Introduction
Lymphangiogenesis is the process by which new
lymphatic vessels form from pre-existing lymphatic vessels or
lymphatic endothelial progenitor cells (1). The process is important in the
normal development of the lymphatic system and is also implicated
in pathological processes such as inflammation, lymphedema and
importantly cancer metastasis. The lymphatic vasculature serves as
a major route for the dissemination of malignant cells from solid
tumours, particularly to regional lymph nodes (2). The extent of lymph node metastasis
is a major determinant of both staging and prognosis in breast
cancer.
The recent discovery of a number of molecular and
cellular markers thought to be specific to lymphatic endothelial
cells has enabled more detailed research into lymphangiogenesis.
The most important lymphangiogenic markers include podoplanin (a
glomerular podocyte membrane mucoprotein) (3), Prox-1 (a homeobox gene involved in
embryonic development of the lymphatic system) (4) and LYVE-1 (a marker shown to be found
exclusively on lymphatic vessels in normal tissues and tumours)
(5). Several studies have
reported that high expression levels of lymphangiogenic markers
(particularly podoplanin and LYVE-1) in breast tumours are
correlated with increased lymphatic vessel density, increased
likelihood of tumour metastasis to regional lymph nodes and
therefore a poor prognosis (6–9).
The process of lymphangiogenesis is thought to be
regulated by the interaction of vascular endothelial growth factor
(VEGF)-C or VEGF-D with the cell surface receptor VEGFR-3 (2). Research suggests that VEGF-C
expression in breast cancer may correlate with increased lymph node
metastases (10) and
significantly poorer disease-free survival (assessed at five years)
(11). In contrast, other studies
have indicated that a high VEGF-C to VEGF-D ratio in breast tumours
may be a more accurate predictor of increased likelihood of lymph
node metastasis (12–14). High expression levels of
lymphangiogenic factors or markers may be important in indicating
an increased potential for lymphangiogenesis and cancer
metastasis.
Interleukin (IL)-24 is a member of the IL-10
cytokine family. The secreted protein is 206 amino acids in length
and has a predicted size of 35–40 kDa (15). Previous studies suggest that IL-24
operates through interaction with IL-20R1/IL-20R2 or
IL-22R1/IL-20R2 heterodimers (15,16). IL-24 was originally detected in
human melanoma cells. However, it appears to be lowly expressed in
cells of the immune system, including spleen cells, thymus cells
and normal melanocytes (16).
IL-24 is thought to possess antitumour
characteristics. By suppressing signals generated by tumour cells
and through interference with tumour vasculature, IL-24 may act to
inhibit tumour growth and metastasis (17). Forced expression of IL-24 in
breast cancer cells was found to cause potent growth suppression
(18). Results from in
vitro assays suggest that IL-24 significantly reduces migration
and motility of breast cancer cells (19).
Immunohistochemical staining has demonstrated that
normal breast tissue contains substantially higher levels of IL-24
compared to malignant breast tissue. Lower transcript levels of
IL-24 were found to be correlated with node-positive breast
tumours, increased likelihood of distant metastasis, a poor
prognosis and shorter disease-free survival. Patients with higher
levels of IL-24 were more likely to have a better prognosis and
remain alive and disease-free (19). The correlation between loss of
IL-24 expression and increased tumour invasion may indicate that
IL-24 has an important role in tumour suppression. However, how
IL-24 is involved in lymphatic metastasis remains unclear.
The aim of the present study was to assess whether
there is an association between levels of IL-24, IL-22R and the
expression of lymphangiogenic factors and markers in breast cancer
tissue samples obtained from a cohort of 127 women. In vitro
studies were then carried out to investigate how IL-24 alters the
expression of lymphangiogenic factors and markers. The effect of
IL-24 on specific functions (growth and microtubule formation) of
endothelial cells was also examined.
Materials and methods
Cohort of tissue samples
Breast cancer tissue samples (n=127) were collected
from patients at the University Hospital of Wales, Cardiff. Ethical
approval and informed consent was obtained. RNA extraction of the
samples was performed using an RNA extraction kit (AbGene Ltd.,
Surrey, UK) along with reverse transcription. Pairs of PCR primers
were designed using the Beacon Designer™ software and synthesised
by Sigma-Aldrich. IcyclerIQ™ (Bio-Rad, Hemel Hempstead, UK) was
used to carry out real-time quantitative PCR [based on previously
described methods (19,20)] to detect transcript levels of
IL-24, IL-22R and lymphangiogenic factors and markers in the breast
cancer samples. The Amplifluor system (Intergen Inc., New York, NY,
USA) was utilized under the following conditions: an initial period
of 15 min at 95°C followed by 60 cycles of 95°C for 15 sec, 55°C
for 60 sec and 72°C for 20 sec together with QPCR Master Mix
(ABgene, Surrey, UK).
Materials and cell lines
HECV cells purchased from Interlab (Milan, Italy)
were maintained in Dulbecco’s modified Eagle’s medium (DMEM)
(Sigma-Aldrich, Poole, UK) supplemented with benzylpenicillin,
amphotericin B, streptomycin and 10% fetal bovine serum
(Sigma-Aldrich). The cells were incubated at 37°C in 5%
CO2 and 95% humidity until confluent. Matrigel
(reconstituted basement membrane) was purchased from Collaborative
Research Products (Bedford, MA, USA).
Treatment of cells with IL-24 and
conventional and quantitative polymerase chain reaction
Six 12.5-cm2 tissue culture flasks were
seeded with 1×106 HECV cells and incubated at 37°C for
24 h. Cells were treated with 25 ng/ml human recombinant IL-24
(R&D Systems Europe) and incubated at 37°C for 1, 2, 4 and 24
h. Total RNA reagent (TRI) was used to extract the RNA from
IL-24-treated cells using the provided protocol (Sigma-Aldrich).
RNA was quantified using a spectrophotometer (WPA UV 1101; Biotech
Photometer, Cambridge, UK) and standardised to a concentration of
500 ng. Reverse transcription was performed using the iScript cDNA
synthesis kit (PrimerDesign Ltd., Southampton, UK).
Conventional PCR was carried out in a T-Cy
Thermocycler (Creacon Technologies Ltd., The Netherlands) using
REDTaq® ReadyMix™ PCR reaction mix (Sigma-Aldrich), cDNA
from cells and the following primers: GAPDH (which served as a
control), VEGF-C and VEGF-D (Table
I). The reaction conditions used were: 5 min at 94°C, 40 sec at
94°C, 40 sec at 55°C, 1 min at 72°C for 35 cycles and 72°C for 10
min. PCR products were then stained with SYBR Safe™ (Invitrogen)
separated on a 1% agarose gel, visualised under UV light and
photographed. ImageJ software was used to semi-quantify the
results.
 | Table IPrimer sequences. |
Table I
Primer sequences.
| Method | Name | Sequences |
|---|
| PCR | VEGF-C F9 |
TTTGCCAATCACACTTCCTG |
| VEGF-C R9 |
CAGGCACATTTTCCAGGATT |
| VEGF-D F9 |
CAGGGCTGCTTCTAGTTTGG |
| VEGF-D R9 |
TTCTTCAGGGATCTGGATGG |
| GAPDH F8 |
ATGATATCGCCGCGCTCGTC |
| GAPDH R8 |
GCTCGGTCAGGATCTTCA |
| QPCR | VEGF-C F12 |
CTACAGATGTGGGGGTTGCT |
| VEGF-C ZR12 |
ACTGAACCTGACCGTACAGTAGCTCGTGCTGGTGTTCA |
| VEGF-D F12 |
TCCACATTGGAACGATCTGA |
| VEGF-D ZR12 |
ACTGAACCTGACCGTACACTCCACAGCTTCCAGTCCTC |
| Prox-1 F11 |
AGAGCAGGAAATGGCTGAAA |
| Prox-1 ZR11 |
ACTGAACCTGACCGTACATCCGGTTGTAAGGAGTTTGG |
| GAPDH F |
CTGAGTACGTCGTGGAGTC |
| GAPDH ZR |
ACTGAACCTGACCGTACAGAGATGATGACCCTTTTG |
Real-time quantitative PCR was carried out using the
iCycler iQ™ Real-Time detection system (Bio-Rad) to determine
transcript levels of VEGF-C, VEGF-D and Prox-1 in IL-24-treated
HECV cells. QPCR was carried out in a 96-well plate using a
previously described method (21)
with 10 pmol sense primer, 1 pmol antisense Z primer (Table I) and 10 pmol FAM-probe, using a
custom Hot-Start QPCR master mix, under the following conditions:
95°C for 15 min, followed by 50 cycles at 95°C for 15 sec, 55°C for
4 sec and 72°C for 15 sec. The levels of transcripts were
calculated as lymphangiogenic factor or lymphangiogenic
marker/GAPDH ratio.
Western blotting
HECV cells (1×106) contained in 25
cm2 flasks were treated with IL-24 (25 ng/ml) for 0, 1,
2 or 4 h. HMCSF buffer containing 1% Triton X-100, 2 mM
CaCl2, 100 μg/ml phenylmethylsulfonyl fluoride, 1 mg/ml
leupeptin, 1 mg/ml aprotinin and 10 mM sodium orthovanadate was
used to detach and lyse the cells. Insoluble components were then
removed by rotating samples on a wheel for 1 h and centrifuging at
13,000 × g. The protein samples were blotted onto Hybond-C Extra
nitrocellulose membranes (Amersham Biosciences UK Ltd., Bucks, UK).
Protein expression of GAPDH and lymphangiogenic factors and markers
in the samples were assessed. Antibodies specific to GAPDH, VEGF-C,
LYVE-1 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) and
VEGF-D (R&D Systems Europe) were used. Visualisation of the
protein bands was carried out using SuperSignal West Dura Extended
Duration substrate chemi-luminescent system (Perbio Science UK
Ltd., Cramlington, UK) and images were captured using a UVIprochem
camera system (UVItec, Cambridge, UK).
The values shown graphically for QPCR, conventional
PCR with semi-quantitative analysis and western blotting are
expressed as the percentage of decrease in lymphangiogenic
transcript expression compared to control cells (the expression in
the cells not treated with IL-24 was regarded as baseline, i.e.
having 100% lymphangiogenic factor/marker expression). The change
in expression was shown as a percentage (%) in comparison with the
control.
In vitro microtubule formation assay
The processes used were modified from previously
published methods (22,23). Briefly, 96-well plates were coated
with 100 μl/well of Matrigel (diluted in a 1 to 1 ratio with
serum-free medium) and incubated for 60 min to allow the thin gel
layer to set. HECV cells (3×104/well) were seeded onto
the Matrigel layer. The cells were treated with IL-24 (2.5, 25 and
250 ng/ml) or a preparation of serum-free medium or normal medium
and incubated for 4–6 h to allow tubule formation to occur.
Subsequently, the microtubule lengths were visualized under bright
light microscopy and quantified (total perimeter length per field)
using ImageJ.
In vitro cell growth assay
The processes used were based on a previously
published method (24). HECV
cells (3,000/well) were plated into 96-well plates followed by
treatment with IL-24 (2.5, 25 and 250 ng/ml) and a period of
incubation (either overnight or 3 days). The cells were fixed in 4%
formaldehyde and stained with 0.5% crystal violet. After washing,
10% acetic acid was added, and the absorbance was determined using
a Bio-Tek ELx800 multiplate spectrophotometer (Bio-Tek Instruments
Inc., Winooski, VT, USA) at a wavelength of 540 nm. Absorbance was
used to represent the cell number.
Results
IL-24 and the expression of
lymphangiogenic factors/markers in breast cancer
Following quantitative determination of the
transcripts of IL-24, IL-22R, lymphangiogenic factors and markers
in the breast cancer tissues (12,19), correlations among these factors
were analysed.
Table II depicts
the results where IL-24 and IL-22R are found in the presence of
CK19 (a specific epithelial cell marker). Levels of IL-24 and
IL-22R were therefore normalised values representative of those
found within epithelial-derived malignant breast tissue. High
levels of IL-22R were associated with significantly high levels of
IL-24 expression (P<0.01). A significant correlation was evident
between increased expression of LYVE-1 and reduced expression of
IL-24 (correlation coefficient -0.288) (P<0.05) in the cohort of
breast cancer tissue samples. Similarly, the expression of another
lymphangiogenic marker, podoplanin, was also significantly
inversely correlated with the expression of IL-24 (P<0.01) and
IL-22R (P<0.05). Although the expression of other examined
lymphangiogenic markers and factors (Prox-1, VEGFR-3 and VEGF-C)
also appeared to increase in the tumours with lower expression of
IL-24, no statistically significant correlation was noted. VEGF-D,
however, a promoter of lymphangiogenesis tended to be positively
associated with the expression of IL-24 (P=0.03).
 | Table IICorrelations between IL-24/IL-22R and
lymphangiogenic factors/markers at their transcript levels in
breast cancer tissues. |
Table II
Correlations between IL-24/IL-22R and
lymphangiogenic factors/markers at their transcript levels in
breast cancer tissues.
| Transcript name | IL-24 | IL-22R |
|---|
| VEGF-C | −0.115
(P=0.358)/−0.047 (P=0.684) | 0.108 (P=0.384)/0.120
(P=0.293) |
| VEGF-D | 0.324 (P=0.03)/−0.068
(P=0.624) | 0.289 (P=0.054)/0.105
(P=0.449) |
| Podoplanin | −0.332
(P=0.001)/−0.183 (P=0.059) | −0.260
(P=0.011)/−0.094 (P=0.33) |
| Prox-1 | −0.199
(P=0.053)/−0.067 (P=0.486) | −0.057
(P=0.578)/0.102 (P=0.291) |
| VEGR3 | −0.156
(P=0.131)/−0.046 (P=0.637) | −0.134
(P=0.192)/0.025 (P=0.792) |
| LYVE-1 | −0.288
(P=0.017)/−0.161 (P=0.145) | −0.039
(P=0.749)/−0.042 (P=0.707) |
| IL-22R | 0.268 (P=0.008)/0.305
(P=0.001) | |
Both epithelial-derived malignant breast tissue and
surrounding stromal cells (including endothelial cells) are
important factors in lymphangiogenesis of tumours. Therefore levels
of IL-24 and IL-22R (non-normalised) in the cohort of breast cancer
tissue samples are also provided in Table II. The expression of all
lymphangiogenic factors (VEGF-C and VEGF-D) and lymphangiogenic
markers (podoplanin, Prox-1, VEGFR-3 and LYVE-1) was inversely
correlated with the expression of IL-24. In contrast VEGF-D, VEGF-C
and VEGFR-3 (promoters of lymphangiogenesis) were positively
associated with expression of IL-22R. None of the non-normalised
results was found to be significant.
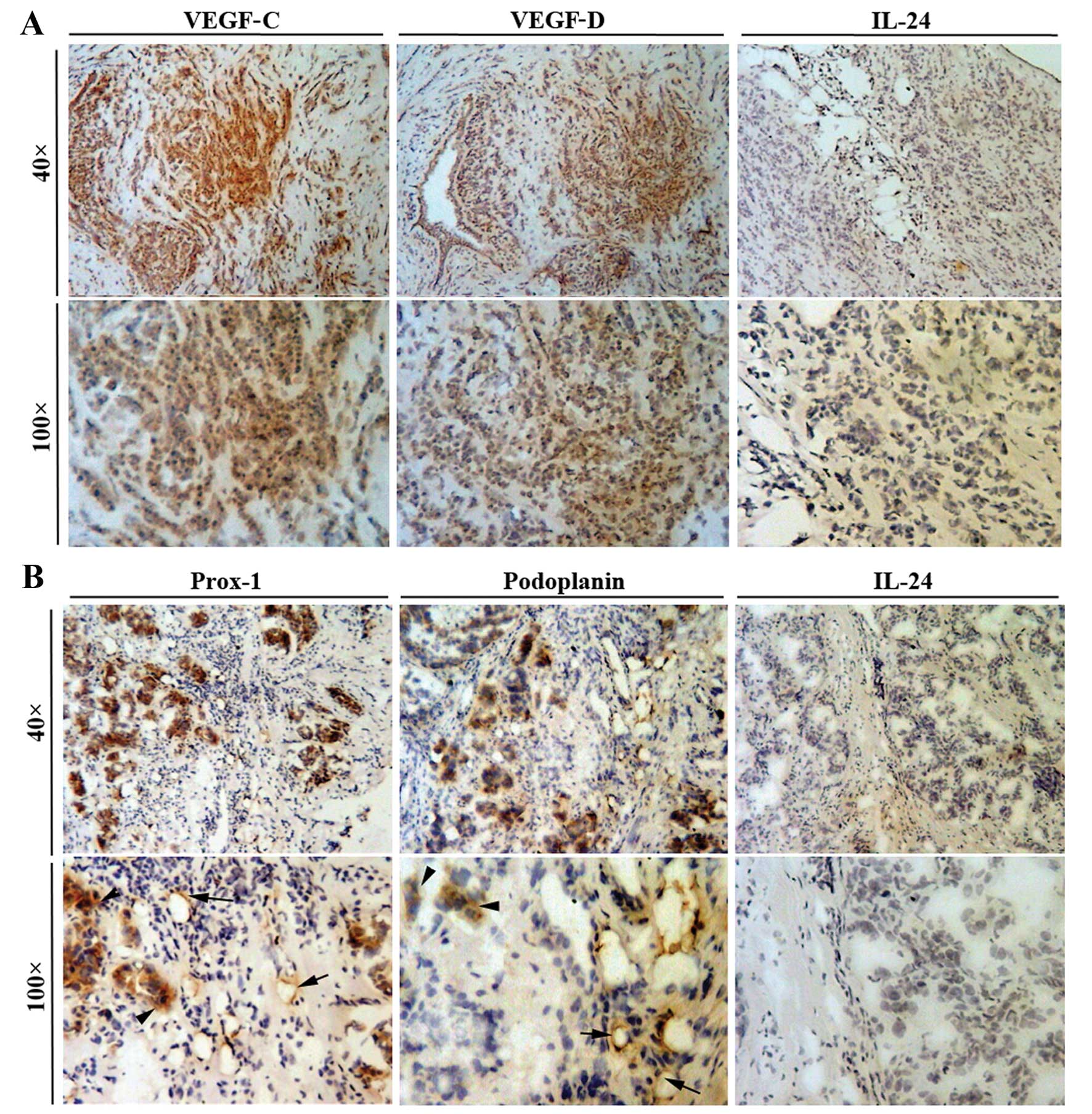
Breast cancer tissue samples were
immunohistochemically stained for levels of IL-24 and
lymphangiogenic factors (VEGF-C and VEGF-D), IL-24 and
lymphangiogenic markers (Prox-1 and podoplanin). A high degree of
VEGF-C and VEGF-D positivity was found to be present in breast
cancer cells (Fig. 1). In
comparison, endothelial cells contained within the tumour tissue
did not stain positively for the lymphangiogenic factors VEGF-C and
VEGF-D. Fig. 1 also shows that
both cancer cells and endothelial cells stained with a high degree
of positivity for the lymphangiogenic markers Prox-1 and
podoplanin. Breast cancer cells appeared to contain low levels of
IL-24 and therefore the staining for this cytokine was very weak or
absent from the cancer cells.
Regulation of lymphangiogenic
factors/markers in endothelial cells by IL-24
Transcript levels of lymphangiogenic factors and
markers in the IL-24-treated HECV cells were detected using
conventional PCR with semi-quantitative analysis and QPCR.
Conventional PCR with semi-quantitative analysis and QPCR
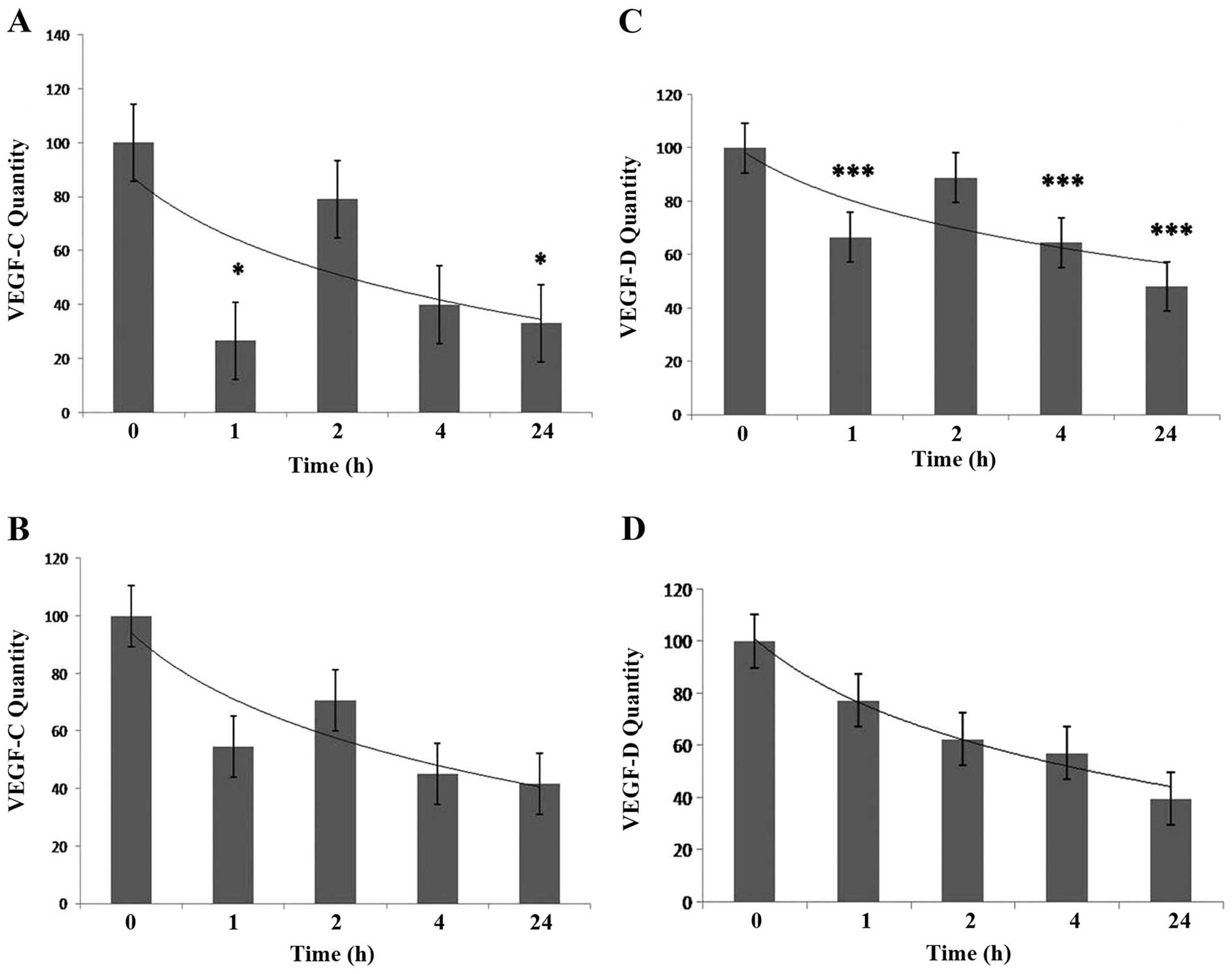
demonstrated that treatment of HECV endothelial cells with IL-24
(at a concentration of 25 ng/ml) resulted in time-dependent
downregulation of VEGF-C and VEGF-D over 24 h. Results obtained
using both methods showed the highest levels of mRNA expression of
VEGF-C and VEGF-D in the control cells (0 h). There was rapid and
significant downregulation of VEGF-C after 1 h as detected using
semi-quantitative PCR (P<0.05). This was followed by increased
expression of VEGF-C after 2 h to levels almost equivalent to those
detected in the control. A gradual decrease in expression of VEGF-C
was then observed after 4 and 24 h of treatment. At 24 h
significantly reduced levels of VEGF-C were detected using
semi-quantitative PCR compared to the control (P<0.05) (Fig. 2A). QPCR results showed the same
trend as those observed using semi-quantitative analysis. No QPCR
results, however, were found to be significant, (Fig. 3B). In a same way,
semi-quantitative analysis showed levels of expression of VEGF-D to
be significantly reduced after 1 h of treatment with IL-24
(P<0.001). Significant downregulation of VEGF-D (when compared
to control) was then observed at 2 h (P<0.001), 4 h (P<0.001)
and 24 h (P<0.001) (Fig. 2C).
Semi-quantitative analysis and QPCR results showed a trend of
gradually decreased expression of VEGF-D over the 24 h period
(Fig. 2C and D).
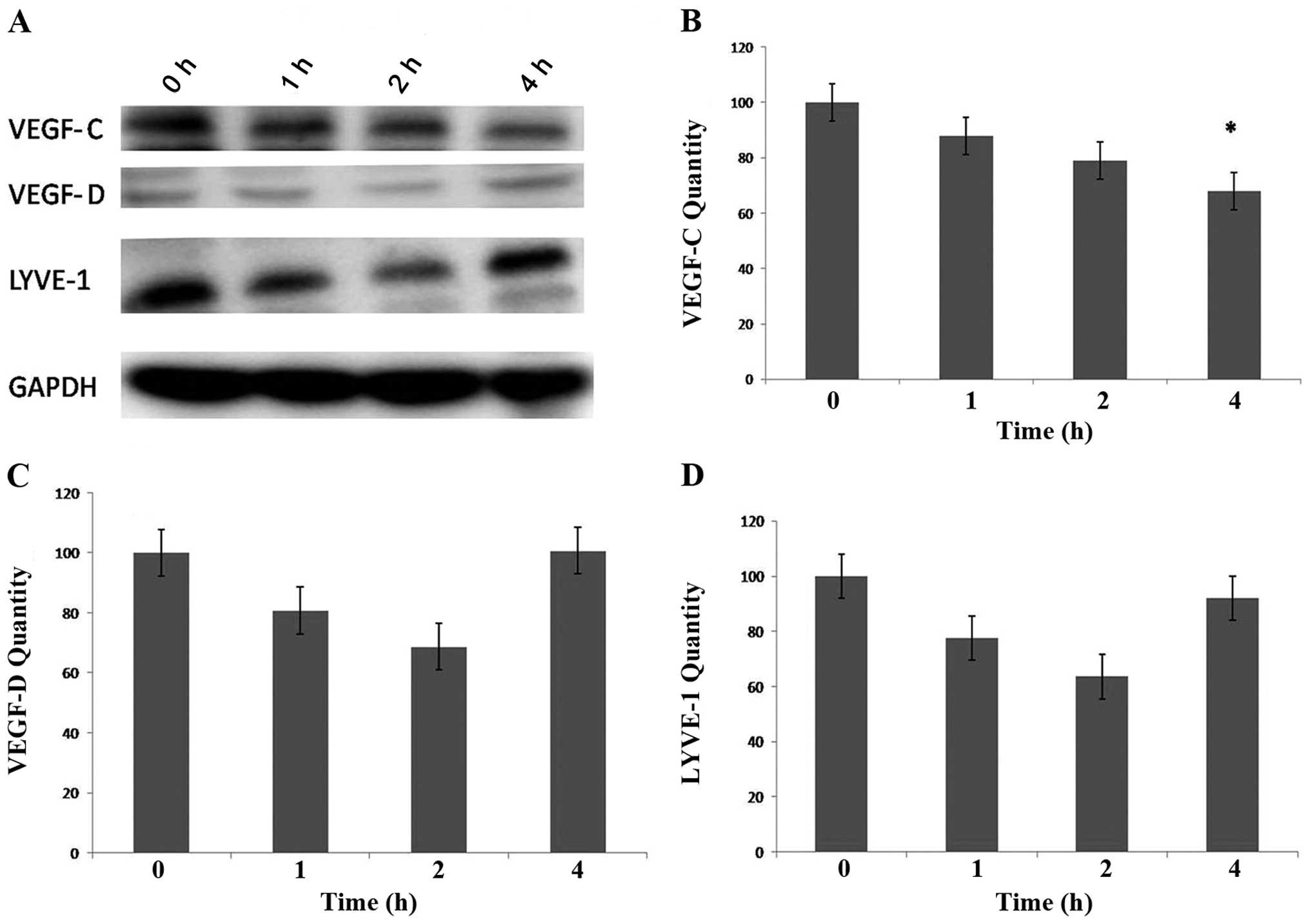
Protein expression of lymphangiogenic factors and
markers in the IL-24-treated HECV cells was detected using western
blot analysis. The results indicate a time-dependent downregulation
of VEGF-C protein expression by IL-24. Levels of VEGF-C protein
expression were highest at 0 h with decreased expression being
detected at 1 and 2 h. Significantly low levels of VEGF-C
expression (compared to control) were detected after 4 h of
treatment with IL-24 (P<0.05) (Fig. 3). In contrast, levels of VEGF-D
and LYVE-1 protein expression appeared to decrease in a
time-dependent manner (at 1 and 2 h). After 4 h, however, VEGF-D
and LYVE-1 expression increased to levels originally detected at
time 0 (Fig. 3). Further research
is required to elucidate whether downregulation of VEGF-D and
LYVE-1 protein expression occurs when HECV cells are treated with
IL-24 for a longer period of time (>4 h).
IL-24 affects in vitro tubule formation
and growth of endothelial cells
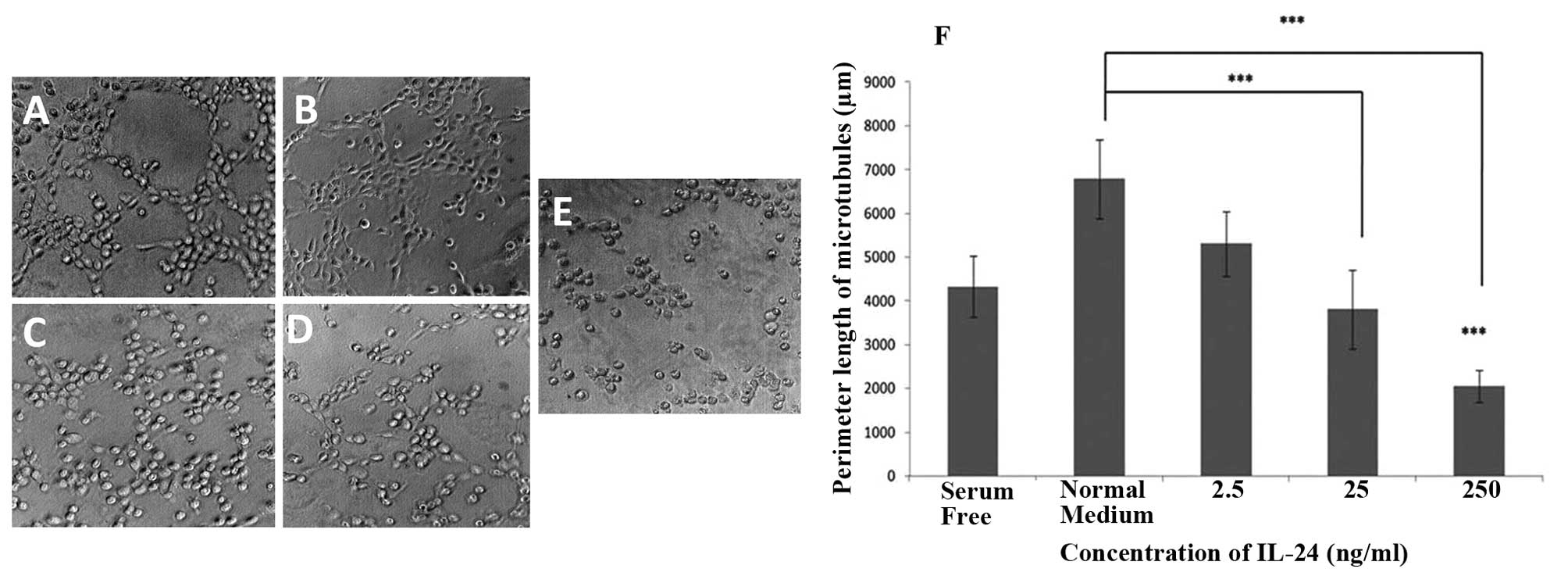
IL-24 exhibited a concentration-dependent inhibition
on microtubule formation of HECV endothelial cells (Fig. 4). HECV cells that were incubated
with normal medium showed the longest perimeter length of
microtubules. The most profound reduction in tubule formation was
observed in cells treated with the highest concentrations of IL-24
(25 and 250 ng/ml). In the presence of IL-24 at a concentration of
250 ng/ml, microtubule formation of HECV cells was significantly
impaired compared to microtubule formation observed when normal
medium was used (P<0.001) and when serum-free medium was used
(P<0.001). In the presence of IL-24 at a concentration of 25
ng/ml, microtubule formation of HECV cells was significantly
impaired compared to microtubule formation in the presence of
normal medium (P<0.001).
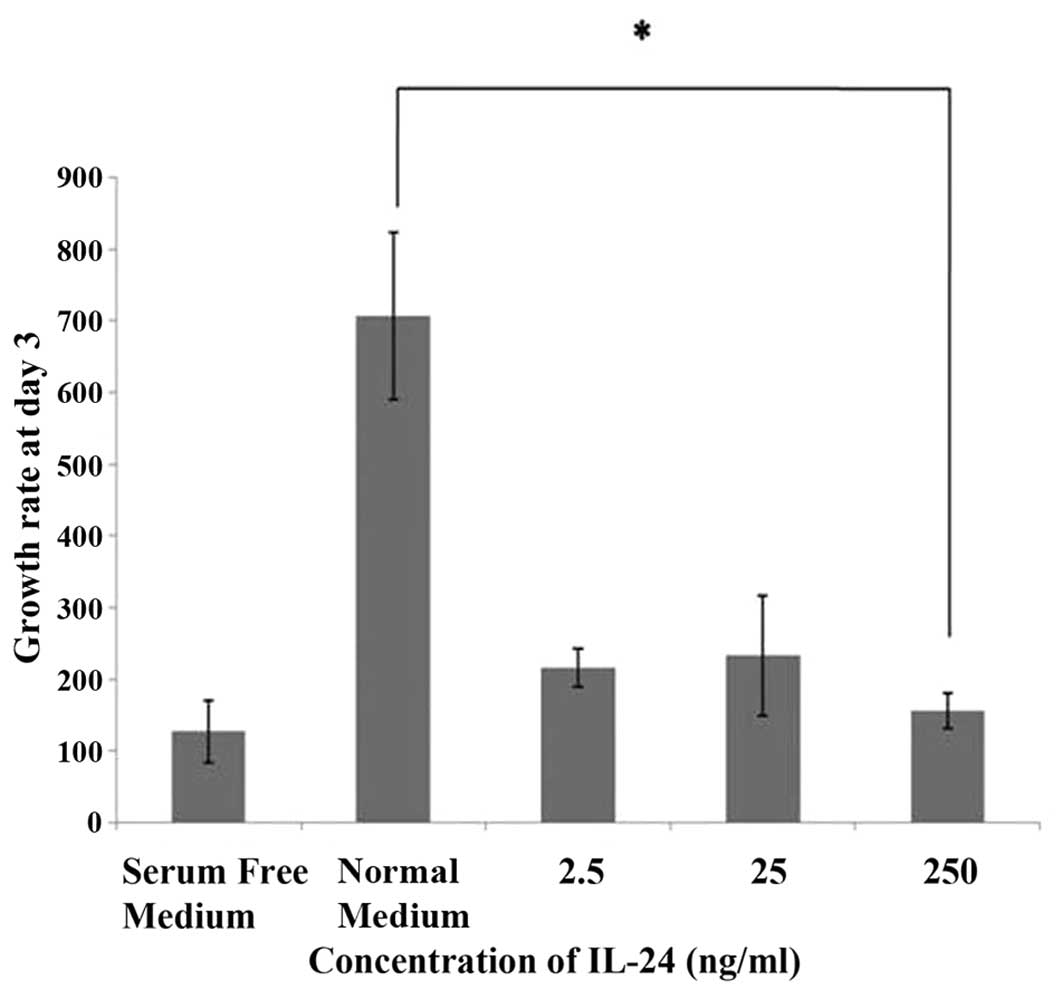
HECV cells incubated with normal medium showed
relatively high rates of growth between day 1 and day 3. In
comparison, HECV endothelial cells exposed to IL-24 at
concentrations of 2.5 and 25 ng/ml showed reduced growth. The
growth rate observed at day 3 was significantly reduced (P<0.05)
compared to the control when HECV endothelial cells were incubated
with a high concentration of IL-24 (250 ng/ml) (Fig. 5).
Discussion
Results obtained in previous studies suggest a link
between loss of IL-24 and increased likelihood of cancer invasion
and metastasis particularly in breast cancer (14). IL-24 exerts its effects via
IL-22R, a cell surface tyrosine kinase receptor. The present study
attempted to ascertain whether loss of IL-24 or IL-22R is
associated with increased levels of expression of lymphangiogenic
markers and factors in a large cohort of women with breast
cancer.
Low expression levels of IL-24 have been found to be
associated with significantly higher levels of expression of the
lymphangiogenic markers podoplanin (P<0.05) and LYVE-1
(P<0.05), and low levels of expression of IL-22R were associated
with significantly increased levels of expression of LYVE-1
(P<0.05). In addition, breast cancer tissue samples were
immunohistochemically stained for lymphangiogenic factors and
markers and IL-24. Within the tissue samples, cancer cells and
endothelial cells stained strongly for VEGF-C and VEGF-D, whereas
endothelial cells dislayed a strong positivity for Prox-1 and
podoplanin. The tissue samples that stained strongly for
lymphangiogenic factors and markers stained very weakly for IL-24.
High levels of expression of lymphangiogenic markers and factors
found within the tissue samples may indicate a high density of
lymphatic capillaries draining the tumour and this may be linked to
reduced levels of expression of IL-24. What remains unclear is
whether IL-24 has a role in inhibiting the process of
lymphangiogenesis of tumours thereby reducing the density of
lymphatic capillaries draining the primary cancer and preventing
the dissemination of malignant cells to regional lymph nodes.
A number of in vitro assays were carried out
to investigate the role of IL-24 in reducing lymphangiogenesis.
Results obtained during the present study suggest that IL-24 acts
in a time-dependent manner to reduce mRNA expression of
lymphangiogenic factors VEGF-C and VEGF-D and the lymphangiogenic
marker Prox-1. Within 24 h of treatment of cells with IL-24,
downregulation in the mRNA levels of lymphangiogenic factor and
marker were detected. Downregulation of protein expression of
VEGF-C was detected after cells were treated with IL-24 for 4 h. In
addition, IL-24 had a significant effect on microtubule formation
and growth of HECV cells.
The mechanism by which IL-24 exerts
anti-lymphangiogenic activity is yet to be fully elucidated.
Previous studies suggest that IL-24 operates through interaction
with IL-20R1/IL-20R2 or IL-22R1/IL-20R2 heterodimers (15,16). Receptor activation is associated
with activation of Janus activated kinase (JAK)/signal transducers
and activators of transcription (STAT) signalling. Ramesh et
al (17) report that
inhibition of microtubule formation is mediated by the interaction
of IL-24 with IL-22R1 resulting in the activation of STAT-3. STAT
proteins are important in cytokine signalling pathways. Al-Rawi and
Jiang (2) suggested that
stimulation of VEGFR-3 results in strong activation of STAT-3. It
is possible therefore that the regulation of VEGFR-3 signalling
(and lymphangiogenesis) can be controlled by other cytokines such
as IL-24, although this requires further investigation.
In conclusion, IL-24 significantly inhibits growth
and microtubule formation of endothelial cells in vitro and
downregulates the expression of some lymphangiogenic factors and
markers. By modifying the ability of endothelial cells to function
normally, IL-24 may act to impair lymphangiogenesis. In addition to
the reduced expression of IL-24 and IL-22R in breast cancer, this
is also correlated with increased expression of specific
lymphangiogenic factors and markers. This suggests that IL-24 plays
a role in vivo to suppress tumour lymphangiogenesis thereby
reducing the likelihood of cancer metastasis via the lymphatic
route. Further research is however required to fully elucidate the
mechanisms by which IL-24 inhibits lymphangiogenesis.
Acknowledgements
The authors wish to thank the Royal College of
Surgeons of England, the Breast Cancer Hope Foundation, the Albert
Hung Foundation and the Cancer Research Wales for supporting their
study.
References
|
1
|
Skobe M, Hawighorst T, Jackson DG, Prevo
R, Janes L, Velasco P, Riccardi L, Alitalo K, Claffey K and Detmar
M: Induction of tumour lymphangiogenesis by VEGF-C promotes breast
cancer metastasis. Nat Med. 7:192–198. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Al-Rawi MA and Jiang WG: Lymphangiogenesis
and cancer metastasis. Front Biosci. 16:723–739. 2011. View Article : Google Scholar
|
|
3
|
Weninger W, Partanen TA,
Breiteneder-Geleff S, Mayer C, Kowalski H, Mildner M, Pammer J,
Stürzl M, Kerjaschki D, Alitalo K and Tschachler E: Expression of
vascular endothelial growth factor receptor-3 and podoplanin
suggests a lymphatic endothelial cell origin of Kaposi’s sarcoma
tumor cells. Lab Invest. 79:243–251. 1999.PubMed/NCBI
|
|
4
|
Wigle JT and Oliver G: Prox1 function is
required for the development of the murine lymphatic system. Cell.
98:769–778. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mandriota SJ, Jussila L, Jeltsch M,
Compagni A, Baetens D, Prevo R, Banerji S, Huarte J, Montesano R,
Jackson DJ, Orc L, Alitalo K, Christofori G and Pepper MS: Vascular
endothelial growth factor-C-mediated lymphangiogenesis promotes
tumour metastasis. EMBO J. 20:672–682. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Choi WW, Lewis MM, Lawson D, Yin-Goen Q,
Birdsong GG, Cotsonis GA, Cohen C and Young AN: Angiogenic and
lymphangiogenic microvessel density in breast carcinoma:
correlation with clinicopathologic parameters and VEGF-family gene
expression. Mod Pathol. 18:143–152. 2005. View Article : Google Scholar
|
|
7
|
Lee SK, Cho EY, Kim WW, Kim S, Hur SM, Kim
S, Choe JH, Kim JH, Kim JS, Lee JE, Nam SJ and Yang JH: The
prediction of lymph node metastasis in ductal carcinoma in situ
with microinvasion by assessing lymphangiogenesis. J Surg Oncol.
102:225–229. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bono P, Wasenius VM, Heikkilä P, Lundin J,
Jackson DG and Joensuu H: High LYVE-1-positive lymphatic vessel
numbers are associated with poor outcome in breast cancer. Clin
Cancer Res. 10:7144–7149. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nakamura Y, Yasuoka H, Tsujimoto M, Imabun
S, Nakahara M, Nakao K, Nakamura M, Mori I and Kakudo K: Lymph
vessel density correlates with nodal status, VEGF-C expression, and
prognosis in breast cancer. Breast Cancer Res Treat. 91:125–132.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Stacker SA, Achen MG, Jussila L, Baldwin
ME and Alitalo K: Lymphangiogenesis and cancer metastasis. Nat Rev
Cancer. 2:573–583. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kinoshita J, Kitamura K, Kabashima A,
Saeki H, Tanaka S and Sugimachi K: Clinical significance of
vascular endothelial growth factor-C (VEGF-C) in breast cancer.
Breast Cancer Res Treat. 66:159–164. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cunnick GH, Jiang WG, Douglas-Jones T,
Watkins G, Gomez KF, Morgan MJ, Subramanian A, Mokbel K and Mansel
RE: Lymphangiogenesis and lymph node metastasis in breast cancer.
Mol Cancer. 7:232008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Weidner N: Tumour vascularity and
proliferation: clear evidence of a close relationship. J Pathol.
189:297–299. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Van den Eynden GG, Van der Auwera I, Van
Laere SJ, Huygelen V, Colpaert CG, van Dam P, Dirix LY, Vermeulen
PB and Van Marck EA: Induction of lymphangiogenesis in and around
axillary lymph node metastases of patients with breast cancer. Br J
Cancer. 95:1362–1366. 2006.PubMed/NCBI
|
|
15
|
Huang EY, Madireddi MT, Gopalkrishnan RV,
Leszczyniecka M, Su Z, Lebedeva IV, Kang D, Jiang H, Lin JJ,
Alexandre D, Chen Y, Vozhilla N, Mei MX, Christiansen KA, Sivo F,
Goldstein NI, Mhashikar AB, Chada S, Huberman E, Pestka S and
Fisher PB: Genomic structure, chromosomal localisation and
expression profile of a novel melanoma differentiation associated
(mda-7) gene with cancer specific growth suppressing and apoptosis
inducing properties. Oncogene. 20:7051–7063. 2001. View Article : Google Scholar
|
|
16
|
Dash R, Bhutia SK, Azab B, Su ZZ, Quinn
BA, Kegelmen TP, Das SK, Kim K, Lee SG, Park MA, Yacoub A, Rahmani
M, Emdad L, Dmitriev IP, Wang XY, Sarkar D, Grant S, Dent P, Curiel
DT and Fisher PB: mda-7/IL/24: a unique member of the IL-10 gene
family promoting cancer-targeted toxicity. Cytokine Growth Factor
Rev. 21:381–391. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ramesh R, Mhashilkar AM, Tanaka F, Saito
Y, Branch CD, Sieger K, Mumm JB, Stewart AL, Boquoi A, Dumoutier L,
Grimm EA, Renauld JC, Kotenko S and Chada S: Melanoma
differentiation-associated gene7/interleukin (IL)-24 is a novel
ligand that regulates angiogenesis via the IL-22 receptor. Cancer
Res. 63:5105–5113. 2003.PubMed/NCBI
|
|
18
|
Jiang H, Lin JJ, Su ZZ, Goldstein NI and
Fisher PB: Subtract ion hybridization identifies a novel melanoma
differentiation associated gene, mda-7, modulated during human
melanoma differentiation, growth and progression. Oncogene.
11:2477–2486. 1995.
|
|
19
|
Patani N, Douglas-Jones A, Mansel R, Jiang
W and Mokbel K: Tumour suppressor function of MDA-7/IL-24 in human
breast cancer. Cancer Cell Int. 10:292010.PubMed/NCBI
|
|
20
|
Jiang WG, Douglas-Jones A and Mansel RE:
Expression of peroxisome-proliferator activated receptor-gamma
(PPARgamma) and the PPARgamma co-activator, PGC-1, in human breast
cancer correlates with clinical outcomes. Int J Cancer.
106:752–757. 2003. View Article : Google Scholar
|
|
21
|
Al-Rawi MA, Watkins G, Mansel RE and Jiang
WG: The effects of interleukin-7 on the lymphangiogenic properties
of human endothelial cells. Int J Oncol. 27:721–730.
2005.PubMed/NCBI
|
|
22
|
Jiang WG, Hiscox SE, Parr C, Martin TA,
Matsumoto K, Nakamura T and Mansel RE: Antagonistic effect of NK4,
a novel hepatocyte growth factor variant, on in vitro angiogenesis
of human vascular endothelial cells. Clin Cancer Res. 5:3695–3703.
1999.
|
|
23
|
Sanders AJ, Ye L, Mason MD and Jiang WG:
The impact of EPLINα (epithelial protein lost in neoplasm) on
endothelial cells, angiogenesis and tumorigenesis. Angiogenesis.
13:317–326. 2010.
|
|
24
|
Jiang WG, Hiscox S, Hallett MB, Horrobin
DF, Scott C and Puntis MCA: Inhibition of hepatocyte growth
factor-induced motility and in vitro invasion of human colon cancer
cells by gamma-linolenic acid. Br J Cancer. 71:744–752. 1995.
View Article : Google Scholar : PubMed/NCBI
|