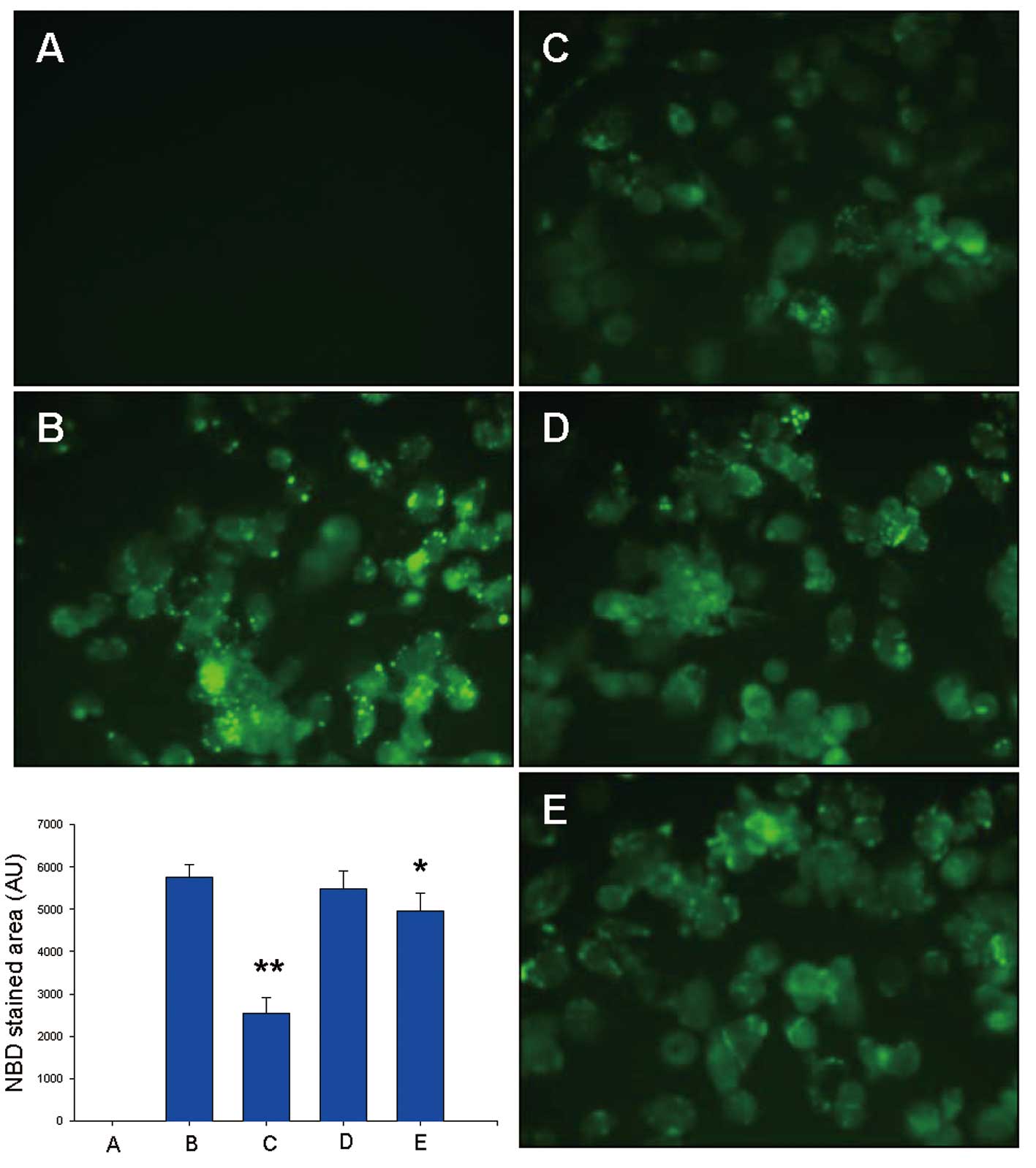
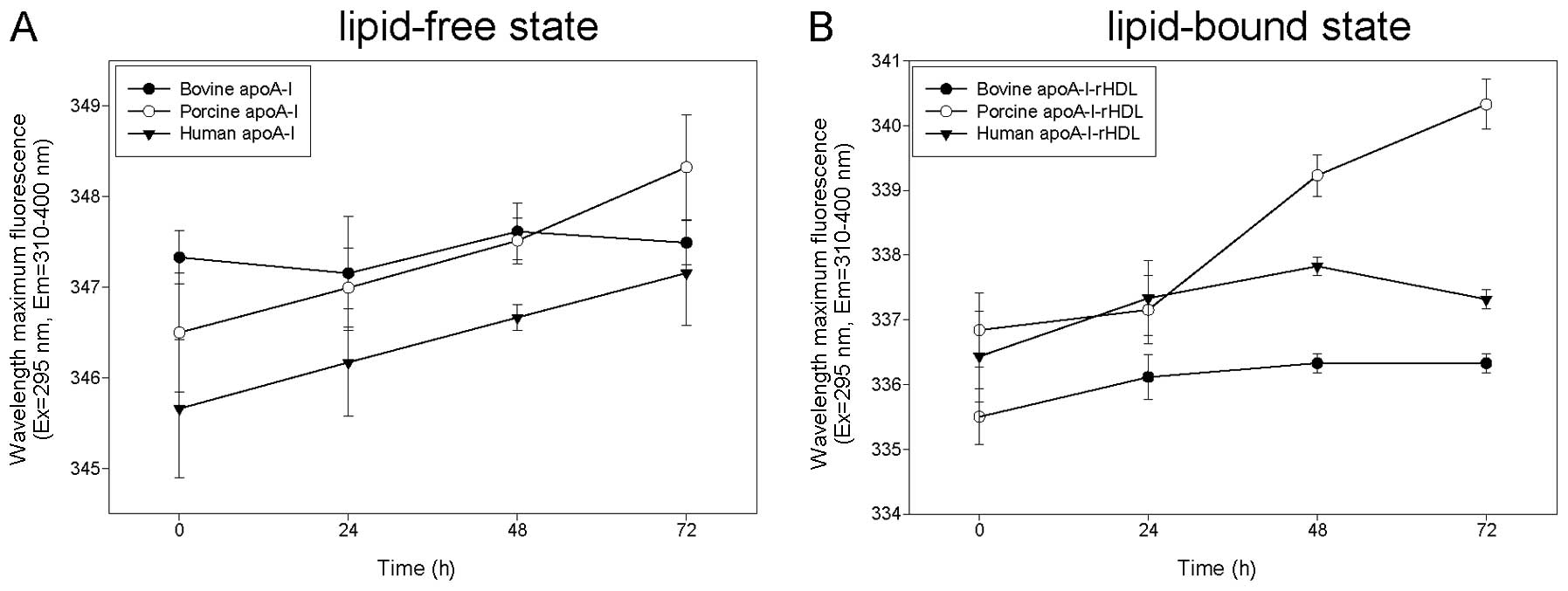
|
1
|
Frank PG and Marcel YL: Apolipoprotein
A-I: Structure-function relationships. J Lipid Res. 41:853–872.
2000.PubMed/NCBI
|
|
2
|
Fielding PE and Fielding CJ: Plasma
membrane caveolae mediate the efflux of cellular free cholesterol.
Biochemistry. 34:14288–14292. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rye KA and Barter PJ: Antiinflammatory
actions of HDL: a new insight. Arterioscler Thromb Vasc Biol.
28:1890–1891. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kelesidis T, Yang OO, Currier JS, Navab K,
Fogelman AM and Navab M: HIV-1 infected patients with suppressed
plasma viremia on treatment have pro-inflammatory HDL. Lipids
Health Dis. 10:352011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fryirs MA, Barter PJ, Appavoo M, Tuch BE,
Tabet F, Heather AK and Rye KA: Effects of high-density
lipoproteins on pancreatic beta-cell insulin secretion.
Arterioscler Thromb Vasc Biol. 30:1642–1648. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Park KH, Yun CO, Kwon OJ, Kim CH, Kim JR
and Cho KH: Enhanced delivery of adenovirus, using proteoliposomes
containing wildtype or V156K apolipoprotein A-I and
dimyristoylphosphatidylcholine. Hum Gene Ther. 21:579–587. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Seo J, Yun CO, Kwon OJ, Choi EJ, Song JY,
Choi I and Cho KH: A Proteoliposome containing apolipoprotein A-I
mutant (V156K) enhances rapid tumor regression activity of human
origin oncolytic adenovirus in tumor-bearing zebrafish and mice.
Mol Cells. 34:143–148. 2012. View Article : Google Scholar
|
|
8
|
Huang R, Silva RA, Jerome WG, Kontush A,
Chapman MJ, Curtiss LK, Hodges TJ and Davidson WS: Apolipoprotein
A-I structural organization in high-density lipoproteins isolated
from human plasma. Nat Struct Mol Biol. 18:416–422. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bashtovyy D, Jones MK, Anantharamaiah GM
and Segrest JP: Sequence conservation of apolipoprotein A-I affords
novel insights into HDL structure-function. J Lipid Res.
52:435–450. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Brouillette CG, Anantharamaiah GM, Engler
JA and Borhani DW: Structural models of human apolipoprotein A-I: a
critical analysis and review. Biochim Biophys Acta. 1531:4–46.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Brewer HB Jr, Ronan R, Meng M and Bishop
C: Isolation and characterization of apolipoproteins A-I, A-II, and
A-IV. Methods Enzymol. 128:223–246. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pownall HJ, Massey JB, Kusserow SK and
Gotto AM Jr: Kinetics of lipid-protein interactions: interaction of
apolipoprotein A-I from human plasma high density lipoproteins with
phosphatidylcholines. Biochemistry. 17:1183–1188. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Blois MS: Antioxidant determinations by
the use of a stable free radical. Nature. 181:1199–1200. 1958.
View Article : Google Scholar
|
|
14
|
Cho KH, Park SH, Han JM, Kim HC, Chung YJ,
Choi I and Kim JR: A point mutant of apolipoprotein A-I, V156K,
exhibited potent anti-oxidant and anti-atherosclerotic activity in
hypercholesterolemic C57BL/6 mice. Exp Mol Med. 39:160–169. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cho KH: Synthesis of reconstituted high
density lipoprotein (rHDL) containing apoA-I and apoC-III: the
functional role of apoC-III in rHDL. Mol Cells. 27:291–297. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Staros JV: Membrane-impermeant, cleavable
cross-linkers: new probes of nearest neighbor relationships at one
face of a membrane. Biophys J. 37:21–22. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen YH, Yang JT and Martinez HM:
Determination of the secondary structures of proteins by circular
dichroism and optical rotatory dispersion. Biochemistry.
11:4120–4131. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Park KH, Jang W, Kim KY, Kim JR and Cho
KH: Fructated apolipoprotein A-I showed severe structural
modification and loss of beneficial functions in lipid-free and
lipid-bound state with acceleration of atherosclerosis and
senescence. Biochem Biophys Res Commun. 392:295–300. 2010.
View Article : Google Scholar
|
|
19
|
Markwell MA, Haas SM, Bieber LL and
Tolbert NE: A modification of the Lowry procedure to simplify
protein determination in membrane and lipoprotein samples. Anal
Biochem. 87:206–210. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Esterbauer H, Striegl G, Puhl H and
Rotheneder M: Continuous monitoring of in vitro oxidation of human
low density lipoprotein. Free Radic Res Commun. 6:67–75. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Noble RP: Electrophoretic separation of
plasma lipoproteins in agarose gel. J Lipid Res. 9:693–700.
1968.PubMed/NCBI
|
|
22
|
Fraenkel-Conrat H: Methods for
investigating the essential groups for enzyme activity. Methods
Enzymol. 4:247–269. 1957. View Article : Google Scholar
|
|
23
|
Cho KH: Biomedicinal implications of
high-density lipoprotein: its composition, structure, functions,
and clinical applications. BMB Rep. 42:393–400. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rubin EM, Krauss RM, Spangler EA,
Verstuyft JG and Clift SM: Inhibition of early atherogenesis in
transgenic mice by human apolipoprotein AI. Nature. 353:265–267.
1991. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Newton RS and Krause BR: HDL therapy for
the acute treatment of atherosclerosis. Atheroscler Suppl. 3:31–38.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nicholls SJ, Uno K, Kataoka Y and Nissen
SE: ETC-216 for coronary artery disease. Expert Opin Biol Ther.
11:387–394. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Collet X, Marcel YL, Tremblay N, Lazure C,
Milne RW, Perret B and Weech PK: Evolution of mammalian
apolipoprotein A-I and conservation of antigenicity: correlation
with primary and secondary structure. J Lipid Res. 38:634–644.
1997.PubMed/NCBI
|
|
28
|
Jonas A and Krajnovich DJ: Effect of
cholesterol on the formation of micellar complexes between bovine
A-I apolipoprotein and L-alpha-dimyristoylphosphatidylcholine. J
Biol Chem. 253:5758–5763. 1978.
|
|
29
|
Oikawa S, Katoh N, Itoh H, Miyamoto T,
Konno M and Kajita T: Decreased serum apolipoprotein A-I
concentrations in cows infected with Salmonella typhimurium. Can J
Vet Res. 61:182–186. 1997.PubMed/NCBI
|
|
30
|
Lee H, Park JE, Choi I and Cho KH:
Enhanced functional and structural properties of high-density
lipoproteins from runners and wrestlers compared to throwers and
lifters. BMB Rep. 42:605–610. 2009. View Article : Google Scholar : PubMed/NCBI
|