Introduction
Glioblastoma is the most common and lethal primary
malignant brain tumor, with an annual incidence of 4.96
cases/100,000 individuals, and accounts for approximately 50% of
all gliomas (1). Glioblastoma is
also an uncommon type of malignancy characterized by localization
in the brain, highly invasive behavior and extremely poor
prognosis. Currently, the treatment strategies for glioblastoma
mainly include surgery, chemotherapy and radiation therapy;
however, the overall median survival time of patients is only ~15
months (2). To improve the
quality of life in patients with glioblastoma, research is focusing
on its pathogenesis and novel therapeutic targets such as microRNAs
(miRNAs) (3).
miRNAs are a class of small, non-coding,
single-stranded RNAs which negatively regulate gene expression at
the post-transcriptional level, mainly by binding to the
3′-untranslated region (3′-UTR) of their target mRNAs. Numerous
studies have demonstrated that aberrant expression of miRNAs is
closely associated with proliferation, invasion, metastasis and
prognosis in various types of cancer (4–6).
The development of glioblastoma is associated with dysregulation of
multiple miRNAs including miR-21 (7), miR-29b, miR-125a (8), miR-137 (9), miR-328 (10), miR-218 (11), miR-124 and others (12). miR-124 is enriched in brain with a
crucial role in neural development and has been shown to be
downregulated in glioma (13),
suggesting it functions as a tumor suppressor in brain tumor
progression. Studies have shown that miR-124 regulates growth,
invasiveness, stem-like traits, differentiation, apoptosis of
glioblastoma cells, and these processes have been correlated to
multiple target genes (3,13–17). miRNAs and their target genes may
represent promising therapeutic targets for glioblastoma.
PPP1R3L, an inhibitory member of the
apoptosis-stimulating protein of p53 family (IASPP), is able to
promote apoptosis through negative regulation of p53 or by
inhibiting the transcriptional activity of p63/p73 on promoters of
proapoptotic genes independent of p53 (18–20) always upregulated in malignant
tumors (21–23). In addition, it is also reported
that PPP1R3L is also involved in tumorigenesis, cell growth, cell
cycle progression, metastasis and chemoresistance (24–27). In glioblastoma cells, silencing of
PPP1R3L leads to cell proliferation inhibition and cell cycle
arrest (28). Analysis indicates
that PPP1R13L is a theoretical target gene of miR-124. But whether
PPP1R13L is a direct target of miR-124 has not been confirmed,
particularly in glioblastoma cells.
Therefore, in the present study, we investigated the
regulation of PPP1R13L expression by miR-124 and their effects on
proliferation, cell cycle transition and invasion in glioblastoma
cell lines U251 and U373. Our results may provide data for
supporting miR-124/PPP1R13L as novel therapeutic, diagnostic or
prognostic tools for glioblastoma.
Materials and methods
Tumor tissue sample preparation
A total of 9 patients diagnosed with glioblastoma
were recruited. The experimental protocols were approved by the
ethics committee of our hospital. RNA or protein samples prepared
from the tumor tissues and normal tissues were then subjected to
quantitative RT-PCR and western blot analysis.
Cell culture and transfection
Glioblastoma cell lines U251 (p53 mutant) and U373
(p53 mutant) were purchased from the China Center for Type Culture
Collection (CCTCC, Wuhan, China) and maintained in RPMI-1640
(HyClone, Logan, UT, USA) containing 10% fetal bovine serum (FBS)
(Hangzhou Sijiqing) in a humidified atmosphere of 5% CO2
at 37°C. For functional analysis, cells were transfected with
miR-124 negative control (scrambled miRNA control), miR-124 mimics,
or miR-124 inhibitor (Ambion), using Lipofectamine® 2000
(Invitrogen Life Technologies, Carlsbad, CA, USA) according to the
manufacturer’s recommendations.
Real-time RT-PCR
Total RNA was extracted from cells with TRIzol
reagent (Invitrogen) following the manufacturer’s instructions. The
relative expression level of miR-124 was determined by quantitative
real-time RT-PCR using the mirVana™ qRT-PCR microRNA Detection kit
(Ambion) following the manufacturer’s instructions. Specific primer
sets for miR-124 and U6 (used as an internal reference) were
obtained from Ambion. Expression of PPP1R13L mRNA was detected by
real-time RT-PCR using the standard SYBR-Green RT-PCR kit (Takara
Bio, Inc., Otsu, Japan) following the manufacturer’s instructions.
The specific primer pairs are as follows: PPP1R13L (131 bp), sense,
5′-GTGGCACGGGTG TTGGCGGA-3′ and antisense, 5′-CGATGGAAGAGGCG
GCTGATG-3′; β-actin (202 bp) as an internal control, sense,
5′-AGGGGCCGGACTCGTCATACT-3′ and antisense, 5′-GG
CGGCACCACCATGTACCCT-3′. The relative expression of PPP1R13L mRNA or
miR-124 was quantified using GraphPad Prism 4.0 software (GraphPad
Software, San Diego, CA, USA) and the 2−ΔΔCt method
(29).
Dual luciferase reporter assay
The 3′-UTR of PPP1R13L (NM_001142502) containing the
miRNA-124 binding sites and its corresponding mutated sequence were
cloned into psi-CHECK2 luciferase reporter vector (Promega)
downstream of Renilla luciferase, named 3′-UTR PPP1R13L and
3′-UTR Mut PPP1R13L, respectively. Using Lipofectamine 2000, U251
and U373 cells were co-transfected with the reporter constructs and
miR-124 mimics, miR-124 inhibitor, negative control (NC) or
negative control inhibitor. Luciferase activity was determined
after 48 h using the Dual-Glo substrate system (Promega) and a
Beckman Coulter LD 400 luminometer. Data are presented as the ratio
of experimental (Renilla) luciferase to control (Firefly)
luciferase.
Cell proliferation assay
U251 and U373 cells transfected with NC, miR-124
mimics or its inhibitor in exponential growth were plated at a
final concentration of 2×103 cells/well in 96-well
plates. The viability of cells was evaluated by MTT assay after 24,
48, 72 and 96 h of seeding. The optical density at 570 nm (OD570)
of each well was measured with an ELISA reader (ELX-800 Type;
BioTek Instruments, Inc., Winooski, VT, USA).
Colony formation assay
The effect of ectopic expression of miR-124 on the
colony formation of U373 and U251 cells was analyzed by colony
formation assay. Parent cells, NC, miR-124 mimics or miR-124
inhibitor-transfected cells in a 6-well plate (200 cells/well) were
cultured for 2 weeks. The cell colonies were washed, fixed and
stained with Giemsa. Individual colonies with more than 50 cells
were counted.
Cell cycle analysis by flow cytometry
(FCM)
The cells were digested and collected after 48 h
post-transfection and washed with PBS twice. The cells were
resuspended in PBS and then fixed in 70% ethanol at 4°C for 18 h.
The cells were washed with PBS and resuspended in staining solution
[50 μg/ml of propidium iodide (PI), 1 mg/ml of RNase A, and
0.1% Triton X-100 in PBS]. The stained cells (1×105)
were then analyzed with a flow cytometer (Beckman Coulter, Miami,
FL, USA).
Cell invasion assay
The cell invasion assay was performed using a cell
invasion assay kit (Chemicon International, Temecula, CA, USA)
according to the manufacturer’s guidelines as described by
Aspenström et al (30).
Briefly, U251 or U373 cells were placed in the upper compartment of
the chambers, and RPMI-1640 containing 10% FBS was added in the
lower chambers. After 24 h of incubation at 37°C, cells on the
upper face of the membrane were wiped off using a cotton swab and
cells on the lower face were fixed, stained and observed under a
microscope. The dye on the membrane was then dissolved with 10%
acetic acid, dispensed into 96-well plates (150 μl/well),
and the optical density at 570 nm (OD570) of each well was measured
with an ELISA reader (ELX-800 Type).
Western blotting
Cells were lysed in cell lysate and then centrifuged
at 12,000 × g for 20 min at 4°C. The supernatant was collected and
denatured. Proteins were separated in 10% SDS-PAGE and blotted onto
polyvinylidene difluoride (PVDF) membranes. The PVDF membranes were
treated with TBST containing 50 g/l skimmed milk at room
temperature for 4 h, followed by incubation with the primary
antibodies, anti-PPP1R13L, anti-MMP-9, anti-MMP-13 and anti-β-actin
(Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) respectively,
at 37°C for 1 h. Membranes were rinsed and incubated for 1 h with
the corresponding peroxidase-conjugated secondary antibodies.
Chemiluminescence detection was performed with the ECL kit (Pierce
Chemical, Rockford, IL, USA). The amount of the protein of
interest, expressed as arbitrary densitometric units, was
normalized to the densitometric units of β-actin.
Statistical analysis
Data are expressed as means ± SD from at least three
separate experiments. Statistical analysis was carried out using
SPSS 15.0 software. The difference between two groups was analyzed
by the Student’s t-test. A value of P<0.05 was considered to
indicate a statistically significant result.
Results
miR-124 is downregulated in glioblastoma
tissues
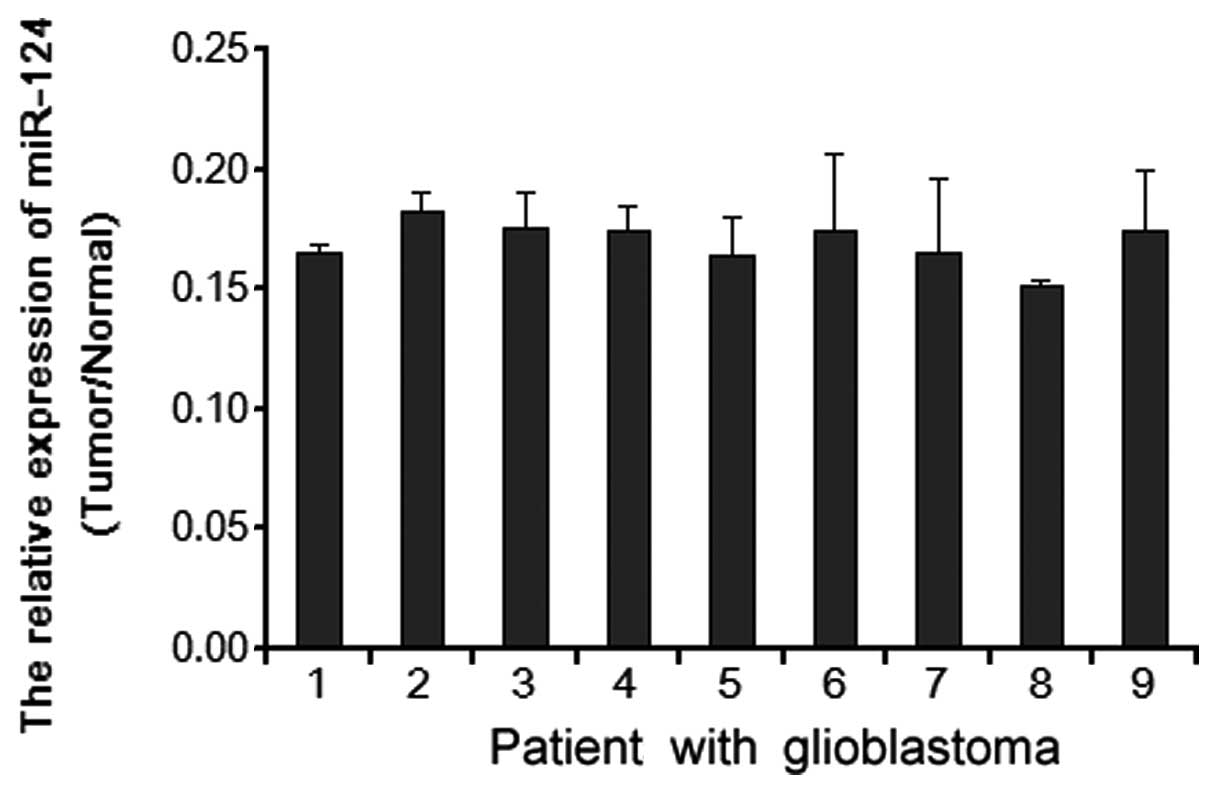
Real-time quantitative RT-PCR analyses revealed that
miR-124 was markedly downregulated in 9 examined tissue samples
paired with adjacent nontumor tissues (normal) from the same
patient (Fig. 1), indicating that
miR-124 is downregulated in human glioblastoma.
miR-124 regulates PPP1R13L expression in
glioblastoma cells
miR-124 and PPP1R13L play an important role in
cancer development. But whether PPP1R13L is the target gene of
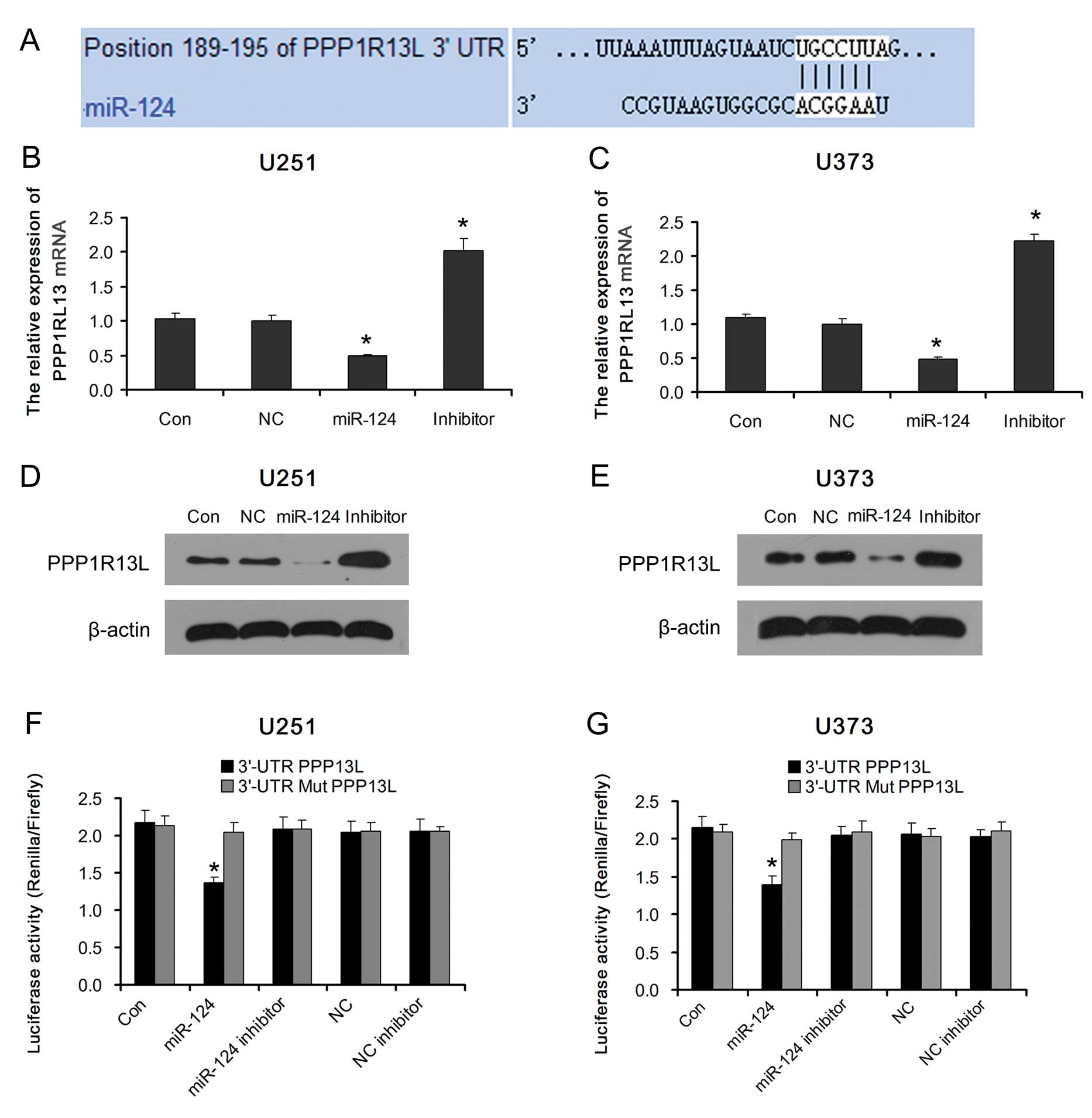
miR-124 in glioblastoma cells is unclear. Analysis using available
algorithms indicated that PPP1R13L is a theoretical target gene of
miR-124 (Fig. 2A). As predicted,
ectopic expression of miR-124 in glioblastoma U251 and U373 cells
decreased the expression of PPP1R13L mRNA and protein. Consistent
with this result, PPP1R13L was upregulated in both types of
glioblastoma cells transfected with the miR-124 inhibitor (Fig. 2B–E).
Furthermore, we subcloned the PPP1R13L
3′-untranslated region (3′-UTR) fragment containing the miR-124
binding site and mutated targeting sequence cloned into psi-CHECK2
dual luciferase reporter vectors (named 3′-UTR PPP1R13L and 3′-UTR
Mut PPP1R13L, respectively). The result showed that ectopic
expression of miR-124 significantly inhibited the luciferase
activity in glioblastoma U251 and U373 cells transfected with the
3′-UTR PPP1R13L reporter vector. The luciferase activity levels in
glioblastoma U251 and U373 cells transfected with the 3′-UTR Mut
PPP1R13L reporter vector or miR-124 inhibitor were restored
(Fig. 2F and G). Taken together,
our results demonstrated that PPP1R13L is a target of miR-124 and
an inverse correlation exists between miR-124 and PPP1R13L in
glioblastoma cells.
miR-124-mediated PPP1R13L regulates
proliferation of glioblastoma cells
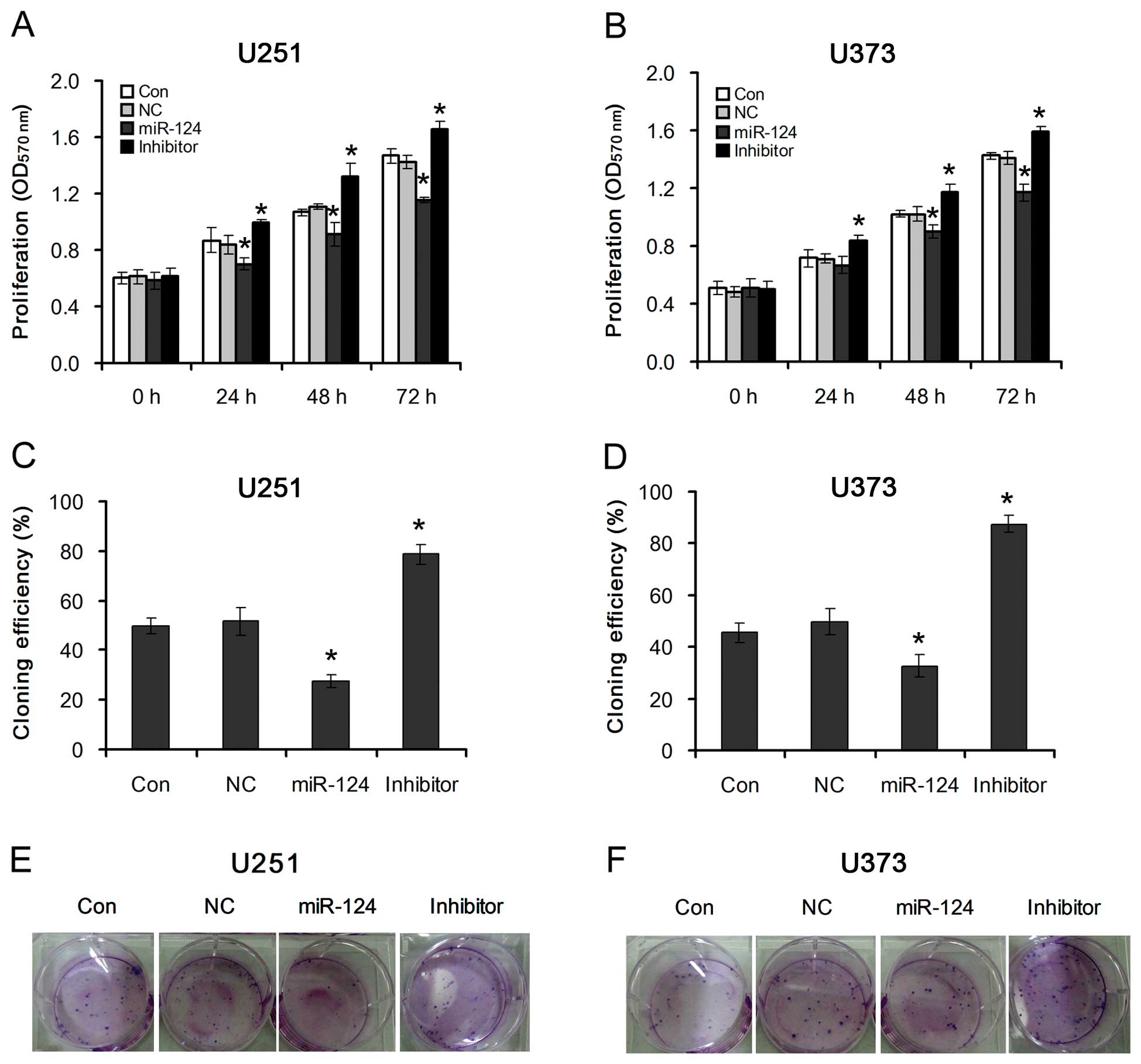
To investigate the effects of miR-124-mediated
PPP1R13L on the proliferation of glioblastoma cells, U251 and U373
cells were transfected with miR-124 mimics or its inhibitor. By
using MTT and colony formation assays, we observed that
overexpression of miR-124 dramatically decreased the growth rate of
both types of glioblastoma cells as compared with that of the
NC-transfected cells. However, inhibition of miR-124 increased the
growth rate of both types of glioblastoma cells as compared with
that of the NC-transfected cells (Fig. 3A and B). This suggests that
downregulation of miR-124, which results in PPP1R13L upregulation,
specifically promotes the proliferation of glioblastoma cells.
miR-124-mediated PPP1R13L regulates G1/S
phase transition of glioblastoma cells
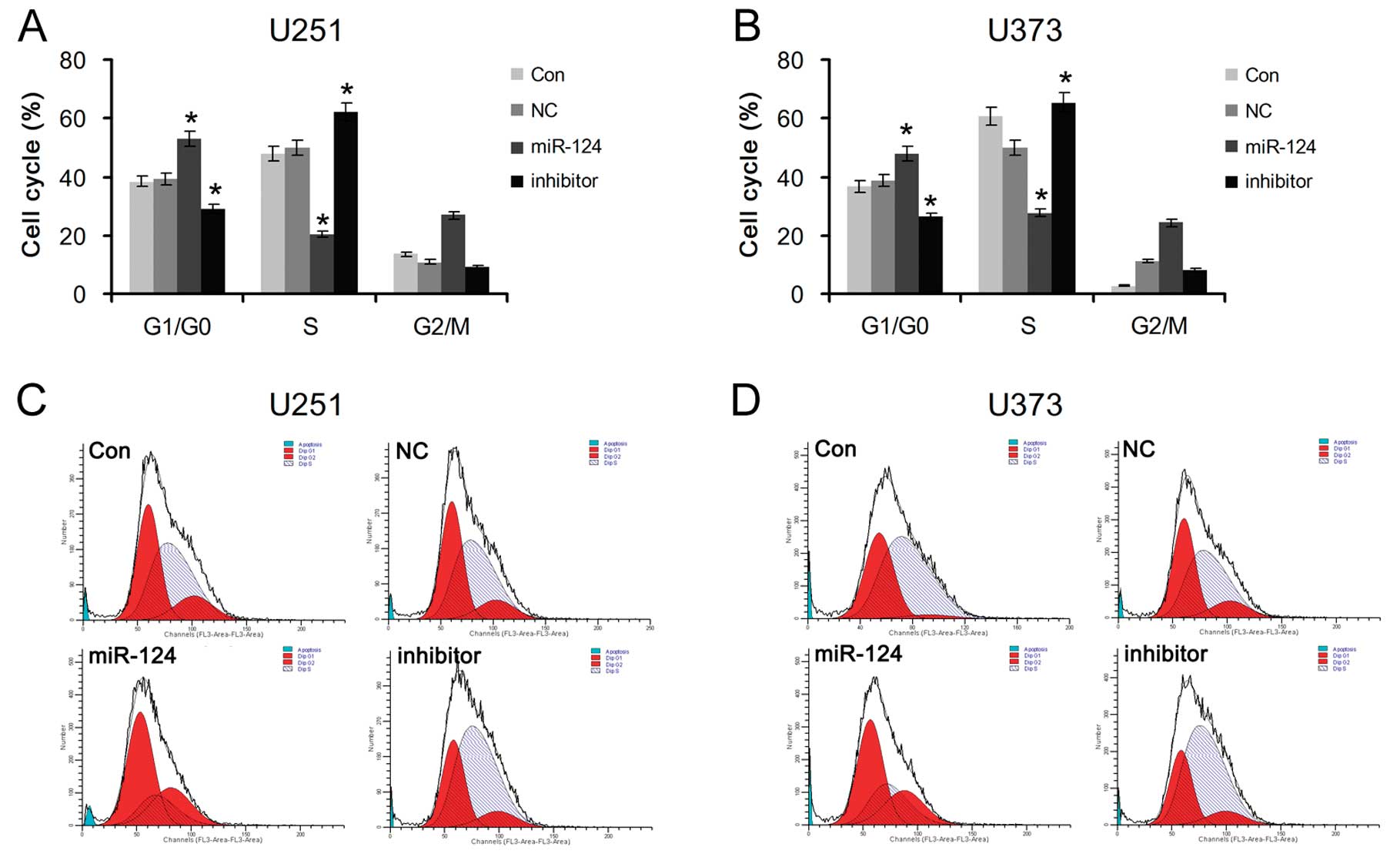
To further explore whether miR-124-mediated PPP1R13L
increases the proliferation of glioblastoma cells by cell cycle
transition, U251 and U373 cells transfected with miR-124 mimics or
its inhibitor were analyzed by flow cytometry. The results showed a
significant increase in the percentage of cells in the G1/G0 phase
and a decrease in the percentage of cells in the S phase in the
miR-124-overexpressing cells, and a decrease in G1/G0 phase cells
and an increase in S-phase cells in the glioblastoma cells
transfected with the miR-124 inhibitor (Fig. 4). The above data suggest that
enhancement of glioblastoma cell growth by downregulation of
miR-124 may be mediated through regulation of cellular entry into
the G1/S transition phase and its target PPP1R13L is possibly
implicated in this process.
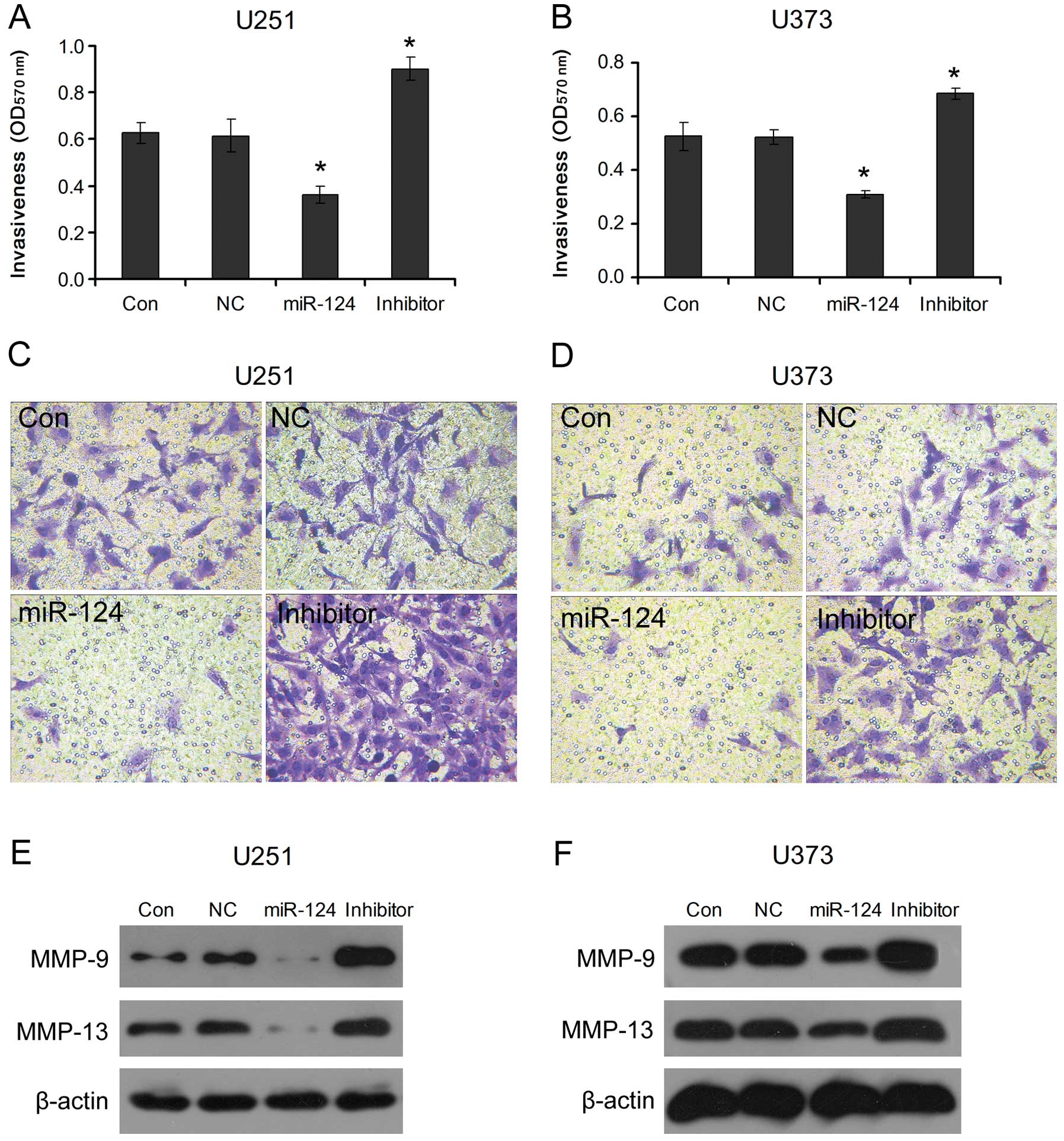
miR-124-mediated PPP1R13L regulates
invasion of glioblastoma cells
To understand the effects of miR-124-mediated
PPP1R13L on glioblastoma cell metastasis, the invasiveness of the
glioblastoma U251 and U373 cells transfected with miR-124 mimics or
its inhibitor was examined. We observed that, compared to NC,
overexpression of miR-124 in U251 and U373 cells obviously
inhibited their invasive ability; however, the invasive ability was
increased after inhibition of miR-124 in U251 and U373 cells
(Fig. 5A–D). Meanwhile, we found
that MMP-9 and MMP-13, molecular markers of cancer metastasis, were
decreased after transfection of the cells with miR-124 mimics, and
the miR-124 inhibitor increased their expression levels as compared
with NC (Fig. 5E and F). This
indicates that loss of miR-124 expression mediates PPP1R13L
upregulation consequently promoting glioblastoma cell invasion.
Discussion
miR-124 is known to play an important role in the
progression of diverse types of cancers (5,31).
In the present study, we found that miR-124 was downregulated in
the examined 9 tissue samples, which is consistent with a previous
report (12). Our data further
illustrate that miR-124 serves as a tumor suppressor in
glioblastoma and its abnormal downregulation facilitates tumor
progression. It is well known that miRNAs function mainly via
specific binding to 3′-UTR of target genes. Theoretic analysis
indicated that the 3′-UTR of PPP1R13L includes the binding sites of
miR-124. To validate this presumption, glioblastoma U251 and U373
cells were transfected with miR-124 mimics or its inhibitor, and
the results showed that the PPP1R13L protein level was accordingly
decreased or increased, suggesting that miR-124 negatively
regulates PPP1R13L expression in glioblastoma cells and that
PPP1R13L is the potential target gene of miR-124. The dual
luciferase reporter assay showed that only miR-124 mimics could
inhibit the luciferase activity in glioblastoma U251 and U373 cells
transfected with the 3′-UTR PPP1R13L reporter vector. Therefore,
for the first time, we demonstrated that PPP1R13L is a target gene
of miR-124 in glioblastoma cells.
The potential effects of the miR-124/PPP1R13L
pathway on glioblastoma U251 and U373 cells was investigated. The
results showed that upregulation of miR-124, which led to decreased
PPP1R13L expression, inhibited cell proliferation, colony
formation, G1/S phase transition and invasive ability of
glioblastoma cells. However, downregulation of miR-124 by its
inhibitor, which led to increased PPP1R13L expression, promoted
these processes. These results suggest that miR-124 downregulation
may play a critical role in malignant progression of glioblastoma,
and its mechanism of action involves PPP1R13L.
The mechanisms underlying the function of miR-124 as
a tumor suppressor in glioma are implicated in the inhibition of
proliferation (e.g. via targeting SLC16A1) (13), cell cycle progression (e.g. via
targeting CDK6) (14,15), invasiveness (17), differentiation (e.g. by
suppressing Twist and SLUG) (16), stem cell characteristics (e.g. via
targeting SNAI2, NRAS or PIM3) (3,17).
In the present study, several of these molecules or others possibly
participated in the process of the effects of miR-124 on the growth
and invasive ability of glioblastoma U251 and U373 cells. We
believe that PPP1R13L, as a target of miR-124, also plays a certain
role in the miR-124-mediated suppression of growth and metastasis
of glioblastoma cells. Studies indicate that PPP1R13L is important
for tumor cell proliferation. Downregulation of PPP1R13L leads to
growth inhibition in various types of cancers (25,32,33). It has been reported that
downregulation of PPP1R13L expression inhibits proliferation and
induces cell cycle arrest at G0/G1 phase, which involves the
increase in cyclin D1 and a decrease in p21waf1/cip1
expression in U251 cells (28).
PPP1R3L is also associated with invasion and lymph node metastasis,
as validated in embedded endometrial endometrioid adenocarcinoma
(34). Overexpression of PPP1R3L
increases the invasiveness of tumor cells through a p53-dependent
or p53-independent pathway (35).
Our results confirmed that miR-124 negatively regulates PPP1R13L
expression in glioblastoma cells with mutant p53. Although miR-124
has numerous target genes, it is logical to deduce that the
promotion of cell proliferation, cell cycle progression and
invasion in glioblastoma cells by miR-124 downregulation is, at
least, partly due to PPP1R13L upregulation.
In conclusion, miR-124 is downregulated in
glioblastoma, and negatively regulates PPP1R13L expression in
glioblastoma U251 and U373 cells. miR-124 downregulation-mediated
malignant progression of glioblastoma is partly attributed to
increased PPP1R13L expression. Consequently, our findings provide a
molecular basis for the role of miR-124/PPP1R13L in the progression
of human glioblastoma cells and suggest a novel target for the
treatment of glioblastoma.
References
|
1.
|
Baldi I, Huchet A, Bauchet L and Loiseau
H: Epidemiology of glioblastoma. Neurochirurgie. 56:433–440. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Preusser M, de Ribaupierre S, Wöhrer A, et
al: Current concepts and management of glioblastoma. Ann Neurol.
70:9–21. 2011. View Article : Google Scholar
|
|
3.
|
Lang MF, Yang S, Zhao C, et al:
Genome-wide profiling identified a set of miRNAs that are
differentially expressed in glioblastoma stem cells and normal
neural stem cells. PLoS One. 7:e362482012. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Skalsky RL and Cullen BR: Reduced
expression of brain-enriched microRNAs in glioblastomas permits
targeted regulation of a cell death gene. PLoS One. 6:e242482011.
View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Sun Y, Zhao X, Zhou Y and Hu Y: miR-124,
miR-137 and miR-340 regulate colorectal cancer growth via
inhibition of the Warburg effect. Oncol Rep. 28:1346–1352.
2012.PubMed/NCBI
|
|
6.
|
Blenkiron C and Miska EA: miRNAs in
cancer: approaches, aetiology, diagnostics and therapy. Hum Mol
Genet. 16:R106–R113. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Li Y, Zhao S, Zhen Y, et al: A miR-21
inhibitor enhances apoptosis and reduces G(2)-M accumulation
induced by ionizing radiation in human glioblastoma U251 cells.
Brain Tumor Pathol. 28:209–214. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Cortez MA, Nicoloso MS, Shimizu M, et al:
miR-29b and miR-125a regulate podoplanin and suppress invasion in
glioblastoma. Genes Chromosomes Cancer. 49:981–990. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Chen L, Wang X, Wang H, et al: miR-137 is
frequently down-regulated in glioblastoma and is a negative
regulator of Cox-2. Eur J Cancer. 48:3104–3111. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Wu Z, Sun L, Wang H, et al: MiR-328
expression is decreased in high-grade gliomas and is associated
with worse survival in primary glioblastoma. PLoS One.
7:e472702012. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Liu Y, Yan W, Zhang W, et al: MiR-218
reverses high invasiveness of glioblastoma cells by targeting the
oncogenic transcription factor LEF1. Oncol Rep. 28:1013–1021.
2012.PubMed/NCBI
|
|
12.
|
Li D, Chen P, Li XY, et al: Grade-specific
expression profiles of miRNAs/mRNAs and docking study in human
grade I–III astrocytomas. OMICS. 15:673–682. 2011.PubMed/NCBI
|
|
13.
|
Li KK, Pang JC, Ching AK, et al: miR-124
is frequently down-regulated in medulloblastoma and is a negative
regulator of SLC16A1. Hum Pathol. 40:1234–1243. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Silber J, Lim DA, Petritsch C, et al:
miR-124 and miR-137 inhibit proliferation of glioblastoma
multiforme cells and induce differentiation of brain tumor stem
cells. BMC Med. 6:142008. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Pierson J, Hostager B, Fan R and Vibhakar
R: Regulation of cyclin-dependent kinase 6 by microRNA 124 in
medulloblastoma. J Neurooncol. 90:1–7. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Xie YK, Huo SF, Zhang G, et al: CDA-2
induces cell differentiation through suppressing Twist/SLUG
signaling via miR-124 in glioma. J Neurooncol. 110:179–186. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Xia H, Cheung WK, Ng SS, et al: Loss of
brain-enriched miR-124 microRNA enhances stem-like traits and
invasiveness of glioma cells. J Biol Chem. 287:9962–9971. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Cai Y, Qiu S, Gao X, Gu SZ and Liu ZJ:
iASPP inhibits p53-independent apoptosis by inhibiting
transcriptional activity of p63/p73 on promoters of proapoptotic
genes. Apoptosis. 17:777–783. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Alsafadi S, Tourpin S, André F, Vassal G
and Ahomadegbe JC: P53 family: at the crossroads in cancer therapy.
Curr Med Chem. 16:4328–4344. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Bergamaschi D, Samuels Y, Sullivan A, et
al: iASPP preferentially binds p53 proline-rich region and
modulates apoptotic function of codon 72-polymorphic p53. Nat
Genet. 38:1133–1141. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Chen J, Xie F, Zhang L and Jiang WG: iASPP
is over-expressed in human non-small cell lung cancer and regulates
the proliferation of lung cancer cells through a p53 associated
pathway. BMC Cancer. 10:6942010. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Lu B, Guo H, Zhao J, et al: Increased
expression of iASPP, regulated by hepatitis B virus X
protein-mediated NF-κB activation, in hepatocellular carcinoma.
Gastroenterology. 139:2183–2194. 2010.PubMed/NCBI
|
|
23.
|
Zhang X, Wang M, Zhou C, Chen S and Wang
J: The expression of iASPP in acute leukemias. Leuk Res.
29:179–183. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Pang MS, Chen X, Lu B, et al: Lentiviral
vector-mediated doxycycline-inducible iASPP gene targeted RNA
interference in hepatocellular carcinoma. Chin J Cancer.
29:796–801. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Lin BL, Xie DY, Xie SB, et al:
Down-regulation of iASPP in human hepatocellular carcinoma cells
inhibits cell proliferation and tumor growth. Neoplasma.
58:205–210. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Liu Z, Zhang X, Huang D, et al: Elevated
expression of iASPP in head and neck squamous cell carcinoma and
its clinical significance. Med Oncol. 29:3381–3388. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Jiang L, Siu MK, Wong OG, et al: iASPP and
chemoresistance in ovarian cancers: effects on paclitaxel-mediated
mitotic catastrophe. Clin Cancer Res. 17:6924–6933. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Li G, Wang R, Gao J, Deng K, Wei J and Wei
Y: RNA interference-mediated silencing of iASPP induces cell
proliferation inhibition and G0/G1 cell cycle
arrest in U251 human glioblastoma cells. Mol Cell Biochem.
350:193–200. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Arocho A, Chen B, Ladanyi M and Pan Q:
Validation of the 2-DeltaDeltaCt calculation as an alternate method
of data analysis for quantitative PCR of BCR-ABL P210 transcripts.
Diagn Mol Pathol. 15:56–61. 2006.PubMed/NCBI
|
|
30.
|
Aspenström P, Fransson A and Saras J: Rho
GTPases have diverse effects on the organization of the actin
filament system. Biochem J. 377:327–337. 2004.PubMed/NCBI
|
|
31.
|
Shi XB, Xue L, Ma AH, et al: Tumor
suppressive miR-124 targets androgen receptor and inhibits
proliferation of prostate cancer cells. Oncogene. Oct 15–2012.(Epub
ahead of print). View Article : Google Scholar
|
|
32.
|
Liu T, Li L, Yang W, et al: iASPP is
important for bladder cancer cell proliferation. Oncol Res.
19:125–130. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Zhang B, Xiao HJ, Chen J, Tao X and Cai
LH: Inhibitory member of the apoptosis-stimulating protein of p53
(ASPP) family promotes growth and tumorigenesis in human
p53-deficient prostate cancer cells. Prostate Cancer Prostatic Dis.
14:219–224. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Liu WK, Jiang XY, Ren JK and Zhang ZX:
Expression pattern of the ASPP family members in endometrial
endometrioid adenocarcinoma. Onkologie. 33:500–503. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Laska MJ, Lowe SW, Zender L, et al:
Enforced expression of PPP1R13L increases tumorigenesis and
invasion through p53-dependent and p53-independent mechanisms. Mol
Carcinog. 48:832–842. 2009. View
Article : Google Scholar : PubMed/NCBI
|