Introduction
Atherosclerosis is a chronic inflammatory disease
that is associated with a variety of inflammatory cytokines and
chemokines that control the balance of pro-inflammation and
anti-inflammation (1,2). This balance plays a critical role in
the prognosis of atherosclerosis. Suppressor of cytokine signaling
(SOCS) proteins are intracellular regulators of receptor signal
transduction (3), which mainly
regulate the Janus kinase/signal transducer and activator of
transduction (JAK/STAT) pathway and play a regulatory role in the
expression and activation of interleukin (IL)-6 (4), tumor necrosis factor-α (TNF-α), as
well as other inflammatory cytokines (5,6).
SOCS protein expression closely correlates with the
occurrence and development of inflammatory diseases through the
regulation of gene expression and cellular activation,
proliferation and differentiation (7). Previous studies have demonstrated
that SOCS1 inhibits inflammation (8); SOCS1 reduces acute inflammation by
regulating the activity of T cells (9), and SOCS1−/− mice suffer
from fatal myocarditis (10).
However, the role of SOCS3 remains controversial (11); intracellular protein therapy with
SOCS3 has been shown to inhibit inflammation and apoptosis
(12). SOCS3 transgenic mice have
a Th2 response (13), while in
mice with experimental autoimmune encephalomyelitis, inflammation
was attenuated upon the intravenous injection of
SOCS3−/− dentritic cells (14). In addition, bone marrow-derived
macrophages differentiate into the M2 phenotype in
SOCS3−/− rats, even under the conditions of classic
activation (15). Macrophages
express different SOCS proteins under various stimulatory
conditions. Interferon-γ mainly induces SOCS1 expression (16) and interleukins, such as IL-6 and
IL-10, induce SOCS3 expression (17). In the SOCS protein family,
different subtypes play different roles in inflammatory diseases
(18,19).
Hyperlipidemia, particularly hypercholesterolemia,
is the most recognized risk factor for atherosclerosis (20). Circulating cholesterol may cause
endothelial cell dysfunction on its own or by its chemical
modifications, thus affecting the chemotaxis and adhesion of
leukocytes and the release of several inflammation-activating
cytokines (21–23), which further promotes the
deposition of cholesterol under the arterial intima. The
interaction between cholesterol and inflammation occurs throughout
the onset and development of atherosclerosis (24). However, the mechanisms by which
hypercholesterolemia influences SOCS expression and the ensuing
effects (i.e., atherosclerosis) remain unclear. Through
observations and quantitative analysis of the expression of SOCS1
and SOCS3 in atherosclerotic plaque in apolipoprotein-deficient
(ApoE−/−) mice at different intervention time points,
this study aimed to clarify the trends and possible mechanisms of
action of SOCS1 and SOCS3 proteins in the formation of
atherosclerotic plaque following exposure to high cholesterol
levels, and to investigate the correlation between blood
cholesterol levels and the expression of SOCS1 and SOCS3. The
objective of this study was to further clarify the role of SOCS1
and SOCS3 in the occurrence and development of atherosclerosis.
Materials and methods
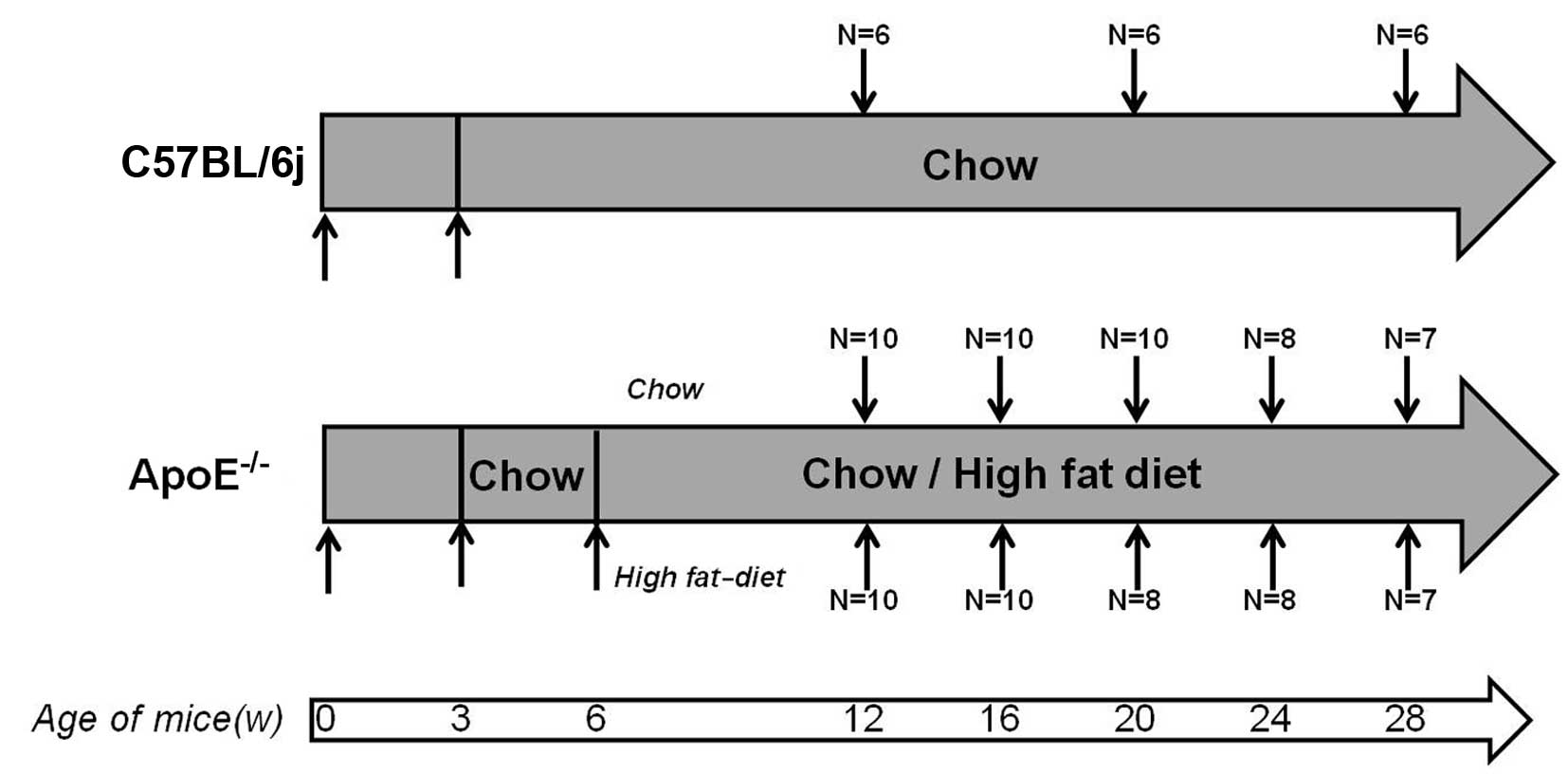
Animals
The animal experiments were carried out in
accordance with the Guide for the Care and Use of Laboratory
Animals published by the US National Institutes of Health (NIH
Publication no. 85-23, revised 1996). The ApoE−/− mice
were kept under constant temperature conditions (18°C) with a 12-h
light-dark cycle (light from 8:00 a.m. to 8:00 p.m.) and allowed
free access to food and water; these mice were a gift from Dr
Edward M. Rubin at the University of California, Berkeley
(Berkeley, CA, USA). C57BL/6j mice were purchased from the Fourth
Military Medical University, Xi’an, China. C57BL/6j and
ApoE−/− mice were fed a chow diet (4% fat and 0%
cholesterol) after being weaned at 3 weeks of age, then half of the
ApoE−/− mice were switched to a high-fat diet containing
21% fat and 0.15% cholesterol from 6 weeks of age. The C57BL/6j
mice were sacrificed at 12, 20 and 28 weeks, while the
ApoE−/− mice were sacrificed at 12, 16, 20, 24 and 28
weeks (Fig. 1). The protocols for
the experiments (sacrifice, blood and main aorta harvest) were
approved by the Institutional Ethics Committee for Animal
Experiments of Xi’an Jiaotong University, Xi’an, China and the mice
were sacrificed after anesthesia, as previously described (25).
Clinical trials
Subjects included 18 male patients who were 35–45
years of age and recruited from the First Affiliated Hospital of
the Medical College of Xi’an Jiaotong University. Coronary heart
disease (CHD) was diagnosed by the American Heart Association (AHA)
2007 criteria (26). Subjects who
were suffering from CHD, hypertension, diabetes, acute or chronic
infection, fever, cancer, autoimmune disease, severe liver, kidney
or other major organ diseases, had surgery within the last 2 weeks,
or were taking anti-inflammatory drugs and/or immune inhibitors
during the month preceding the study, were excluded. Informed
consent was obtained from all subjects. The study was carried out
according to the principles outlined in the Declaration of Helsinki
and the study protocol was approved by the Xi’an Jiaotong
University Ethics Committee. Peripheral blood mononuclear cells
(PBMCs) were separated by density gradient centrifugation with
lymphocyte isolation solution (Shanghai Huajing Biotech Co.,
Shanghai, China) and stored at −80°C.
Detection of lipid and glucose in
serum
Serum was separated by the centrifugation of venous
blood from mice and patients at 3,000 rpm for 15 min, and total
cholesterol (TC), triglyceride (TG), high-density lipoprotein
cholesterol (HDL-C) and low-density lipoprotein cholesterol (LDL-C)
levels were measured using assay kits (Dongou Bioengineering,
Beijing, China) while glucose levels were measured using an
Accu-Chek1 Advantage Glucometer (Roche Diagnostics, Inc.,
Indianapolis, IN, USA), according to the manufacturer’s
instructions. Each sample was examined 3 times.
Chemical and immunohistochemical
staining
The root of the aorta was dissected under a
microscope and frozen in optimal cutting temperature embedding
medium for serial cryosectioning at 6 mm, covering 500 mm of the
root. The first section was harvested when the 3 aortic valve cusps
became visible in the lumen of the aorta, and every 5th section was
harvested on 1 slide (8 sections/slide). Oil Red O staining was
used to detect lipids in the plaque and the sections were analyzed
using polarization microscopy. Immunohistochemistry was performed
with antibodies against SOCS1 (1:100; Abcam, Cambridge, MA, USA)
and SOCS3 (1:50; Santa Cruz Biotechnology, Inc., Santa Cruz, CA,
USA). Negative controls in the absence of primary antibodies were
also used. Section images were captured digitally using an Olympus
BX51 imaging system and were quantified with Image-Pro Plus 6.0
software. The cross-sectional surface area of the lesion and total
cross-sectional vessel area were also quantified, as previously
described (27).
RNA extraction and quantitative PCR
SOCS1 and SOCS3 mRNA expression in the main aorta of
the mice and in the PBMCs of patients were determined using
quantitative PCR. Total RNA was isolated from the main aorta of the
mice and PBMCs of patients with TRIzol reagent (Invitrogen,
Carlsbad, CA, USA); the NanoDrop 1000 spectrophotometer (Thermo
Scientific) was used to quantify the total RNA. The resulting RNA
was reverse transcribed and analyzed by quantitative PCR using the
SYBR PrimeScript™ RT-PCR Kit II (Takara Bio, Inc.). All real-time
reactions were performed on the iQ5™ Multicolor Real-time PCR
detection system (Bio-Rad, Hercules, CA, USA). A three-step PCR
procedure of 5 sec at 95°C, 20 sec at 63.5°C and 10 sec at 72°C was
applied for 45 cycles. GAPDH was used as a housekeeping gene. The
primer sequences are shown in Table
I. The data were analyzed using the 2−ΔΔCT method,
as previously described (28).
Each sample was examined 3 times.
 | Table IPrimers used for real-time PCR. |
Table I
Primers used for real-time PCR.
| Gene name | Forward
(5′-3′) | Reverse
(5′-3′) | NM code |
|---|
| Mouse |
| GAPDH |
TCAACGGCACAGTCAAGG |
ACTCCACGACATACTCAGC | NM_008084.2 |
| SOCS1 |
TCCGATTACCGGCGCATCACG |
CTCCAGCAGCTCGAAAAGGCA | NM_009896.2 |
| SOCS3 |
CACAGCAAGTTTCCCGCCGCC |
GTGCACCAGCTTGAGTACACA | NM_007707.2 |
| IL-6 |
AGCCAGAGTCCTTCAGAGAGATAC |
GCTAAGGACCAAGACCATCCAATT | NM_031168.1 |
| TNF-α |
GCTCTTCTGTCTACTGAACTTCGG |
CCAGACCCTCACACTCAGATCAT | NM_013693.2 |
| TNF-β |
GCAACATGTGGAACTCTACCAGAA |
GACGTCAAAAGACAGCCACTCA | NM_011577.1 |
| Human |
| GAPDH |
AACATCATCCCTGCCTCTACTG |
CTCCGACGCCTGCTTCACC | NM_002046.3 |
| SOCS1 |
GCAGCCGACAATGCAGTCT |
CGAACGGAATGTGCGGAAGTG | NM_003745.1 |
| SOCS3 |
GCCACCTACTGAACCCTCCTC |
TCCGACAGAGATGCTGAAGAGTG | NM_003955.3 |
Western blot analysis
Proteins from the main aorta were extracted by RIPA
buffer (Cybrdi, Inc.) according to the manufacturer’s instructions,
and a protease inhibitor cocktail (Roche Diagnostics, Inc.) was
added to all samples. The BCA protein assay reagent kit (Pierce)
was used to quantify the total amount of protein. Equal amounts of
protein extracts were separated by 10% SDS-PAGE gel and then
transferred onto nitrocellulose membranes using a Bio-Rad transfer
blotting system (Bio-Rad). Skim milk of 5% was used for blocking
non-specific binding for 1 h at room temperature with slow shaking.
The blots were incubated overnight at 4°C with anti-SOCS3 (1:500;
Santa Cruz Biotechnology, Inc.), anti-SOCS1 (1:500; Abcam) or
anti-GAPDH (1:1,000; Santa Cruz Biotechnology, Inc.). A horseradish
peroxidase-conjugated anti-goat (1:10,000; Abcam) or anti-rabbit
secondary antibody (1:5,000; Abcam) and enhanced chemiluminescent
substrate (Pierce) were used for detection, as previously described
(29). Each sample was examined 3
times.
Statistics analysis
Data were expressed as the means ± SD. The Student’s
t-test or one-way ANOVA were used to analyze the differences among
groups. Post hoc comparisons between groups were performed using
the Newman-Keuls multiple comparison test. A value of P<0.05 was
considered to indicate a statistically significant difference.
Results
General condition of the experimental
animals
After being fed with different diets for 6 weeks,
the total serum cholesterol levels of the ApoE−/− mice
fed a high-fat diet were significantly higher than those of the
mice fed the chow diet (2.52-fold) and the C57Bl/6j mice
(31.05-fold) (P<0.01); however, the total serum cholesterol
levels did not change significantly with the prolonged feeding time
in each group. The TG, HDL-C and LDL-C levels in the serum of the
ApoE−/− mice were higher than those in the serum of the
C57BL/6j mice (P<0.01); however, the ApoE−/− mice
with different dietary interventions showed no significant
differences in the aforementioned indexes. Body weight increased
significantly in each group with the increased feeding duration. No
significant difference in serum glucose levels was observed among
the groups (Table II).
 | Table IISubject characteristics. |
Table II
Subject characteristics.
| ApoE−/−
mice fed CD | ApoE−/−
mice fed HD | C57BL/6j mice fed
CD |
|---|
|
|
|
|
|---|
| 12 W | 16 W | 20 W | 24 W | 28 W | 12 W | 16 W | 20 W | 24 W | 28 W | 12 W | 20 W | 28 W |
|---|
| N | 10 | 10 | 10 | 8 | 7 | 10 | 10 | 8 | 8 | 7 | 6 | 6 | 6 |
| Male | 5 | 5 | 5 | 4 | 4 | 5 | 5 | 4 | 5 | 4 | 3 | 3 | 3 |
| Female | 5 | 5 | 5 | 4 | 3 | 5 | 5 | 4 | 3 | 3 | 3 | 3 | 3 |
| BW | 16.2±0.8 | 20.9±1.2 | 24.6±1.7 | 26.8±1.5 | 29.2±1.4 | 27.0±1.5 | 28.0±1.9 | 28.8±1.0 | 30.8±1.9 | 32.6±3.0 | 16.7±0.9 | 23.5±1.7 | 26.3±1.9 |
| CHO | 720.2±48.0a | 726.6±124.0 | 653.9±112.3 | 764.8±56.0 | 622.9±68.1 |
1813.4±243.5a | 1936.4±316.4 | 2043.5±425.7 | 1774.3±326.0 | 1947.3±374.6 | 58.4±12.5 | 63.5±21.7 | 60.3±20.7 |
| TG | 132.5±34.2a | 173.5±63.2 | 153.6±37.4 | 180.4±47.3 | 147.2±36.9 | 159.3±32.5a | 170.1±50.3 | 120.8±44.3 | 191.6±37.9 | 128.7±21.7 | 64.2±19.7 | 59.6±23.5 | 63.5±20.4 |
| HDL-C | 102.7±19.6a | 127.3±23.5 | 102.5±26.9 | 137.6±36.2 | 117.4±27.5 | 136.5±11.5a | 146.3±19.5 | 148.9±30.4 | 140.3±19.6 | 150.4±28.0 | 70.3±16.7 | 89.4±21.5 | 85.2±29.4 |
| LDL-C | 104.2±21.5a | 132.5±24.3 | 127.3±32.6 | 132.5±29.0 | 127.3±25.3 | 139.0±80.2a | 156.5±77.5 | 121.3±43.3 | 144.8±66.7 | 65.4±27.1 | 7.4±2.4 | 6.9±3.2 | 7.6±3.3 |
| GLU | 6.7±0.8 | 6.3±0.6 | 7.2±0.8 | 6.7±0.5 | 7.0±0.6 | 6.8±0.7 | 7.5±0.9 | 7.8±0.5 | 7.2±0.4 | 6.9±0.5 | 6.3±0.3 | 6.4±0.6 | 6.1±0.3 |
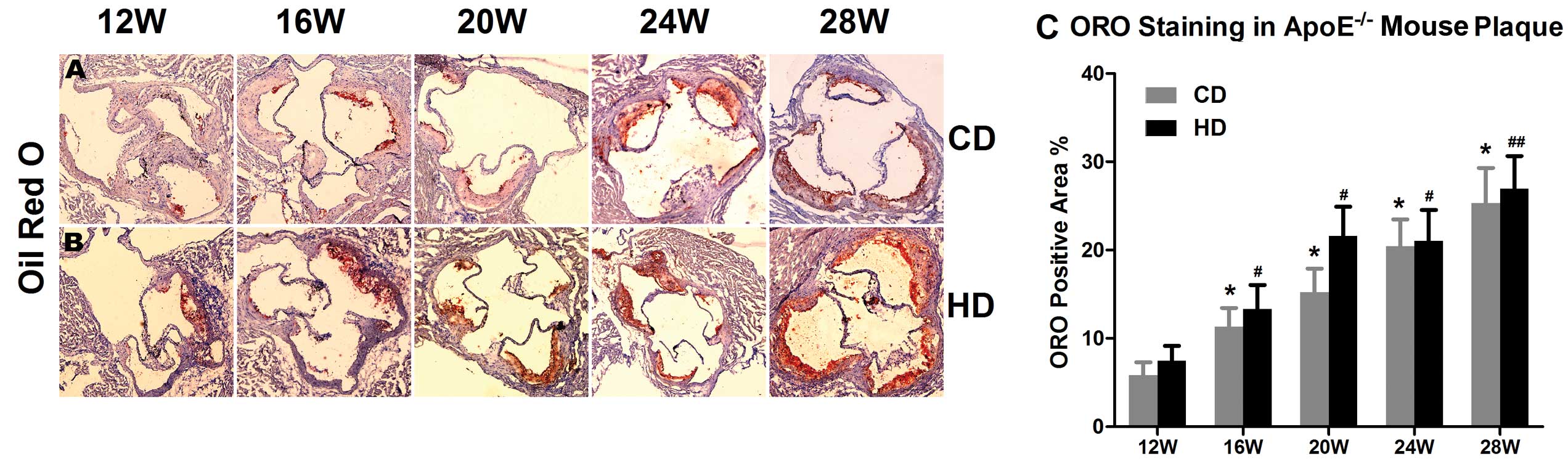
Positive Oil Red O staining showed that the
atherosclerotic plaque size at the aortic root and lipid deposition
in the plaque of the ApoE−/− mice were consistently
growing as the feeding duration increased. At 28 weeks, the Oil Red
O positive area was 4.33-fold larger than that at 12 weeks in the
group fed the chow diet (P<0.05) and 3.62-fold larger in the
group fed the high-fat diet (P<0.01). The area of lipid deposit
in the atherosclerotic plaque in the group fed the high-fat diet
was larger than that in the group fed the chow diet in
ApoE−/− mice of the same age (Fig. 2).
Trend in SOCS1 expression in the
ApoE−/− mice
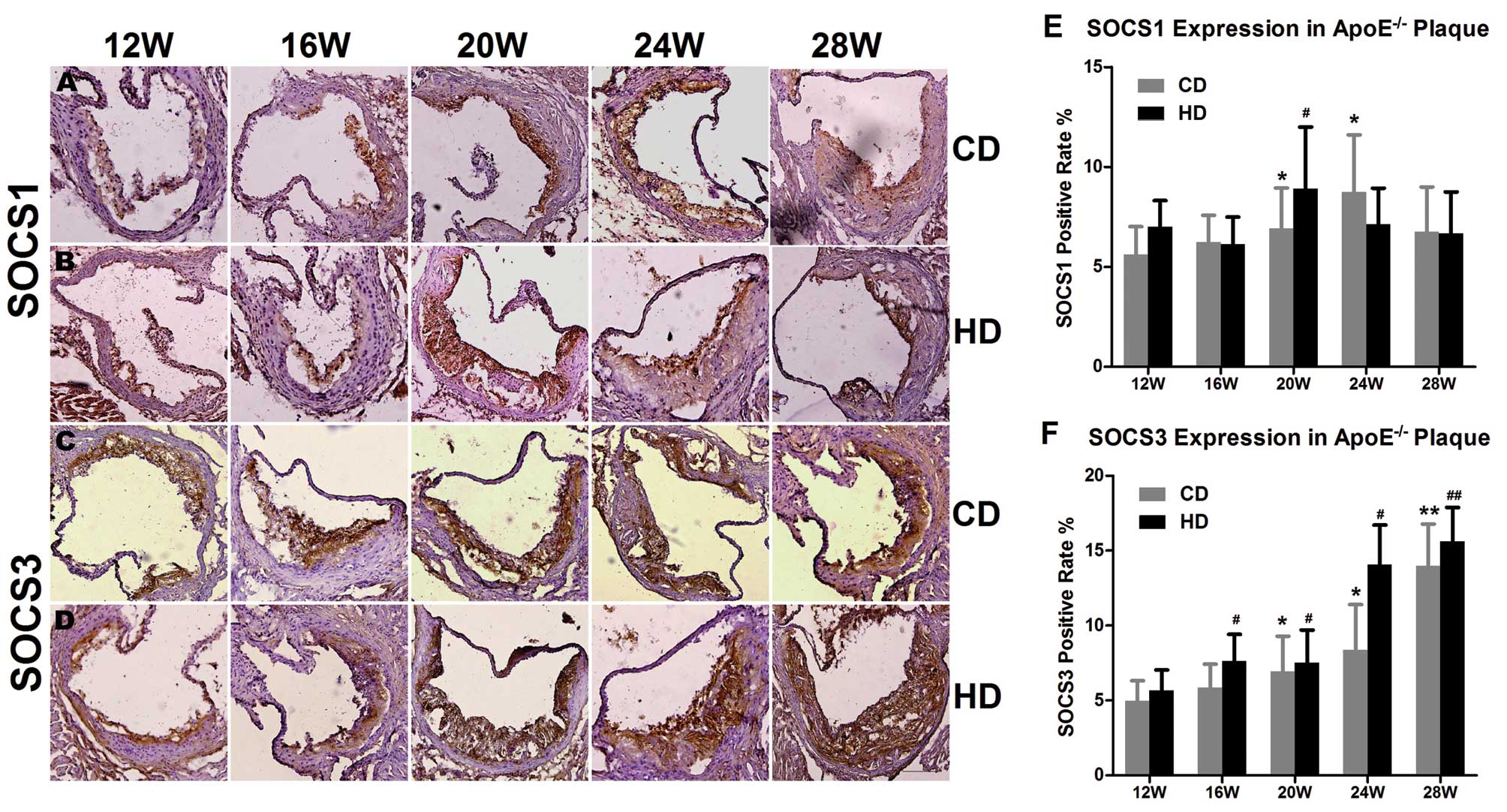
The results of immunohistochemical staining revealed
that in the group fed the chow diet, SOCS1 expression in the plaque
at the aortic root of the ApoE−/−mice showed an
increasing trend first (eventually peaking at 24 weeks) and then
decreased as the feeding duration increased (P<0.05). The same
single peak trend appeared earlier in the group fed the high-fat
diet at 20 weeks (P<0.05) (Fig.
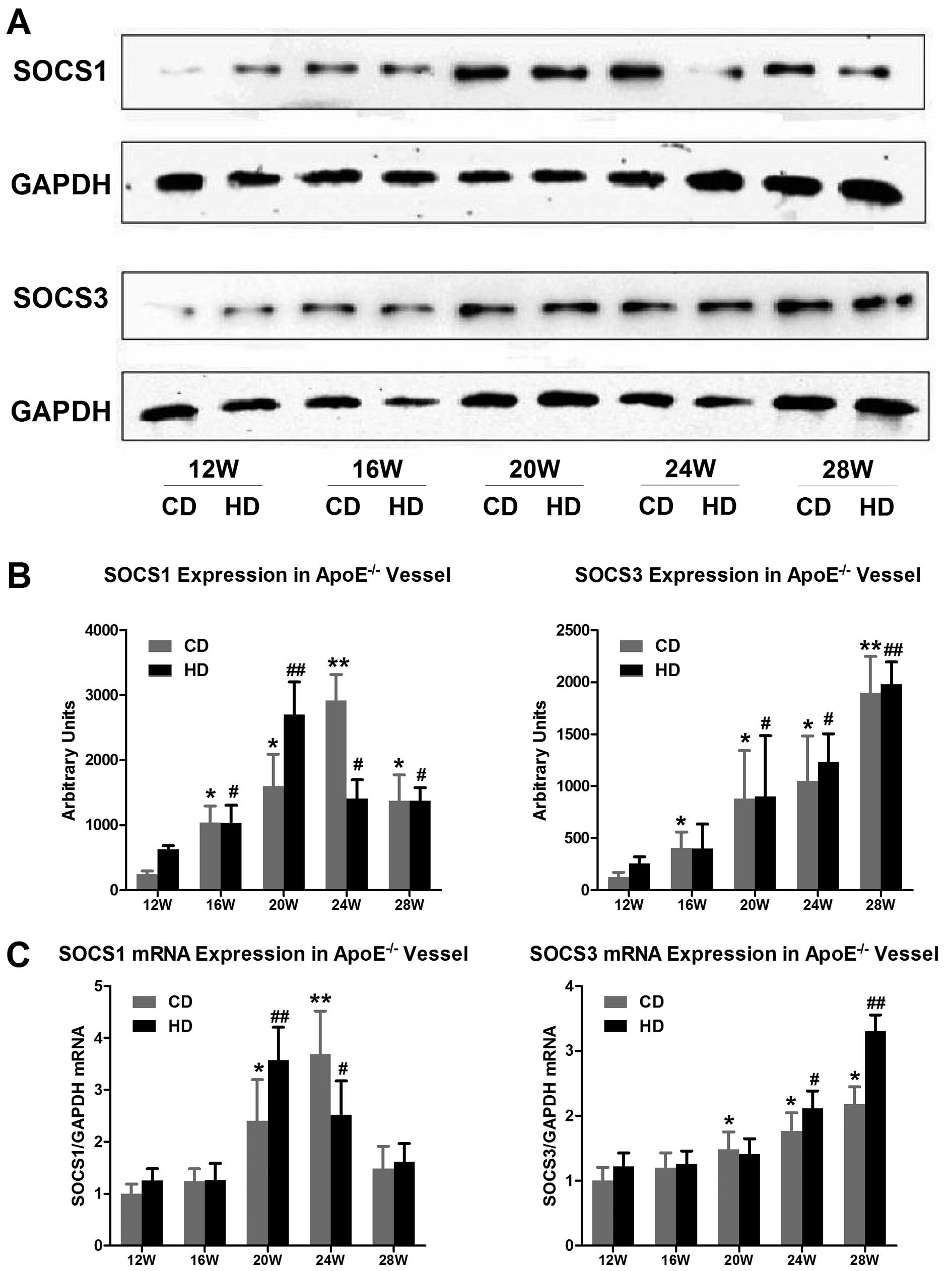
3A, B and E). Surprisingly, the results of the real-time PCR
and western blot analyses after the extraction of RNA and protein
levels showed a similar trend in the expression of SOCS1 in the
animals fed the different diets. In the group fed the chow diet,
the SOCS1 protein expression level was 11.85-fold higher and the
mRNA expression level was 3.68-fold higher at 24 weeks than at 12
weeks (P<0.01) (Fig. 4A and
B). In the group fed the high-fat diet, the SOCS1 protein
expression level was 4.32-fold higher and the mRNA expression level
was 2.02-fold higher at 20 weeks than at 12 weeks (P<0.01)
(Fig. 4C).
Upregulation of SOCS3 in the
ApoE−/− mice
SOCS1 and SOCS3 belong to the SOCS family of
proteins. To confirm whether they have the same expression tendency
in ApoE−/− mice, we also detected the SOCS3 mRNA and
protein level. Immunohistochemical staining showed that in the
groups fed the chow and high-fat diet, the SOCS3-positive areas
within the plaque of the ApoE−/− mice were enlarged with
the increased feeding duration. The results of the real-time PCR
and western blot analyses were consistent with the
immunohistochemical results. The expression of SOCS3 mRNA and
protein in the aorta of the ApoE−/− mice increased
significantly after 20 weeks, regardless of the type of diet, and
at 28 weeks, the expression of SOCS3 mRNA had significantly
increased (P<0.01) (Figs. 3C, D
and F and Fig. 4). At the
same time point, the SOCS3 expression level was higher in the group
fed the high-fat diet than in the group fed the chow diet. The
differential expression of SOCS1 and SOCS3 suggests that they play
different roles in atherosclerosis.
Expression levels of SOCS1 and SOCS3 in
the aorta of C57Bl/6j mice
ApoE−/− mice are used in classic animal
models of atherosclerosis, due to hyperlipidemia. We also used a
wild-type control to identify the correlation between blood lipids
and inflammatory cytokines. No formation of plaque was found in the
aortic root in the C57Bl/6j mice of different ages in the group fed
the chow diet (12, 20 and 28 weeks), and Oil Red O staining
displayed no lipid deposition (data not shown). Immunohistochemical
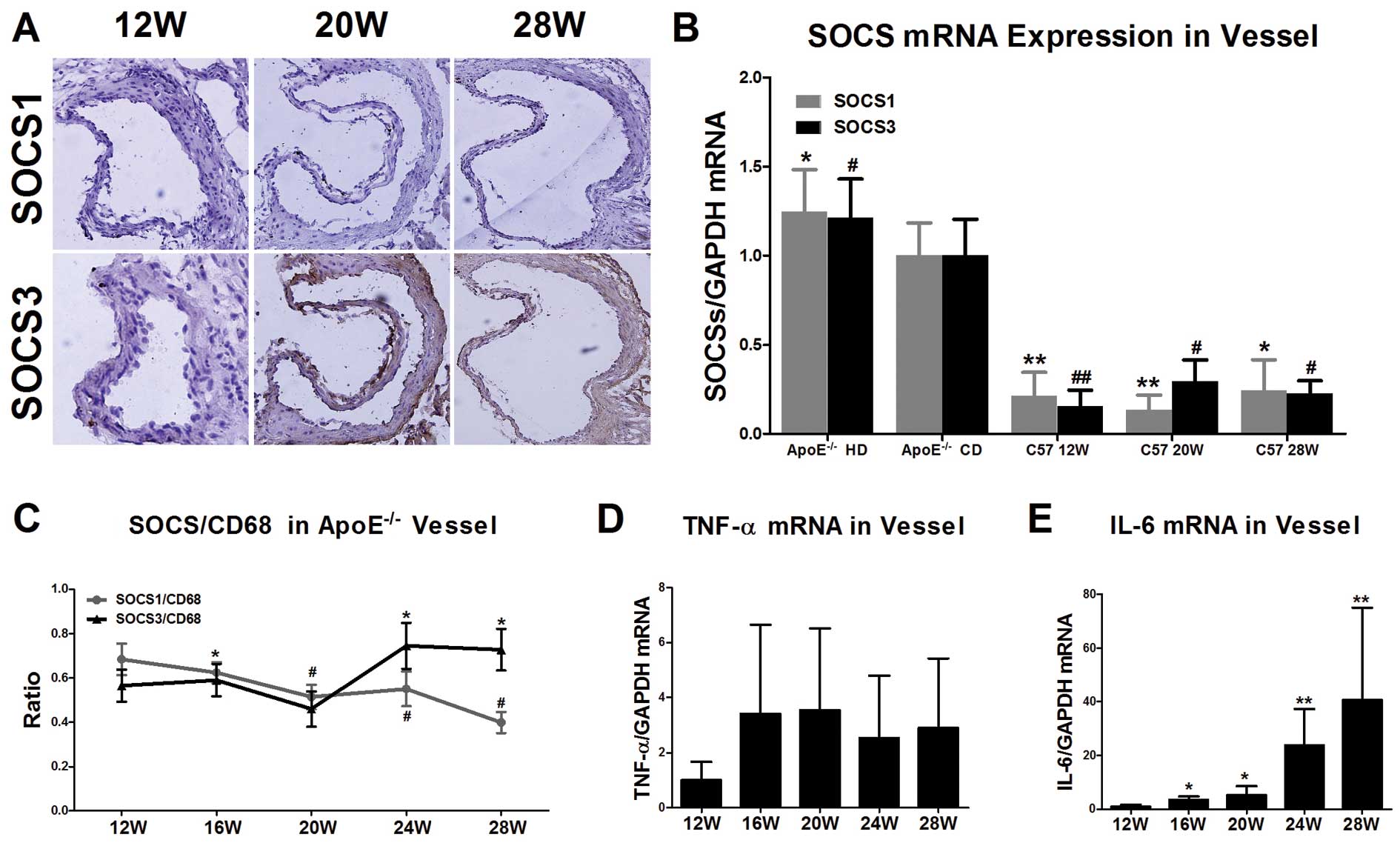
staining showed that the expression of SOCS1 and SOCS3 at the
aortic root of C57Bl/6j mice of different ages was very limited
(Fig. 5A). Real-time PCR
conducted with RNA extracted from the aorta of C57Bl/6j mice
revealed that the SOCS1 and SOCS3 expression level was 22% and 9%
in the ApoE−/− mice in the group fed the chow diet at 12
weeks, respectively (P<0.01) (Fig.
5B).
SOCS1/CD68 and SOCS3/CD68 show opposite
trends in expression with the increasing feeding duration of the
high-fat diet
As SOCS proteins are expressed in several cell types
but mainly in macrophages, which induce the majority of
inflammatory cytokines in disease, we detected the ratio of SOCS1/3
and macrophages (CD68) to clarify the contribution of macrophages
in disease progression. According to the immunohistochemical
staining results of the ApoE−/− mice fed the high-fat
diet, the CD68-positive area in the aortic root plaque increased in
size as the feeding period increased, and the ratio of the
SOCS1/CD68 stained areas decreased gradually over time (from
0.68±0.32 at 12 weeks to 0.39±0.21 at 28 weeks, P<0.05). The
ratio of the stained area of SOCS3/CD68 showed an inverse trend as
compared to SOCS1 (0.56±0.32 at 12 weeks to 0.73±0.42 at 28 weeks,
P<0.05) (Fig. 5C). These
results suggest that SOCS1 and SOCS3 play different roles in
disease progression.
Expression of inflammatory cytokines in
vascular tissue of ApoE−/− mice
It is well known that SOCS protein expression
correlates with the expression of inflammatory cytokines. To
confirm whether a correlation exists between SOCS3 and IL-6
expression in atherosclerosis, as shown in other diseases, we
detected the expression levels of a number of inflammatory
cytokines. As the high-fat diet feeding duration increased, the
expression levels of IL-6 mRNA in the aorta of the
ApoE−/− mice increased continuously (the expression
levels at 28 weeks were 40.64-fold higher than those at 12 weeks),
whereas no change in the mRNA expression of TNF-α was observed
(Fig. 5D and E).
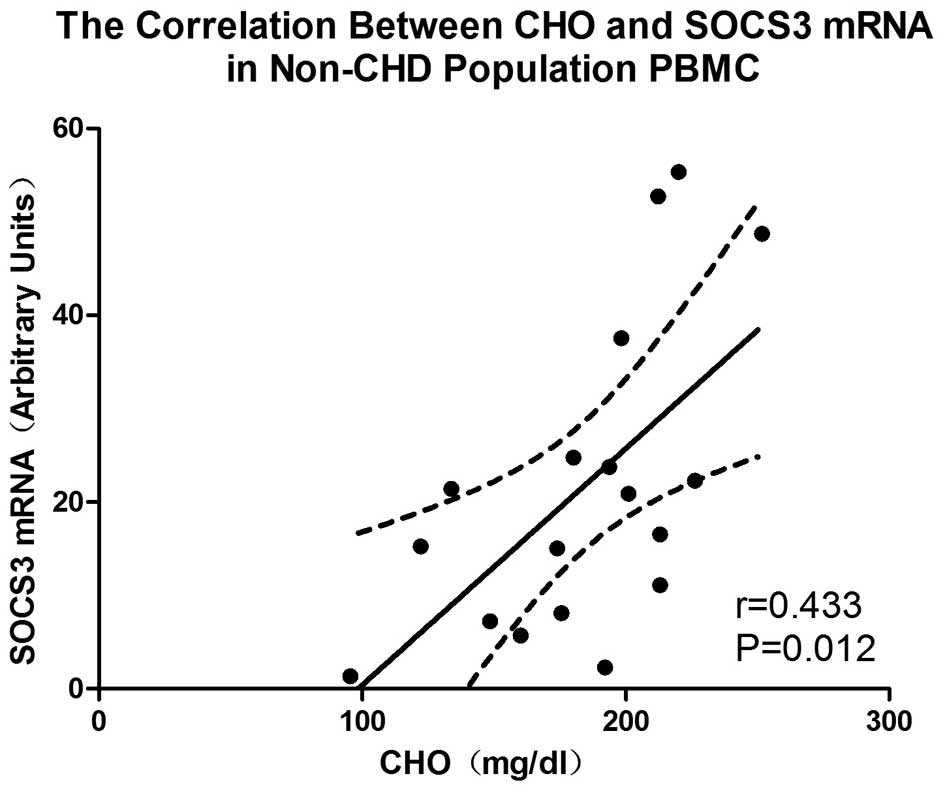
Positive correlation between total serum
cholesterol levels and SOCS3 mRNA expression in PBMCs of the
non-CHD population
To confirm our above results, we also investigated
the correlation between SOCS expression and cholesterol levels in
humans. In the PBMCs of the non-CHD population (n=18, males, 35–45
years of age), a positive correlation was observed between the
total serum cholesterol levels and SOCS3 mRNA expression (r=0.433,
P=0.012) (Fig. 6). However, no
correlation was observed between the total serum cholesterol levels
and the expression of SOCS1 mRNA in the PBMCs (r=0.061, P=0.45).
Further analysis revealed no correlation between the expression of
SOCS1 and SOCS3 mRNA, age, body mass index (BMI) and other factors
(P>0.05) (data not shown).
Discussion
In the present study, we investigated SOCS1 and
SOCS3 expression in ApoE−/− mice exposed to high levels
of cholesterol for increasing periods of time. SOCS1 expression
showed a single peak change, whereas SOCS3 expression showed a
continuous increase. In the animals exposed to different levels of
cholesterol, high cholesterol levels increased SOCS1 and SOCS3
expression in ApoE−/− mice of the same age but had no
effect on the respective change oinf SOCS1 and SOCS3 expression
with the increased feeding duration. IL-6 expression in the aortas
of the ApoE−/− mice increased with the increase in SOCS3
expression. In addition, SOCS3 mRNA expression positively
correlated with cholesterol levels in the non-CHD population. In
conclusion, SOCS1 and SOCS3 expression differ in ApoE−/−
mice with the progression of the disease and SOCS3 may play a
pro-atherosclerotic role.
In this study, the trend in SOCS1 and SOCS3
expression, which was similar to that shown in the study by Tang
et al (30), demonstrated
that although these 2 proteins belong to the SOCS family, they play
opposing roles in the initiation and development of
atherosclerosis. According to previous studies, the existence of
the SH2 domain in the SOCS protein family may compete with JAK to
bind STAT, thus inhibiting the phosphorylation of STAT and
subsequent inflammatory response (31). As a result, studies on the SOCS
protein family have focused on inflammatory disease or a variety of
inflammatory pathways (32).
Previous studies have demonstrated a consistent view on the role of
SOCS1 (33), which has been shown
to inhibit the progression of inflammatory disease. However, the
role of SOCS3 remains controversial, as described in the
Introduction. The trend in the expression of SOCS1 which exerts an
anti-inflammation effect was consistent with that of
pathophysiological processes. Although the role of SOCS3 in
atherosclerosis remains controversial, the results of this study
revealed that SOCS3 may play the following roles in
atherosclerosis: i) SOCS3 may exert a pro-inflammatory effect,
which is consistent with the progression of the disease; its
expression increased with increasing cholesterol levels and the
prolonged feeding duration; ii) SOCS3 may play multiple roles in
atherosclerosis and other inflammatory diseases; in various types
of inflammatory diseases, the reflected net effect may differ or
involve a more complex mechanism of immune regulation; thus, its
role cannot simply be explained as a balance of anti-inflammation
and pro-inflammation; iii) SOCS3 is expressed during the late
stages of atherosclerotic lesions, indicating that it plays an
important role in the late stages of the disease and may play a
crucial role in maintaining plaque stability, which requires
further validation studies; and iv) SOCS3 expression may be
subjected to the regulation of other inflammatory factors during
the progression of atherosclerosis. When the
pro-inflammatory/anti-inflammatory balance tilted in favor of
pro-inflammation, the expression of SOCS3 also increased. In the
long process of the onset and development of human atherosclerosis,
immune-inflammatory mechanisms play an important role, and both
pro-inflammatory and anti-inflammatory factors are present in this
complex network (34). Overall,
SOCS proteins ultimately inhibit the progression of inflammation by
inhibiting the phosphorylation of STAT, and the JAK/STAT pathway
influences the progression of atherosclerosis. Based on the results
obtained in this study, we hypothesized that SOCS1 and SOCS3,
through changes in their expression, affect the downstream
signaling pathway, thus influencing the development of
atherosclerosis. Upon exposure to high cholesterol levels, these 2
factors show differential expression patterns, suggesting that
during the development of atherosclerosis, SOCS1 and SOCS3 may play
different roles in addition to the common mechanisms of SOCS
proteins (i.e., SOCS1 has a clear anti-inflammatory effect while
SOCS3 may play a pro-inflammatory role).
The SOCS protein family is mainly expressed in
activated macrophages (3), and
under the induction of high cholesterol, the number of macrophages
in the atherosclerotic plaque of ApoE−/− mice
continuously increased. Descriptive studies on cholesterol and SOCS
expression in disease are limited. During the prolonged high
cholesterol exposure in our study, the differential expression of
SOCS1 and SOCS3 was independent of the changes in the number of
macrophages. The expression of anti-inflammatory SOCS1 per
macrophage decreased with the increase in the exposure time to high
cholesterol levels, while an opposite trend was observed with
SOCS3. The difference in the expression trends of these 2 proteins
further suggests that the 2 proteins play different roles in the
occurrence and development of atherosclerosis. If these 2 factors
antagonize each other, then the expression of SOCS1, with
anti-inflammatory effects, would gradually decrease, while that of
SOCS3, which may have pro-inflammatory effects, would steadily
increase in line with the accelerated disease process. These
different trends were mainly observed in the differential
expression patterns of these 2 proteins in plaque.
In the C57Bl/6j mice of the control group, the
expression of SOCS1 and SOCS3 in the aorta remained low, and no
plaque was generated, regardless of the age of the mice. In the
ApoE−/− mice, even when those fed a normal diet, the
total serum cholesterol level was 10-fold higher than that in the
C57Bl/6j mice; when the ApoE−/− mice were fed the
high-fat diet, their plasma cholesterol level increased by
>29-fold compared to the C57Bl/6j mice. This indicates that high
cholesterol is not only an important factor leading to the
generation of atherosclerotic plaque, but also an important
condition that induces the expression of SOCS1 and SOCS3 proteins.
This also indicates that the changes in SOCS1 and SOCS3 expression
over time are related to the exposure to a high-fat diet as opposed
to age. Hypercholesterolemia plays a crucial role in the occurrence
and development of atherosclerosis, promotes subintimal lipid
deposition, and this metabolic disorder also contributes to
immune-inflammatory response changes in the body. The change in the
metabolic level, represented by high levels of cholesterol, affects
a variety of cytokines and chemotactic proteins that are involved
in the inflammatory response and alters the inflammatory network
equilibrium, which affects the speed of subendocardial lipid
deposition through the inflammatory cascade reaction. SOCS1 and
SOCS3 are one link in the aforementioned inflammatory network,
through which a high-fat diet can affect the progress of disease
development; therefore, it is important to clarify the role of SOCS
proteins in atherosclerosis. In addition, in non-CHD human
populations, we observed that SOCS3 mRNA expression in PBMCs
positively correlated with total serum cholesterol levels, which
further suggests that a high-fat diet is an important factor in the
induction of SOCS3 protein expression. With the increase in
cholesterol levels, the expression of SOCS3 also increased. In the
circulation, SOCS3 mRNA could only be observed in the PBMCs when
the cholesterol reached a certain level (~100 mg/dl). We can
therefore hypothesize that the SOCS3 protein, as a pro-inflammatory
cytokine, may mediate inflammatory activation in response to high
cholesterol levels; that is, high cholesterol plays a role in
pro-inflammation by stimulating SOCS3 expression. This theory has
never been mentioned in previous studies. There was no correlation
observed between SOCS1, cholesterol and TG in our human study
population, which was consistent with our findings in the animal
experiments; the effect of a high-fat diet on SOCS1 expression
cannot be summarized using a single linear correlation.
When analyzing mouse aortic RNA, we found that the
mRNA expression levels of IL-6 but not those of TNF-α significantly
increased when the mice were exposed to high cholesterol levels.
The expression of IL-6, a pro-inflammatory cytokine thought to be
most closely associated with chronic inflammation caused by
metabolic abnormalities, was increased continuously with prolonged
exposure to high cholesterol. This change in the expression levels
of this indicator suggests that as the effect of
hypercholesterolemia accumulated, vascular inflammation also
increased. Together with our observation of SOCS3, this suggests
that the SOCS3 protein in ApoE−/− mice with
atherosclerosis may be regulated by the induction of IL-6 in the
disease. Li et al (35)
found that free cholesterol-loaded mouse peritoneal macrophages are
an abundant resource of TNF-α and IL-6. In addition, Frisdal et
al (36) showed that lipid
loading in human macrophages was accompanied by a strong increase
of IL-6 secretion, followed by the activation of the JAK2/STAT3
signaling pathway by IL-6 to reduce lipid accumulation. Taking into
account our results, as well as those of the above studies, we
hypothesized that the high level of cholesterol increased IL-6
expression, subsequently activating JAK2/STAT3. In addition, SOCS3,
as a classic negative regulator of JAK2/STAT3, was upregulated with
the progression of inflammation aggravated by hyperlipidemia.
By examining the changes in aortic SOCS1 and SOCS3
expression in ApoE−/− mouse models exposed to high
cholesterol for different lengths of time, this study observed the
differential expression patterns of SOCS1 and SOCS3 in plaque.
These results suggest that SOCS1 and SOCS3 play different roles in
the development of atherosclerosis in ApoE−/− mice.
SOCS3 induced by IL-6 upregulated during hyperlipidemia, may
promote the development of atherosclerosis. It is of great
importance that we clarify the specific mechanisms of action of
SOCS proteins in atherosclerosis.
Acknowledgements
This study was supported by the Natural Science
Foundation of China (30570732 and 30871043 to Z.Y. and 30700320 to
Y.L.), the National Science Fund for Distinguished Young Scholars
(81025002 to Z.Y.).
References
|
1
|
Ross R: Atherosclerosis - an inflammatory
disease. N Engl J Med. 340:115–126. 1999. View Article : Google Scholar
|
|
2
|
Tedgui A and Mallat Z: Cytokines in
atherosclerosis: pathogenic and regulatory pathways. Physiol Rev.
86:515–581. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ortiz-Munoz G, Martin-Ventura JL,
Hernandez-Vargas P, et al: Suppressors of cytokine signaling
modulate JAK/STAT-mediated cell responses during atherosclerosis.
Arterioscler Thromb Vasc Biol. 29:525–531. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yan C, Cao J, Wu M, et al: Suppressor of
cytokine signaling 3 inhibits LPS-induced IL-6 expression in
osteoblasts by suppressing CCAAT/enhancer-binding protein {beta}
activity. J Biol Chem. 285:37227–37239. 2010.PubMed/NCBI
|
|
5
|
Albanesi C, Fairchild HR, Madonna S, et
al: IL-4 and IL-13 negatively regulate TNF-alpha- and
IFN-gamma-induced beta-defensin expression through STAT-6,
suppressor of cytokine signaling (SOCS)-1, and SOCS-3. J Immunol.
179:984–992. 2007. View Article : Google Scholar
|
|
6
|
Alexander WS and Hilton DJ: The role of
suppressors of cytokine signaling (SOCS) proteins in regulation of
the immune response. Annu Rev Immunol. 22:503–529. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yoshimura A, Naka T and Kubo M: SOCS
proteins, cytokine signalling and immune regulation. Nat Rev
Immunol. 7:454–465. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Davey GM, Heath WR and Starr R: SOCS1: a
potent and multifaceted regulator of cytokines and cell-mediated
inflammation. Tissue Antigens. 67:1–9. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Egan PJ, Lawlor KE, Alexander WS and Wicks
IP: Suppressor of cytokine signaling-1 regulates acute inflammatory
arthritis and T cell activation. J Clin Invest. 111:915–924. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Metcalf D, Di Rago L, Mifsud S, et al: The
development of fatal myocarditis and polymyositis in mice
heterozygous for IFN-gamma and lacking the SOCS-1 gene. Proc Natl
Acad Sci USA. 97:9174–9179. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ilangumaran S, Ramanathan S and Rottapel
R: Regulation of the immune system by SOCS family adaptor proteins.
Semin Immunol. 16:351–365. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jo D, Liu D, Yao S, et al: Intracellular
protein therapy with SOCS3 inhibits inflammation and apoptosis. Nat
Med. 11:892–898. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li Y, Chu N, Rostami A and Zhang GX:
Dendritic cells transduced with SOCS-3 exhibit a tolerogenic/DC2
phenotype that directs type 2 Th cell differentiation in vitro and
in vivo. J Immunol. 177:1679–1688. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Matsumura Y, Kobayashi T, Ichiyama K, et
al: Selective expansion of foxp3-positive regulatory T cells and
immunosuppression by suppressors of cytokine signaling 3-deficient
dendritic cells. J Immunol. 179:2170–2179. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu Y, Stewart KN, Bishop E, et al: Unique
expression of suppressor of cytokine signaling 3 is essential for
classical macrophage activation in rodents in vitro and in vivo. J
Immunol. 180:6270–6278. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
O’Keefe GM, Nguyen VT, Ping Tang LL and
Benveniste EN: IFN-gamma regulation of class II transactivator
promoter IV in macrophages and microglia: involvement of the
suppressors of cytokine signaling-1 protein. J Immunol.
166:2260–2269. 2001.
|
|
17
|
Croker BA, Krebs DL, Zhang JG, et al:
SOCS3 negatively regulates IL-6 signaling in vivo. Nat Immunol.
4:540–545. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yoshimura A, Suzuki M, Sakaguchi R, et al:
SOCS, inflammation, and autoimmunity. Front Immunol. 3:202012.
View Article : Google Scholar
|
|
19
|
Wormald S and Hilton DJ: Inhibitors of
cytokine signal transduction. J Biol Chem. 279:821–824. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Brown MS and Goldstein JL: Lipoprotein
metabolism in the macrophage: implications for cholesterol
deposition in atherosclerosis. Annu Rev Biochem. 52:223–261. 1983.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gower RM, Wu H, Foster GA, et al:
CD11c/CD18 expression is upregulated on blood monocytes during
hypertriglyceridemia and enhances adhesion to vascular cell
adhesion molecule-1. Arterioscler Thromb Vasc Biol. 31:160–166.
2011. View Article : Google Scholar
|
|
22
|
Gokalp D, Tuzcu A, Bahceci M, et al:
Levels of proinflammatory cytokines and hs-CRP in patients with
homozygous familial hypercholesterolaemia. Acta Cardiol.
64:603–609. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sima AV, Stancu CS and Simionescu M:
Vascular endothelium in atherosclerosis. Cell Tissue Res.
335:191–203. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Steinberg D: Atherogenesis in perspective:
hypercholesterolemia and inflammation as partners in crime. Nat
Med. 8:1211–1217. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tian Y, Yuan Z, Liu Y, et al: Pioglitazone
modulates the balance of effector and regulatory T cells in
apolipoprotein E deficient mice. Nutr Metab Cardiovasc Dis.
21:25–32. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Anderson JL, Adams CD, Antman EM, et al:
ACC/AHA 2007 guidelines for the management of patients with
unstable angina/non-ST-Elevation myocardial infarction: a report of
the American College of Cardiology/American Heart Association Task
Force on Practice Guidelines (Writing Committee to Revise the 2002
Guidelines for the Management of Patients With Unstable
Angina/Non-ST-Elevation Myocardial Infarction) developed in
collaboration with the American College of Emergency Physicians,
the Society for Cardiovascular Angiography and Interventions, and
the Society of Thoracic Surgeons endorsed by the American
Association of Cardiovascular and Pulmonary Rehabilitation and the
Society for Academic Emergency Medicine. J Am Coll Cardiol.
50:e1–e157. 2007.
|
|
27
|
Zhao Y, Yuan Z, Liu Y, et al: Activation
of cannabinoid CB2 receptor ameliorates atherosclerosis associated
with suppression of adhesion molecules. J Cardiovasc Pharmacol.
55:292–298. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xue JH, Yuan Z, Wu Y, et al: High glucose
promotes intracellular lipid accumulation in vascular smooth muscle
cells by impairing cholesterol influx and efflux balance.
Cardiovasc Res. 86:141–150. 2010. View Article : Google Scholar
|
|
29
|
He L, He M, Lv X, et al: NF-kappaB binding
activity and pro-inflammatory cytokines expression correlate with
body mass index but not glycosylated hemoglobin in Chinese
population. Diabetes Res Clin Pract. 90:73–80. 2010. View Article : Google Scholar
|
|
30
|
Tang J, Kozaki K, Farr AG, et al: The
absence of platelet-derived growth factor-B in circulating cells
promotes immune and inflammatory responses in atherosclerosis-prone
ApoE−/− mice. Am J Pathol. 167:901–912. 2005. View Article : Google Scholar
|
|
31
|
Endo TA, Masuhara M, Yokouchi M, et al: A
new protein containing an SH2 domain that inhibits JAK kinases.
Nature. 387:921–924. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Park SJ, Oh EJ, Yoo MA and Lee SH:
Involvement of DNA-dependent protein kinase in regulation of
stress-induced JNK activation. DNA Cell Biol. 20:637–645. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kinjyo I, Hanada T, Inagaki-Ohara K, et
al: SOCS1/JAB is a negative regulator of LPS-induced macrophage
activation. Immunity. 17:583–591. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Libby P, Ridker PM and Hansson GK:
Inflammation in atherosclerosis: from pathophysiology to practice.
J Am Coll Cardiol. 54:2129–2138. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li Y, Schwabe RF, DeVries-Seimon T, et al:
Free cholesterol-loaded macrophages are an abundant source of tumor
necrosis factor-alpha and interleukin-6: model of NF-kappaB- and
map kinase-dependent inflammation in advanced atherosclerosis. J
Biol Chem. 280:21763–21772. 2005.
|
|
36
|
Frisdal E, Lesnik P, Olivier M, et al:
Interleukin-6 protects human macrophages from cellular cholesterol
accumulation and attenuates the proinflammatory response. J Biol
Chem. 286:30926–30936. 2011. View Article : Google Scholar : PubMed/NCBI
|