Introduction
Adipose tissue is not only a simple energy store,
but is increasingly being recognized as an important organ in the
regulation of metabolism and pathological processes. Adipokines are
a series of soluble factors secreted by adipose tissue. There are
several known types of adipokines, such as relatively well known
leptin, adiponectin, interleukin-6 and resistin, as well as
visfatin and retinol-binding protein 4 (RBP4) (1–3).
Visfatin is a 52-kDa cytokine secreted by visceral fat and its
expression level in plasma increases with the severity of obesity.
Visfatin exerts insulin-mimetic effects and its plasma levels
increase in overweight and obese patients with metabolic syndrome
(2,4). RBP4 was initially thought to
function only in the delivery of retinol to tissues (5). However, the transgenic
overexpression of human RBP4 causes insulin resistance and glucose
intolerance through a retinol-independent mechanism, whereas the
normalization of serum RBP4 induces insulin sensitivity. In obese
patients with type 2 diabetes mellitus, serum levels of RBP4 are
increased (1,6). Substances involved in the regulation
of adipokine expression in adipocytes are currently being
investigated. Such substances may participate in energy elevation,
sugar or lipid metabolic processes and the overall process of
obesity within the body.
Estrogen deficiency during menopause causes
excessive visceral adipose tissue accumulation which is known to be
linked to metabolic syndrome (7).
Loss of ovarian function is related to both an increase in total
fat and an accumulation of central fat, which increases the risk of
cardiovascular and metabolic disease (8–11).
Adipose tissue metabolism is directly influenced by sex hormones,
particularly estrogen, and the estrogen receptor (ER) is expressed
at the mRNA and protein level in human adipose tissues (12). ERα and ERβ are both expressed in
adipose tissue and bind estrogen with different affinities
(13,14). The physiological role of ERβ
appears to be a modulator of ERα activity in vitro (15). However, very little is known about
the effects of the two subtypes of ERs on the expression of
visfatin and RBP4.
In this study, we aimed to demonstrate the effects
of estrogen via ERs on visfatin and RBP4 expression by manipulating
the concentration of estradiol (E2) and ERα- and β-selective
agonists and antagonists in 3T3-L1 adipocytes.
Materials and methods
Materials
We used 17β-estradiol (1,3,5[10]-estratriene-3,
17β-diol, cell culture tested) purchased from Sigma (St. Louis, MO,
USA). The ERα selective agonist,
4,4′,4″-(4-propyl-[1H]-pyrazole-1,3,5-triyl)trisphenol (PPT), the
ERβ selective agonist, 2,3-bis(4-hydroxyphenyl)-propionitrile
(DPN), the ERα selective antagonist,
1,3-bis(4-hydroxyphenyl)-4-methyl-5-[4-
(2-piperidinylethoxy)phenol]-1H-pyrazole dihydrochloride (MPP), and
the ERβ pure antagonist and partial ERα agonist, (5R,
11R)-5,11-diethyl-5,6,11,12-tetrahydro-2,8-chrysenediol
[(R,R)-THC], were purchased from Tocris Bioscience (Ellisville, MO,
USA). Anti-mouse RBP4 and anti-mouse visfatin antibodies were
obtained from R&D Systems (Minneapolis, MN, USA). Anti-GAPDH
antibody was purchased from Bio-Rad Laboratories (Hercules, CA,
USA).
Cell culture
Mouse 3T3-L1 fibroblasts (American Type Culture
Collection, Manassas, VA, USA) were plated at 5×104
cells in Dulbecco’s modified Eagle’s medium (DMEM) supplemented
with 25 mM glucose, 10% fetal bovine serum (FBS), 500 units/ml
penicillin and 500 μg/ml streptomycin (medium A) at 37°C in a 5%
CO2 humidified atmosphere. The cells were grown in the
same medium until three days after confluence and were then
differentiated into mature adipocytes by treatment with 500 μM
3-isobutyl-1-methylxanthine (IBMX), 250 nM dexamethasone and 330 nM
insulin in medium A. The cells were then incubated for two days in
medium A containing 330 nM insulin, followed by four days of
incubation in medium A. The medium was changed every two days.
After eight days of incubation, the intracytoplasmic accumulation
of lipid droplets was observed in the fully differentiated 3T3-L1
adipocytes and they were stained with Oil Red O solution (0.5% Oil
Red O in isopropanol). For all the experiments, mature adipocytes
were serum-starved for 12 h and then incubated with or without
chemical reagents at various concentrations. At first, the cells
were incubated in sterile medium containing various concentrations
of E2. To modulate ERα and ERβ expression in adipocytes, the ER
agonists, PPT or DPN, were added to the cells at several
concentrations. In subsequent experiments, the ER antagonists, MPP
or (R,R)-THC, were added to cells along with a constant dose of E2
for stimulation. These cells were used for the measurement of RBP4
and visfatin expression.
Assessment of cell viability and cell
number
Cultured cells were detached from the culture dishes
with 0.05% trypsin-EDTA (Gibco BRL, Life Technologies, Merelbeke,
Belgium) at 72 h of culture under different culture conditions. The
cells were stained with trypan blue (Gibco BRL, Life Technologies)
and viable cells were counted on a hemocytometer without
staining.
Total RNA isolation and reverse
transcription reaction
RNA was extracted and purified using an RNeasy lipid
tissue mini kit as suggested by the manufacturer (Qiagen, Valencia,
CA, USA). The RNA concentration was measured using a
spectrophotometer (DU®530; Beckman Coulter, Fullerton,
CA, USA) and RNA quality was confirmed on agarose gels. A total RNA
sample (2 μg/sample) was used for cDNA synthesis in a volume of 20
μl using a SuperScript™ III First-Strand Synthesis System for
RT-PCR kit (Invitrogen, Milano, Italy). RNA was reverse-transcribed
under the following conditions: 25 mM MgCl2, 10 mM dNTP
mix, 10X RT buffer, 0.1 M DTT, 200 U of SuperScript™ III
(Invitrogen), 40 U of RNaseOut and 50 μM oligo(dT) primers in a
final volume of 20 μl. The reaction was incubated at 65°C for 5 min
and 50°C for 50 min and the enzyme was then heat-inactivated at
85°C for 5 min. Four microliters of the reaction product were used
for quantitative PCR.
Quantitative PCR
Quantitative PCR was used to quantify the mRNA
expression of RBP4 and visfatin. The expression was normalized
using the GAPDH housekeeping gene product as an internal reference.
The primers and probes were designed for mouse RBP4 and visfatin
using Primer Express® Software version 2.0 (Applied
Biosystems, Foster City, CA, USA). RBP4 and visfatin mRNA levels
were quantified using TaqMan Real-Time PCR with an ABI 7700 system
(Applied Biosystems). Gene-specific probes and primer pairs for
RBP4 (Assays-on-Demand, Mm00803264_m1; Applied Biosystems) and
visfatin (Assays-on-Demand, Mm00451938_m1; Applied Biosystems) were
used. For each probe/primer set, a standard curve was generated,
which was confirmed to increase linearly with increasing amounts of
cDNA. The amplification conditions were 2 min at 50°C, 10 min at
95°C and a two-step cycle of 95°C for 15 sec and 60°C for 60 sec
for a total of 45 cycles.
Western blot analysis
The cells were lysed using a buffer containing 50 mM
HEPES (pH 7.5), 150 mM NaCl, 1.5 mM MgCl2, 1 mM EDTA,
10% glycerol, 1% Triton X-100 and a mixture of protease inhibitors
[aprotinin, phenylmethyl sulfonyl fluoride (PMSF) and sodium
orthovanadate]. Equal amounts of total protein were resolved on a
12% SDS-polyacrylamide gel and proteins were transferred onto a
nitrocellulose membrane. After blocking (TBS, 0.1% Tween-20) at 4°C
overnight, the membranes were incubated with primary antibodies for
anti-mouse RBP4 (dilution 1:1000) or anti-mouse visfatin (dilution
1:1000) for 2 h followed by incubation with secondary antibodies
linked to HRP, anti-mouse GAPDH (dilution 1:2000). Immunoreactive
proteins were visualized by chemiluminescence using
SuperSignal® West Dura Extended Duration Substrate
(Pierce Chemical Co., Rockford, IL, USA) and a Fujifilm Luminescent
Image Analyzer LAS-3000 with a charge-coupled device camera
(Science Imaging Scandinavia AB).
Statistical analysis
To compare the mRNA expression levels of RBP4 and
visfatin in adipocytes, analysis of variance (ANOVA) with a
post-hoc Dunnett’s test was used. Data are presented as the means ±
standard error of the mean. To evaluate the presence of a
correlation, Pearson’s correlation coefficient and linear
regression analysis were used. Null hypotheses of no difference
were rejected if P<0.05. All statistical analyses were performed
using the SPSS statistical package version 10.0 (SPSS, Inc.,
Chicago, IL, USA).
Results
Effects of estradiol on expression of
RBP-4 and visfatin in adipocytes
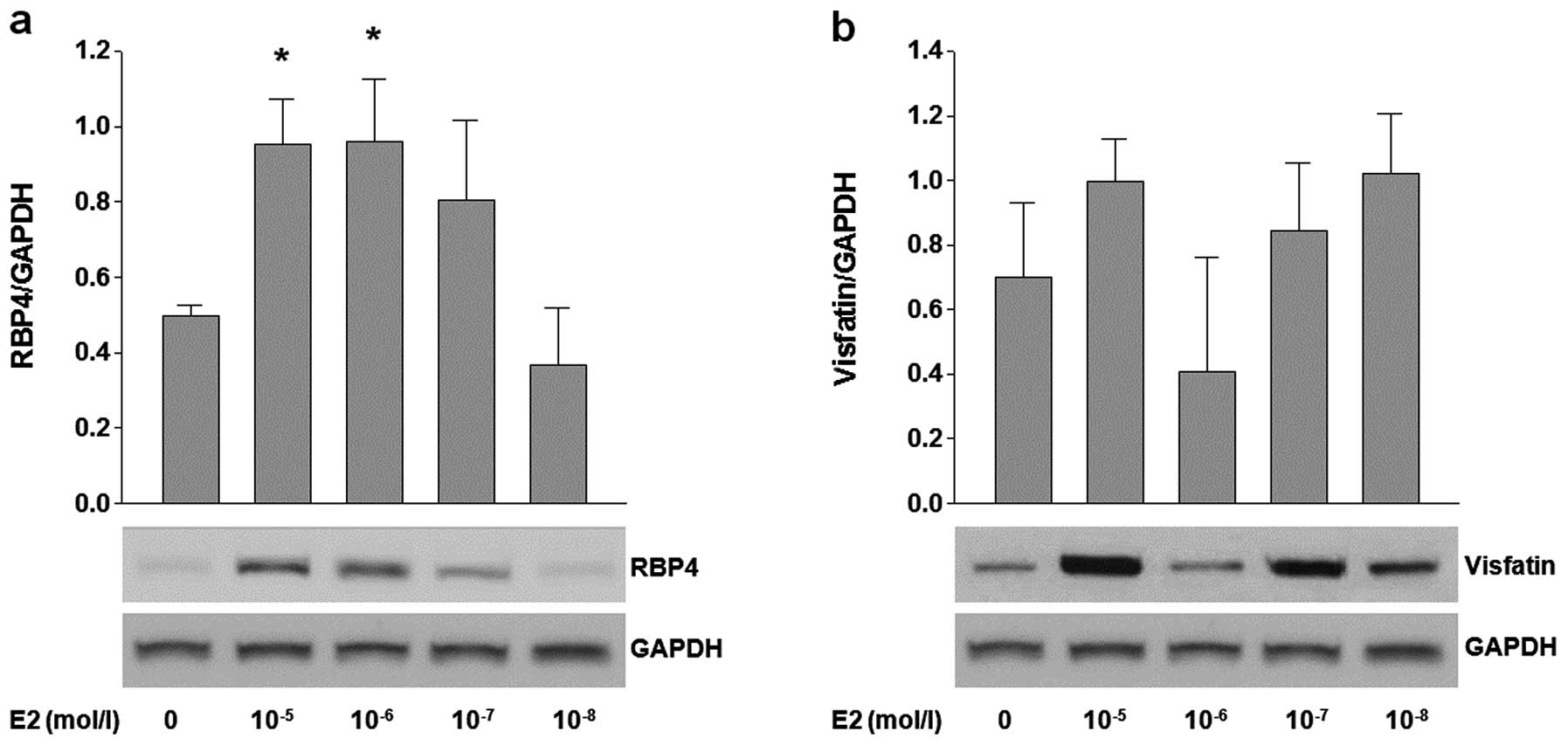
To investigate the effects of E2 on the expression
of RBP4 and visfatin, 3T3-L1 adipocytes were treated with various
concentrations (10−5–10−9 mol/l) of E2.
Treatment with high concentrations (10−5 and
10−6 mol/l) of E2 significantly increased the RBP4 mRNA
levels (P=0.012, P=0.011, respectively), as well as RBP4 protein
expression (Fig. 1a). However,
the expression of visfatin was not influenced by any tested
concentration of E2 (Fig.
1b).
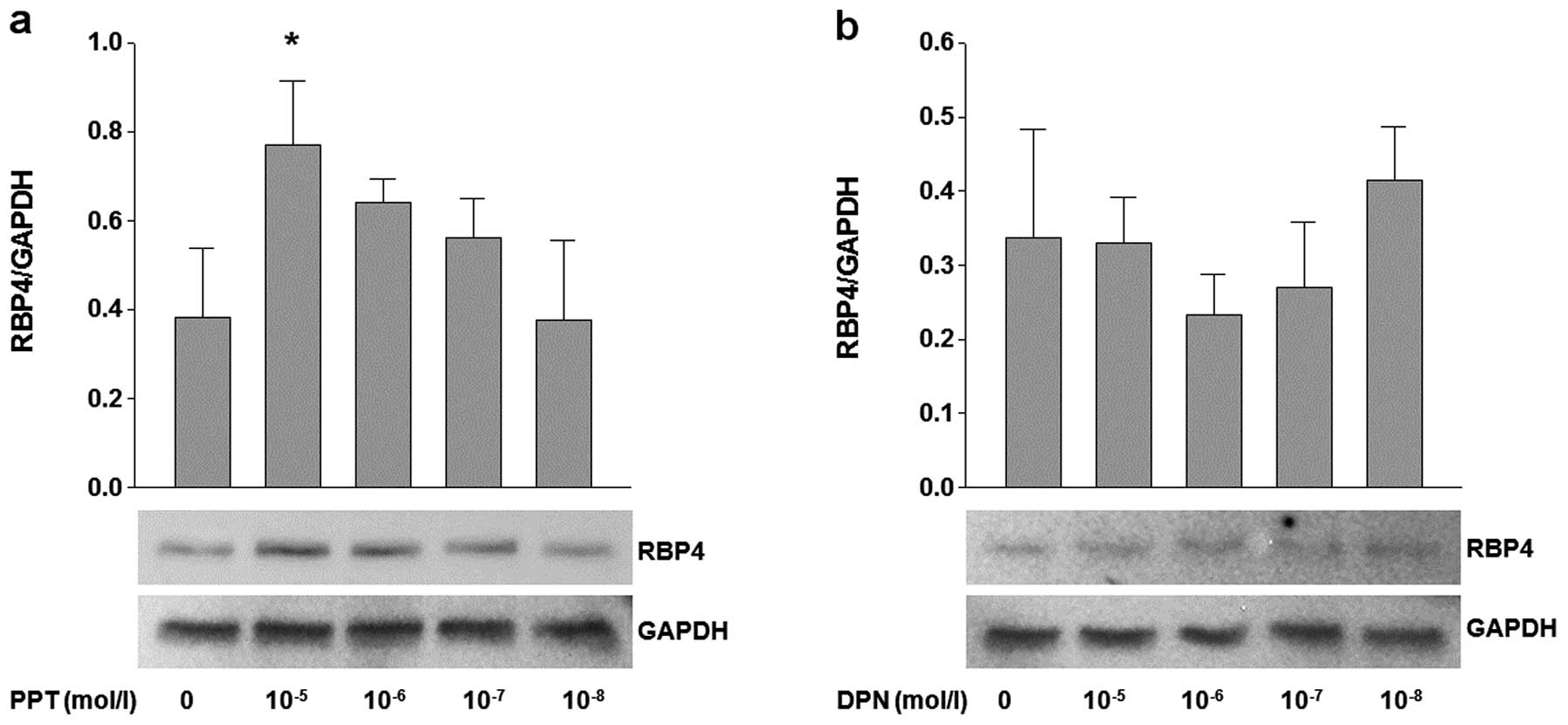
Effects of ERα and ERβ agonists (PPT and
DPN) on the expression of RBP4 and visfatin
The adipocytes were treated with various
concentrations of PPT (ERα agonist) and DPN (ERβ agonist) to
investigate the effects of ERα and ERβ on the expression of RBP4
and visfatin. The cells treated with 10−5 mol/l PPT
showed a significant and dose-dependent increase in RBP4 mRNA
(P<0.05) and protein expression (Fig. 2a). On the other hand, the
adipocytes treated with DPN showed no difference in expression
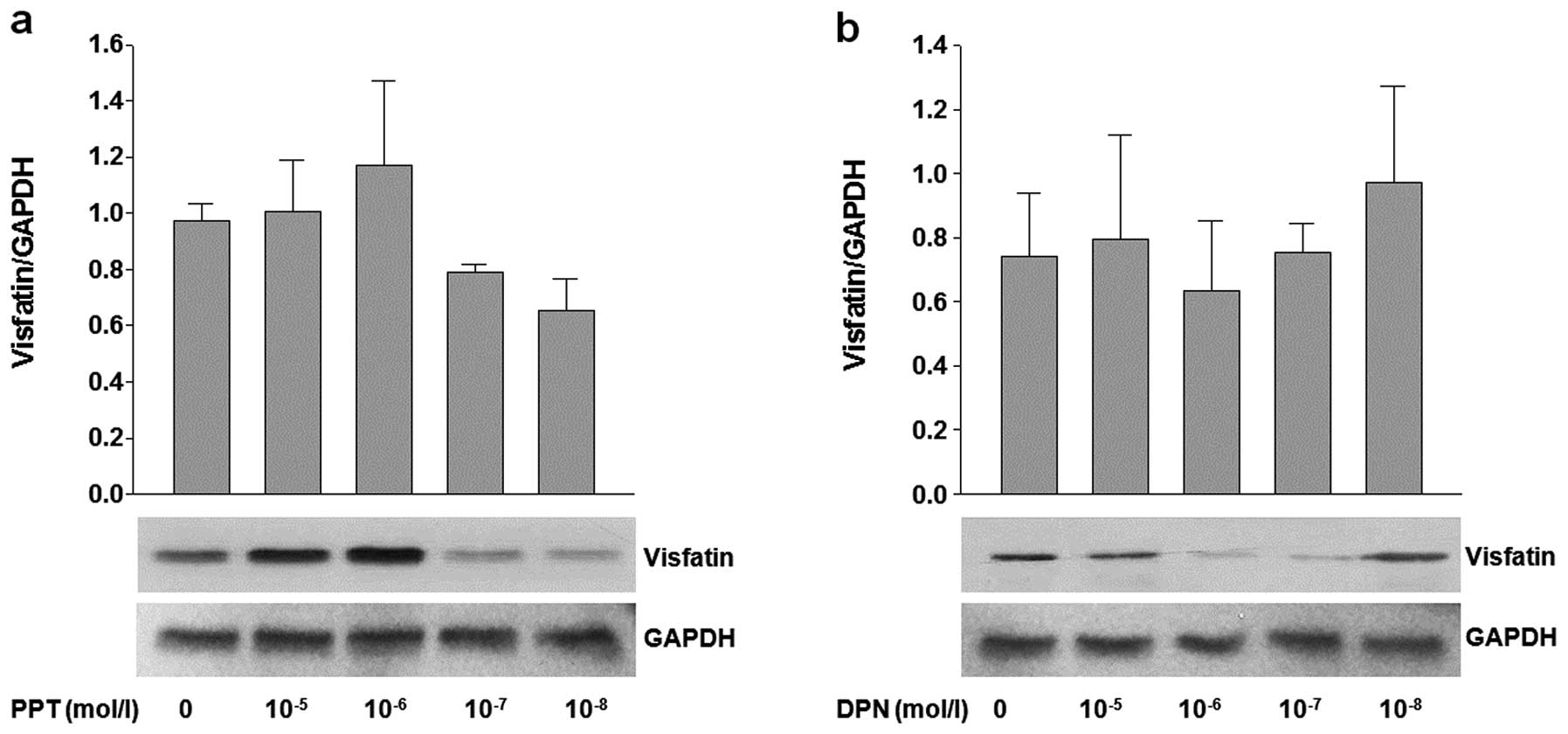
(Fig. 2b). Of note, the mRNA and
protein expression of visfatin was not influenced by treatment with
PPT and DPN (Fig. 3a and b).
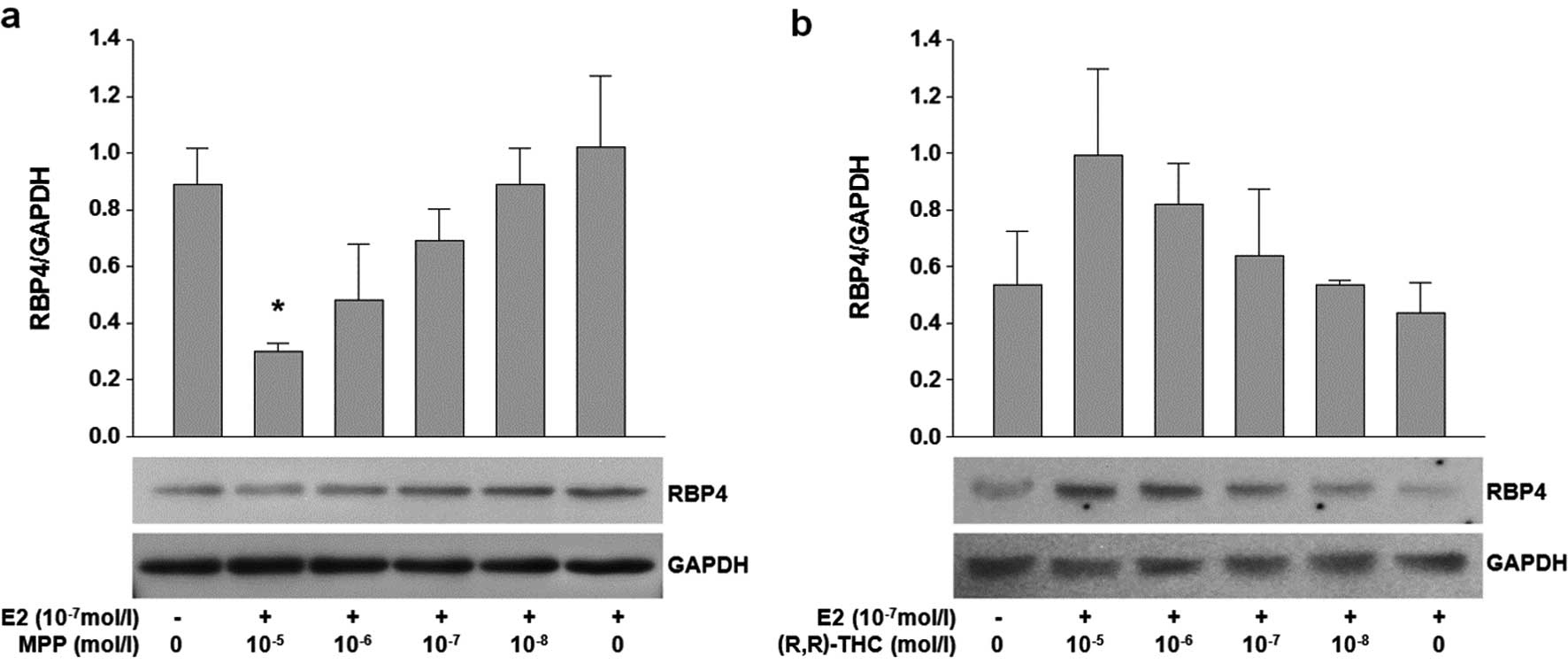
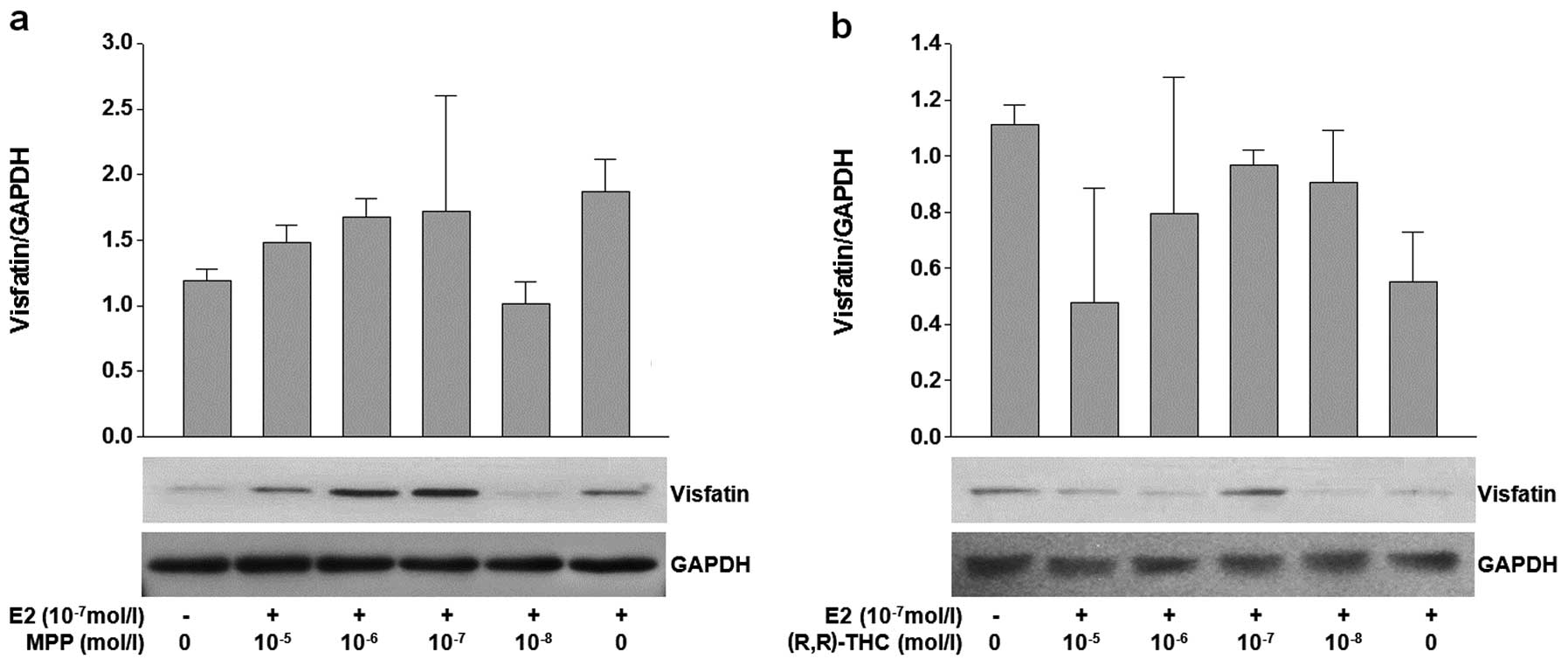
Effects of ERα and ERβ antagonists [MPP
and (R,R)-THC] on the expression of RBP4 and visfatin
In parallel with the ER agonist experiment, ER
antagonists [MPP and (R,R)-THC] were used to verify the functions
of ER subtypes in fat cells. The aim of this experiment was to
isolate the effects of each ER subtype. The cells were treated
simultaneously with a fixed concentration of E2 (10−7
mol/l) to provide an appropriate stimulus. Treatment with high
concentrations (10−5 mol/l) of MPP and E2
(10−7 mol/l) resulted in the reduced expression of RBP4
at the mRNA and protein level (P=0.032). No significant change in
the mRNA level of RBP4 was observed following treatment with
variable concentrations of (R,R)-THC (Fig. 4a and b). There was no significant
change in the expression of visfatin upon treatment with various
concentrations of MPP or (R,R)-THC (Fig. 5a and b).
Discussion
In this study, we evaluated the effects of
17β-estradiol on RBP4 and visfatin expression in 3T3-L1 adipocytes.
RBP4 mRNA expression and protein production was increased in a
dose-dependent manner in adipocytes by estradiol. RBP4 does not
reach adult plasma levels until puberty, after which plasma levels
are changeable, based on the menstrual cycle (16–18). This variation in the plasma RBP
level appears to correlate with peak levels of estradiol and
menopausal status in females. Previous studies have reported that
plasma RBP4 levels in post-menopausal women are higher than those
in pre-menopausal women, which is associated with insulin
resistance (19,20). After menopause, women develop
increased amounts of visceral fat due to fat redistribution. RBP4
expression is highly elevated, not just in serum, but also in
visceral fat, and serum RBP4 protein levels are considered a marker
of intra-abdominal fat mass (21). Thus, we initially assumed that a
correlation may exist between specific adipokines in visceral fat
and estrogen. ERα and β densities are more dependent on the
location of adipose deposition than on gender, with visceral depots
showing higher mRNA densities (22). Our results demonstrated that
17β-estradiol significantly increased the secretion of RBP4 and
upregulated the expession of RBP4 in 3T3-L1 adipocytes, which is
consistent with the results of previous studies (23,24).
In a previous study, Janke et al reported
that RBP4 gene expression in adipose tissue was decreased in obese
menopausal subjects and that there were no differences in serum
RBP4 levels among lean, overweight and obese menopausal subjects
(25). However, their experiment
used human subcutaneous adipose tissue in vivo and the RBP4
level is not related to subcutaneous fat mass. The serum RBP4 level
does not correlate with the subcutaneous fat diameter, but rather
with the visceral fat diameter (26).
The reason estrogen significantly increases RBP4
expression in adipose tissues is unclear. Vitamin A (retinol) is
taken up by peripheral tissue, such as the genital tracts in the
form of free retinol by passive diffusion based on the
concentration gradient between the blood and cytosol. Retinol is
therefore thought to play a pivotal role in the female reproductive
organs. In addition, estrogen itself appears to control retinoic
acid biosynthesis. Estrogen has been shown to markedly increase the
cellular RBP4 mRNA content in rat vagina and uterus tissues, which
participate in the uptake and/or intracellular metabolism of
retinol (27). We found that
estrogen significantly upregulated the expression of RBP4 in 3T3-L1
adipocytes. Thus, the estrogen-induced upregulation of RBP4
expression in adipose tissue is expected to result in a shift in
the equilibrium of retinoic acid in the reproductive organs. Our
results suggest that estrogen mediates retinoic acid metabolism by
the regulation of RBP4 expression in adipose tissue. The systemic
deficiency of serum estrogen may activate a specific regulatory
process and consequently, may induce the overexpression of RBP4
through ER in visceral adipocytes. These data, together with our
finding that estrogen stimulates RBP4 expression in adipocytes,
this process may be partially due to a decrease in serum estrogen
levels after menopause.
Visfatin may play an important role in adipocyte
metabolism in association with metabolic syndrome-related diseases.
Visfatin is regulated by conditions associated with metabolic
syndrome and visfatin mRNA expression is regulated by sex hormones
in 3T3-L1 pre-adipocytes (28).
The estriol treatment of 3T3-L1 cells has been shown to increase
visfatin gene expression, but estradiol had insignificant effects
on visfatin gene expression (29). These results are partly consistent
with our finding that visfatin was not directly influenced by
estrodiol in 3T3-L1 adipocytes.
Estradiol selectively influences adipose tissue,
according to the type of adipokine. ERα and β control the
expression of leptin in different ways (30). However, our results verified that
adipokines can be influenced independently by ERα without being
affected by ERβ. There are many existing studies on the effect of
sex hormones on adipokine expression, but the results of these
studies differ according to the type of adipokine or the
experimental design (31–33). These differences suggest that the
pathway may be dependent on adipokine type and may be established
through the specific ER type. However, a comprehensive analysis of
the effects of steroidal hormones on adipokine expression is
warranted.
We found that estrogen significantly increased RBP4
expression via ERα in 3T3-L1 adipocytes without influencing
visfatin expression, suggesting a novel role for ERα in the
regulation of RBP4 expression. To the best of our knowledge, this
is the first demonstration that 17β-estradiol significantly
increases the secretion of RBP4 and upregulates RBP4 expression via
ERα, but not ERβ, in 3T3-L1 adipocytes. RBP4 may have a specific
function in visceral fat redistribution in menopausal women.
Through the control of sex hormones in menopausal women, the
regulation of RBP4 expression in adipose tissue may potentially
inhibit visceral fat redistribution and ultimately protect
post-menopausal women from obesity-related diseases. Further in
vivo studies are required to investigate the link between RBP4
expression and estrogen, while considering the effects of estrogen
status, including the menstrual cycle. In addition, the results of
this study need to be clinically verified. The development of novel
therapeutics for metabolic syndrome-related diseases will require
further understanding of the correlation between the effects of
estrogen and adipokines.
Acknowledgements
This study was supported in part by the Konyang
University Myunggok Research Fund of 2009.
References
|
1
|
Yang Q, Graham TE, Mody N, et al: Serum
retinol binding protein 4 contributes to insulin resistance in
obesity and type 2 diabetes. Nature. 436:356–362. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fukuhara A, Matsuda M, Nishizawa M, et al:
Visfatin: a protein secreted by visceral fat that mimics the
effects of insulin. Science. 307:426–430. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Klein J, Perwitz N, Kraus D and Fasshauer
M: Adipose tissue as source and target for novel therapies. Trends
Endocrinol Metab. 17:26–32. 2006.PubMed/NCBI
|
|
4
|
Filippatos TD, Derdemezis CS, Kiortsis DN,
Tselepis AD and Elisaf MS: Increased plasma levels of
visfatin/pre-B cell colony-enhancing factor in obese and overweight
patients with metabolic syndrome. J Endocrinol Invest. 30:323–326.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tilg H and Moschen AR: Adipocytokines:
mediators linking adipose tissue, inflammation and immunity. Nat
Rev Immunol. 6:772–783. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Masaki T, Anan F, Tsubone T, et al:
Retinol binding protein 4 concentrations are influenced by renal
function in patients with type 2 diabetes mellitus. Metabolism.
57:1340–1344. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wajchenberg BL: Subcutaneous and visceral
adipose tissue: their relation to the metabolic syndrome. Endocr
Rev. 21:697–738. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bjorntorp P: Adipose tissue distribution
and function. Int J Obes. 15(Suppl 2): 67–81. 1991.
|
|
9
|
Tchernof A and Poehlman ET: Effects of the
menopause transition on body fatness and body fat distribution.
Obes Res. 6:246–254. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Carr MC: The emergence of the metabolic
syndrome with menopause. J Clin Endocrinol Metab. 88:2404–2411.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tchernof A, Desmeules A, Richard C, et al:
Ovarian hormone status and abdominal visceral adipose tissue
metabolism. J Clin Endocrinol Metab. 89:3425–3430. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mizutani T, Nishikawa Y, Adachi H, et al:
Identification of estrogen receptor in human adipose tissue and
adipocytes. J Clin Endocrinol Metab. 78:950–954. 1994.PubMed/NCBI
|
|
13
|
Kuiper GG, Carlsson B, Grandien K, et al:
Comparison of the ligand binding specificity and transcript tissue
distribution of estrogen receptors alpha and beta. Endocrinology.
138:863–870. 1997.PubMed/NCBI
|
|
14
|
Pedersen SB, Bruun JM, Hube F, Kristensen
K, Hauner H and Richelsen B: Demonstration of estrogen receptor
subtypes alpha and beta in human adipose tissue: influences of
adipose cell differentiation and fat depot localization. Mol Cell
Endocrinol. 182:27–37. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu MM, Albanese C, Anderson CM, et al:
Opposing action of estrogen receptors alpha and beta on cyclin D1
gene expression. J Biol Chem. 277:24353–24360. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vahlquist A, Rask L, Peterson PA and Berg
T: The concentrations of retinol-binding protein, prealbumin, and
transferrin in the sera of newly delivered mothers and children of
various ages. Scand J Clin Lab Invest. 35:569–575. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Michaelsson G, Vahlquist A, Juhlin L,
Mellbin T and Bratt L: Zinc and vitamin A: serum concentrations of
zinc and retinol-binding protein (RBP) in healthy adolescents.
Scand J Clin Lab Invest. 36:827–832. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Vahlquist A, Johnsson A and Nygren KG:
Vitamin A transporting plasma proteins and female sex hormones. Am
J Clin Nutr. 32:1433–1438. 1979.PubMed/NCBI
|
|
19
|
Suh JB, Kim SM, Cho GJ, Choi KM, Han JH
and Taek Geun H: Elevated serum retinol-binding protein 4 is
associated with insulin resistance in older women. Metabolism.
59:118–122. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
An C, Wang H, Liu X, et al: Serum
retinol-binding protein 4 is elevated and positively associated
with insulin resistance in postmenopausal women. Endocr J.
56:987–996. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kloting N, Graham TE, Berndt J, et al:
Serum retinol-binding protein is more highly expressed in visceral
than in subcutaneous adipose tissue and is a marker of
intra-abdominal fat mass. Cell Metab. 6:79–87. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rodriguez-Cuenca S, Monjo M, Proenza AM
and Roca P: Depot differences in steroid receptor expression in
adipose tissue: possible role of the local steroid milieu. Am J
Physiol Endocrinol Metab. 288:E200–E207. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tan BK, Chen J, Lehnert H, Kennedy R and
Randeva HS: Raised serum, adipocyte, and adipose tissue
retinol-binding protein 4 in overweight women with polycystic ovary
syndrome: effects of gonadal and adrenal steroids. J Clin
Endocrinol Metab. 92:2764–2772. 2007. View Article : Google Scholar
|
|
24
|
Whitman MM, Harnish DC, Soprano KJ and
Soprano DR: Retinol-binding protein mRNA is induced by estrogen in
the kidney but not in the liver. J Lipid Res. 31:1483–1490.
1990.PubMed/NCBI
|
|
25
|
Janke J, Engeli S, Boschmann M, et al:
Retinol-binding protein 4 in human obesity. Diabetes. 55:2805–2810.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tschoner A, Sturm W, Engl J, et al:
Retinol-binding protein 4, visceral fat, and the metabolic
syndrome: effects of weight loss. Obesity (Silver Spring).
16:2439–2444. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Matsuda M, Masui F and Mori T: Neonatal
estrogenization leads to increased expression of cellular retinol
binding protein 2 in the mouse reproductive tract. Cell Tissue Res.
316:131–139. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
MacLaren R, Cui W and Cianflone K:
Visfatin expression is hormonally regulated by metabolic and sex
hormones in 3T3-L1 pre-adipocytes and adipocytes. Diabetes Obes
Metab. 9:490–497. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhou J and Seidel ER: Estrogens induce
visfatin expression in 3T3-L1 cells. Peptides. 31:271–274. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yi KW, Shin JH, Seo HS, et al: Role of
estrogen receptor-alpha and -beta in regulating leptin expression
in 3T3-L1 adipocytes. Obesity (Silver Spring). 16:2393–2399. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Watanobe H and Suda T: A detailed study on
the role of sex steroid milieu in determining plasma leptin
concentrations in adult male and female rats. Biochem Biophys Res
Commun. 259:56–59. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kristensen K, Pedersen SB and Richelsen B:
Regulation of leptin by steroid hormones in rat adipose tissue.
Biochem Biophys Res Commun. 259:624–630. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chen YH, Lee MJ, Chang HH, Hung PF and Kao
YH: 17 beta-estradiol stimulates resistin gene expression in 3T3-L1
adipocytes via the estrogen receptor, extracellularly regulated
kinase, and CCAAT/enhancer binding protein-alpha pathways.
Endocrinology. 147:4496–4504. 2006. View Article : Google Scholar
|