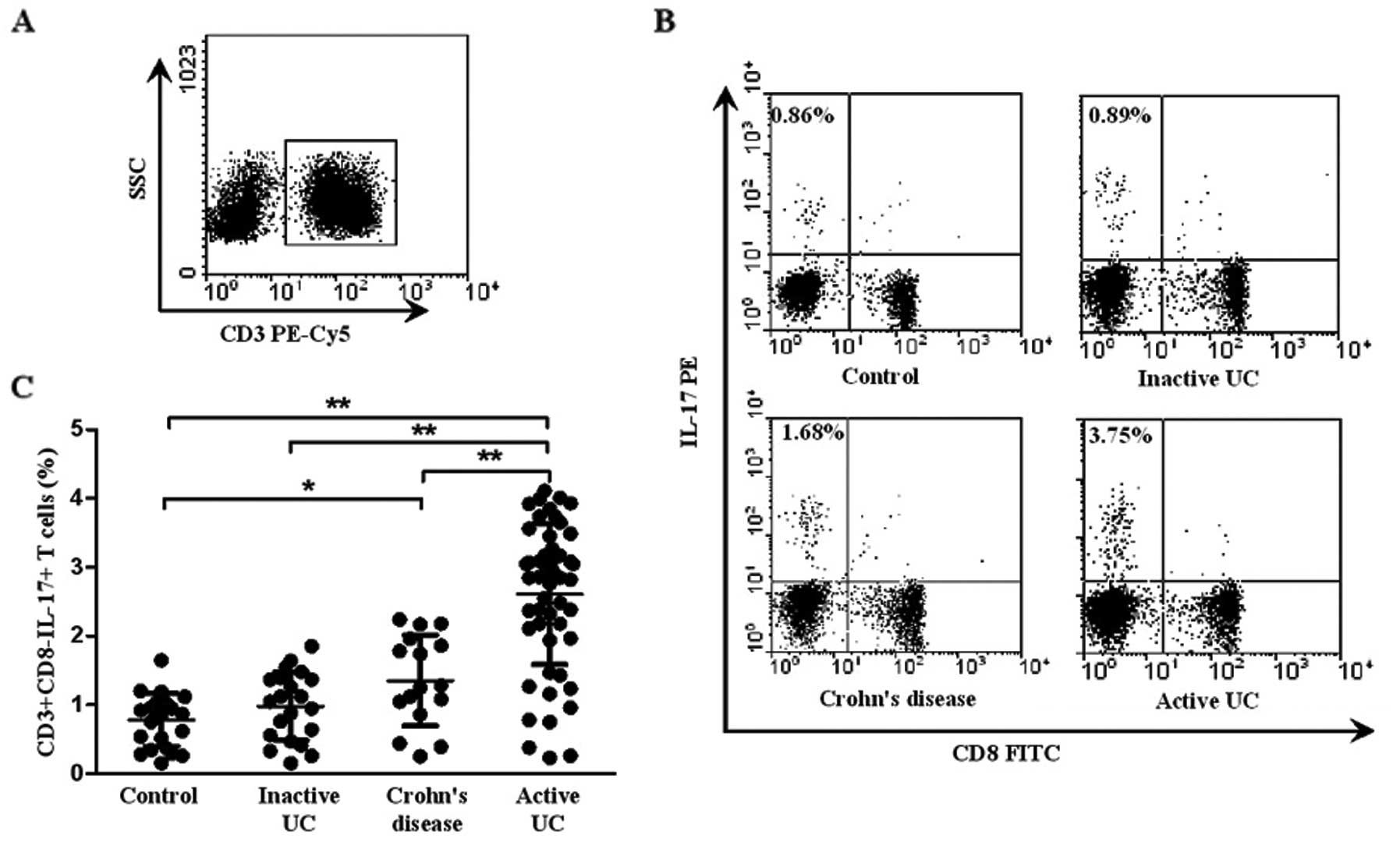
Fig. 1A and B shows a
representative dot plot of IL-17 gated on CD3+ T cells
in patients with active UC, inactive UC, CD and controls. As shown
in Fig. 1C, the percentage of
circulating Th17 cells
(CD3+CD8−IL-17+ T cells) was
significantly elevated in patients with active UC (2.90±0.73%)
compared with that in inactive UC (0.98±0.49%, P<0.001), CD
(1.46±0.67%, P=0.008) and healthy controls (0.87±0.47%,
P<0.001). Patients with CD displayed an increased percentage of
Th17 cells when compared with that of the healthy controls
(P=0.014). However, there was no significant difference between
inactive UC and healthy controls (P>0.05). Taken together, these
results suggest that Th17 cells play an important role in the
progression of UC.
Alteration of the levels of circulating
Th1 and Tc1 cells in patients with active and inactive UC
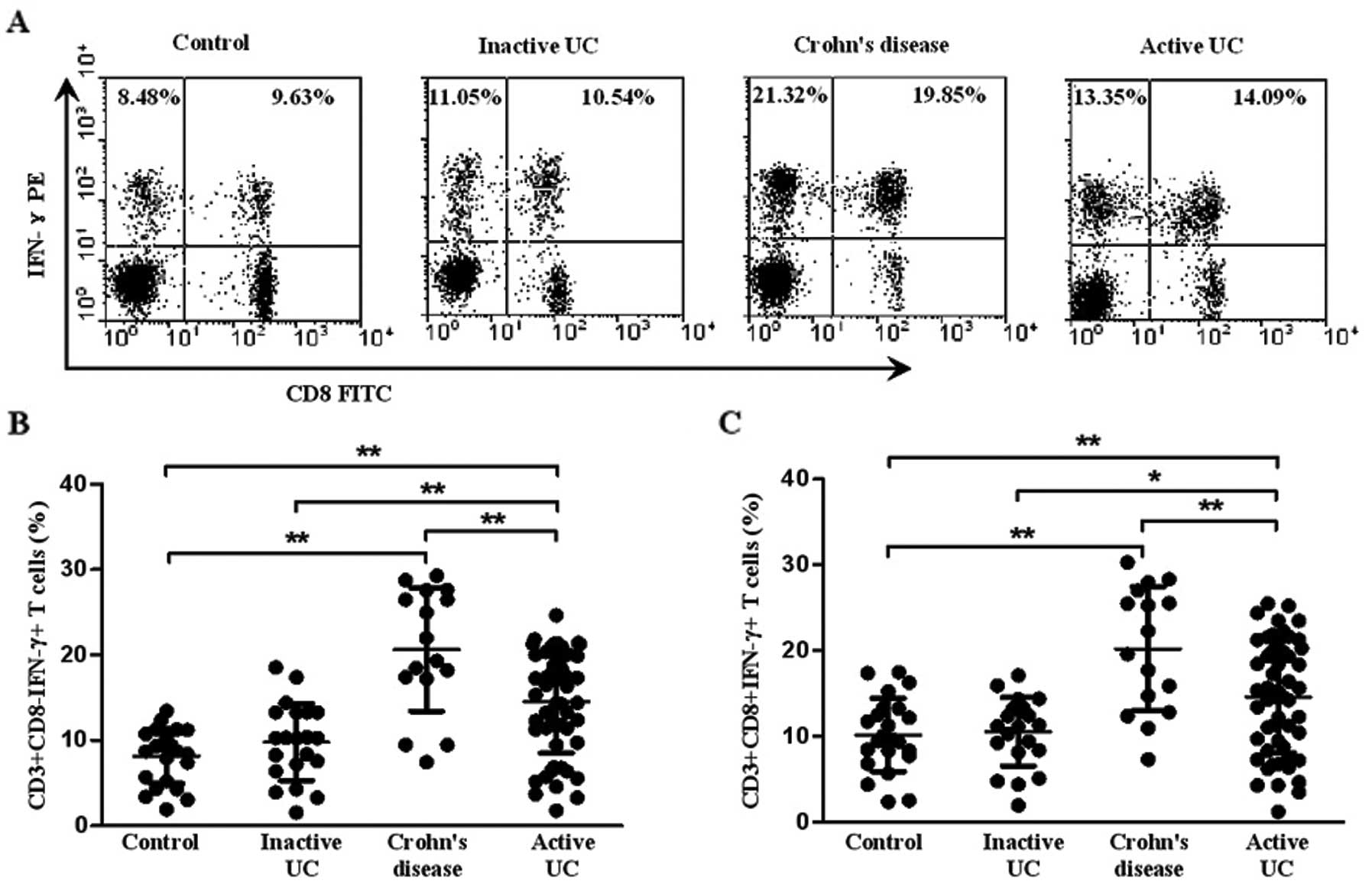
Th1 and Tc1 cells were identified as
CD3+CD8−IFN-γ+ and
CD3+CD8+IFN-γ+, respectively. A
representative dot plot of IFN-γ gated on CD3+ T cells
in all the subject groups is shown in Fig. 2A. The percentage of circulating
Th1 cells in the patients with active UC was significantly higher
(14.45±5.89%) than that in the patients with inactive UC
(11.27±3.34%, P=0.002) and healthy controls (9.85±1.59%,
P<0.001), although it was lower than that in CD (20.64±7.22%,
P=0.004). Meanwhile, the percentage of Th1 cells was higher in the
patients with CD than that in healthy controls (P<0.001)
(Fig. 2B). However, there was no
significant difference between inactive UC and healthy controls
(P>0.05). Similarly, an increased percentage of Tc1 cells was
also found in patients with active UC (14.61±6.52%) compared with
inactive UC (11.32±3.21%, P=0.013) and healthy controls
(10.16±2.11%, P=0.006). Yet, the level of circulating Tc1 cells in
CD (20.22±7.22%) was higher than that in active UC (P=0.008). No
significant difference was observed between inactive UC and healthy
controls (P>0.05) (Fig.
2C).
Correlation among Th17, Th1 and Tc1 cells
in patients with active UC
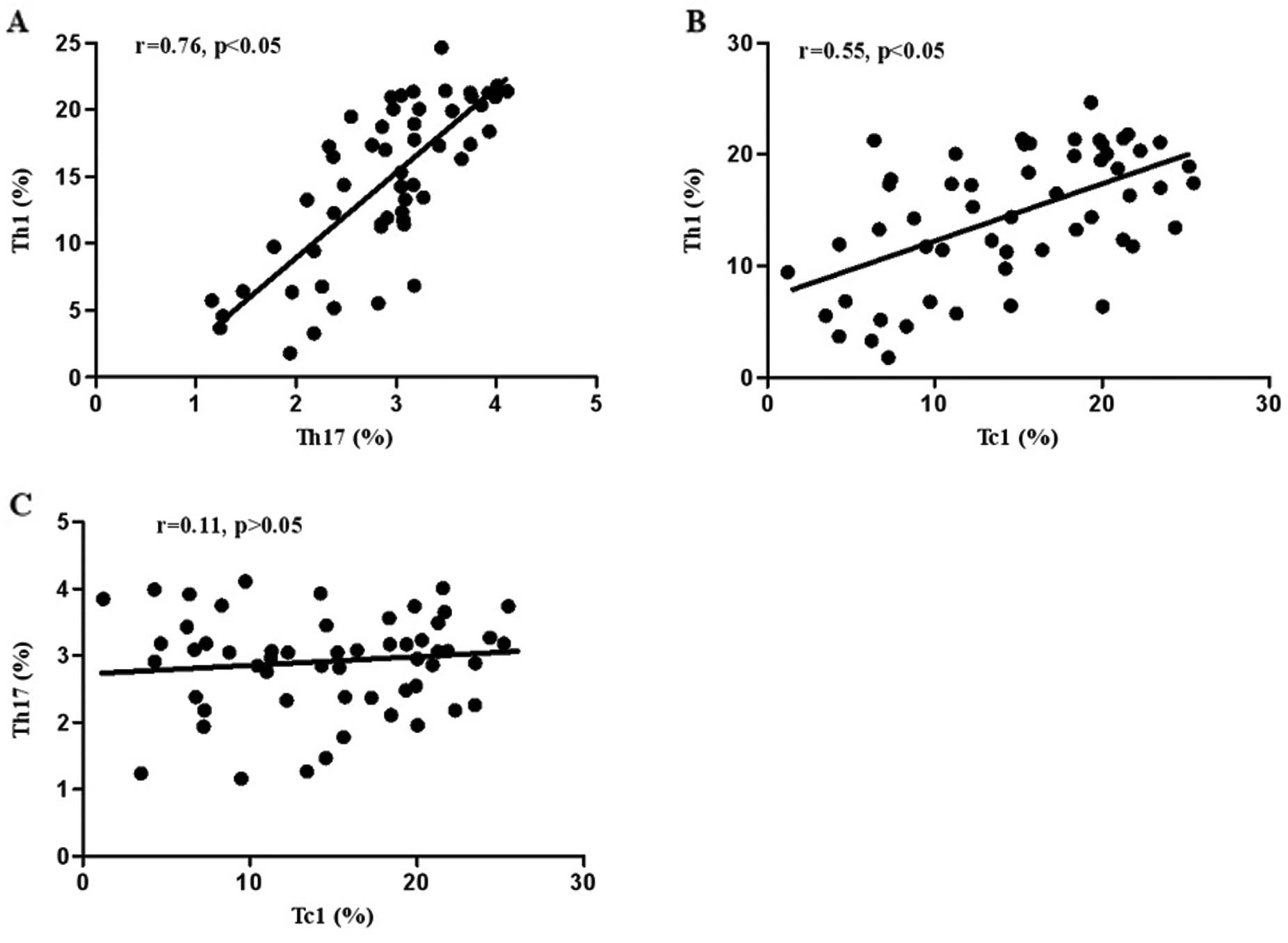
The correlation of Th17, Th1 and Tc1 cells in UC was
further investigated. As shown in Fig. 3, the percentage of circulating Th1
cells had a significant positive correlation with Th17 (r=0.76,
P<0.05) (Fig. 3A) and Tc1
cells (r=0.55, P<0.05) (Fig.
3B) in patients with active UC. However, no significant
correlation between Th17 and Tc1 cells was observed (r=0.11,
P>0.05) (Fig. 3C).
Correlation of Th17, Th1 and Tc1 cells
with clinical parameters in patients with active UC
To investigate whether these cells were involved in
UC progression, the correlation of these cells with clinical and
laboratory features of patients with active UC was analyzed. As
shown in Table I, the percentage
of circulating Th17 and Th1 cells had a positive correlation with
disease activity (P=0.007 for Th17, P=0.014 for Th1), extent of
disease (P=0.001 for Th17, P=0.002 for Th1), ESR (P=0.001 for Th17,
P=0.01 for Th1) and CRP (P=0.005 for Th17, P=0.001 for Th1) in the
patients with active UC, while the percentage of circulating Tc1
cells had a correlation with disease activity of patients with
active UC (P=0.014).
Levels of Th17 and Th1 cytokines in
patients with active UC, inactive UC, CD and healthy controls
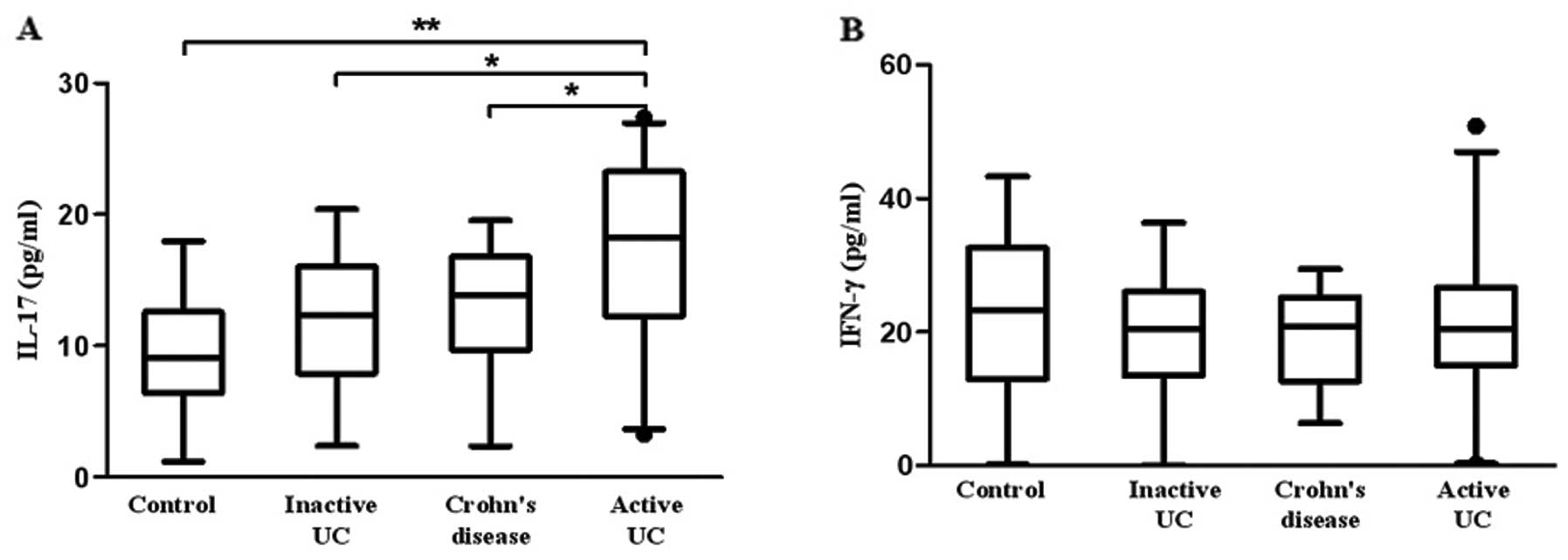
We further investigated the concentration of Th17
and Th1 cytokines in plasma. Fig.
4A shows that the plasma level of IL-17 was higher in patients
with active UC (17.08±6.44 pg/ml) when compared with the level in
patients with inactive UC (12.00±4.12 pg/ml, P=0.034), CD
(13.12±3.07 pg/ml, P=0.011) and controls (9.46±4.34 pg/ml,
P=0.001). However, no significant difference in plasma IFN-γ was
observed between active UC (23.14±11.72 pg/ml), inactive UC
(22.57±7.82 pg/ml), CD (20.42±7.42 pg/ml) and controls (24.09±10.39
pg/ml) (Fig. 4B).
RORγt and T-bet are upregulated in
patients with active UC
Previous studies have indicated that Th1 and Th17
cell differentiation requires T-bet and RORγt, respectively
(21). Therefore, we assessed the
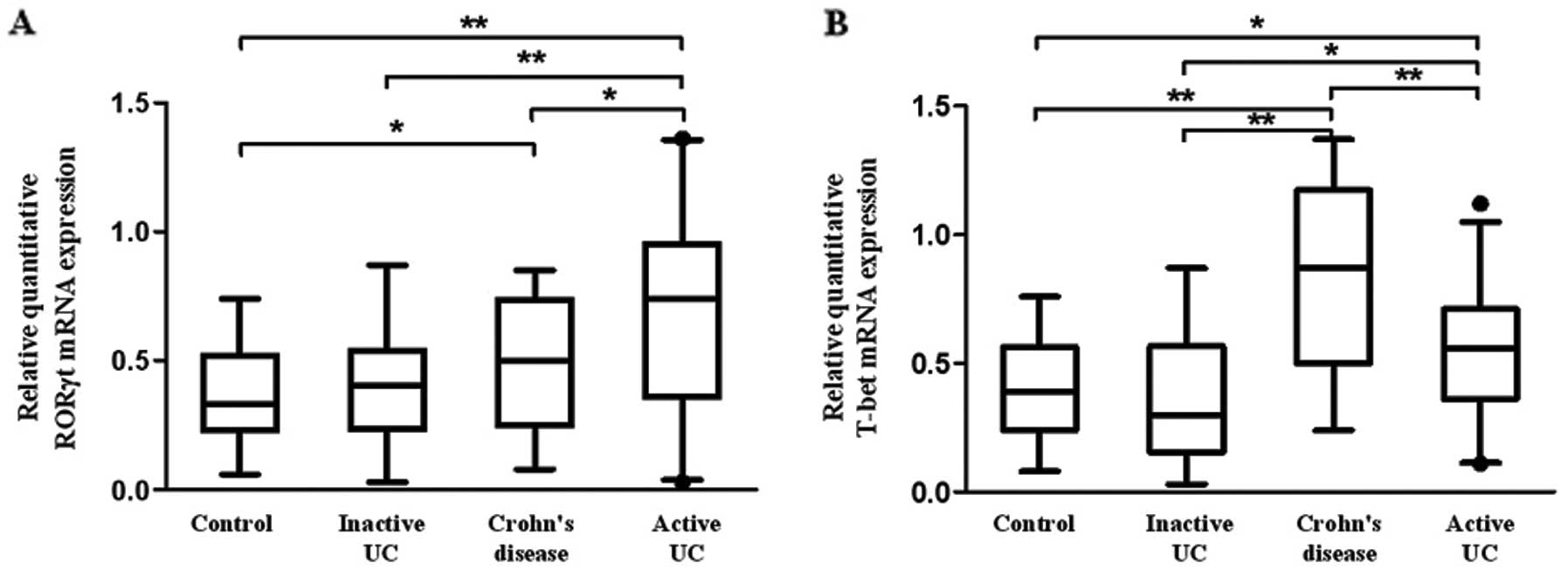
expression of T-bet and RORγt in PBMCs. Fig. 5A shows that the mRNA level of
RORγt in patients with active UC was significantly higher when
compared with that in patients with inactive UC, CD and healthy
controls (P=0.020, 0.011, 0.001, respectively). Moreover, a
significantly increased RORγt mRNA was observed in patients with CD
compared with healthy controls (P=0.032). Similarly, T-bet mRNA was
upregulated in patients with active UC compared with that in
inactive UC and healthy controls, although it was lower than that
in CD (P<0.01) (Fig. 5B).
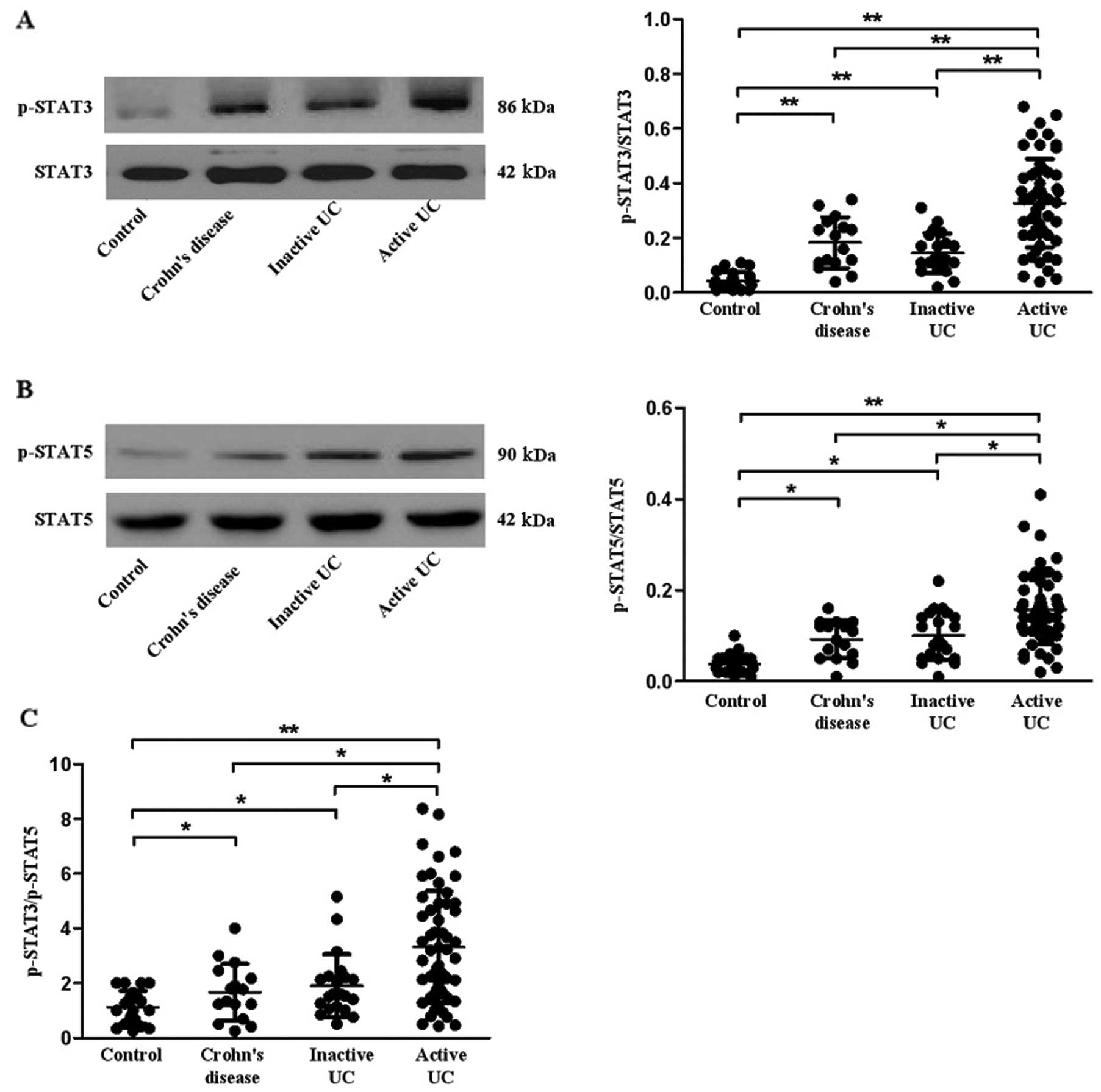
Expression of STAT3 and STAT5 in patients
with active UC, inactive UC, CD and controls
STAT3 and STAT5 have been reported to play an
important role in Th17 cell differentiation (33,34). Here, we investigated the
expression of STAT3 and STAT5 in all subjects. Our data revealed
that the phosphorylation of STAT3 and STAT5 was increased in PBMCs
of patients with active UC, inactive UC and CD when compared with
the healthy controls (P=0.001, 0.007, 0.001 for p-STAT3; P=0.020,
0.014, 0.015 for p-STAT5, respectively) (Fig. 6A and B). To further investigate
which STAT was dominant in the regulation of Th17 differentiation,
we calculated the ratio of p-STAT3/p-STAT5 (Fig. 6C) and found that the ratio was
higher in patients with inactive UC (P=0.021) and CD (P=0.011) than
that in the healthy controls. Furthermore, the ratio was higher in
patients with active UC when compared with the ratio in patients
with inactive UC, CD and healthy controls (P=0.005, 0.040, 0.005,
respectively).
Discussion
Ulcerative colitis is a global health issue.
Research findings in recent years has revealed that the imbalance
of T lymphocytes plays an important role in the pathogenesis of UC
(6). In the present study, our
results revealed that levels of circulating Th17, Th1 and Tc1 cells
were significantly increased in patients with active UC, and the
percentage of Th1 cells was correlated with that of Th17 and Tc1
cells. These cells were positively correlated with disease activity
or extent of disease, ESR and CRP in active UC patients. Moreover,
the mRNA levels of RORγt and T-bet, and the phosphorylation of
STAT3 and STAT5, were upregulated in PBMCs of patients with active
UC. Therefore, we hypothesized that aberrant expression of
circulating Th17, Th1 and Tc1 cells, and the abnormal activity of
the STAT pathway play important roles in the pathogenesis of UC.
These findings may help to broaden our knowledge concerning the
immunopathological role of these cells in the progression of
UC.
Dysregulated immune activity involving the
CD4+ T cell component is suspected to be an initial or
additional factor for the progression of UC (6). Most studies have demonstrated a Th1
polarization of the immune response based on the findings that
pro-inflammatory cytokines IL-6 and TNF-α are the predominant
cytokines in UC (12,13). In contrast, other studies have
yielded inconsistent results, indicating that IL-10 is
overexpressed in mucosal T cells, and suggesting that UC is a
disease with a Th2-type immune response (7). It should be noted that these studies
examined the expression of cytokines only in a local environment,
and are insufficient to evaluate the general immune status of UC.
Increased levels of circulating T lymphocytes and related cytokines
have been recently reported in several autoimmune diseases
(15,16). Therefore, we focused on the
peripheral immune status of UC, and our findings revealed that the
level of circulating Th1 cells was increased in patients with
active UC, although the level was lower than that in CD. A similar
trend also applied to T-bet mRNA in these groups. Further analysis
showed that Th1 cells were positively associated with disease
activity and extent of disease, ESR and CRP in active UC patients.
These results indicate that UC may be characterized by a mixed
profile rather than a Th2 cytokine profile, which may help to
advance our understanding of the immune status of UC.
IL-17 is an ~20-kDa glycoprotein of 155 amino acids.
It has been reported that IL-17 induces pro-inflammatory cytokine
production by macrophages, which subsequently creates a link
between innate and adaptive immunity (35). IL-17 is theoretically prone to
Th17 differentiation and averse to Treg cell differentiation, and
plays a role in host defense against bacteria and fungi (19). A previous study showed that IL-17
expression is correlated with increased Th1 cells in the
pathogenesis of IgG4-related sclerosing sialadenitis (36). To date, information is scarce
concerning the precise profile of circulating Th17 and Th1 cells,
and the cytokines generated by them in the whole blood of UC
patients. Our findings revealed that levels of circulating Th17 and
Th1 cells in active UC patients were significantly upregulated,
which is consistent with the fact that elevated expression of IFN-γ
and Th17 cytokine genes in mucosa was found to be positively
correlated with the disease activity of UC (9,37).
We further found that the elevated levels of Th17 and Th1 cells
were positively correlated with disease activity and extent, plasma
CRP and ESR level, and laboratory parameters that are commonly
utilized to reflect disease activity of active UC (30,38). Moreover, the percentage of
circulating Th17 was higher in active UC than that in CD, although
the cytokine profile of CD is characterized by a Th1 pattern
(39). All of these data suggest
that Th1 and Th17 cells play an important role in the development
and progression of UC, and their close relationship with clinical
parameters and laboratory features implicate a potential parameter
for the diagnosis and differential diagnosis of UC. Further studies
involving a greater number of patients may clarify their usefulness
in UC.
Th17 cell differentiation requires the activity of
RORγt, one subtype of RORγ (21).
Our results revealed that the mRNA level of RORγt in PBMCs was
increased in active UC when compared with levels in inactive UC and
CD. A previous study demonstrated that immune-deficient
(RAG1-deficient) mice receiving T cells transferred from
RORγt-deficient mice, which lack a transcription factor necessary
for production of Th17 cytokines (ie, IL-17A, IL-17F or IL-22),
failed to develop colitis (40).
Notably, when treated with exogenous IL-17A, mice may develop
colitis, suggesting that RORγt plays a preliminary role to IL-17 in
the development of UC. This hypothesis is also supported by a
further experimental model. RAG1-deficient mice that received T
cells with normal RORγt expression but lacking the capacity to
produce the individual Th17 cytokine presented with full-blown
colitis (40). It has been
reported that when appropriate stimuli (such as IL-12) exist, then
cells of the Th17 lineage are plastic and differentiate into
Th1-type cells (41). Thus, one
possible explanation is that transfer of RORγt-deficient T cells
may lead to a great reduction in IFN-γ production, and consequently
induce the development of UC. These studies provide a basic
framework with which to understand the immunopathogenesis of UC. In
addition, several upregulated inflammatory factors in patients with
UC, such as IL-6, TGF-β and IL-23, may induce RORγt expression
(13,23). However, their expression levels
were not explored in this study.
Cytokine signaling pathways involving transcription
factors of the STAT family play a key role in the pathogenesis of
UC. STAT proteins are latent cytoplasmic transcription factors that
induce transcription upon phosphorylation, dimerization and nuclear
translocation (25). As two major
members of the STAT family, the relative activation of STAT3 and
STAT5 directly dictates the outcome of IL-17 production (42). Our results demonstrated that the
level of p-STAT3 was higher in patients with active UC when
compared with the level in patients with inactive UC. This is
consistent with observations that upregulation of STAT3 is
associated with aggravation of UC (25). Notably, in the present study, the
level of p-STAT5 was also increased, although it has been reported
that action of STAT5 mediated by IL-2 opposes Th17 cell
differentiation (34). This may
be because STAT5 is also expressed in other blood cells, such as B
cells and CD8+ T cells (43). Further experiments must be design
to analyze the expression of STAT5 in different subgroups of T
cells. However, the ratio of p-STAT3/p-STAT5 was higher in active
UC patients than that in inactive UC and healthy controls, thereby
suggesting that STAT3 is predominant in active UC. The ratio was
still higher in patients with inactive UC when compared with the
ratio in the controls and this may be a reason for the relapse of
UC. Taken together, our findings indicate that i) STAT3, but not
STAT5, predominates in the progression of UC, and ii) the role of
the STAT family in Th17 cells are disease specific. In addition,
the JAK-STAT pathway and activated STAT3, induced by IL-6, IL-21
and IL-23, are implicated in Th17 differentiation and function by
binding to the IL-17A promoter directly as shown by ChIP, and are
regarded as a critical component of Th17-dependent autoimmune
processes (22,23). This suggests that other molecules
may be required for this process, and further experiments are
required to test these possibilities.
Plasma IL-17 and IFN-γ levels were also evaluated in
our study. IL-17 was elevated in active UC, and these results are
compatible with flow cytometric data. Notably, our findings showed
that plasma IL-17 was higher in active UC than in CD. However,
Fujino et al (44)
revealed that IL-17 is higher in CD than in UC. This discrepancy is
possibly due to the ethnic distinction or the differences in
procedure and patients in our experiments. Notably, there was no
significant difference in plasma IFN-γ expression, although a
difference was observed by flow cytometry. The reason may be due to
the fact that: i) cytokines in the plasma were at a low level; ii)
the cells measured by flow cytometry were stimulated with PMA and
ionomycin while the blood used for ELISA was not treated as
described in Materials and methods; and iii) the methods used had
different sensitivities, i.e., flow cytometry may be more sensitive
than ELISA.
Our findings suggest that levels of circulating Tc1
cells are also increased in UC and are positively related to Th1
cells, which is in line with a previous study that found that the
predominance of Th1 and Tc1 cells contributes to the pathogenic
mechanism in IgG4-related sclerosing sialadenitis (36). This indicates that Tc
cell-mediated cytotoxicity is an alternative mechanism for active
UC, and that predominant Tc1 cells, probably accompanying a Th1
response, may play a cooperative or synergetic function through
production of IFN-γ under the influence of a particular
microenvironment. This is in line with previous findings that
IFN-γ-producing CD8+ lymphocytes are increased in the
PBMCs of patients with UC by co-incubation with epithelial cells
(45). In addition, an aberrant
T-cell function has been indicated in UC with abnormal cytokine
profiles correlated to loss of immune tolerance (46). A detailed phenotypic and
functional analysis of this subset is warranted.
In conclusion, elevated levels of circulating Th17,
Th1 and Tc1 cells, as well as abnormal activity of the STAT
pathway, may be implicated in the progression of UC. Our findings
may help to broaden our understanding of the immunopathological
role of circulating T lymphocytes in the progression of UC.
Acknowledgements
The study was supported by the China National
Natural Science Foundation Projects (grant nos. 81271916, 31270971,
81072406 and 30672010) and the Graduate Independent Innovation
Foundation of Shandong University (grant no. yzc10133). The authors
thank Dr Edward C. Mignot, Shandong University, for linguistic
advice.
References
|
1
|
Wang YF, Ouyang Q and Hu RW: Progression
of inflammatory bowel disease in China. J Dig Dis. 11:76–82. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Eaden JA, Abrams KR and Mayberry JF: The
risk of colorectal cancer in ulcerative colitis: a meta-analysis.
Gut. 48:526–535. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Choi PM and Zelig MP: Similarity of
colorectal cancer in Crohn’s disease and ulcerative colitis:
implications for carcinogenesis and prevention. Gut. 35:950–954.
1994.
|
|
4
|
Targan SR and Karp LC: Defects in mucosal
immunity leading to ulcerative colitis. Immunol Rev. 206:296–305.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ishiguro Y: Mucosal proinflammatory
cytokine production correlates with endoscopic activity of
ulcerative colitis. J Gastroenterol. 34:66–74. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Strober W and Fuss IJ: Proinflammatory
cytokines in the pathogenesis of inflammatory bowel diseases.
Gastroenterology. 140:1756–1767. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Melgar S, Yeung MM, Bas A, et al:
Over-expression of interleukin 10 in mucosal T cells of patients
with active ulcerative colitis. Clin Exp Immunol. 134:127–137.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Strober W, Fuss IJ and Blumberg RS: The
immunology of mucosal models of inflammation. Annu Rev Immunol.
20:495–549. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rismo R, Olsen T, Cui G, Christiansen I,
Florholmen J and Goll R: Mucosal cytokine gene expression profiles
as biomarkers of response to infliximab in ulcerative colitis.
Scand J Gastroenterol. 47:538–547. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Komatsu M, Kobayashi D, Saito K, et al:
Tumor necrosis factor-alpha in serum of patients with inflammatory
bowel disease as measured by a highly sensitive immuno-PCR. Clin
Chem. 47:1297–1301. 2001.PubMed/NCBI
|
|
11
|
Akazawa A, Sakaida I, Higaki S, Kubo Y,
Uchida K and Okita K: Increased expression of tumor necrosis
factor-alpha messenger RNA in the intestinal mucosa of inflammatory
bowel disease, particularly in patients with disease in the
inactive phase. J Gastroenterol. 37:345–353. 2002. View Article : Google Scholar
|
|
12
|
Olsen T, Goll R, Cui G, et al: Tissue
levels of tumor necrosis factor-alpha correlates with grade of
inflammation in untreated ulcerative colitis. Scand J
Gastroenterol. 42:1312–1320. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bernardo D, Vallejo-Díez S, Mann ER, et
al: IL-6 promotes immune responses in human ulcerative colitis and
induces a skin-homing phenotype in the dendritic cells and T cells
they stimulate. Eur J Immunol. 42:1337–1353. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rovedatti L, Kudo T, Biancheri P, et al:
Differential regulation of interleukin 17 and interferon gamma
production in inflammatory bowel disease. Gut. 58:1629–1636. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang J, Ma D, Zhu X, Qu X, Ji C and Hou
M: Elevated profile of Th17, Th1 and Tc1 cells in patients with
immune thrombocytopenic purpura. Haematologica. 94:1326–1329. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang L, Li JM, Liu XG, et al: Elevated
Th22 cells correlated with Th17 cells in patients with rheumatoid
arthritis. J Clin Immunol. 31:606–614. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Harrington LE, Hatton RD, Mangan PR, et
al: Interleukin 17-producing CD4+ effector T cells
develop via a lineage distinct from the T helper type 1 and 2
lineages. Nat Immunol. 6:1123–1132. 2005.PubMed/NCBI
|
|
18
|
Park H, Li Z, Yang XO, et al: A distinct
lineage of CD4 T cells regulates tissue inflammation by producing
interleukin 17. Nat Immunol. 6:1133–1141. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Monteleone I, Pallone F and Monteleone G:
Th17-cytokine blockers as a new approach for treating inflammatory
bowel disease. Ann Med. 43:172–178. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bettelli E, Korn T, Oukka M and Kuchroo
VK: Induction and effector functions of T(H)17 cells. Nature.
453:1051–1057. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ivanov II, McKenzie BS, Zhou L, et al: The
orphan nuclear receptor RORgammat directs the differentiation
program of proinflammatory IL-17+ T helper cells. Cell.
126:1121–1133. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li Y, de Haar C, Chen M, et al:
Disease-related expression of the IL6/STAT3/SOCS3 signalling
pathway in ulcerative colitis and ulcerative colitis-related
carcinogenesis. Gut. 59:227–235. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bi Y and Yang R: Direct and indirect
regulatory mechanisms in TH17 cell differentiation and functions.
Scand J Immunol. 75:543–552. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Monteleone I, Pallone F and Monteleone G:
Th17-related cytokines: new players in the control of chronic
intestinal inflammation. BMC Med. 9:1222011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li F, Zou Y and Li X: Up-regulation of
signal transducer and activator of transcription-3 is associated
with aggravation of ulcerative colitis. Surgeon. 8:262–266. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sandborn WJ and Faubion WA: Biologics in
inflammatory bowel disease: how much progress have we made? Gut.
53:1366–1373. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sands BE: From symptom to diagnosis:
clinical distinctions among various forms of intestinal
inflammation. Gastroenterology. 126:1518–1532. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Schroeder KW, Tremaine WJ and Ilstrup DM:
Coated oral 5-aminosalicylic acid therapy for mildly to moderately
active ulcerative colitis. A randomized study. N Engl J Med.
317:1625–1629. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hart AL, Kamm MA, Knight SC and Stagg AJ:
Prospective evaluation of intestinal homing memory T cells in
ulcerative colitis. Inflamm Bowel Dis. 10:496–503. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bitton A, Peppercorn MA, Antonioli DA, et
al: Clinical, biological, and histologic parameters as predictors
of relapse in ulcerative colitis. Gastroenterology. 120:13–20.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ge J, Wang K, Meng QH, Qi ZX, Meng FL and
Fan YC: Implication of Th17 and Th1 cells in patients with chronic
active hepatitis B. J Clin Immunol. 30:60–67. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Pelchen-Matthews A, Parsons IJ and Marsh
M: Phorbol ester-induced downregulation of CD4 is a multistep
process involving dissociation from p56lck, increased association
with clathrin-coated pits, and altered endosomal sorting. J Exp
Med. 178:1209–1222. 1993. View Article : Google Scholar
|
|
33
|
Yang XP, Ghoreschi K, Steward-Tharp SM, et
al: Opposing regulation of the locus encoding IL-17 through direct,
reciprocal actions of STAT3 and STAT5. Nat Immunol. 12:247–254.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Laurence A, Tato CM, Davidson TS, et al:
Interleukin-2 signaling via STAT5 constrains T helper 17 cell
generation. Immunity. 26:371–381. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kolls JK and Lindén A: Interleukin-17
family members and inflammation. Immunity. 21:467–476. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ohta N, Makihara S, Okano M, et al: Roles
of IL-17, Th1, and Tc1 cells in patients with IgG4-related
sclerosing sialadenitis. Laryngoscope. 122:2169–2174. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Olsen T, Rismo R, Cui G, Goll R,
Christiansen I and Florholmen J: TH1 and TH17 interactions in
untreated inflamed mucosa of inflammatory bowel disease, and their
potential to mediate the inflammation. Cytokine. 56:633–640. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Turner D, Mack DR, Hyams J, et al:
C-reactive protein (CRP), erythrocyte sedimentation rate (ESR) or
both? A systematic evaluation in pediatric ulcerative colitis. J
Crohns Colitis. 5:423–429. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Dong C: TH17 cells in development: an
updated view of their molecular identity and genetic programming.
Nat Rev Immunol. 8:337–348. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Leppkes M, Becker C, Ivanov II, et al:
RORgamma-expressing Th17 cells induce murine chronic intestinal
inflammation via redundant effects of IL-17A and IL-17F.
Gastroenterology. 136:257–267. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Boniface K, Blumenschein WM, Brovont-Porth
K, et al: Human Th17 cells comprise heterogeneous subsets including
IFN-γ-producing cells with distinct properties from the Th1
lineage. J Immunol. 185:679–687. 2010.PubMed/NCBI
|
|
42
|
Garg SK, Voelmle MK, Beatson CR, et al:
Use of continuous glucose monitoring in subjects with type 1
diabetes on multiple daily injections versus continuous
subcutaneous insulin infusion therapy: a prospective 6-month study.
Diabetes Care. 34:574–579. 2011. View Article : Google Scholar
|
|
43
|
Johnston RJ, Choi YS, Diamond JA, Yang JA
and Crotty S: STAT5 is a potent negative regulator of TFH cell
differentiation. J Exp Med. 209:243–250. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Fujino S, Andoh A, Bamba S, et al:
Increased expression of interleukin 17 in inflammatory bowel
disease. Gut. 52:65–70. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Bisping G, Lugering N, Lutke-Brintrup S,
et al: Patients with inflammatory bowel disease (IBD) reveal
increased induction capacity of intracellular interferon-gamma
(IFN-gamma) in peripheral CD8+ lymphocytes co-cultured
with intestinal epithelial cells. Clin Exp Immunol. 123:15–22.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kamikozuru K, Fukunaga K, Hirota S, et al:
The expression profile of functional regulatory T cells,
CD4+CD25high+/forkhead box protein
P3+, in patients with ulcerative colitis during active
and quiescent disease. Clin Exp Immunol. 156:320–327.
2009.PubMed/NCBI
|