Introduction
Stroke is the second most common cause of death and
the major cause of disability worldwide (1). Inflammatory reaction has been shown
to play an important role in secondary brain damage after stroke,
which is believed to be the consequence of microglial activation
(2,3). Microglial cells are generally
considered to be the resident immunocompetent cells of the central
nervous system (CNS), and over-activation of microglia can induce
significant and highly detrimental neurotoxic effects through
excessive production of a large array of cytotoxic factors such as
superoxide, nitric oxide (NO), tumour necrosis factor-α (TNF-α),
interleukin (IL)-1β and IL-6 (4).
In addition, previous studies have demonstrated that a decrease in
the secretion of pro-inflammatory mediators in microglia attenuates
the severity of neuro-degenerative diseases including ischemic
stroke (5,6). LPS, an inducer of inflammation, has
been frequently employed to stimulate microglia to construct a
useful model for the study of mechanisms that underlie neuronal
injury (7). Toll-like receptor 4
(TLR4) is well known as the unique receptor for lipopolysac-TLR4)
is well known as the unique receptor for lipopolysaccharide (LPS),
which transduces immune-related signals to the nucleus via
activating transcription factors such as nuclear factor-κB (NF-κB).
In unstimulated cells, NF-κB is sequestered in the cytosol via
interaction with inhibitory IκB proteins. However, when cells
receive pathological stimuli, IκB protein is phosphorylated
resulting in its ubiquitination and degradation, which in turn
releases sequestered NF-κB, leading to its translocation to the
nucleus where it positively regulates the expression of various
pro-inflammatory mediators (8–10).
Therefore, anti-inflammatory treatment may reduce ischemic brain
injury and enhance stroke recovery.
Gua Lou Gui Zhi decoction (GLGZD) is a classical
traditional Chinese formula that was first prescribed in Eastern
Han Dynasty, around 210 AD, which consists of a combination of six
herbs, including Trichosanthis Radix, Ramulus
Cinnamomi, Paeonia lactiflora, Glycyrrhiza,
Zingiber officinale Roscoe and Fructus Jujubae. GLGZD
has long been used in China to clinically treat post-stroke
disabilities such as muscular spasticity (11–13). However, the mode of its action
remains largely unclear. Using LPS-stimulated microglial BV-2 cells
as an in vitro inflammatory model of neural cells, in the
present study we evaluated the anti-inflammatory effect of GLGZD
and investigated the underlying molecular mechanisms.
Materials and methods
Materials and reagents
LPS (from Escherichia coli 055:B5) and
3-[4,5-dimethyl-2-thiazolyl]-2,5-diphenyltetrazolium bromide (MTT)
were obtained from Sigma-Aldrich (St. Louis, MO, USA). Fetal bovine
serum (FBS), Dulbecco’s modified Eagle’s medium (DMEM), penicillin,
streptomycin, 0.05% (w/v) trypsin/EDTA, and phosphate-buffered
saline (PBS) were obtained from Gibco-BRL (Gaithersburg, MD, USA).
Cytokine (IL-6, TNF-α and IL-1β) ELISA kits were purchased from
R&D Systems (Minneapolis, MN, USA). Antibodies for western blot
analysis included: i) TLR4 antibody (rodent-specific) rabbit
polyclonal antibody; ii) MyD88 (D80F5) rabbit mAb; iii) inhibitor
of κBα (IκBα) (L35A5) mouse mAb (amino-terminal antigen); iv)
phospho-IκBα (Ser32/36) (5A5) mouse mAb; v) anti-β-actin (8H10D10)
mouse mAb; vi) horseradish peroxidase (HRP)-conjugated secondary
antibodies (all from Cell Signaling Technology, Beverly, MA, USA).
The antibody for immunofluorescence was NF-κB p65 (F-6) (Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA).
Preparation of water extract from
GLGZD
Medicinal plants were supplied by Guo Yi Tang
Chinese Herbal Medicine Store (Fujian, China) for the preparation
of the GLGZD water extract. The preparation was a mixture of six
crude plant extract ingredients soaked in (5-fold of the plant)
double distilled water (DDW) for 30 min. The mixture was heated to
100°C for 1 h, and the decoction was filtrated. The filtrates
obtained from two cycles of the procedures were mixed, then
filtered and concentrated using a rotary evaporator (Model RE-2000;
Yarong Biochemistry Instrument Co., Shanghai, China). The extract
of GLGZD was obtained by a spraying desiccation method using a
spray dryer (Model B-290; Büchi Labortechnik AG, Flawil,
Switzerland). The stock and working concentrations of GLGZD were
prepared by dissolving the extract in culture media to a
concentration of 50 mg/ml and 50, 100, 200 μg/ml.
Cell culture and treatment
A murine BV-2 microglial cell line [purchased from
the American Type Culture Collection, Manassas, VA, USA] was
maintained in DMEM supplemented with FBS (10%), 100 U/ml penicillin
and 100 μg/ml streptomycin. Cells were incubated in culture
medium at 37°C in a 95% atmospheric air and 5% CO2
humidified atmosphere. Briefly, the numbers of viable cells were
evaluated by counting trypan blue-excluding cells that were then
plated at a density of 1×105 cells/well in 96-well
trays, 2×105 cells/well in 24-well trays, or plated at a
density of 5×105 cells/well in 6-well trays (for
remaining experiments), incubated at 37°C for 24 h, and given a
fresh change of medium. Cells were then incubated with or without
LPS (1 μg/ml) in the presence of various concentrations of
GLGZD (50, 100, 200 or 500 μg/ml) at 37°C for an additional
24 h, and in all experiments, cells were serum starved for 4 h
prior to the treatment. The cultured cells were treated with LPS
with or without GLGZD for 24 h (for ELISA), or for 12 h (for
RT-PCR), or for 1 h for NF-κB translocation activity and western
blotting for relative protein expression.
Cytotoxity assay
Microglial BV-2 cells were grown in 96-well plates
and then incubated with or without LPS (1 μg/ml) in the
presence of GLGZD at various concentrations for 24 h. MTT assay was
used to measure the viability of the cells. MTT is a pale yellow
substrate that produces a dark blue formazan product when incubated
with living cells; an MTT ring is cleaved in active mitochondria,
which occurs only in living cells. After the supernatants were
removed for nitrite determination, cells were incubated at 37°C
with MTT (0.05 mg/ml) for 4 h. The resulting color was assayed at
570 nm using a microplate absorbance reader (EXL 8008,
Germany).
Determination of nitrite production
Inducible NO synthase (iNOS)-derived NO release is
one of the major contributing factors during the inflammatory
process in cerebral ischemic injury (14,15). Nitrite production was assessed by
the Griess reaction (16). In
brief, each 50 μl aliquot of the above-mentioned culture
supernatants was collected and mixed with an equal volume of Griess
reagent [0.1% N-(1-naphthyl)-ethylenediamine, 1% sulfanilamide in
5% phosphoric acid] and incubated at room temperature (RT) for 10
min. Absorbance at 540 nm was measured in a microplate absorbance
reader. Nitrite concentration was determined from a sodium nitrite
standard curve.
Measurement of inflammatory
cytokines
BV-2 cells were plated in a 24-well cell culture
plate and incubated with GLGZD water extract (50, 100 and 200
μg/ml) in the presence or absence of LPS (1 μg/ml)
for 24 h. Following the manufacturer’s instructions, a volume of 1
ml of culture-medium supernatant was collected for measurement of
the concentration levels of IL-6, TNF-α and IL-1β by the relevant
ELISA kit.
Reverse transcription-polymerase chain
reaction (RT-PCR)
Total RNA was extracted from cultured microglial
cells by using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) and
following the standard protocol. Purity and integrity of the RNA
were assessed using a NanoDrop reader. Subsequently, first-strand
cDNA synthesis was performed with 2 μg total RNA using
RevertAid™ H Minus First Strand cDNA Synthesis Kit (Fermentas, St.
Leon-Rot, Germany) according to the manufacturer’s instructions.
The obtained cDNA was used to determine the mRNA levels of TNF-α,
IL-6, IL-1β and iNOS by a PCR kit (Fermentas). β-actin was used as
an internal control.
Western blot analysis
BV-2 cells were plated overnight in 6 culture flasks
and were then further incubated in serum-free medium for at least 4
h before treatments. After washing with cold PBS for three times,
cells were lysed in RIPA buffer containing protease inhibitor PMSF
(Roche Diagnostics, Mannheim, Germany). The content of the cells
was pooled and centrifuged for 10 min at 12,000 × g and stored at
−80°C. The protein concentration of the lysates was measured using
BCA quantification assay (Pierce, Rockford, IL, USA). Proteins (50
μg) were separated using 12% (10% for TLR4 analysis)
SDS-PAGE and transferred to PVDF membranes with a 0.45-μm
pore size (IPVH00010; Millipore, Billerica, MA, USA). The membranes
were incubated with primary antibodies overnight at 4°C: rabbit
TLR4 monoclonal Ab, rabbit myd88, mouse IκBα, phospho-IκBα, β-actin
monoclonal Ab (1:1,000) diluted in immunoblot buffer (TBS
containing 0.05% Tween-20 and 5% non-fat dry milk). Following
washing with TBST three times, membranes were incubated with the
secondary antibody HRP-conjugated anti-mouse (or rabbit) IgG
(1:1,000) for 1 h at RT. After washing, the blots were detected
with Chemiluminescence (ECL)-Plus (RPN2132; GE Healthcare Life
Sciences, Pittsburgh, PA, USA) for 1 min using a camera along with
the VersaDoc System (Bio-Rad, Hercules, CA, USA). The pixel
intensities of the immunoreactive bands were quantified using the
percentage adjusted volume feature of Quantity One 5.4.1 software
(Bio-Rad). Data are expressed as a ratio of the intensity of the
band of the protein of interest over the intensity of the band of
the loading control protein (β-actin).
Double-immunofluorescence labeling
assay
For the detection of intracellular location of NF-κB
p65, BV-2 cells were cultured on sterile glass coverslips and
treated with GLGZD and LPS as described above. Following the
various treatments, BV-2 cells were fixed with 4% paraformaldehyde
in PBS for 30 min. After rinsing with PBS for three times, the cell
membrane was permeabilized with 0.1% Triton X-100 for 2 min, and
the coverslips with adherent cells were submitted to the
double-immunofluorescence labeling assay. BV-2 cells were incubated
with Hoechst (dilution 1:50,000; Sigma-Aldrich) plus NF-κB p65
(sc-8008, dilution 1:50; Santa Cruz Biotechnology, Inc.). The
fluorescence signals were detected and analyzed using laser scan
confocal microscopy (LSM 700; Carl Zeiss, Göttingen, Germany).
Statistical analysis
Data are expressed as means ± SD. One-way ANOVA was
used when comparing the data obtained from the different
experimental conditions. In vitro experiments were conducted
in triplicates; representative results are shown. A P-value of
<0.05 was considered to indicate a statistically significant
result.
Results
GLGZD inhibits the LPS-induced
inflammatory response in microglial BV-2 cells
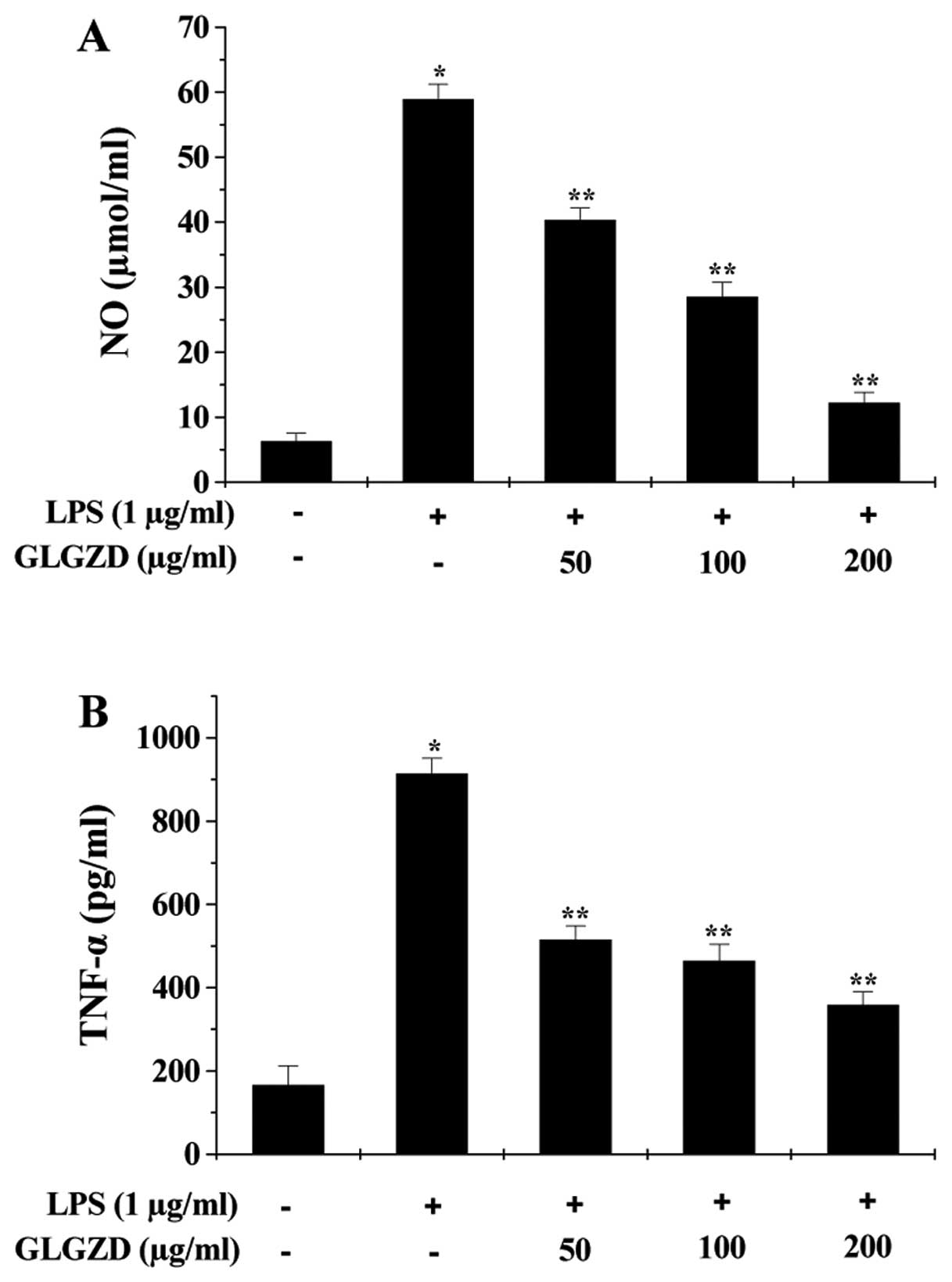
We first evaluated the effect of GLGZD on
LPS-induced inflammation in BV-2 cells by measuring the production
of NO and secretion levels of pro-inflammatory cytokines (TNF-α,
IL-6 and IL-1β). As shown in Fig.
1, LPS stimulation for 24 h significantly induced the release
of NO, TNF-α, IL-6 and IL-1β, indicating an inflammatory response
in BV-2 cells. However, the LPS-induced inflammation was
significantly inhibited by GLGZD treatment in a dose-dependent
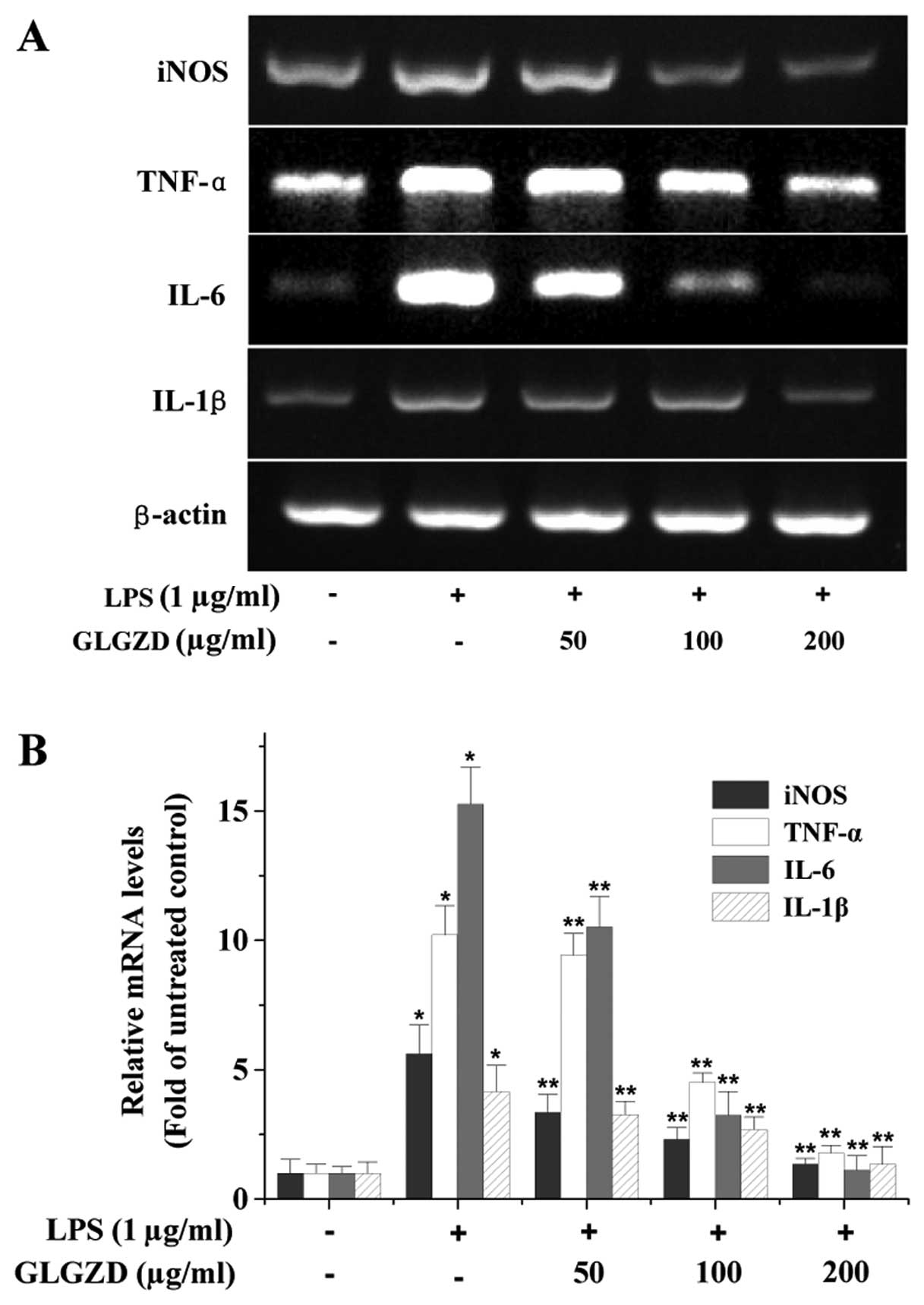
manner. To further verify these observations, we determined the
effect of GLGZD on the mRNA expression of the pro-inflammatory
factors. As shown in Fig. 2, LPS
stimulation profoundly increased the mRNA expression of iNOS,
TNF-α, IL-6 and IL-1β in BV-2 cells, which was dose-dependently and
significantly suppressed by GLGZD treatment.
GLGZD does not display a cytotoxic effect
in BV-2 cells
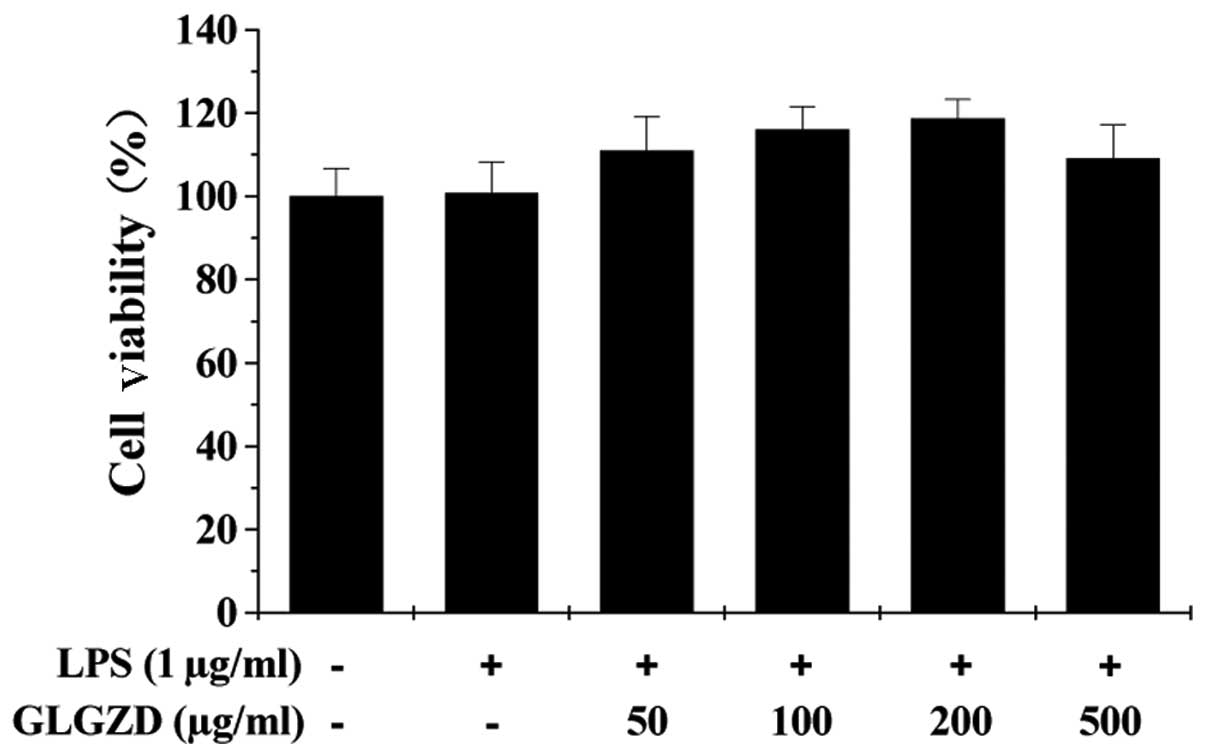
We next performed an MTT assay to evaluate the
effect of GLGZD on cell viability. As shown in Fig. 3, treatment with various
concentrations of GLGZD and/or LPS had no effect on the viability
of the BV-2 cells, suggesting that inhibition of the LPS-induced
inflammatory response in BV-2 cells did not result from the
cytotoxic action of GLGZD.
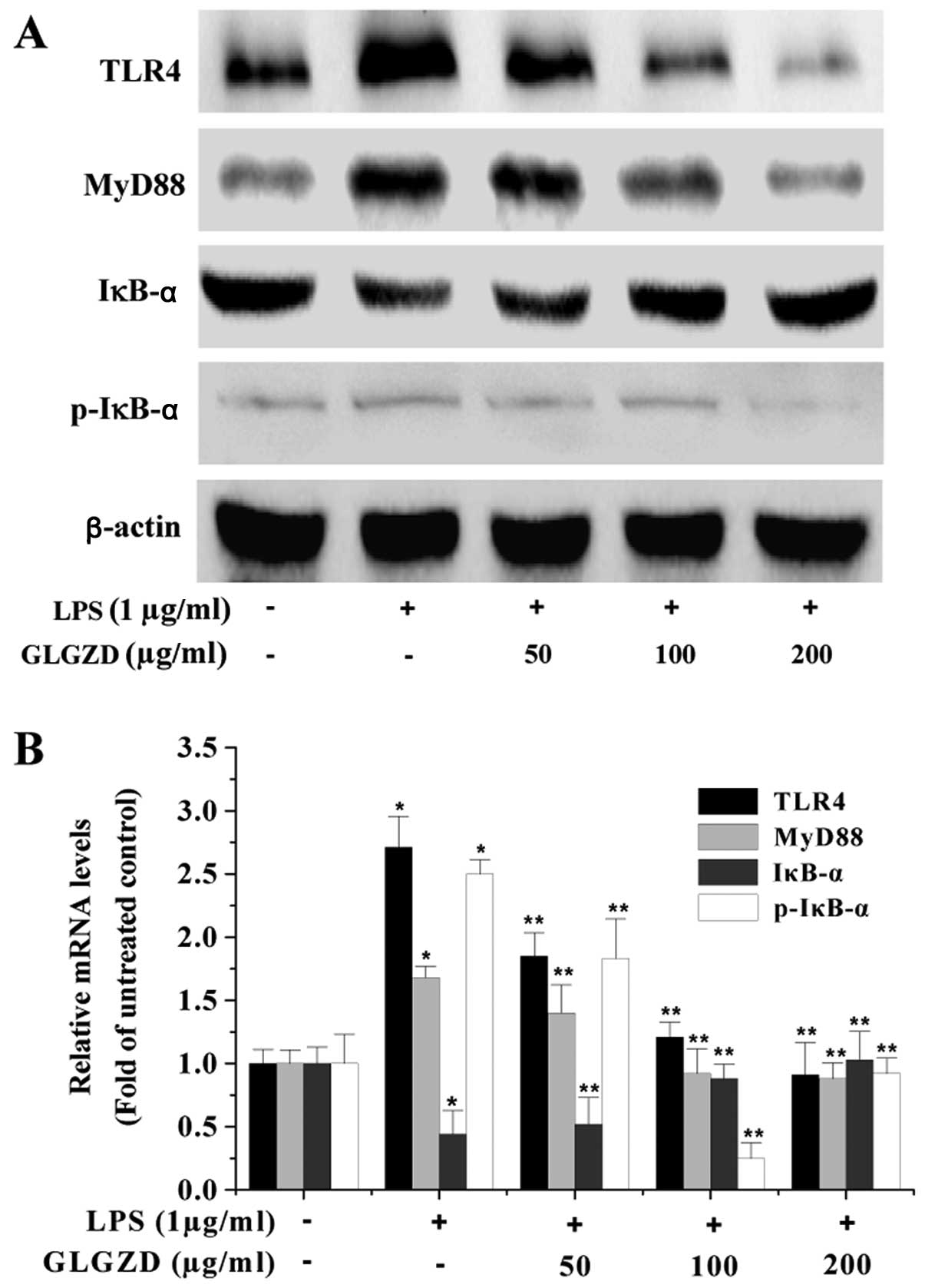
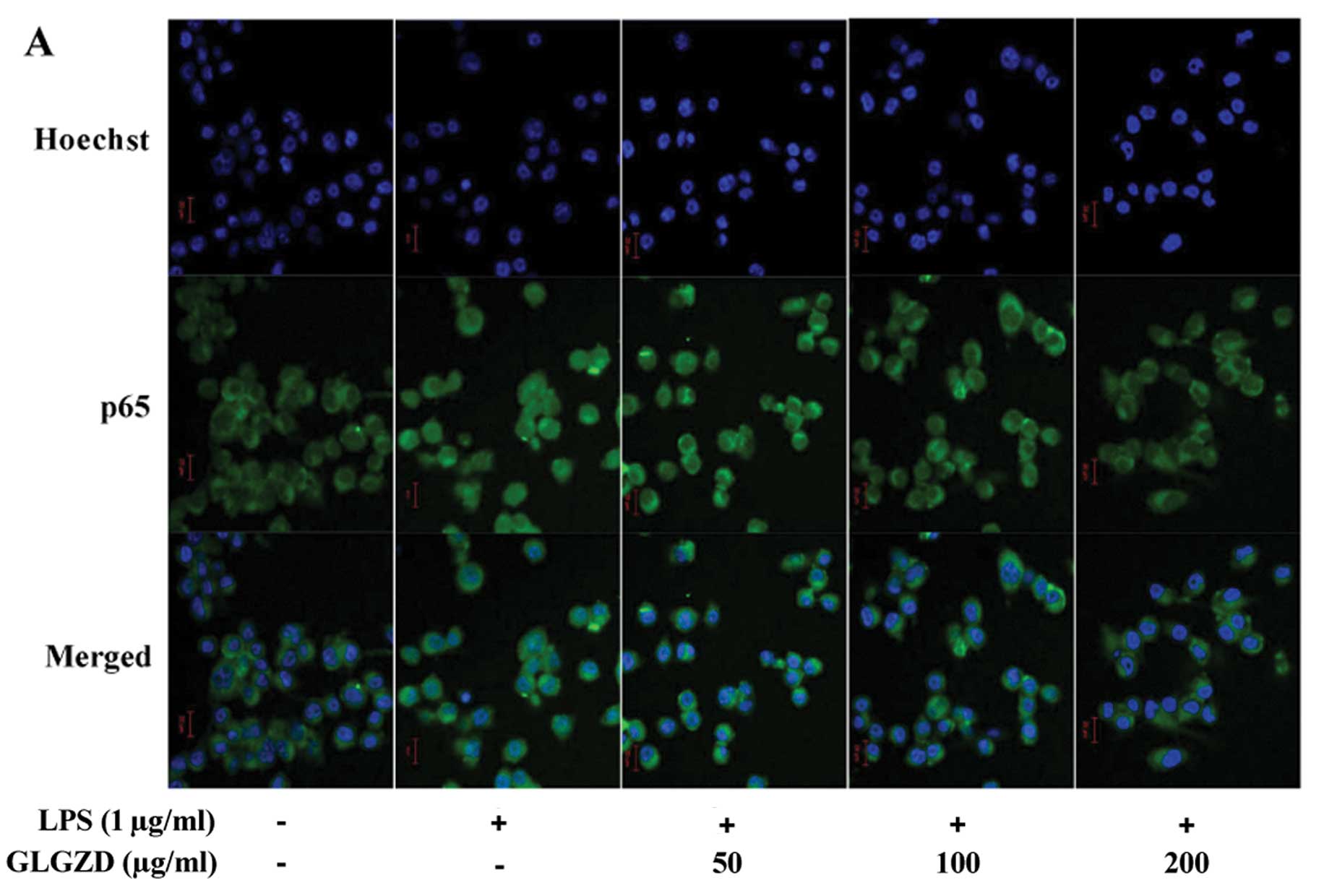
GLGZD suppresses LPS-induced activation
of the TLR4/NF-κB pathway in BV-2 cells
TLR4/NF-κB signaling is one of the major pathways
mediating immune and inflammation responses, which are involved in
the expression of inflammatory mediators. The process of NF-κB
activation includes several key links such as TLR4 activation, the
presence of MyD88, phosphorylation/degradation of IκB, and
subsequent nuclear translocation of NF-κB. To elucidate the
mechanism of the anti-inflammatory activity of GLGZD, we examined
its effect on the TLR4/NF-κB signaling pathway in microglial cells.
As shown in Fig. 4, upon LPS
stimulation, the expression of TLR4 and MyD88 as well as the
phosphorylation level of IκB were significantly increased. However,
GLGZD significantly neutralized the effect of LPS stimulation,
dose-dependently reducing TLR4 and MyD88 protein expression and IκB
phosphorylation levels in BV-2 cells. To further verify these
results, we evaluated the effect of GLGZD on the nuclear
translocation of NF-κB which is a critical step for NF-κB
activation. The NF-κB p65 subunit was visualized by
immunofluorescence staining. The cells were counterstained with
DAPI, and NF-κB nuclear translocation was recognized by the
co-localization of the p65 subunit with DAPI. As shown in Fig. 5, LPS stimulation caused the
nuclear translocation of the NF-κB p65 subunit, which was not
observed in unstimulated cells. However, GLGZD treatment
significantly blocked LPS-induced NF-κB nuclear translocation in
the BV-2 cells, in a dose-dependent manner. Collectively, these
data suggest that the anti-inflammatory effect of GLGZD is mediated
by suppression of TLR4/NF-κB signaling in microglial BV-2
cells.
Discussion
Post-stroke neuroinflammation triggered by microglia
cells is now believed to be a crucial mechanism leading to
secondary injury of the CNS. Although an appropriate inflammatory
response should be considered as a process to keep the CNS under
dynamic surveillance, its injurious property has to be taken into
account, including the release of pro-inflammatory mediators that
are responsible for neurotoxic processes (17–19). Therefore, anti-inflammation
therapy has been an attractive strategy to combat cerebral lesion.
Gua Lou Gui Zhi decoction (GLGZD), a classical traditional Chinese
medicine prescription, has been demonstrated to be effective for
the clinic treatment of ischemic stroke. However, little is known
about the mechanism of its neuroprotective action.
Pro-inflammatory mediators produced in microglia,
such as NO, TNF-α, IL-6 and IL-1β, play an important role in
neuro-inflammation; release of pro-inflammatory cytokines is
therefore considered as an indicator for inflammatory response.
Using LPS-stimulated microglial BV-2 cells as an in vitro
inflammatory model of neural cells, in the present study we found
that GLGZD significantly and dose-dependently reduced LPS-induced
secretion of NO, TNF-α, IL-6 and IL-1β, indicating that it inhibits
the inflammatory reaction in neural cells. Neuro-inflammation is
highly regulated by a family of pattern-recognition receptors, the
Toll-like receptors (TLRs) (20–22). To date, over 13 members of the TLR
family have been identified in mammals, of which TLR4 is the best
studied (23–25). After activation via binding to
specific ligands such as LPS, TLR4 mediates immune signals via
recruitment of adaptor proteins (such as MyD88, Mal, TRIF and
TRAM), resulting in the activation of transcription factors
including NF-κB. Under physiological conditions, NF-κB is
sequestered in the cytosol by IκB proteins via direct interaction.
On the contrary, after cells receive pathological stimuli, IκB
protein is phosphorylated, a process leading to its ubiquitination
and degradation. Consequently, released NF-κB translocates to the
nucleus where it induces the expression of pro-inflammatory
mediators (26). Using western
blotting and immunofluorescence staining analyses, we found that
GLGZD treatment significantly decreased the protein expression of
TLR4 and MyD88, inhibited the phosphorylation of IκB and blocked
the nuclear translocation of NF-κB in BV-2 cells, suggesting that
GLGZD suppresses the activation of the TLR4/NF-κB signaling
pathway.
In conclusion, we demonstrated for the first time
that GLGZD exerts its anti-inflammatory actions via interference
with TLR4/NF-κB signaling, which may be one of the mechanism
whereby GLGZD ameliorates the damage in ischemic cerebral
tissues.
Abbreviations:
|
GLGZD
|
Gua Lou Gui Zhi decoction;
|
|
CNS
|
central nervous system;
|
|
LPS
|
lipopolysaccharide;
|
|
NO
|
nitric oxide;
|
|
iNOS
|
inducible NO synthase;
|
|
IL-1β
|
interleukin-1β;
|
|
TLR4
|
Toll-like receptor 4;
|
|
TNF-α
|
tumour necrosis factor-α;
|
|
NF-κB
|
nuclear factor-κB;
|
|
MyD88
|
myeloid differentiation factor 88;
|
|
IκBα
|
inhibitor of κBα
|
Acknowledgements
This study was sponsored by the
Guidance Project of the Fujian Provincial Department of Science and
Technology (no. 2012D012), the Key Project of the Department of
Health of Fujian Province (no. zlckf01), the Key Project of Fujian
Provincial Department of Science and Technology (no. 2012Y0041) and
the Project of Fujian Education Department (no. JA12176).
References
|
1
|
Donnan GA, Fisher M, Macleod M and Davis
SM: Stroke. Lancet. 371:1612–1623. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yenari MA, Kauppinen TM and Swanson RA:
Microglial activation in stroke: therapeutic targets.
Neurotherapeutics. 7:378–391. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lehnardt S: Innate immunity and
neuroinflammation in the CNS: the role of microglia in Toll-like
receptor-mediated neuronal injury. Glia. 58:253–263.
2010.PubMed/NCBI
|
|
4
|
Tambuyzer BR, Ponsaerts P and Nouwen EJ:
Microglia: gate-keepers of central nervous system immunology. J
Leukoc Biol. 85:352–370. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fan T, Jiang WL, Zhu J and Feng Zhang Y:
Arctigenin protects focal cerebral ischemia-reperfusion rats
through inhibiting neuroinflammation. Biol Pharm Bull.
35:2004–2009. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Park JS, Shin JA, Jung JS, et al:
Anti-inflammatory mechanism of compound K in activated microglia
and its neuroprotective effect on experimental stroke in mice. J
Pharmacol Exp Ther. 341:59–67. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Park HY, Kim GY and Choi YH: Naringenin
attenuates the release of pro-inflammatory mediators from
lipopolysaccharide-stimulated BV2 microglia by inactivating nuclear
factor-κB and inhibiting mitogen-activated protein kinases. Int J
Mol Med. 30:204–210. 2012.PubMed/NCBI
|
|
8
|
Mattson MP: NF-κB in the survival and
plasticity of neurons. Neurochem Res. 30:883–893. 2005.
|
|
9
|
Wang J, Hou J, Zhang P, Li D, Zhang C and
Liu J: Geniposide reduces inflammatory responses of oxygen-glucose
deprived rat microglial cells via inhibition of the TLR4 signaling
pathway. Neurochem Res. 37:2235–2248. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dalloneau E, Pereira PL, Brault V, Nabel
EG and Hérault Y: Prmt2 regulates the lipopolysaccharide- induced
responses in lungs and macrophages. J Immunol. 187:4826–4834. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sun X: Research on formula treating
paralysis and spasticity From ‘Treatise on febrile and
miscellaneous diseases’. Zhongguo Zhong Yi Ji Chu Yi Xue Za Zhi.
8:644–645. 2010.(In Chinese).
|
|
12
|
Zhang L and Ai H: Effects of Gua Lou Gui
Zhi Decoction on c-fos and c-jun on epileptic rats. Shi Yong Zhong
Yi Yao Za Zhi. 23:21–22. 2005.(In Chinese).
|
|
13
|
Yang C, Chen L and Tao J: New usage of a
classical formula - Gua Lou Gui Zhi Decoction. Liaoning J Tradit
Chin Med. 8:166–167. 2012.(In Chinese).
|
|
14
|
Samdani AF, Dawson TM and Dawson VL:
Nitric oxide synthase in models of focal ischemia. Stroke.
28:1283–1288. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Iadecola C: Bright and dark sides of
nitric oxide in ischemic brain injury. Trends Neurosci. 20:132–139.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kovac A, Erickson MA and Banks WA: Brain
microvascular pericytes are immunoactive in culture: cytokine,
chemokine, nitric oxide, and LRP-1 expression in response to
lipopolysaccharide. J Neuroinflammation. 8:1392011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
McColl BW, Allan SM and Rothwell NJ:
Systemic infection, inflammation and acute ischemic stroke.
Neuroscience. 158:1049–1061. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
McColl BW, Rothwell NJ and Allan SM:
Systemic inflammatory stimulus potentiates the acute phase and CXC
chemokine responses to experimental stroke and exacerbates brain
damage via interleukin-1- and neutrophil-dependent mechanisms. J
Neurosci. 27:4403–4412. 2007. View Article : Google Scholar
|
|
19
|
Jin R, Yang G and Li G: Inflammatory
mechanisms in ischemic stroke: role of inflammatory cells. J Leukoc
Biol. 87:779–789. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Beg AA: Endogenous ligands of Toll-like
receptors: implications for regulating inflammatory and immune
responses. Trends Immunol. 23:509–512. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Arroyo DS, Soria JA, Gaviglio EA,
Rodriguez-Galan MC and Iribarren P: Toll-like receptors are key
players in neurodegeneration. Int Immunopharmacol. 11:1415–1421.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Downes CE and Crack PJ: Neural injury
following stroke: are Toll-like receptors the link between the
immune system and the CNS? Br J Pharmacol. 160:1872–1888. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lehnardt S, Lachance C, Patrizi S, et al:
The Toll-like receptor TLR4 is necessary for
lipopolysaccharide-induced oligodendrocyte injury in the CNS. J
Neurosci. 22:2478–2486. 2002.PubMed/NCBI
|
|
24
|
Lehnardt S, Massillon L, Follett P, et al:
Activation of innate immunity in the CNS triggers neurodegeneration
through a Toll-like receptor 4-dependent pathway. Proc Natl Acad
Sci USA. 100:8514–8519. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tanga FY, Nutile-McMenemy N and DeLeo JA:
The CNS role of Toll-like receptor 4 in innate neuroimmunity and
painful neuropathy. Proc Natl Acad Sci USA. 102:5856–5861. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jung HW, Chung YS, Kim YS and Park YK:
Celastrol inhibits production of nitric oxide and proinflammatory
cytokines through MAPK signal transduction and NF-κB in
LPS-stimulated BV-2 microglial cells. Exp Mol Med. 39:715–721.
2007.PubMed/NCBI
|