Introduction
Age-related macular degeneration (AMD) is a leading
cause of irreversible visual loss, affecting 5–10% of the
population over 60 years of age in Western countries (1). Drusen represent the earliest sign of
AMD and are also high risk factors for the development of AMD
complications (2). Amyloid-β
(Aβ), a known constituent of drusen, is a contributor to the
development of AMD (3–5). Accumulated evidence has confirmed
that the Aβ-mediated inflammatory response is associated with the
pathology of AMD (6–8).
The retina is an immune-privileged site due to local
tissue barrier (9). Yet, the
manner by which immune cells pass through this barrier and
accumulate in the retina remains unclear. Retinal pigment
epithelial (RPE) cells in the retina form a monolayer between
photoreceptors and choroidal vessels which is a barrier to maintain
the structural and functional integrity of the retina. The
blood-retinal barrier (BRB) is composed of the inner BRB (iBRB)
consisting of retinal microvascular endothelial cells and the outer
BRB (oBRB) consisting of RPE cells. Alterations in the BRB play a
crucial role in the development of AMD (10,11). The formation of the oBRB by RPE
cells is dependent on the structure of tight junction (TJ)
proteins, including the transmembrane protein occludin, claudins
and zonula occludens-1 (ZO-1). Zonula occludens interact with the
transmembrane proteins at the cytoplasmic face of the cell membrane
and serve to anchor them to the actin cytoskeleton (actin
filaments, F-actin). Abnormal expression or localization of
occludin, F-actin, and ZO-1 in RPE cells contributes to barrier
dysfunction (12,13). Matrix metalloproteinase (MMP)-2
and 9 were reported to cause barrier disruption by abnormal
degradation of TJ proteins (14,15).
A recent study reported that Aβ caused morphological
alterations and barrier dysfunction in ARPE-19, a spontaneously
transformed cell line (16). Yet,
the underlying mechanism is poorly understood. Therefore, we aimed
to ascertain whether Aβ-induced RPE barrier disruption is mediated
by MMPs. To note, the immortalized human retinal pigment epithelial
cell line ARPE-19 may not exhibit important properties similar to
the native tissue; the cell line is not ideal for studying outer
retinal barrier function because of its abnormal junctional
complexes and the lower transepithelial electrical resistance (TER)
(17). Therefore, isolated human
adult RPE cells were used, for the first time to the best of our
knowledge, to explore the effect of Aβ on epithelial barrier
integrity.
Although Aβ-induced disruption of the RPE structure
and breakdown of the barrier function were observed in our previous
study, little is known concerning the mechanism that contributes to
Aβ-induced RPE alterations. Since Aβ-induced chronic inflammation
is strongly linked to the pathology of AMD (7,8),
inflammatory cytokines are speculated to be one of the primary
causative events that contribute to Aβ-induced barrier dysfunction.
In addition to causing inflammation, some pro-inflammatory factors
such as IL-6 can alter tissue microenvironments by the modulation
of epithelial TJ proteins (18).
It is worth mentioning that MMPs have been reported to act broadly
in inflammation to regulate barrier function (19–21). Microarray analysis of the gene
expression profile of ARPE-19 cells in response to Aβ stimulation
suggests that IL-8 is significantly upregulated by Aβ (8). Higher intraocular concentrations of
IL-6, IL-8 and MMP-9 have been measured in AMD patients and are
significantly associated with the severity of the disease (22,23). Therefore, in the present study
expression levels of IL-6, IL-8, MMP-2 and MMP-9 were analyzed in
the Aβ-stimulated RPE cells. An increase in MMP activity has been
causally linked to epithelial barrier disruption and severe
symptoms of inflammatory diseases (24–26). To demonstrate the key role of MMPs
in modulating epithelial barrier structure and function, general
proteinase agonist 4-aminophenylmercuric acetate (APMA) was used to
induce MMP activation. For inhibitory studies, MMP-2 and MMP-9
activities were inhibited by RNA interference strategy or by the
MMP inhibitor GM6001.
Materials and methods
Aβ1–42 oligomerization and dot
blot assay
Lyophilized Aβ1–42 peptide
(Sigma-Aldrich) was dissolved to a concentration of 1.5 M in
hexafluoroisopropanol (HFIP) on ice and aliquoted at −20°C. Aβ
monomers were spin-vacuumed just prior to the experiment, diluted
to 250 μM in HFIP solution, and maintained at room temperature (RT)
for 3 days to synthesize Aβ oligomers. To note, In the present
study, references to Aβ or OAβ are to the oligomeric form of
Aβ1–42, unless otherwise stated.
Several lines of evidence have demonstrated that
soluble oligomers of Aβ may be better correlated with the severity
of the disease than are monomers or insoluble amyloid fibrils
(3,27). Therefore, a dot blot assay was
performed to determine the oligomeric form of Aβ1–42.
Five microliters of oligomeric Aβ1–42 solution and
monomers of Aβ1–42 were spotted onto a nitrocellulose
membrane. The membrane was then blocked with 5% dry milk in TTBS
(50 mM Tris, 0.05% Tween-20) for 1 h at RT, and washed three times
before being incubated for 1 h at RT with the A11 (27), an anti-oligomer antibody (1:1,000;
Invitrogen, Carlsbad, CA, USA).The membrane was then washed and
incubated for 1 h at RT with a HRP-conjugated secondary antibody
(1:1,000 goat anti-rabbit IgG; Santa Cruz Biotechnology, Inc. Santa
Cruz, CA, USA). The blots were washed three times in TTBS and
incubated with chemiluminescent reagent, and finally exposed to
ImageQuant LAS 4,000.
Ethics
Informed consent for tissue donation was obtained
from the relatives of the donors, and the protocol of the study was
approved by the local ethics committee and adhered to the tenets of
the Declaration of Helsinki for experiments involving human
tissue.
Isolation of human RPE cells
Five human donor eyes were obtained from the eye
bank of the Eye and ENT Hospital of Fudan University, Shanghai,
China. The donors ranged in age from 30 to 40 years. None of the
donors had a history of eye disease. In brief, whole eyes were
cleansed in 0.9% NaCl solution, immersed in 5% polyvinylpyrrolidone
iodine and rinsed again in NaCl solution. Then the anterior segment
from each eye was removed. The neural retina was peeled away from
the RPE-choroid-sclera. The eyecup was rinsed with Ca2+
and Mg2+-free Hank’s balanced salt solution and treated
with 0.25% trypsin for 1 h at 37°C. The trypsin was aspirated and
replaced with DMEM/F12 (HyClone) supplemented with 20% fetal calf
serum (FCS).
Cell viability assay
To measure cytotoxicity, haRPE cells seeded in a
96-well plate were treated with 0.3 μM of OAβ (Sigma-Aldrich,
China), 20 μg/ml of GM6001 (Calbiochem, Germany), 100 μM of APMA
(Sigma-Aldrich) for 24 h. Cell viability was measured by the
addition of
3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenol)-2-(4-sulphophenyl)-2H-tetrazolium
(MTS; Promega, USA) for 3 h at 37°C. The optical density was
measured spectrophotometrically at 490 nm on a microplate
reader.
Enzyme-linked immunosorbent assay
The protein concentrations of IL-8 and IL-6 were
determined using ELISA kits (R&D Systems, Minneapolis, MN, USA)
and were calculated based on standard curves. The optical density
was measured at 490 nm on a microplate reader.
Transfection of haRPE cells with small
interfering RNA (siRNA)
Human MMP-9-specific siRNAs were purchased from
Santa Cruz Biotechnology, Inc. For RNA interference experiments,
cells were plated at a density of 4×105 cells/well in a
6-well plate. After 24 h, cells were transfected with siRNA using
Lipofectamine 2000 (Invitrogen), according to the manufacturer’s
instructions.
Gelatin zymography
To examine whether Aβ induces MMP secretion, gelatin
zymography was carried out as previous described (19). Briefly, the supernatant was
collected after treatment and subjected to SDS-PAGE in 10%
polyacrylamide gels with 1 mg/ml gelatin. After electrophoresis,
gels were incubated in 2.5% Triton X-100 (1 h, 37°C) followed by
overnight incubation in 50 mM Tris-HCl (pH 7.8), 5 mM
CaCl2, 0.02% NaN3, 0.02% Brij gels, and were
stained with 2.5% Coomassie Blue R-250 (Bio-Rad) for 45 min
followed by destaining in deionized water with 10% acetic acid and
20% methanol. Gels were scanned and the density analyses of the
bands were performed using Photoshop CS4.0.
Cell morphology and immunofluorescence
staining
The barrier function of RPE cells is considered
highly dependent on the integrity of TJ proteins and F-actin. To
investigate the effects of OAβ, RPE cell morphology, structures of
F-actin, and location of TJ proteins were examined by light
microscopy and confocal microscopy, respectively. The haRPE cells
were treated with Aβ (0.3 μM) for 72 h. We hypothesized that
Aβ-induced MMP-9 secretion causes disruption of epithelial barrier
integrity. To test this hypothesis, cells were treated with APMA
(100 μM) for 6 h to induce MMP activation. For inhibitory studies,
MMP-9 activity was inhibited by MMP-9 siRNA or by the MMP inhibitor
GM6001. Then cells were fixed in 4% paraformaldehyde (PFA) for 30
min and blocked with 1% BSA in TBS for 1 h, then incubated with
rabbit anti-occludin antibody or mouse anti-ZO-1 antibody (Abcam,
Hong Kong) for 1 h. After washing, they were incubated with
AlexaFluor 488-conjugated secondary antibody (1:500; Invitrogen)
and DAPI (1:1,000) for 1 h. Changes in F-actin structures were
detected by FITC-labeled phalloidin (1:200; Beyotime, China). Then
slides were viewed using a Leica SP5 scanning confocal
microscope.
Measurement of TER
RPE cell cultures were grown on a microporous filter
to form monolayers. TER was measured using Millicell-ERS
(Millicell; Millipore, Bedford, MA, USA) and calculated by
subtracting the value of a blank filter without cells from the
experimental value. Final resistance-area products (Ω ×
cm2) were obtained by multiplying the TER by the
effective growth area. Fifteen days after TER stabilization, the
monolayers were incubated with or without one of the stimuli: Aβ
(0.3 μM), APMA (100 μM), GM6001 (20 μg/ml). To demonstrate the key
role of MMP-9 in regulating barrier function, MMP-9 silenced haRPE
cells were exposed to Aβ (0.3 μM). Measurements were repeated at
least four times for each filter, and each experiment was repeated
at least four times.
Permeability assay
The permeability assays were performed by measuring
the passive permeation of FITC-dextran (4 kDa; Sigma-Aldrich)
across confluent cells grown on filters. Fifteen days later, the
monolayers were treated as previously described. FITC-dextran (500
mg/ml) was added to the upper chamber on day 21. Samples (100 μl)
were taken from the upper and lower chamber 24 h after addition of
FITC-dextran. The concentration of FITC-dextran in these samples
was quantified by a microplate reader (ex1800; Biotek, Winooski,
VT, USA). Each experiment was repeated four times.
Statistical analysis
Data were analyzed with the software SPSS 11.5.
Results were expressed as means ± SEM. Values were processed for
statistical analysis (unpaired t-test or by ANOVA), and differences
were considered statistically significant at P<0.01 and
P<0.05.
Results
Characterization of Aβ1–42
oligomers and morphology of haRPE cells
Samples were examined after incubation for 12 or 72
h. Dot blot assay with the anti-oligomeric specific antibody (A11)
further confirmed the presence of oligomeric structures as it
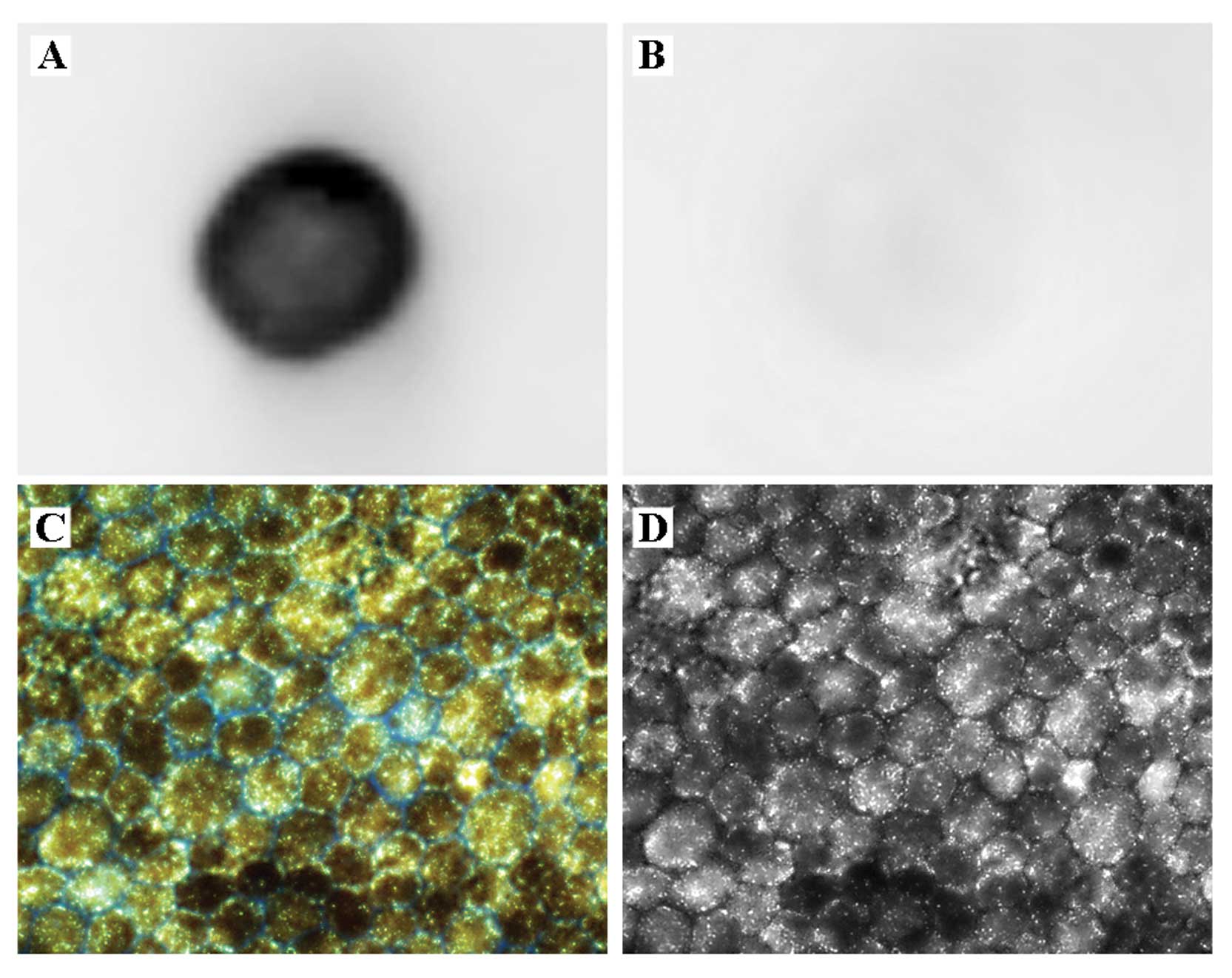
reacted positively with our OAβ sample (Fig. 1A). Furthermore, the A11 antibody
did not react against a monomer (Fig.
1B) confirming the specificity of the antibody to the
oligomeric form.
Structural integrity is the basis of good function.
To confirm that the isolated haRPE cells displayed classical
morphology (uniform hexagonal arrays of cells), confluence and
uniform pigmentation as in native tissue, the morphology of the
cells was evaluated using fluorescence microscopy (Fig. 1C). Images captured by fluorescence
microscopy were analyzed using image editing software (Photoshop
CS4) in the ‘a’ channel of LAB color mode. Apparent regular
hexagonal morphology of the cells was observed (Fig. 1D). This result confirmed that the
isolated haRPE cells exhibited heavy pigmentation (Fig. 1C) and hexagonal epithelial
morphology (Fig. 1C and D)
similar to these features in native tissue (17).
MTS assay of cytotoxicity
Effects of GM6001 (20 μg/ml) and APMA (100 μM) on
cell viability were demonstrated at 24 h. None of the measurements
showed significant cytotoxicity for the treatments at the
concentrations used in the present study (data not shown).
Expression of MMP-9 and proinflammatory
cytokines in Aβ-stimulated haRPE cells
Since the inflammatory response is speculated to be
one of the primary causative events that contributes to OAβ-induced
RPE degeneration, haRPE cells were treated with 0.3 μM of OAβ for
24 h, and the conditioned medium was collected. Expression of IL-6
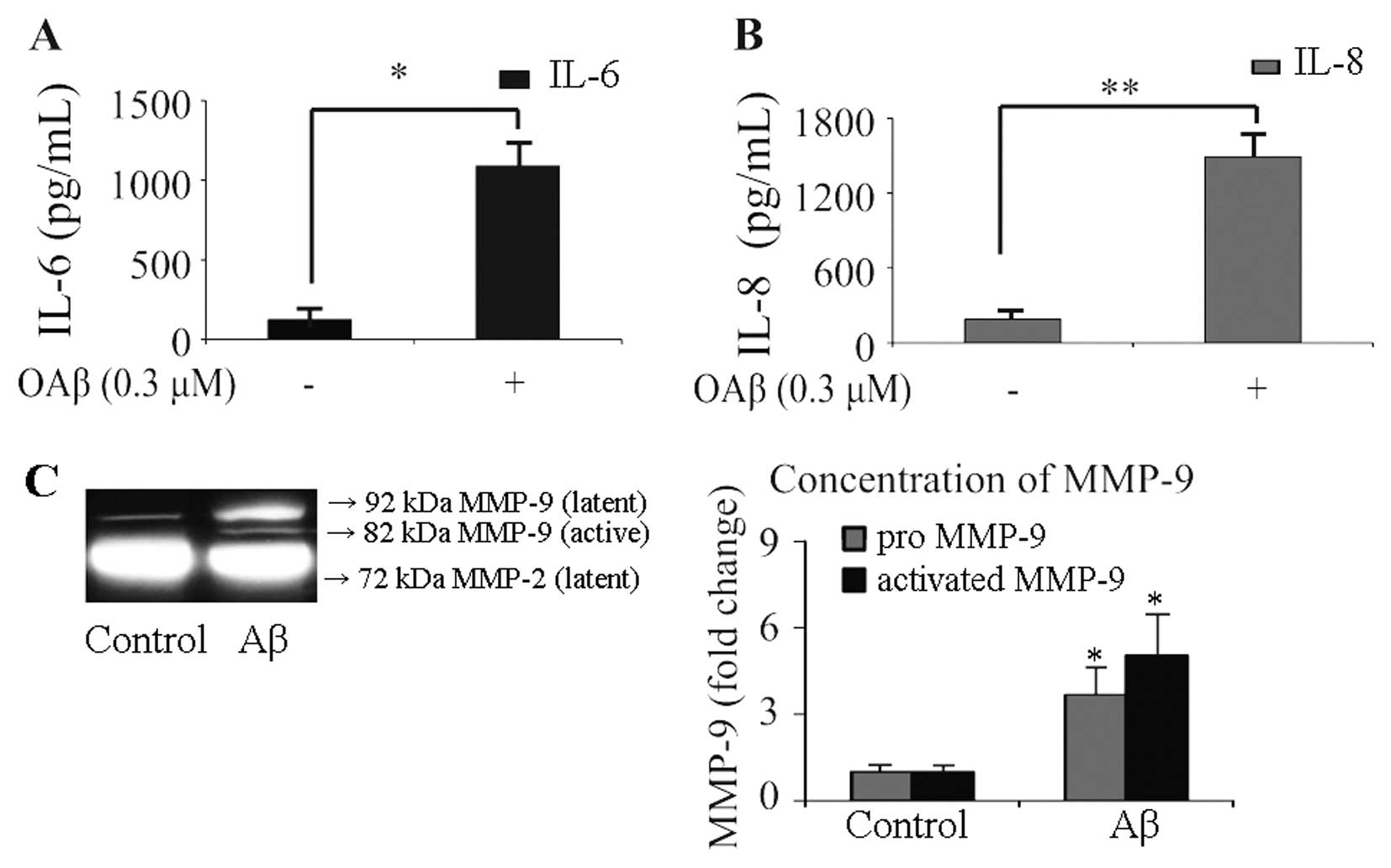
and IL-8 was analyzed using ELISA (Fig. 2A and B), and the secreted levels
of MMP-2 and MMP-9 were detected by gelatin zymography (Fig. 2C). The protein levels of IL-6 and
IL-8 in the culture media of unstimulated RPE cells were 123.6±62.5
and 190.9±68.2 pg/ml, respectively. In OAβ-stimulated cells, the
protein level of IL-6 increased to 1086.2±153.3 pg/ml (Fig. 2A), and the concentration of IL-8
increased to 1493.2±182.3 pg/ml (Fig.
2B). MMP release from the control cells was characterized by
low levels of pro-MMP-9, absent levels of active-MMP-9,
considerable output of pro-MMP-2, and absence of active-MMP-2.
Exposure to OAβ significantly increased secreted levels of
pro-MMP-9 (3.8±0.8-fold, P<0.05) and active-MMP-9 (5.1±1.2-fold,
P<0.05), while treatment with OAβ did not induce any change in
the secreted levels of pro-MMP-2. Active foms of MMP-2 were not
observed in the OAβ-stimulated cells. These results suggest that
OAβ induces haRPE cells to secrete higher concentrations of IL-6
and IL-8, which are critical chemoattractants responsible for
immune-cell recruitment. In addition, the results revealed that OAβ
induced the release of pro-MMP-9 and active-MMP-9 from haRPE
cells.
Influence of APMA, GM6001 and MMP-9 siRNA
on MMP release from RPE cells
Aβ has been shown to affect the TJs of ARPE-19 cells
(16). Uncontrolled increase in
MMP-9 activity has been causally linked to epithelial barrier
disruption as the result of abnormal degradation of TJ proteins
(21). Increased MMP-9 activity
on the ocular surface disrupted corneal epithelial barrier function
due to proteolytic cleavage of occludin (26). We hypothesized that Aβ-induced RPE
disruption is mediated by activation of MMP-9. Therefore, MMP
agonist APMA (100 μM) and MMP inhibitor GM6001 (20 μg/ml) were used
to stimulate haRPE cells for 6 h. The MMP-9 gene was silenced by
transfection of haRPE cells with 40 nM of MMP-9 siRNA for 24 h.
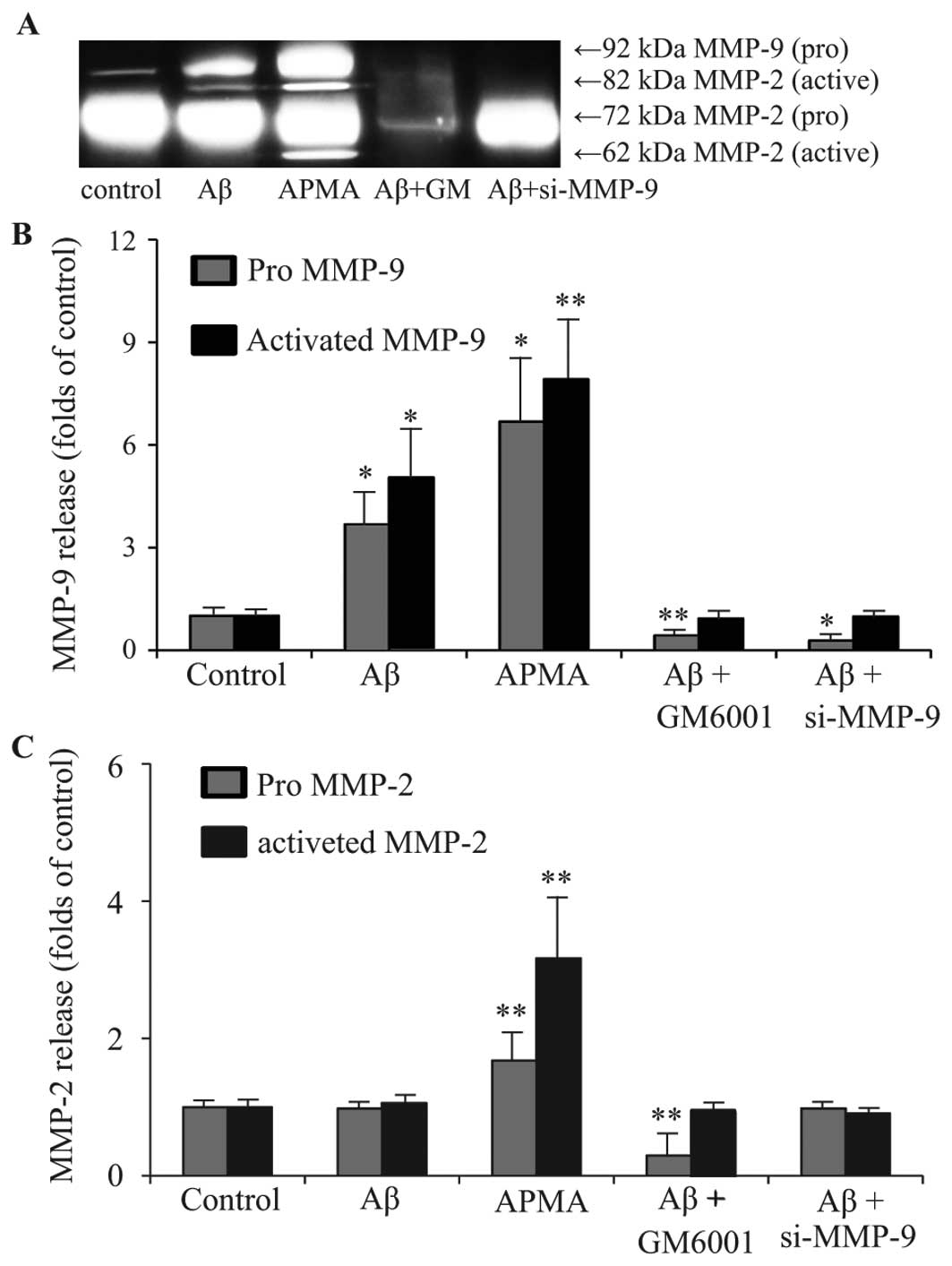
Release of MMP-9 and MMP-2 from haRPE cells was then detected by
gelatin zymography (Fig. 3A), and
the secreted levels of MMP-2 and MMP-9 were quantified by
densitometry (Fig. 3B and C).
Secretion of MMPs from control cells was characterized by low
levels of pro-MMP-9, absent levels of active-MMP-9, considerable
output of pro-MMP-2 and absence of active-MMP-2. Treatment with
APMA significantly increased secretion of pro-MMP-2 and pro-MMP-9,
as well as the activated form of MMP-2 and MMP-9. Treatment with
GM6001 or silencing of MMP-9 reduced the increase in pro-MMP-9 and
active-MMP-9 resulting from Aβ treatment.
Aβ-induced disruption of barrier
integrity is mediated by MMP-9 activity
Recent studies involving several CNS diseases
suggest that MMP-9 is involved in the permeability of the
blood-brain barrier by disrupting junction complexes (15,20). Giebel et al (28) found that RPE cells treated with
purified MMP-9 displayed alterations in tight junction function as
shown by decreased TER. Therefore, we hypothesized that OAβ-induced
RPE barrier disruption is mediated by activation of MMP-9. To test
this hypothesis, haRPE cells were treated with OAβ (0.3 μM) or with
APMA (100 μM) to induce MMP activation, and the barrier structure
was evaluated by immunostaining of TJ proteins. The barrier
function was evaluated by measuring TER and passive permeation of
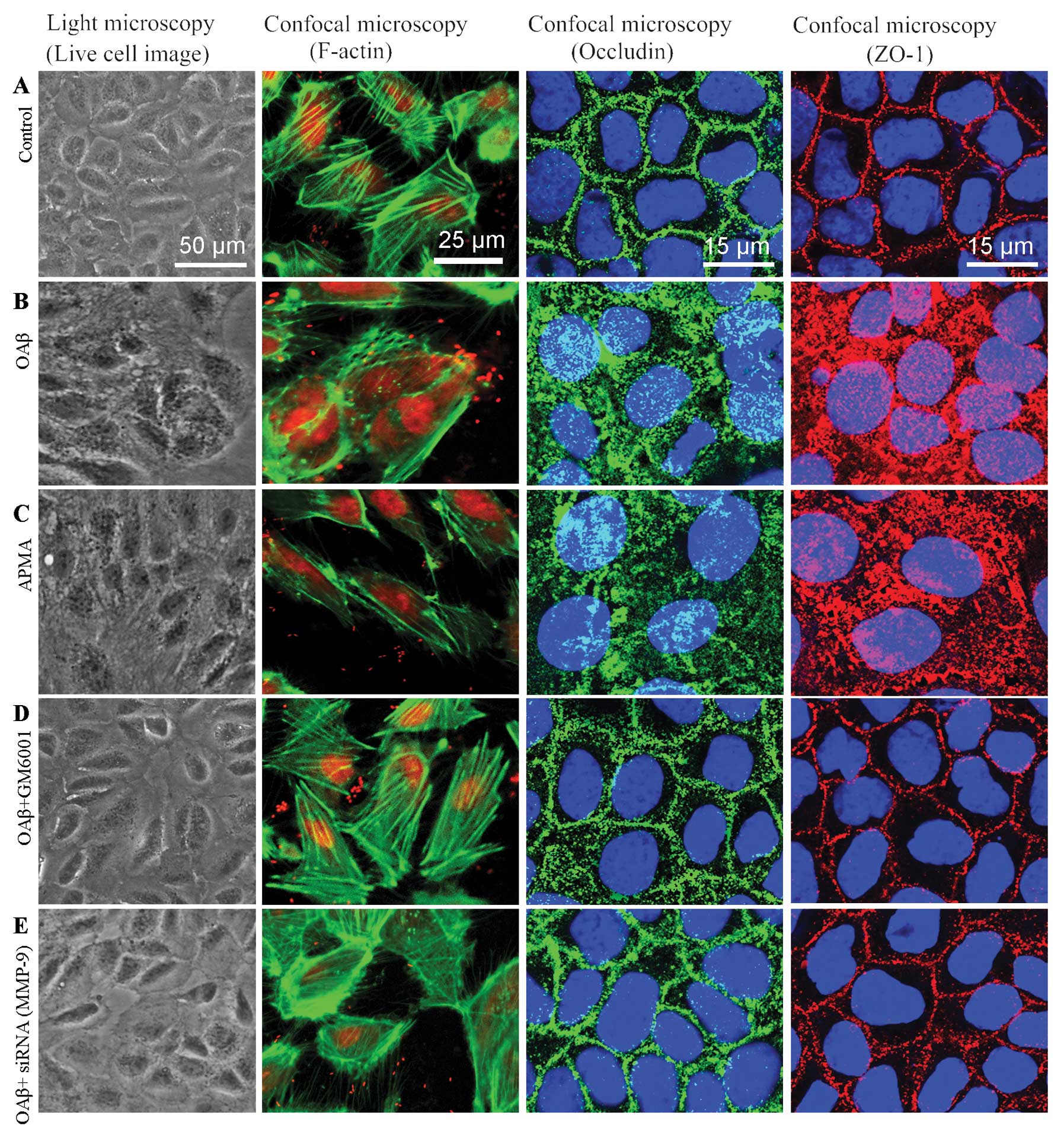
FITC-dextran. In the haRPE monolayers cultured with DMEM/F12 as
control (Fig. 4A), no
morphological change was observed; actin filaments were regular
with no breaks in the staining pattern, and the distribution of
occludin and ZO-1 was continuous and regular around the cells.
Exposure to Aβ (Fig. 4B) resulted
in an irregular morphology and a disturbed distribution of F-actin,
ZO-1 and occludin; the staining of F-actin was interrupted in the
intercellular space. The staining of occludin showed a diffuse
cytoplasmic distribution, and the abnormal distribution of ZO-1 was
typically manifested as fragmental staining. The alterations caused
by Aβ (Fig. 4B) suggest that Aβ
may be associated with RPE dysfunction in AMD. Notably, the
deleterious effects of Aβ were reproduced by stimulation with APMA
(Fig. 4C) which is known as an
MMP agonist. Further study was performed to examine whether this
abnormality was associated with a compromised barrier function
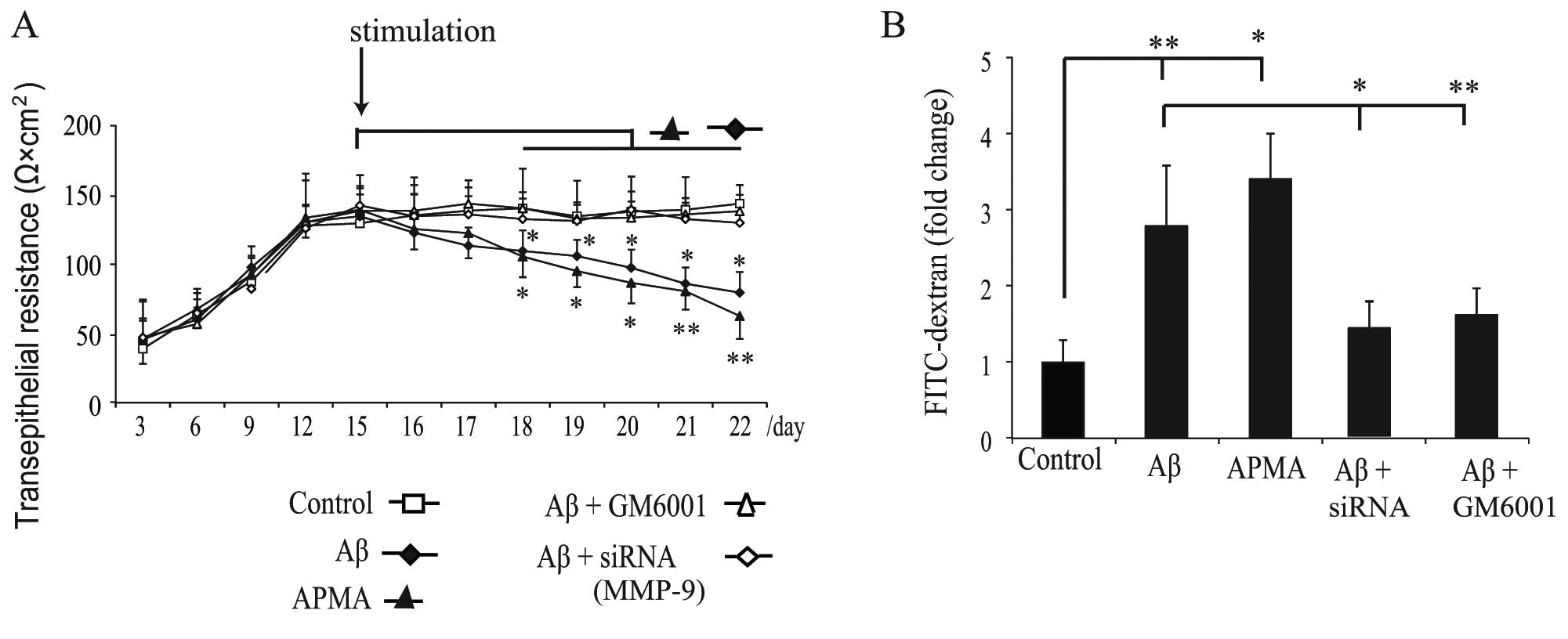
following exposure to Aβ or APMA. The TER (Fig. 5A) was recorded to determine the
stability of TJs, and the transepithelial diffusion rate of
FITC-dextran (Fig. 5B) was
measured to evaluate the permeability of the monolayers. The result
revealed that the TER increased rapidly during the initial 12 days
of standard culture and reached a plateau within the following 3
days (Fig. 5A). A mean level of
138±8.5 Ω × cm2 TER was recorded on day 15, and then the
monolayer was stimulated as previously described and the TER was
measured every day. We observed that exposure of haRPE cells to Aβ
or APMA gradually decreased the TER (Fig. 5A) and increased the diffusion rate
of FITC-dextran (Fig. 5B). These
results suggest that activation of MMP-9 by Aβ or APMA caused
disruption of TJ proteins and transepithelial permeability
dysfunction.
siRNA targeting MMP-9 and GM6001 prevents
Aβ-induced disruption of barrier integrity
To confirm whether MMP-9 is responsible for
disruption of epithelial barrier integrity, haRPE cells were
transfected with 40 nM MMP-9 siRNA or were pretreated with GM6001
(20 μg/ml) to inhibit MMP activation prior to Aβ stimulation.
Pretreatment with GM6001 inhibited the deleterious effects of Aβ on
TJ proteins in the RPE cells (Fig.
4D), and partially attenuated the decrease in TER (Fig. 5A) and the increase in permeability
resulting from Aβ treatment (Fig.
5B). These results suggest that inhibition of MMP activation
may reverse the deleterious effects of Aβ. Exposure of
MMP-9-silenced haRPE cells to Aβ did not induce abnormal staining
of TJ proteins (Fig. 4E).
Furthermore, transfection with MMP-9 siRNA in haRPE cells
attenuated Aβ-induced transepithelial permeability dysfunction
(Fig. 5). We demonstrated for the
first time that inhibition of MMP-9 activity using RNA interference
strategy attenuated Aβ-induced disruption of TJ proteins and
permeability dysfunction.
Discussion
The BRB plays an important role in the homeostatic
regulation of the microenvironment in the retina. Disruption of
oBRB is associated with development of AMD. Our study showed for
the first time that Aβ-induced secretion of MMP-9 is associated
with disruption of barrier integrity in primary human adult RPE
cells. Stimulation with Aβ promoted release of pro-MMP-9 and
active-MMP-9 from haRPE cells (Fig.
2). Exposure of haRPE cells to OAβ resulted in a disruption of
F-actin, ZO-1 and occludin (Fig.
4), loss of TER (Fig. 5A) and
increased permeability (Fig. 5B).
The results suggest that Aβ may be associated with the reduced
barrier function of RPE cells in AMD. We then compared the
morphological change (Fig. 4) and
barrier functional analysis of monolayers (Fig. 5) in the APMA- and Aβ-stimulated
group, and similar effects were observed. This suggests that
activation of MMP is associated with Aβ-induced barrier disruption.
Furthermore, inhibition of MMP-9 activity by GM6001 or by MMP-9
siRNA abolished the deleterious effects of Aβ on TJ ptoreins
(Fig. 4) and barrier function of
RPE cell monolayers (Fig. 5).
MMPs are calcium-requiring, zinc-containing
endopeptidases which constitute a major component of the enzyme
cascade responsible for degradation of extracellular matrix
proteins such as collagen, proteoglycan and laminin. MMPs can be
secretory or cell surface bound. Under normal conditions, MMP
activity is required for tissue remodeling, but altered MMP
activity has been observed in diseases. Higher concentration of
MMP-9 was measured in aqueous humour (22) and plasma (29) of AMD patients. The vitreous MMP-9
level was positively correlated with the severity of AMD (23). A recent study on genome-wide
association scan (GWAS) identified AMD susceptibility loci near
TIMP3, a metalloproteinase involved in degradation of the
extracellular matrix and implicated in early-onset AMD (30).
The TJ proteins of RPE cells are dynamic structures
with a variable permeability depending on local factors secreted by
RPE cells (31). Accumulated
evidence suggests that MMPs may induce barrier breakdown by
regulating the structures of TJ proteins. Knockdown of MMP-2 and
MMP-9 in leukemic cells reversed the disruption of TJ proteins
(19). Activation of MMP-9
disrupted corneal epithelial barrier function by proteolytic
cleavage of occludin (26).
Although the level of MMP-9 is correlated with the development of
AMD, it is unclear what controls MMP secretion from RPE cells and
subsequent activation. Complement activation resulted in a loss of
TER and accompanying elevated expression of MMP-2 and MMP-9 in RPE
cells (32). A recent study
demonstrated that Aβ-induced increase in MMP secretion from
endothelial cells contributed to disruption of ZO-1 expression
(33), but the role of MMP-9 in
regulating RPE barrier integrity is largely unknown. The present
study demonstrated for the first time that OAβ caused a significant
increase in secretion of MMP-9 from RPE cells (Figs. 2 and 3), which was associated with Aβ-induced
disruption of barrier integrity (Figs. 4 and 5). Recent evidence suggests that
Ca2+ deregulation may mediate the cytotoxicity of Aβ on
cellular function and viability (33–35). Actually, Aβ in glia can directly
increase Ca2+ signals (36). The MMPs constitute a family of
proteolytic enzymes that require the binding of Zn2+ and
Ca2+ for their enzymatic activity. Therefore, Aβ-induced
deregulation of Ca2+ homeostasis may explain excessive
activation of MMP-9 in cells (37).
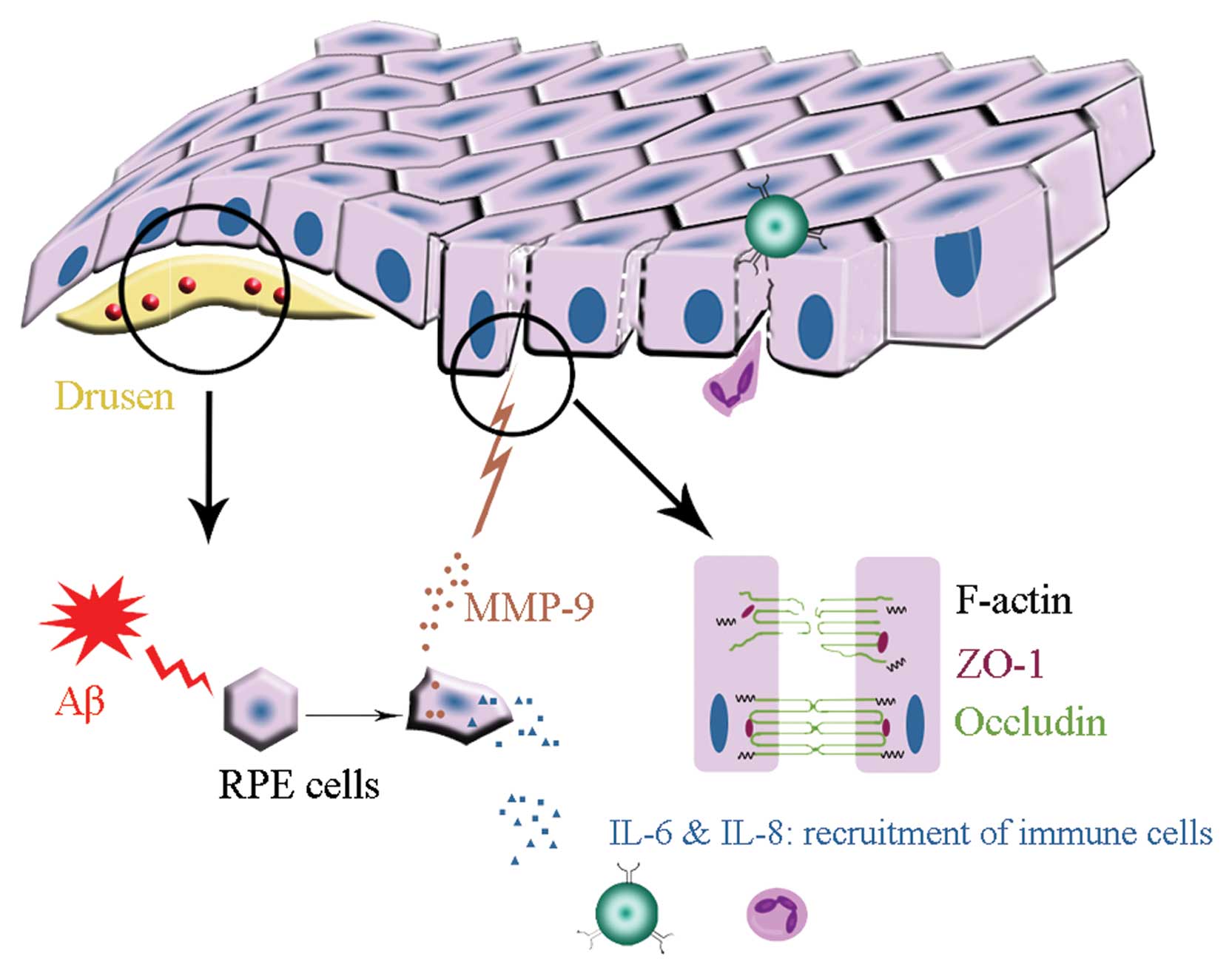
Aβ, detected in the drusen of AMD, may trigger
inflammatory responses in the RPE/choroidal layers of the eye.
Aβ-induced expression of IL-6, IL-8 and MMP-9 by RPE cells may
cause continuous chronic inflammation in AMD for the following
reasons (Fig. 6). i) The retina
is an immune-privileged site where inflammatory responses are
suppressed, but opening of epithelial barriers by MMP activity may
be a mechanism that allows passage of plasma proteins and
inflammatory cells into this privileged compartment. ii) The
aqueous humour of AMD patients contain higher concentrations of
IL-6 and IL-8 (22), which play
pivotal roles in leukocyte recruitment. iii) Continued presence
(sometimes over many years) of pro-inflammatory factors and immune
cells in the retina may cause chronic inflammation. iv) Cytokines
secreted by RPE cells may accelerate inflammatory response and cell
damage. Together with these observations, our present study
suggests that Aβ-induced release of MMP-9 contributes to epithelial
barrier disruption. Maintenance of the structural integrity in RPE
cell monolayers by blocking the action of Aβ or Aβ-mediated MMP-9
secretion may thus represent a new approach to the treatment of
AMD.
Acknowledgements
This study was supported by the Science and
Technology Commission of Shanghai (11JC1409900). We gratefully
thank the Biochemistry and Molecular Biology Institute of Shanghai
Tenth People’s Hospital for the technological support, the Xiaoqing
Liu’s Laboratory of Tongji University for the experimental
facilities and equipment and Xiujuan Shi for the excellent
technical assistance.
References
|
1
|
Klein R, Peto T, Bird A and Vannewkirk MR:
The epidemiology of age-related macular degeneration. Am J
Ophthalmol. 137:486–495. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Johnson LV, Forest DL, Banna CD, et al:
Cell culture model that mimics drusen formation and triggers
complement activation associated with age-related macular
degeneration. Proc Natl Acad Sci USA. 108:18277–18282. 2011.
View Article : Google Scholar
|
|
3
|
Luibl V, Isas JM, Kayed R, Glabe CG,
Langen R and Chen J: Drusen deposits associated with aging and
age-related macular degeneration contain nonfibrillar amyloid
oligomers. J Clin Invest. 116:378–385. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mullins RF, Russell SR, Anderson DH and
Hageman GS: Drusen associated with aging and age-related macular
degeneration contain proteins common to extracellular deposits
associated with atherosclerosis, elastosis, amyloidosis, and dense
deposit disease. FASEB J. 14:835–846. 2000.
|
|
5
|
Isas JM, Luibl V, Johnson LV, et al:
Soluble and mature amyloid fibrils in drusen deposits. Invest
Ophthalmol Vis Sci. 51:1304–1310. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang J, Ohno-Matsui K, Yoshida T, et al:
Amyloid-beta up-regulates complement factor B in retinal pigment
epithelial cells through cytokines released from recruited
macrophages/microglia: another mechanism of complement activation
in age-related macular degeneration. J Cell Physiol. 220:119–128.
2009. View Article : Google Scholar
|
|
7
|
Wang J, Ohno-Matsui K, Yoshida T, et al:
Altered function of factor I caused by amyloid beta: implication
for pathogenesis of age-related macular degeneration from drusen. J
Immunol. 181:712–720. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kurji KH, Cui JZ, Lin T, et al: Microarray
analysis identifies changes in inflammatory gene expression in
response to amyloid-beta stimulation of cultured human retinal
pigment epithelial cells. Invest Ophthalmol Vis Sci. 51:1151–1163.
2010. View Article : Google Scholar
|
|
9
|
Streilein JW: Immune privilege as the
result of local tissue barriers and immunosuppressive
microenvironments. Curr Opin Immunol. 5:428–432. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jo DH and Kim JH and Kim JH: How to
overcome retinal neuropathy: the fight against angiogenesis-related
blindness. Arch Pharm Res. 33:1557–1565. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cunha-Vaz J, Bernardes R and Lobo C:
Blood-retinal barrier. Eur J Ophthalmol. 21:3–9. 2010. View Article : Google Scholar
|
|
12
|
Bailey TA, Kanuga N, Romero IA, Greenwood
J, Luthert PJ and Cheetham ME: Oxidative stress affects the
junctional integrity of retinal pigment epithelial cells. Invest
Ophthalmol Vis Sci. 45:675–684. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Peng S, Gan G, Rao VS, Adelman RA and
Rizzolo LJ: Effects of proinflammatory cytokines on the claudin-19
rich tight junctions of human retinal pigment epithelium. Invest
Ophthalmol Vis Sci. 53:5016–5028. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu J, Jin X, Liu KJ and Liu W: Matrix
metalloproteinase-2-mediated occludin degradation and
caveolin-1-mediated claudin-5 redistribution contribute to
blood-brain barrier damage in early ischemic stroke stage. J
Neurosci. 32:3044–3057. 2012. View Article : Google Scholar
|
|
15
|
Martins T, Baptista S, Gonçalves J, et al:
Methamphetamine transiently increases the blood-brain barrier
permeability in the hippocampus: role of tight junction proteins
and matrix metalloproteinase-9. Brain Res. 1411:28–40. 2011.
|
|
16
|
Bruban J, Glotin AL, Dinet V, et al:
Amyloid-beta(1–42) alters structure and function of retinal
pigmented epithelial cells. Aging Cell. 8:162–177. 2009.
|
|
17
|
Maminishkis A, Chen S, Jalickee S, et al:
Confluent monolayers of cultured human fetal retinal pigment
epithelium exhibit morphology and physiology of native tissue.
Invest Ophthalmol Vis Sci. 47:3612–3624. 2006. View Article : Google Scholar
|
|
18
|
Suzuki T, Yoshinaga N and Tanabe S:
Interleukin-6 (IL-6) regulates claudin-2 expression and tight
junction permeability in intestinal epithelium. J Biol Chem.
286:31263–31271. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Feng S, Cen J, Huang Y, et al: Matrix
metalloproteinase-2 and -9 secreted by leukemic cells increase the
permeability of blood-brain barrier by disrupting tight junction
proteins. PLoS One. 6:e205992011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bauer AT, Bürgers HF, Rabie T and Marti
HH: Matrix metalloproteinase-9 mediates hypoxia-induced vascular
leakage in the brain via tight junction rearrangement. J Cereb
Blood Flow Metab. 30:837–848. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Manicone AM and McGuire JK: Matrix
metalloproteinases as modulators of inflammation. Semin Cell Dev
Biol. 19:34–41. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jonas JB, Tao Y, Neumaier M and Findeisen
P: Cytokine concentration in aqueous humour of eyes with exudative
age-related macular degeneration. Acta Ophthalmol. 90:e381–e388.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ecker SM, Pfahler SM, Hines JC, Lovelace
AS and Glaser BM: Sequential in-office vitreous aspirates
demonstrate vitreous matrix metalloproteinase 9 levels correlate
with the amount of subretinal fluid in eyes with wet age-related
macular degeneration. Mol Vis. 18:1658–1667. 2012.
|
|
24
|
Jeong S, Ledee DR, Gordon GM, et al:
Interaction of clusterin and matrix metalloproteinase-9 and its
implication for epithelial homeostasis and inflammation. Am J
Pathol. 180:2028–2039. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Huet E, Vallée B, Delbé J, et al: EMMPRIN
modulates epithelial barrier function through a MMP-mediated
occludin cleavage: implications in dry eye disease. Am J Pathol.
179:1278–1286. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pflugfelder SC, Farley W, Luo L, et al:
Matrix metalloproteinase-9 knockout confers resistance to corneal
epithelial barrier disruption in experimental dry eye. Am J Pathol.
166:61–71. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kayed R, Head E, Thompson JL, et al:
Common structure of soluble amyloid oligomers implies common
mechanism of pathogenesis. Science. 300:486–489. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Giebel SJ, Menicucci G, McGuire PG and Das
A: Matrix metalloproteinases in early diabetic retinopathy and
their role in alteration of the blood-retinal barrier. Lab Invest.
85:597–607. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chau KY, Sivaprasad S, Patel N, Donaldson
TA, Luthert PJ and Chong NV: Plasma levels of matrix
metalloproteinase-2 and -9 (MMP-2 and MMP-9) in age-related macular
degeneration. Eye. 22:855–859. 2008. View Article : Google Scholar
|
|
30
|
Chen W, Stambolian D, Edwards AO, et al:
Genetic variants near TIMP3 and high-density lipoprotein-associated
loci influence susceptibility to age-related macular degeneration.
Proc Natl Acad Sci USA. 107:7401–7406. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Balda MS, Gonzalez-Mariscal L, Contreras
RG, et al: Assembly and sealing of tight junctions: possible
participation of G-proteins, phospholipase C, protein kinase C and
calmodulin. J Membr Biol. 122:193–202. 1991. View Article : Google Scholar
|
|
32
|
Bandyopadhyay M and Rohrer B: Matrix
metalloproteinase activity creates pro-angiogenic environment in
primary human retinal pigment epithelial cells exposed to
complement. Invest Ophthalmol Vis Sci. 53:1953–1961. 2012.
View Article : Google Scholar
|
|
33
|
Kook SY, Hong HS, Moon M, Ha CM, Chang S
and Mook-Jung I: Aβ1–42-RAGE interaction disrupts tight
junctions of the blood-brain barrier via
Ca2+-calcineurin signaling. J Neurosci. 32:8845–8854.
2012.
|
|
34
|
Thibault O, Gant JC and Landfield PW:
Expansion of the calcium hypothesis of brain aging and Alzheimer’s
disease: minding the store. Aging Cell. 6:307–317. 2007.PubMed/NCBI
|
|
35
|
Grolla AA, Fakhfouri G, Balzaretti G, et
al: Aβ leads to Ca2+ signaling alterations and
transcriptional changes in glial cells. Neurobiol Aging.
34:511–522. 2013.
|
|
36
|
Kuchibhotla KV, Lattarulo CR, Hyman BT and
Bacskai BJ: Synchronous hyperactivity and intercellular calcium
waves in astrocytes in Alzheimer mice. Science. 323:1211–1215.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kobayashi T, Hattori S, Nagai Y and Tajima
S: Differential regulation of the secretions of matrix
metalloproteinase-9 and tissue inhibitor of metalloproteinases-1
from human keratinocytes in culture. IUBMB Life. 50:221–226. 2000.
View Article : Google Scholar
|