Introduction
Ricin is a 65-kDa glycoprotein toxin produced by the
seeds of Ricinus communis and has been classified as a
ribosome-inactivating protein (RIP) (1). Ricin consists of a cytotoxic A chain
(RTA) and a galactose-binding B chain (RTB) linked by a disulfide
bond (2). The A-chain is 32 kDa,
targets the ribosome and inhibits protein synthesis in mammalian
cells; the B-chain is 34 kDa and is a galactose-binding lectin
protein that binds to the eukaryotic cell membrane by interacting
with cell-surface molecules, such as galactose and glycolipids
(3,4). RTB mediates ricin endocytosis and
the delivery of RTA to the cytosol of target cells, and RTB has
attracted attention due to its well-characterized endocytotic
trafficking and efficacy over a wide range of cell types (5,6).
Studies have shown that RTB can be successfully used as a carrier
fused to other molecules. When RTB was fused to rotavirus
nonstructural protein 4 (NSP4), the molecule stimulated a Th1
lymphocyte response (7). In
addition, an RTB-GFP fusion protein expressed in tobacco has been
shown to generate humoral immune responses in immunized mice,
indicating a Th2 response (8).
However, an understanding of the immune response induced by RTB is
necessary to determine the mechanisms by which RTB acts as an
immunostimulant.
Macrophages play an important role in the activation
of the adaptive immune system by responding to pathogens, tumor
cells and toxicants by changing from a resting to an activated
state (9–11). Upon activation, macrophages
produce many types of inflammatory mediators, such as interleukin
(IL)-6, tumor necrosis factor (TNF)-α, and nitric oxide (NO)
molecules that are central to the immunoregulatory function of
macrophages (12–14).
The NO produced by inducible NOS (iNOS) is a major
acute and chronic inflammatory mediator (15,16). Indeed, the NO released by
activated macrophages mediates a number of host-defense functions
and contributes to immune-mediated tissue destruction disorders
(17,18).
In the present study, we investigated the effects of
RTB on iNOS, IL-6 and TNF-α, as well as the signal transduction
mechanisms involved in recombinant RTB-induced macrophage
activation. This may help to identify the most important target
molecules for the development of novel drug therapies.
Materials and methods
Materials
RTB was cloned and express in Escherichia
coli. The protein was purified using nickel-NTA column
chromatography. Lipopolysaccharide (LPS) contamination was <0.03
pg/μg protein for recombinant RTB as determined by the Limulus
amebocyte lysate assay (BioWhittaker, Inc., Walkersville, MD, USA).
L-N monomethyl arginine (L-NMMA), genistein, SB203580, LY294002 and
BAY 11–7082 were obtained from Sigma (St. Louis, MO, USA).
Antibodies against iNOS were purchased from Cell Signaling
Technology, Inc. (Danvers, MA, USA) and the one-step RT-PCR kit was
purchased from Takara Bio, Inc. (Shiga, Japan).
Cell culture
RAW264.7 cells were cultured in RPMI-1640 medium
supplemented with 10% fetal calf serum (FCS) (HyClone, Logan, UT,
USA), 100 U/ml penicillin and 100 U/ml streptomycin at 37°C in a
humidified atmosphere of 5% CO2. Cells were seeded in
24-well plates and allowed to grow overnight, then treated with
fresh medium or different concentrations of RTB. In another set of
cultures, the cells were co-incubated with RTB (10 μg/ml) and the
tyrosine kinase inhibitor, genistein (10 μM), the
phosphatidylinositol 3-kinase (PI3K) inhibitorm LY294002 (10 μM),
the p42/44 inhibitor, PD98059 (50 μM), the p38 inhibitor, SB203580
(4 μM), the JNK inhibitor, SP600125 (10 μM), the protein kinase C
(PKC) inhibitor, staurosporine (50 nM), the JAK2 inhibitor,
tyrphostin (AG490) (25 μM), or the NOS inhibitor, L-NMMA (500
μM).
Western blot analysis
The RAW264.7 cells were treated with RTB for the
indicated periods of time, collected and lysed in 100 μl RIPA lysis
buffer (Beyotime Biotechnology, Shanghai, China) for 30 min at 4°C.
The lysates were centrifuged at 15,000 × g for 20 min at 4°C, and
the protein content of the lysates was determined using a BCA assay
kit (Beyotime Biotechnology). The protein supernatants were
separated by 10% SDS-PAGE and transferred onto polyvinylidene
fluoride (PVDF) membranes (Millipore, Billerica, MA, USA). The
membranes were blocked with 5% non-fat milk (Becton, Dickinson and
Co., Oxford, UK) in PBS for 1 h at room temperature and incubated
with anti-iNOS and then secondary antibodies. Immunodetection was
performed using Amersham ECL™ Western Blotting Detection Reagents
(GE Healthcare, Chalfont St. Giles, UK) according to the
manufacturer’s instructions.
RT-PCR analysis
Total RNA was isolated from 1×106
RAW264.7 cells using TRIzol reagent. RT-PCR analysis of the mRNA
expression of iNOS, IL-6 and TNF-α was performed using one-step
RT-PCR kits. GADPH was used as the housekeeping gene. The primer
sequences used were as follows: iNOS forward,
5′-CTGCAGCACTTGGATCAGGAAGCTG-3′ and reverse,
5′-GGGAGTAGCCTGTGTGCACCTCGAA-3′; IL-6 forward,
5′-TTCCCTACTTCACAAGTC-3′ and reverse, 5′-ACTAGGTTTGCCGAGTAG-3′; and
TNF-α forward, 5′-TTCTGTCTACTGAACTTCGGGGTGATCGGTCC-3′ and reverse,
5′-GTATGAGATAGCAAATCGGCTGACGGTGT GGG-3′. The conditions for PCR
were 30 cycles of 94°C denaturation for 30 sec, 60°C annealing for
30 sec and 72°C extension for 60 sec. The PCR products were
analyzed by electrophoresis on 1.5% agarose gels stained with
ethidium bromide. The relative quantification of mRNA expression
was performed using β-actin as an internal control to normalize the
gene expression for the PCR templates.
Cytokine ELISA
The RAW264.7 cells were treated with RTB in the
presence or absence of different inhibitors, and the expression of
IL-6 and TNF-α in the culture supernatant was measured using ELISA
kits (BioLegend, San Diego, CA, USA).
NO production assay
The RAW264.7 cells were treated with RTB in the
presence of different inhibitors. NO generation was determined
using Griess reagent (Beyotime Biotechnology), with nitrite
production measured as the absorbance at 540 nm.
Phospho-specific protein microarray
analysis
Phosphoprotein arrays were obtained from Full Moon
Biosystems (Sunnyvale, CA, USA) and 228 site-specific tyrosine
phosphorylation profiles were detected with 6 replicates each. The
protein samples were biotinylated using Tyrosine Phosphorylation
ProArray (Full Moon BioSystems), and the biotin-labeled protein
samples were conjugated to antibodies using the Array Assay Kit
(Full Moon BioSystems). The conjugated, labeled protein was
detected using Cy3-streptavidin. Antibody array slides were scanned
using the GenePix 4000 B microarray scanner (Axon Instruments,
Union City, CA, USA). For data analysis, the fold change in
intensity of the 6 replicates was measured using GenePix Pro 6.0
software (Axon Instruments). After being divided by the averaged
actin value in each experiment, the changes in the phosphorylation
rate following treatment with RTB and tyrosine kinase inhibitor
(genistein) was computed as follows: increasing phosphorylation
ratio = (average value with RTB treatment)/average value without
RTB treatment; decreasing phosphorylation ratio = (average value
with inhibitor treatment - average value without inhibitor
treatment)/average value without inhibitor treatment.
Statistical analysis
The data are presented as the means ± SD. The
differences between the groups were analyzed using a paired
Student’s t-test. A p-value <0.05 was considered to indicate a
statistically significant difference; and p<0.01 a highly
statistically significant difference.
Results
Effects of RTB on iNOS expression in
RAW264.7 cells
In the RAW264.7 cells treated with RTB, the mRNA and
protein expression levels of iNOS increased in a dose-dependent
manner, as observed by RT-PCR (Fig.
1A) and western blot analysis (Fig. 1B). The mRNA and protein expression
of iNOS in the cells treated with LPS (50 ng/ml) was used as the
positive control. The addition of the iNOS inhibitor, L-NMMA (500
μM), decreased the NO production and iNOS mRNA synthesis induced by
RTB (Fig. 2 and 3A).
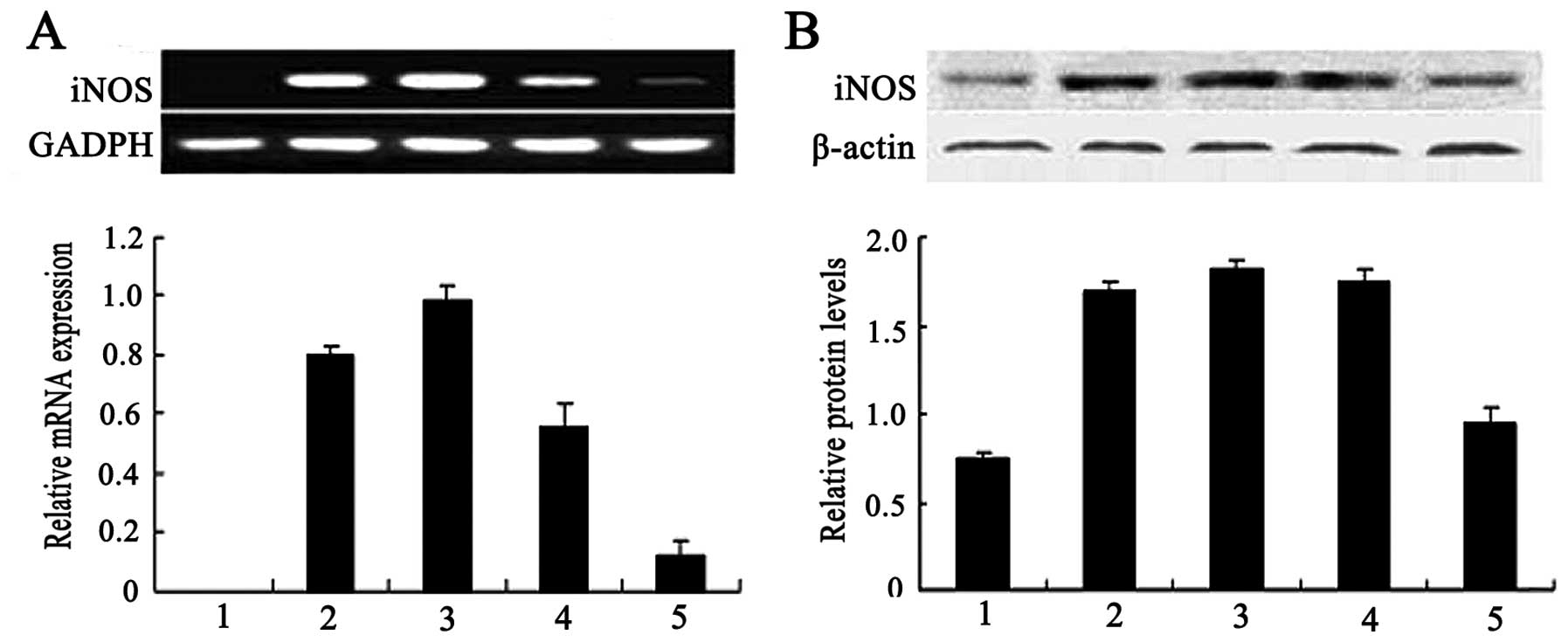 | Figure 1Effect of ricin toxin-binding subunit
B (RTB) on the mRNA and protein expression of increased inducible
nitric oxide (NO) synthase (iNOS) in RAW264.7 cells. Cells were
incubated with RTB (1, 10, 100 μg/ml) or lipopolysaccharide (LPS)
(50 ng/ml) for 24 h. (A) Total RNA was isolated and the mRNA
expression was examined by RT-PCR. Lane 1, untreated macrophages;
lane 2, LPS (50 ng/ml); lane 3, RTB (100 g/ml); lane 4, RTB (10
μg/ml); lane 5, RTB (1 μg/ml). (B) Cell lysates were prepared and
iNOS protein expression was examined by western blot analysis. The
lower panel represents the expression of GADPH and actin. Lane 1,
untreated macrophages; lane 2, LPS (50 ng/ml); lane 3, RTB (100
μg/ml); lane 4, RTB (10 μg/ml); lane 5, RTB (1 μg/ml). Shown are
representative results from 1 of 3 experiments with similar
results. Densitometric analysis showing the relative intensity of
the bands; relative ratios vs. GADPH or actin. |
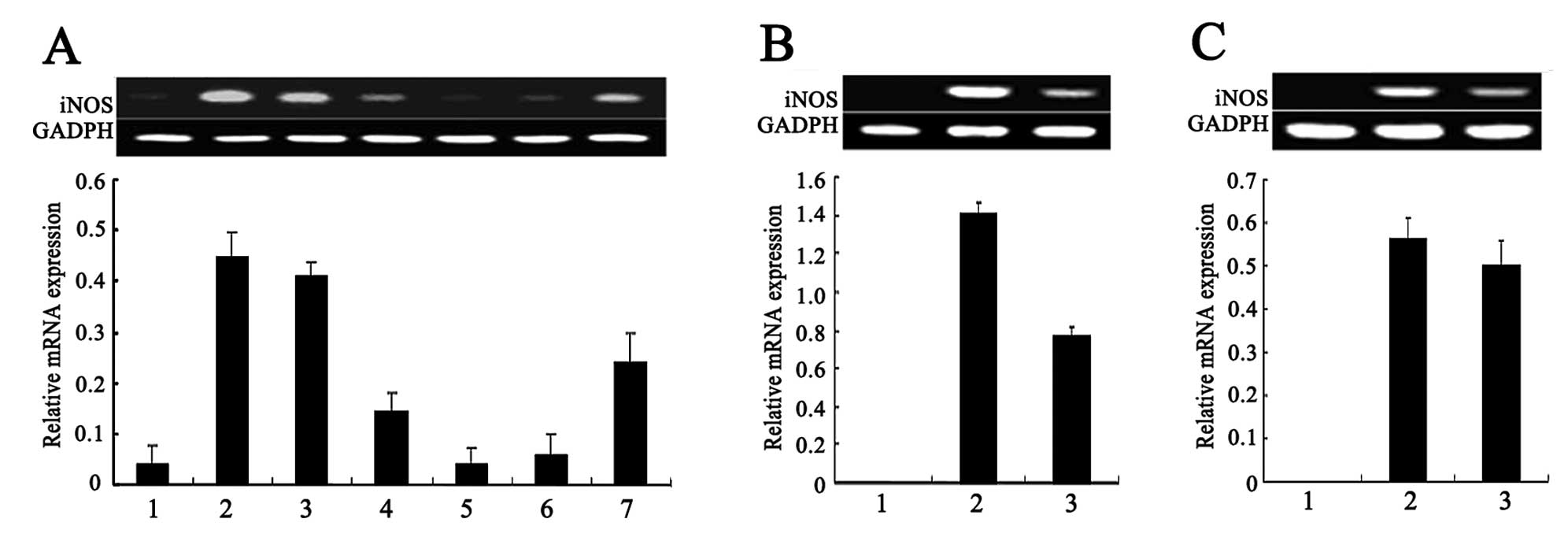 | Figure 3Effect of different inhibitors on the
mRNA expression of increased inducible nitric oxide (NO) synthase
NOS (iNOS) in ricin toxin-binding subunit B (RTB)-treated RAW264.7
cells. Following pre-treatment with various inhibitors for 1 h, the
RAW264.7 cells were treated with RTB (10 μg/ml) for 24 h. iNOS mRNA
expression were examined by RT-PCR. (A) Effect of SB203580,
genistein, LY294002, L-N monomethyl arginine (L-NMMA) and BAY on
the mRNA expression of iNOS in the RTB-treated RAW264.7 cells. Lane
1, untreated macrophages; lane 2, RTB (10 μg/ml); lane 3, RTB +
SB203580 (500 μM); lane 4, RTB + genistein (10 μM); lane 5, RTB +
LY294002 (10 μM); lane 6, RTB + L-NMMA (500 μM); lane 7, RTB + BAY
(100 μM). (B) Effect of staurosporine on the mRNA expression of
iNOS in RTB-treated RAW264.7 cells. Lane 1, untreated macrophages;
lane 2, RTB (10 μg/ml); lane 3, RTB + staurosporine (50 nM). (C)
Effect of AG490 on the mRNA expression of iNOS in RTB-treated
RAW264.7 cells. Lane 1, untreated macrophages; lane 2, RTB (10
μg/ml); lane 3, RTB + AG490 (25 μM). The lower panel represents the
expression of GADPH and actin. The results are representative of 1
of 3 experiments with similar results. Densitometric analysis
showing the relative intensity of the bands; relative ratios vs.
GADPH. |
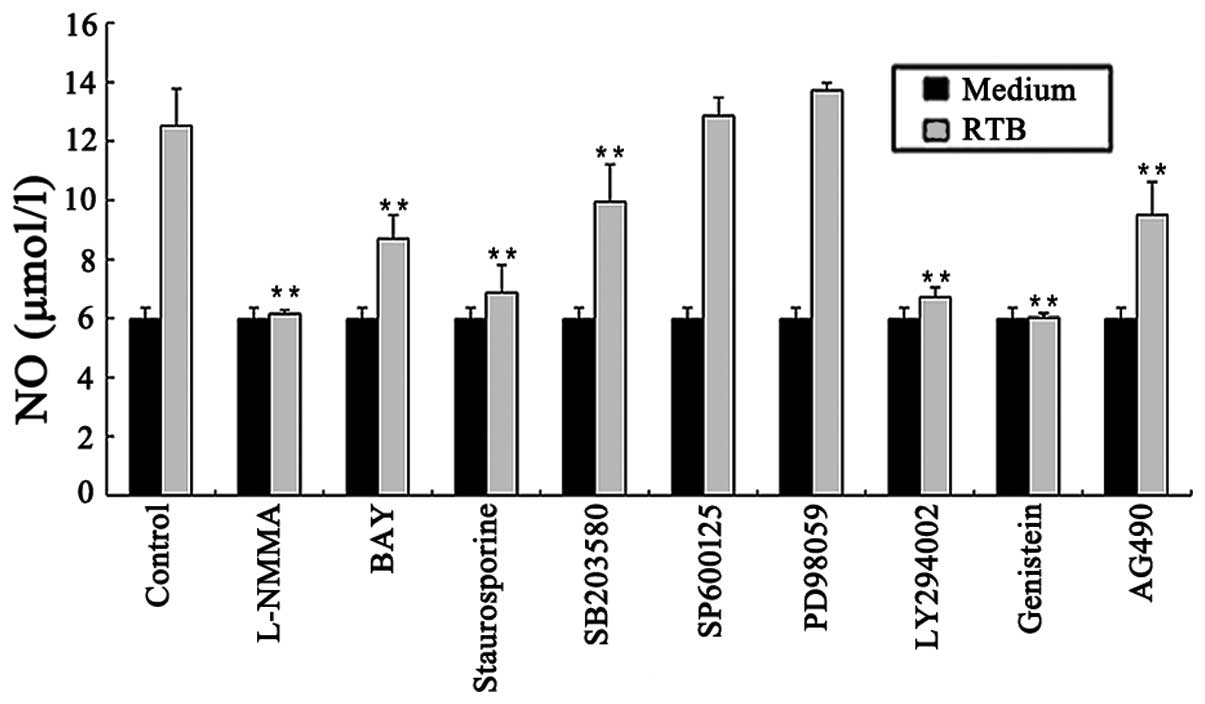
Role of protein kinases in NO production
in RTB-treated RAW264.7 cells
The RAW264.7 cells treated with RTB (10 μg/ml) in
the presence of genistein, SB203580, LY294002, or staurosporine for
24 h exhibited reduced NO production (Fig. 2). Similar results were obtained
for the mRNA expression of iNOS by RT-PCR (Fig. 3A and B). Of note, the
mitogen-activated protein kinase (MAPK) inhibitors, PD98059 and
SP600125, did not affect the observed RTB-induced NO production
(Fig. 2).
Role of the JAK-STAT pathway in NO
production in RTB-treated RAW264.7 cells
The RAW264.7 cells treated with RTB (10 μg/ml) in
the presence of the JAK2 inhibitor, tyrphostin (AG490), for 24 h
showed a decreased NO production (Fig. 2). Similar results were obtained
for the mRNA expression of iNOS (Fig.
3C).
Role of the nuclear factor (NF)-κB
pathway in NO production in RTB-treated RAW264.7 cells
The treatment of RAW264.7 cells with RTB (10 μg/ml)
in the presence of the NF-κB inhibitor, BAY 11–7082, for 24 h
resulted in the inhibition of NO production (Fig. 2). Similar results were obtained
for the mRNA expression of iNOS (Fig.
3A).
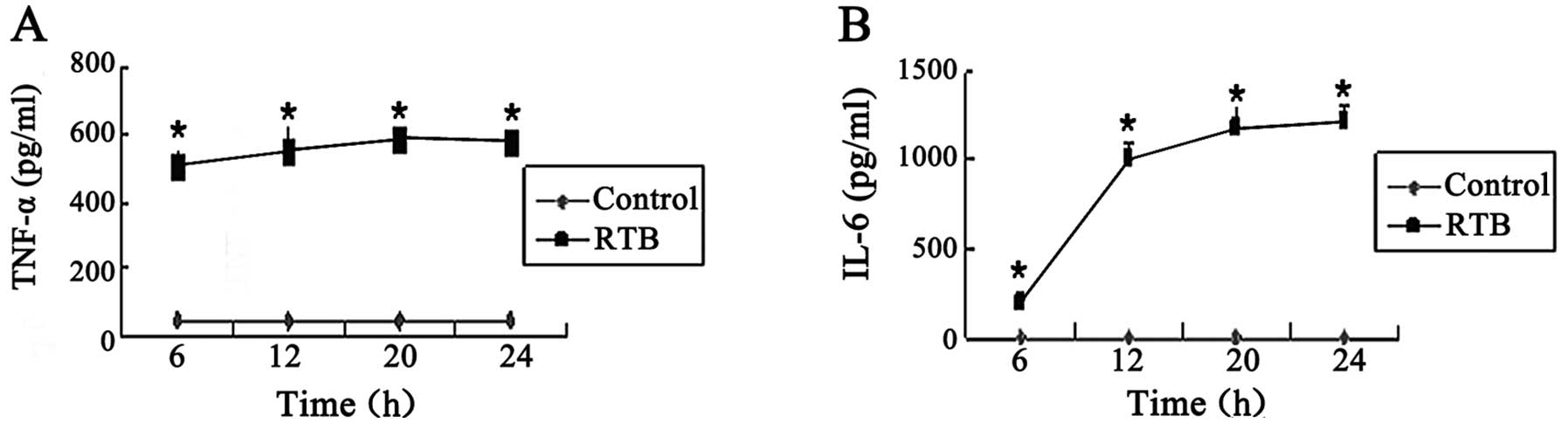
Time kinetics of TNF-α and IL-6
expression induced by RTB
The concentrations of TNF-α and IL-6 stimulated by
RTB (10 μg/ml) were increased at different time intervals, with
TNF-α maximum production at 20 h and IL-6 maximum production at 24
h (Fig. 4).
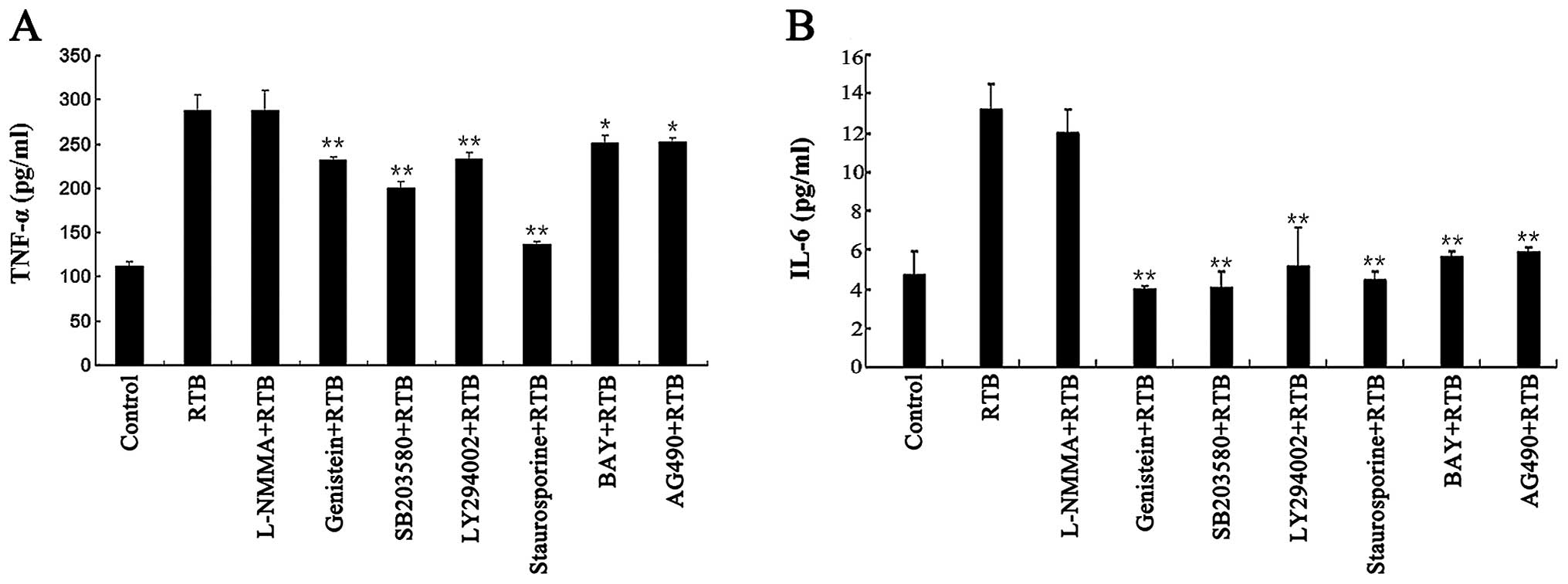
Role of protein tyrosine kinases,
JAK-STAT and NF-κB signaling in the production of TNF-α and IL-6 in
RTB-treated RAW264.7 cells
The production of TNF-α and IL-6 induced by RTB was
inhibited by the co-treatment with genistein, SB203580, LY294002,
BAY 11–7082, staurosporine and AG490 (Fig. 5). Similar effects were observed as
regards TNF-α and IL-6 gene transcription (Fig. 6).
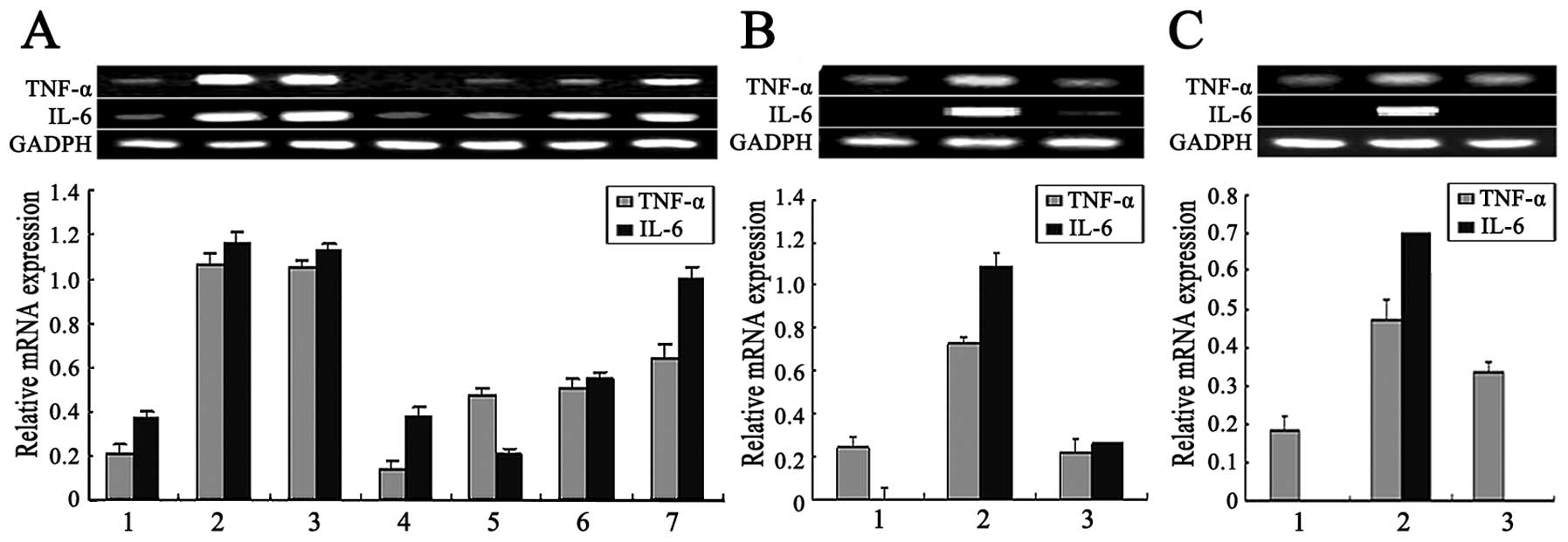 | Figure 6Effect of different inhibitors on the
mRNA expression of tumor necrosis factor (TNF)-α and interleukin-6
(IL-6) in ricin toxin-binding subunit B (RTB)-treated RAW264.7
cells. Following pre-treatment with various inhibitors for 1 h, the
RAW264.7 cells were treated with RTB (10 μg/ml) for 24 h. TNF-α and
IL-6 mRNA expression were examined by RT-PCR. (A) Effect of L-N
monomethyl arginine (L-NMMA), genistein, SB203580, LY294002 and BAY
11–7082 on the mRNA expression of TNF-α and IL-6 in the RTB-treated
RAW264.7 cells. Lane 1, untreated macrophages; lane 2, RTB (10
μg/ml); lane 3, RTB + L-NMMA (500 μM); lane 4, RTB + genistein (10
μM); lane 5, RTB + SB203580 (500 μM); lane 6, RTB + LY294002 (10
μM); lane 7, RTB + BAY (100 μM). The lower panel represents the
expression of GADPH. The results are representative of 3
experiments with similar results. (B) Effect of staurosporine on
the mRNA expression of TNF-α and IL-6 in the RTB-treated RAW264.7
cells. Lane 1, untreated macrophages; lane 2, RTB (10 μg/ml); lane
3, RTB + staurosporine (50 nM). (C) Effect of AG490 on the mRNA
expression of TNF-α and IL-6 in the RTB-treated RAW264.7 cells.
Lane 1, untreated macrophages; lane 2, RTB (10 μg/ml); lane 3, RTB
+ AG490 (25 μM). The lower panel represents the expression of GADPH
and actin. The results are representative of 1 of 3 experiments
with similar results. Densitometric analysis showing the relative
intensity of the bands; relative ratios vs. GADPH. |
Role of NO in the modulation of TNF-α and
IL-6 production in RTB-treated RAW264.7 cells
The treatment of RAW264.7 cells with RTB (10 μg/ml)
in the presence of the NOS inhibitor, L-NMMA, had no affect on the
mRNA and protein expression of TNF-α and IL-6 (Fig. 5 and 6A).
The viability of the RAW264.7 cells was not affected
by the dose of the inhibitor used in any of these experiments (data
not shown).
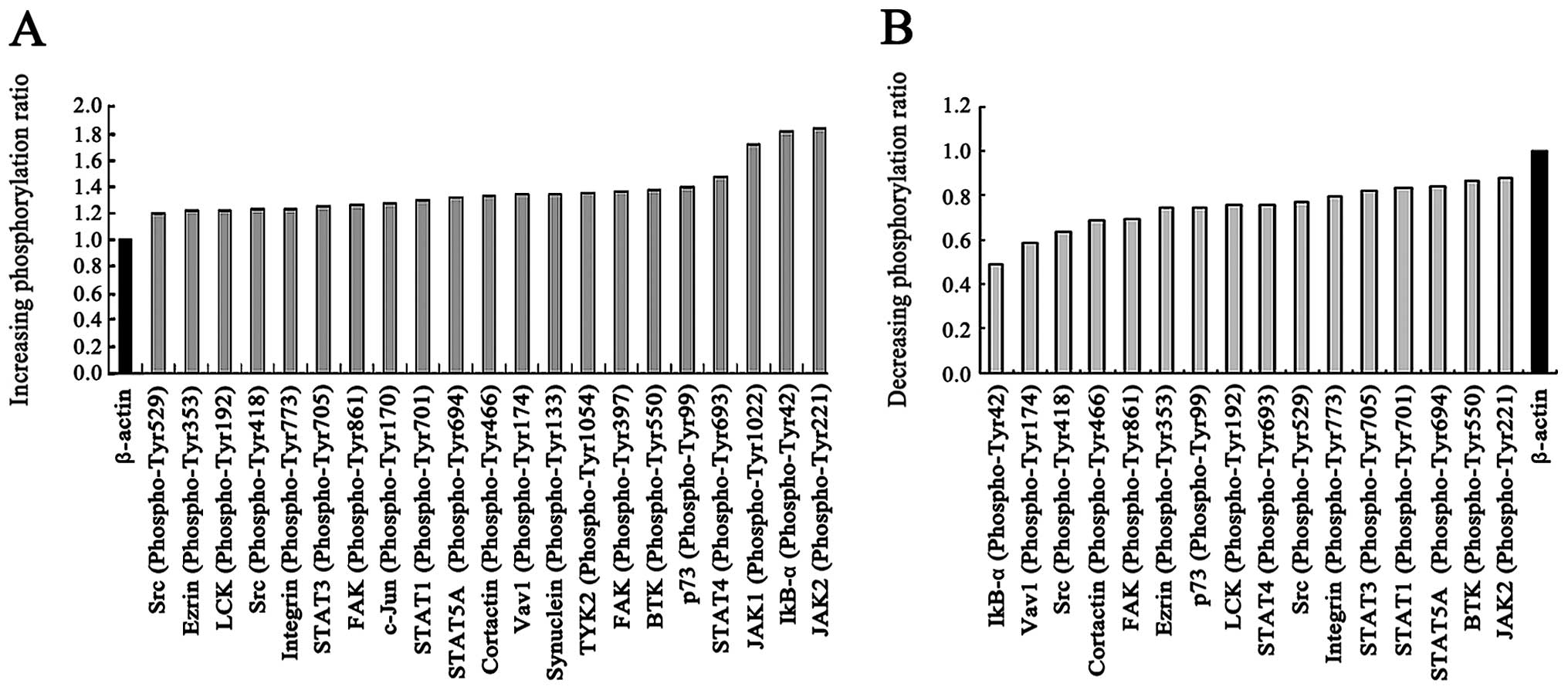
Tyrosine phosphorylation levels in
RTB-treated RAW264.7 cells
Using a phosphoarray, we analyzed the tyrosine
phosphorylation profiles of RTB-treated RAW264.7 cells with or
without the protein tyrosine kinase inhibitor, genistein, and
assessed which kinases were inhibited in association with the
suppressive effects in culture. A spectrum of proteins whose
phosphorylation levels were increased or decreased by >20 or
15%, with low 95% CI values were identified. Without the inhibitor,
20 of the 228 sites were positive for phosphorylation, and the
highest value was observed for JAK2 Tyr221. Phosphorylation was
found to be decreased at 52 sites (data not shown). Two tyrosines
(Tyr418 and Tyr529) of the Src family were phosphorylated. The JAK1
Tyr1022, JAK2 Tyr221, STAT1 Tyr701, STAT3 Tyr705, STAT4 Tyr693,
STAT5A Tyr694 and IκB-α Tyr42 phosphorylation levels were increased
(Fig. 7A). Genistein reduced the
phosphorylation of 16/288 tyrosine sites (Fig. 7B). Some of the identified proteins
are associated with the downstream cascades of activated JAK-STAT
and NF-κB receptors.
Discussion
RTB is a galactose/N-acetyl-galactosamine-specific
lectin (19). Although RTB alone
is not toxic (20), it directly
facilitates the translocation of RTA to the cytosol (21). In the present study, we
demonstrate that recombinant RTB stimulates iNOS, TNF-α and IL-6
expression; this involves the activation of protein tyrosine
kinase, NF-κB and JAK-STAT signaling.
Macrophages respond to extracellular stimuli through
phagocytosis. During the process of macrophage activation, many
types of inflammatory mediators, such as IL-6, TNF-α and NO, are
induced. The cellular responses to external intruders are commonly
a complex array of phosphorylation cascades (22).
Previously, we found that the treatment of RAW264.7
cells with recombinant RTB results in a time- and dose-dependent
production of NO (unpublished data), a finding that inspired us to
study the in-depth mechanism of macrophage activation. The
production of NO mediated by iNOS contributes to the killing of
virally infected cells, tumor cells and certain pathogens (23,24). The NO pathway, which is one of the
major mechanisms involved, can be used to evaluate various
immunomodulators of the innate immune system (25). It was observed that recombinant
RTB directly induced the transcription of the iNOS gene and the
expression of its protein, whereas this NO production by
recombinant RTB was blocked by L-NMMA. We also found that
recombinant RTB led to a time-dependent production of TNF-α and
IL-6.
Previous studies have suggested the involvement of
tyrosine kinases, PI3K and PKC in the NO production and macrophage
activation induced by different agents (26,27). In this study, we found that the
co-treatment of macrophages with the tyrosine kinase inhibitor,
genistein, the PI3K inhibitor, LY294002, and the PKC inhibitor,
staurosporine, inhibited the production of RTB-induced NO to
varying degrees. We also observed that the RTB-induced NO
production was reduced in the cells co-treated with the JAK2
inhibitor, AG490. These results are in accordance with the
reduction in RTB-induced iNOS gene transcription when the cells
were co-treated with the pharmacological inhibitors, genistein,
LY294002, staurosporine and AG490. These data suggest the possible
role of tyrosine kinases, PI3K, PKC and JAK2 in the RTB-mediated
macrophage activation. Among signaling molecules, MAPKs are
recognized as important versatile signaling kinases that link
upstream signaling events to macrophage activation (22,28), and the activation of MAPKs
involves changes in the expression of the AP-1 transcription
factors, c-Fos and c-Jun (29).
In our study, we also used the P42/44 inhibitor, PD98059, the p38
inhibitor, SB203580, and the JNK inhibitor, SP600125, and found
that the RTB-induced NO production was sensitive to SB203580,
although PD98059 and SP600125 had no effect. These results suggest
the involvement of the p38 MAPK pathway in RTB-induced NO
production. The activation of MAPKs controls the activation of
NF-κB and plays a significant role in NO production (30,31). The activation of NF-κB has been
shown to be involved in the induction of iNOS production and the
expression of various genes that are associated with immune and
inflammatory responses (32,33). LPS and various polysaccharides
induce the activation of ERK and p38, whereas only p38 is involved
in the induction of NF-κB DNA binding and the subsequent expression
of iNOS and NO release in macrophages (30). In this study, we found that
RTB-induced NO production was inhibited by the treatment of
macrophages with the NF-κB inhibitor, BAY 11–7082, and iNOS gene
expression was also inhibited by SB203580 and BAY 11–7082,
suggesting that the p38 MAPK and NF-κB pathways are involved in the
RTB-induced NO production. Recombinant RTB was found to induce a
time-dependent production of TNF-α and IL-6, important mediators of
immune modulation and inflammation. The maximum production of TNF-α
was observed at 20 h of treatment with RTB, whereas that for IL-6
was observed at 24 h. The inhibition of the protein production and
gene expression of TNF-α and IL-6 was observed in the macrophages
treated with genistein, SB203580, LY294002, staurosporine, BAY
11–7082 and AG490, suggesting the possible involvement of tyrosine
kinases, p38 MAPK, PI3K, PKC, NF-κB and JAK2. To investigate the
role of NO in the modulation of TNF-α and IL-6 production in the
RTB-treated macrophages, TNF-α and IL-6 expression levels were
measured following treatment with the iNOS inhibitor, L-NMMA. The
result that the production of TNF-α and IL-6 was not significantly
downregulated suggests that NO does not regulate TNF-α and IL-6
production in macrophages following treatment with RTB.
Protein phosphorylation mediated by tyrosine kinases
and the serine/threonine kinase involved in the production of NO,
TNF-α and other cytokines in macrophages activated by agents such
as LPS, interleukins and cisplatin has been reported (34,35). To explore the tyrosine
phosphorylation profiles of recombinant RTB-treated RAW264.7 cells
with or without the protein tyrosine kinase inhibitor, genistein,
we performed a phospho-proteomics-based study using a
phospho-antibody microarray. This technique provides a
high-throughput platform for efficient protein phosphorylation
status profiling, with the detection and analysis of
phosphorylation events at specific sites to identify whether the
phosphorylation and activation levels in RAW264.7 cells were
regulated by RTB. Based on the enhancement spectrum, our results
indicated that signaling cascades, including the JAK-STAT and NF-κB
pathways, were involved in the activation of phosphorylation
induced by RTB in macrophages; furthermore, the activation of the
phosphorylation of the JAK-STAT and NF-κB pathways induced by RTB
was inhibited by genistein. These observations indicate the
involvement of different signaling cascades in the activation of
macrophages following treatment with recombinant RTB in
vitro.
References
|
1
|
Falnes P and Sandvig K: Penetration of
protein toxins into cells. Curr Opin Cell Biol. 12:407–413. 2000.
View Article : Google Scholar
|
|
2
|
Stirpe F and Barbieri L:
Ribosome-inactivating proteins up to date. FEBS Lett. 195:1–8.
1986. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Audi J, Belson M, Patel M, Schier J and
Osterloh J: Ricin poisoning a comprehensive review. JAMA.
294:2342–2351. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tran H, Leong C, Loke WK, Dogovski C and
Liu CQ: Surface plasmon resonance detection of ricin and
horticultural ricin variants in environmental samples. Toxicon.
52:582–588. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rapak A, Falnes PO and Olsnes S:
Retrograde transport of mutant ricin to the endoplasmic reticulum
with subsequent translocation to cytosol. Proc Natl Acad Sci USA.
94:3783–3788. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sandvig K and van Deurs B: Delivery into
cells: lessons learned from plant and bacterial toxins. Gene Ther.
12:865–872. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Choi NW, Estes MK and Langridge WH:
Mucosal immunization with a ricin toxin B subunit-rotavirus NSP4
fusion protein stimulates a Th1. J Biotechnol. 121:272–283. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Medina-Bolivar F, Wright R, Funk V, Sentz
D, Barroso L, Wilkins TD, Petri W Jr and Cramer CL: A non-toxic
lectin for antigen delivery of plant-based mucosal vaccines.
Vaccine. 21:997–1005. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Adams DO and Nathan CF: Molecular
mechanisms in tumor-cell killing by activated macrophages. Immunol
Today. 4:166–177. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hamilton TA, Ohmori Y, Lebo JM and Kishore
R: Regulation of macrophage gene expression by pro- and
anti-inflammatory cytokines. Pathobiology. 67:241–244. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Schorey JS and Cooper AM: Macrophage
signalling upon mycobacterial infection the MAP kinases lead the
way. Cell Microbiol. 5:133–142. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gordon S: Pattern recognition receptors:
doubling up for the innate immune response. Cell. 111:927–930.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bolander FF Jr: The role of nitric oxide
in the biological activity of prolactin in the mouse mammary gland.
Mol Cell Endocrinol. 174:91–98. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kozlowska K, Cichorek M, Wachulska M and
Bautembach I: Role of interleukins and nitric oxide secretion by
peritoneal macrophages in differential tumoricidal effect to
transplantable melanomas as regarding their biological properties.
Immunopharmacol Immunotoxicol. 28:305–317. 2006. View Article : Google Scholar
|
|
15
|
Michel T and Feron O: Nitric oxide
synthases: which, where, how, and why? J Clin Invest.
100:2146–2152. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mayer B and Hemmens B: Biosynthesis and
action of nitric oxide in mammalian cells. Trends Biochem Sci.
22:477–481. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Korhonen R, Lahti A, Kankaanranta H and
Moilanen E: Nitric oxide production and signaling in inflammation.
Curr Drug Targets Inflamm Allergy. 4:471–479. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kolb H and Kolb-Bachofen V: Nitric oxide
in autoimmune disease: cytotoxic or regulatory mediator? Immunol
Today. 19:556–561. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gräslund S, Eklund M, Falk R, Uhlén M,
Nygren PÅ and Ståhl S: A novel affinity gene fusion system allowing
protein A-based recovery of non-immunoglobulin gene products. J
Biotechnol. 99:41–50. 2002.PubMed/NCBI
|
|
20
|
Olsnes S and Pihl A: Different biological
properties of the two constituent peptide chains of ricin, a toxic
protein inhibiting protein synthesis. Biochemistry. 12:3121–3126.
1973. View Article : Google Scholar
|
|
21
|
Sharma S, Podder SK and Karande AA:
Comparative studies on kinetics of inhibition of protein synthesis
in intact cells by ricin and a conjugate of ricin B-chain with
momordin. Mol Cell Biochem. 200:133–141. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Robinson MJ and Cobb MH: Mitogen-activated
protein kinase pathways. Curr Opin Cell Biol. 9:180–186. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lim S, Kang KW, Park SY, Kim SI, Choi YS,
Kim ND, Lee KU, Lee HK and Pak YK: Inhibition of
lipopolysaccharide-induced inducible nitric oxide synthase
expression by a novel compound, mercaptopyrazine, through
suppression of nuclear factor-kappaB binding to DNA. Biochem
Pharmacol. 68:719–728. 2004. View Article : Google Scholar
|
|
24
|
Juang SH, Xie K, Xu L, Shi Q, Wang Y,
Yoneda J and Fidler IJ: Suppression of tumorigenicity and
metastasis of human renal carcinoma cells by infection with
retroviral vectors harboring the murine inducible nitric oxide
synthase gene. Hum Gene Ther. 9:845–854. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pan D, Bera AK, Das S, Bandyopadhyay S,
Rana T, Bandyopadhyay S, Das SK and Bhattacharya D: Use of zinc
chloride as alternative stimulant for in vitro study of nitric
oxideproduction pathway in avian splenocyte culture. Mol Biol Rep.
37:2223–2226. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dong Z, Qi X, Xie K and Fidler IJ: Protein
tyrosine kinase inhibitors decrease induction of nitric oxide
synthase activity in lipopolysaccharide-responsive and
lipopolysaccharide-nonresponsive murine macrophages. J Immunol.
151:2717–2724. 1993.
|
|
27
|
Shishodia S, Shrivastava A and Sodhi A:
Protein kinase C: a potential pathway of macrophage activation with
cisplatin. Immunol Lett. 61:179–186. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lewis TS, Shapiro PS and Ahn NG: Signal
transduction through MAP kinase cascades. Adv Cancer Res.
74:49–139. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Angel P and Karin M: The role of Jun, Fos
and AP-1 complex in the cell-proliferation and transformation.
Biochem Biophys Acta. 1072:129–157. 1991.PubMed/NCBI
|
|
30
|
Chen CC and Wang JK: P38 but not p44/42
mitogen-activated protein kinase is required for nitric oxide
synthase induction mediated by lipopolysaccharide in RAW264.7
macrophages. Mol Pharmacol. 55:481–488. 1999.PubMed/NCBI
|
|
31
|
Yoon YD, Kang JS, Han SB, Park SK, Lee HS
and Kim HM: Activation of mitogen-activated protein kinases and
AP-1 by polysaccharide isolated from the radix of Platycodon
grandiflorum in RAW264.7 cells. Int Immunopharmacol.
4:1477–1487. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mendes AF, Carvalho AP, Caramona MM and
Lopes MC: Role of nitric oxide in the activation of NF-kappaB, AP-1
and NOS II expression in articular chondrocytes. Inflamm Res.
51:369–375. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Connelly L, Palacios-Callender M, Ameixa
C, Moncada S and Hobbs AJ: Biphasic regulation of NF-kappa B
activity underlies the pro- and anti-inflammatory actions of nitric
oxide. J Immunol. 166:3873–3881. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Eason SW and Martin W: Involvement of
tyrosine kinase and protein kinase C in the induction of nitric
oxide synthase by LPS and IFN-γ in J774 macrophages. Arch Int
Pharmacodyn Ther. 330:225–236. 1995.
|
|
35
|
Foulkes JG, Chow M, Gorka C, Frackelton AR
Jr and Baltimore D: Purification and characterization of a protein
tyrosine kinase encoded by the Abelson murine leukemia virus. J
Biol Chem. 260:8070–8077. 1985.PubMed/NCBI
|