Introduction
Tumors are characterized by high geometric and
viscous resistance to blood flow, high microvascular hydrostatic
pressure, low resistance to transcapillary fluid flow, high
resistance to interstitial fluid flow, impaired lymphatic drainage
and an abnormal extracellular matrix (1,2).
Excess fluid leaks from the vasculature into the interstitium,
where it accumulates and distends the elastic extracellular matrix
(ECM), elevating interstitial fluid pressure (IFP). Solid tumors
present with an IFP that is elevated above that of normal tissues.
A number of studies have shown that solid malignant tumors have IFP
values of 5 to 40 mmHg, whereas the IFP of most normal tissues
ranges from −3 to +3 mmHg (3–6).
Gutmann et al (3) measured
IFP in squamous cell carcinomas of the head and neck region in
humans using the wick-in-needle technique and identified that the
IFP ranged from 4 to 33 mmHg. The increase in IFP leads to a
positive pressure gradient, which is a driving force for a
convective transport back into the capillaries or to adjacent
regions with low IFP. Such convective forces inhibit the transfer
of drugs to the tumor interstitium and facilitate tumor cell
intravasation into the vascular or lymphatic circulation, and hence
promote metastasis (7).
Furthermore, there are clinical data showing that tumor IFP
correlates with response to treatment, with strong evidence for
high IFP as a poor prognostic indicator in patients with cervical
cancer treated with radiotherapy (6,8).
Patients are significantly more likely to develop distant
recurrence if they present with a tumor IFP value above the group
median (19 mmHg), which suggests a role for IFP in metastatic
spread (9).
Although local oral squamous cell carcinoma (OSCC)
can be effectively controlled by surgical excision and
radiotherapy, metastasis to the lymph nodes and distant organs
significantly decreases the survival rate (10). Metastasis is the final step in
solid tumor progression and is the most common cause of mortality
in cancer patients (11). How
tumor cells become metastatic is largely unknown. However, several
mechanisms have been suggested, including the possibility that
tumors with a high IFP may have a high rate of cell proliferation
and metastasis (2). It was widely
believed that metastatic cells are rare and evolve during thelate
stages of tumor progression from a series of changes in gene
expression that enable the cells to progress through the sequential
steps that finally result in growth in distant organ
microenvironments.
Experimental studies attempting to relate the
outcome of cancer treatment to IFP are rare. Thus, the mechanisms
linking an elevated IFP to poor survival rates in patients with
OSCC are not yet well understood. In this study, we subjected SCC-4
and SCC-9 OSCC cells to conditions mimicking IFP in vitro.
The cells were incubated for 24 h under 0, 15 and 30 mmHg increased
extracellular pressure and we then investigated the alterations in
malignant phenotypes and the relevant molecular mechanisms.
Furthermore, we identified that an elevated extracellular pressure
significantly promotes cancer cell proliferation and invasion by
altering the expression of >1,800 genes, which are involved in
invasion and metastasis, the heat shock pathway, the p38 and JNK
signaling pathway, apoptosis and the cell growth and
differentiation signaling pathway using microarray analysis.
Materials and methods
Cell culture and reagents
SCC-4 and SCC-9 human tongue squamous cell carcinoma
cell lines were obtained from ATCC. The cells were cultured at 37ºC
in 5% CO2 in RPMI-1640 containing 10% fetal bovine serum
(FBS; Gibco Life Technologies, Carlsbad, CA, USA), 2 mM glutamine,
1 mM sodium pyruvate, 10 mM HEPES, 100 U/ml penicillin G and 100
mg/ml streptomycin (Sigma, USA). The cell proliferation and
cytotoxicity assay kit was from Dojindo Laboratories (Kumamoto,
Japan). The real-time PCR primers were synthesized commercially
(Takara, Kyoto, Japan). The Invasion Assay kit was from Millipore
(Billerica, MA, USA). All tissue culture media and sera were from
Gibco Life Technologies. Peroxidase-labeled anti-rabbit antibody
and enhanced chemiluminescence (ECL) system were from
Millipore.
Pressure regulation
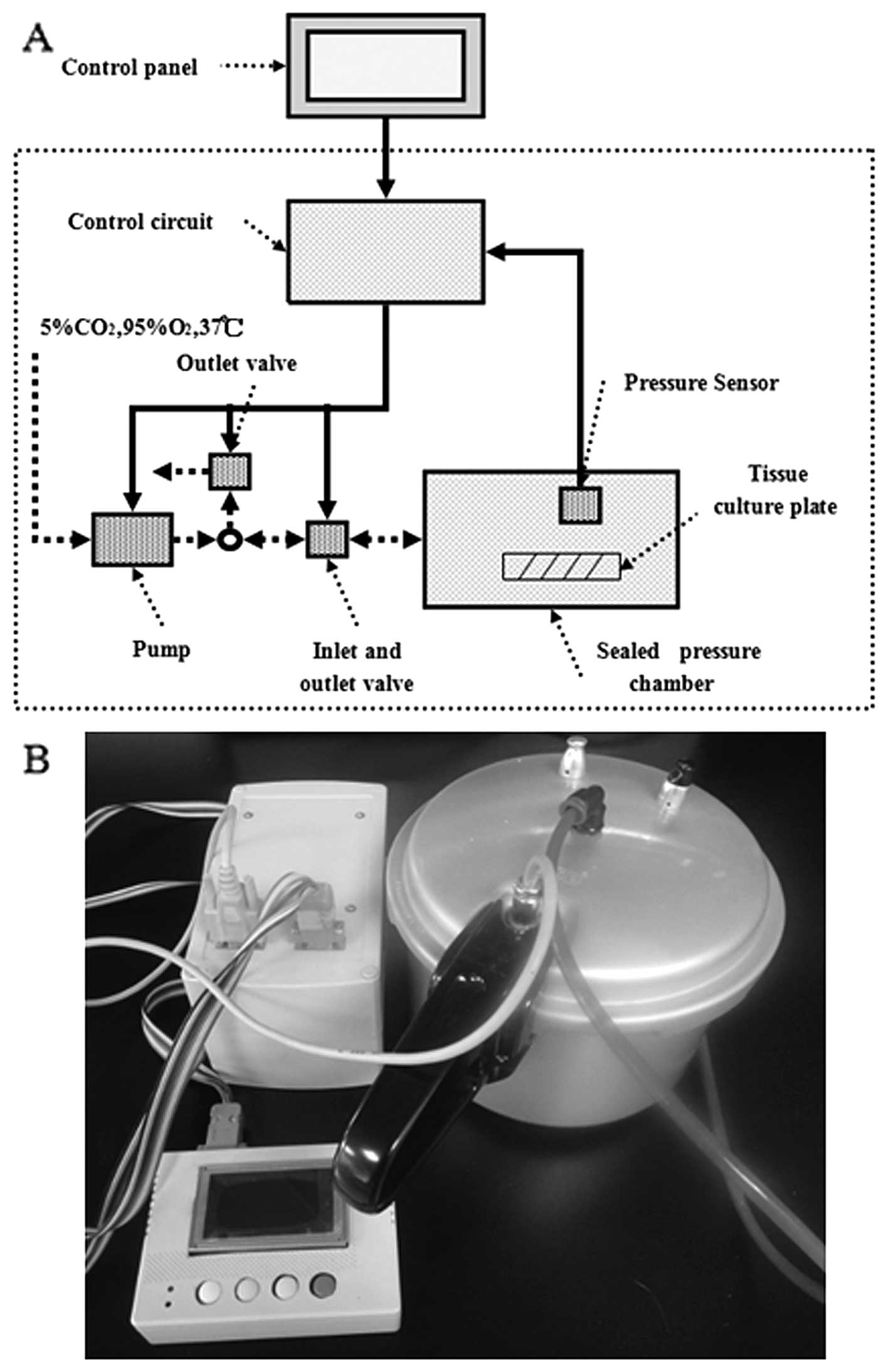
The pressure system used in the present study was
custom designed (Sichuan University, Chengdu, China) to expose
cultured tumor cells to well-defined stable pressure regimes
appropriate for modeling IFP levels in tumors. The cells were
placed in an airtight stainless steel box with an inlet valve
through which the air flowed into the box and an air pump was
connected to this box. There is an absolute pressure sensor
(measuring range 30–120 kPa; VTI Technologies, Finland) in the box,
which can promptly sense and respond to variations ins pressure.
Control and data acquisition programs utilized a pre-calibrated
differential pressure transducer to acquire pressure data (needed
to control the opening and closing of the appropriate solenoid
valves). The pressure box was warmed and maintained at a
temperature of 37ºC for at least 1 h prior to each experiment to
prevent pressure fluctuations due to temperature shifts of the
pressurizing gas. Temperature was maintained within ±2ºC and
pressure within ±1.5 mmHg. The whole experimental facility apart
from the electronic control panel was placed in a 5% CO2
incubator. The desired pressure was achieved within 1 min for the
experiments with the cells. Cells subjected to pressure were placed
inside this pressure box for 24 h with the pressure set at 15 and
30 mmHg above atmospheric pressure. The blank group cells were
incubated at 37ºC in 5% CO2 under ambient pressure for
the same amount of time (Fig.
1).
Cell proliferation assay
Cell proliferation assays were performed using the
Cell Counting kit-8 (CCK-8; Dojindo Laboratories). Cells were
plated in 96-well plates at 1×104 cells/well and
incubated for five days. Each day, at a certain time, 10 μl of
CCK-8 solution were added to each well and the cells were incubated
for a further 2 h. The optical density (OD) value was obtained by
the differences in absorbance at a wavelength of 450 nm using a
microplate reader (Varioskan Flash 3001; Thermo Scientific,
Waltham, MA, USA). The amount of formazan dye, generated by the
activities of dehydrogenases in the cells, is directly proportional
to the number of living cells. In addition, we used plate colony
formation assay to evaluate the colony forming ability of the tumor
cells. The tumor cells were cultured at 500 cells/5 ml with
RPMI-1640 medium and 10% FBS in a 6-well plate. After ten days in
culture, the cells were fixed with methanol for 10 min and stained
with 1% crystal violet solution for 20 min to visualize the
colonies for cell counting.
Cell invasion assay
The cell invasion assay was performed using the QCM™
12-well Invasion Assay kit (Chemicon International, Inc., Temecula,
CA, USA). This cell invasion assay is performed in an invasion
chamber, based on the Boyden chamber principle. This kit contains
12 inserts; each insert contains an 8-μm pore size polycarbonate
membrane coated with a thin layer of ECMatrix™. Briefly, the cells
were resuspended in serum-free RPMI-1640, and 2×105
cells were added to the interior of the inserts, which had been
previously rehydrated at room temperature for 30 min. A total of
500 μl of RPMI-1640 containing 10% FBS was added to the lower
chamber as a chemoattractant. The cells were incubated for 48 h at
37ºC in a CO2 incubator (5% CO2).
Subsequently, the upper surface of the chamber was scraped to
remove non-migratory cells. The migrated cells were fixed and
stained with 0.1% crystal violet and photographed under a light
microscope (x100). The stained insert was transferred to a clean
well containing 500 μl of 10% acetic acid for 15 min at room
temperature. The OD of the stained cells was measured at 560
nm.
Microarray analysis
The 44K Human Genome Oligo Microarray including
44,000 60-mer oligonucleotide probes representing 41,000 unique
genes and transcripts was purchased from Agilent Technologies (Palo
Alto, CA, USA). In brief, total RNA from the SCC-4 and SCC-9 cells
treated with normal ambient pressure, 15 and 30 mmHg, were
harvested using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) and
the RNeasy kit (Qiagen, Hilden, Germany). The amplification and
labeling of 500 ng of total RNA was performed according to the
protocol provided with the Agilent Quick Amp labeling kit using
Cy3. Hybridization was performed for 16 h at 50ºC. Following
hybridization and washing, the processed slides were scanned using
the Agilent DNA microarray scanner (part no. G2505B; Agilent
Technologies). The resulting text files extracted using Agilent
Feature Extraction Software (version 10.5.1.1) were imported into
the Agilent GeneSpring GX software (version 10.0) for further
analysis. To identify the genes that were differentially expressed,
we performed a fold-change screening between the two groups
obtained from the experiment. The threshold we used to screen the
upregulated or downregulated genes was a fold-change of >2.
Hierarchical clustering and a tree diagram were generated using
Cluster 3.0 software.
Quantitative reverse transcription PCR
(qRT-PCR)
Total RNA was isolated from 1×106 cells
using TRIzol reagent (Invitrogen). Total RNA was subsequently
reverse transcribed into cDNA using the SuperScript First-Strand
cDNA Synthesis kit (Invitrogen). qRT-PCR was performed using the
SYBR premix Ex Taq™ II kit (Takara). The specific primers used in
this study are listed in Table I.
The comparative threshold cycle (CT) method was used to calculate
the amplification fold. The GAPDH gene was used as a reference
control gene to normalize the expression value of the target genes.
Triple replicates were performed for each gene and the average
expression value was computed for subsequent analysis. The relative
expression level of the genes was calculated using the
2−ΔΔCt method as previously described (12).
 | Table IGenes and primer sequences used for
quantitative RT-PCR. |
Table I
Genes and primer sequences used for
quantitative RT-PCR.
| Genes | Forward primer
(5′-3′) | Reverse primer
(5′-3′) |
|---|
| HSPA4 |
GGAGGAACCACATGTTGAAG |
TGGATCCAGCTTGAGAGGTC |
| VEGFC |
AACCTCCATGTGTGTCCGTC |
TGGCAAAACTGATTGTTACTGG |
| VEGFR3 |
CTACAAAGACCCCGACTACG |
CGTAGTCGGGGTCTTTGTAG |
| KISS1 |
CTTGGCAGCTACTGCTTTTC |
GTAGCAGCTGGCTTCCTCTC |
| TWIST1 |
GGCTCAGCTACGCCTTCTC |
CTAGTGGGACGCGGACAT |
| VEGFD |
GTATGGACTCTCGCTCAGCAT |
AGGCTCTCTTCATTGCAACAG |
| MAPK8 |
ACGCCTTATGTAGTGACTCGCTACT |
TTGTAGCCCATGCCAAGGA |
| PAI2 |
GCATCCACTGGCTTGGAA |
GGGAATGTAGACCACAACATCAT |
| FOS |
TCGGGCTTCAACGCAGACTACG |
TGACCGTGGGAATGAAGTTGGC |
| HIF1A |
CATAAAGTCTGCAACATGGAAGGT |
ATTTGATGGGTGAGGAATGGGTT |
| CDKN1A |
CTGCCCAAGCTCTACCTTCC |
CAGGTCCACATGGTCTTCCT |
| BAX |
GCCCTTTTGCTTCAGGGTTT |
TCCAATGTCCAGCCCATGAT |
| FOSL1 |
ATCCCCGACCTCTGACCTAT |
CAAGGCGTTCCTTCTGCTT |
| CD44 |
CCTCCAGTGAAAGGAGCAGCAC |
GTGTCTTGGTCTCTGGTAGCAGGGAT |
| CCND1 |
ACAAGTGTGTCTTACGTGCCACCAC |
ACGACAGACAAAGCGTCCCTCAAG |
| STAB1 |
GTTTGTCACTCACACACCCTGT |
ATAGCGGCAGTCCAGAAGTATC |
| BAK1 |
GAACAGGAGGCTGAAGGGGT |
TCAGGCCATGCTGGTAGACG |
| CASP1 |
AATGATTGAGAAACTCTTCACTGTGT |
CGGGGTACCAAGCCTAGGAAACACAAGGAGA |
| ERBB2 |
AGCAGACCCAGTACCTGTCC |
AGGGTTGGTCCTTCTATGAGAAT |
| NM23 |
ATGGCCAACTGTGAGCGTACC |
TTCATAGATCCAGTTCTGAGCACAAGC |
| BCL2 |
GACAGAAGATCATGCCGTCC |
GGTACCAATGGCACTTCAAG |
| ANKK1 |
CGGCTGGCCAAGTTGTCTAA |
AGCACCTTCCTGAGTGTCATCA |
| TNFRSF6 |
TATGCTTCTTCGTGCAGCAGTT |
GCTGCCACACGCTCCTCTAG |
| SMAD2 |
CCAGGTCTCTTGATGGTCGT |
TATATCCAGGAGGTGGCGTT |
| GAPDH |
GAAGGTGAAGGTCGGAGTC |
GAAGATGGTGATGGGATTTC |
Statistical analysis
Data are expressed as the means ± standard deviation
(SD), when normally distributed. The statistical significance of
differences was determined using the Student's two-tailed t-test
for two groups and one-way ANOVA for multiple groups. A P-value
<0.05 was considered to indicate a statistically significant
difference. All data were analyzed using SPSS 15.0 software.
Results
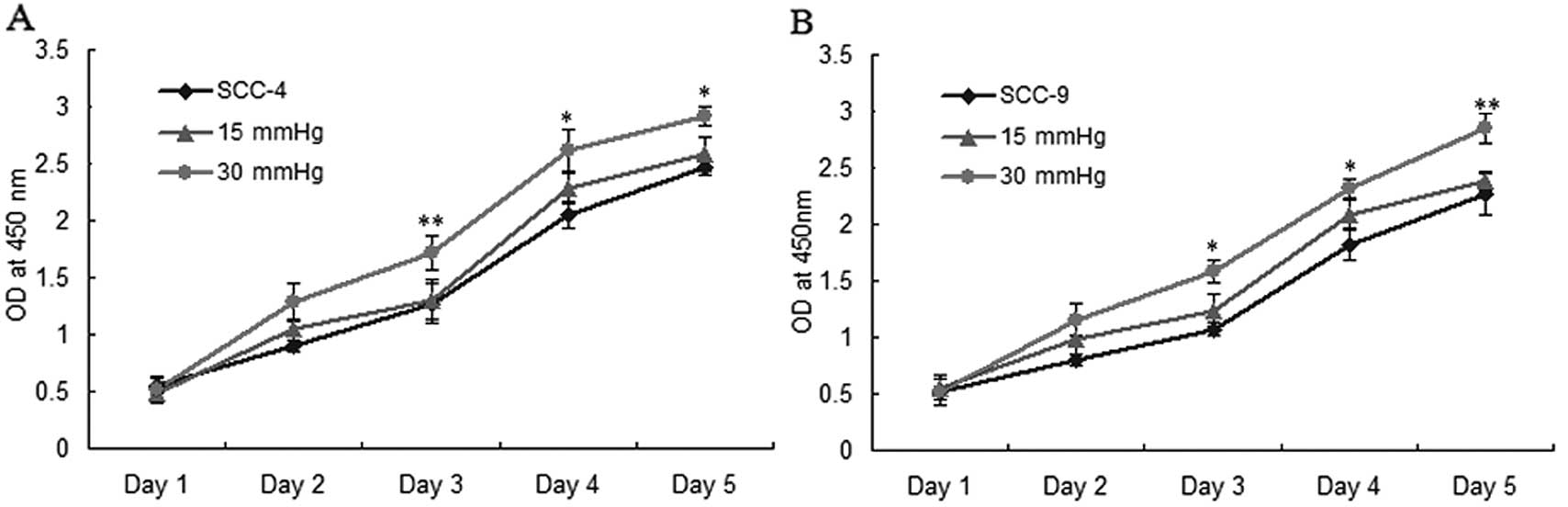
Elevated extracellular pressure promotes
OSCC cell proliferation and survival in vitro
To detect the effects of extracellular pressure on
OSCC cell proliferation and survival, we used the CCK-8 and plate
colony formation assays to assess the effect of extracellular
pressure on the proliferation and survival of SCC-4 and SCC-9 cells
(0, 15 and 30 mmHg). The results revealed that elevated
extracellular pressure significantly increased cancer cell
proliferation and colony formation, particularly in the group
exposed to 30 mmHg pressure (P<0.05) (Fig. 2A–E).
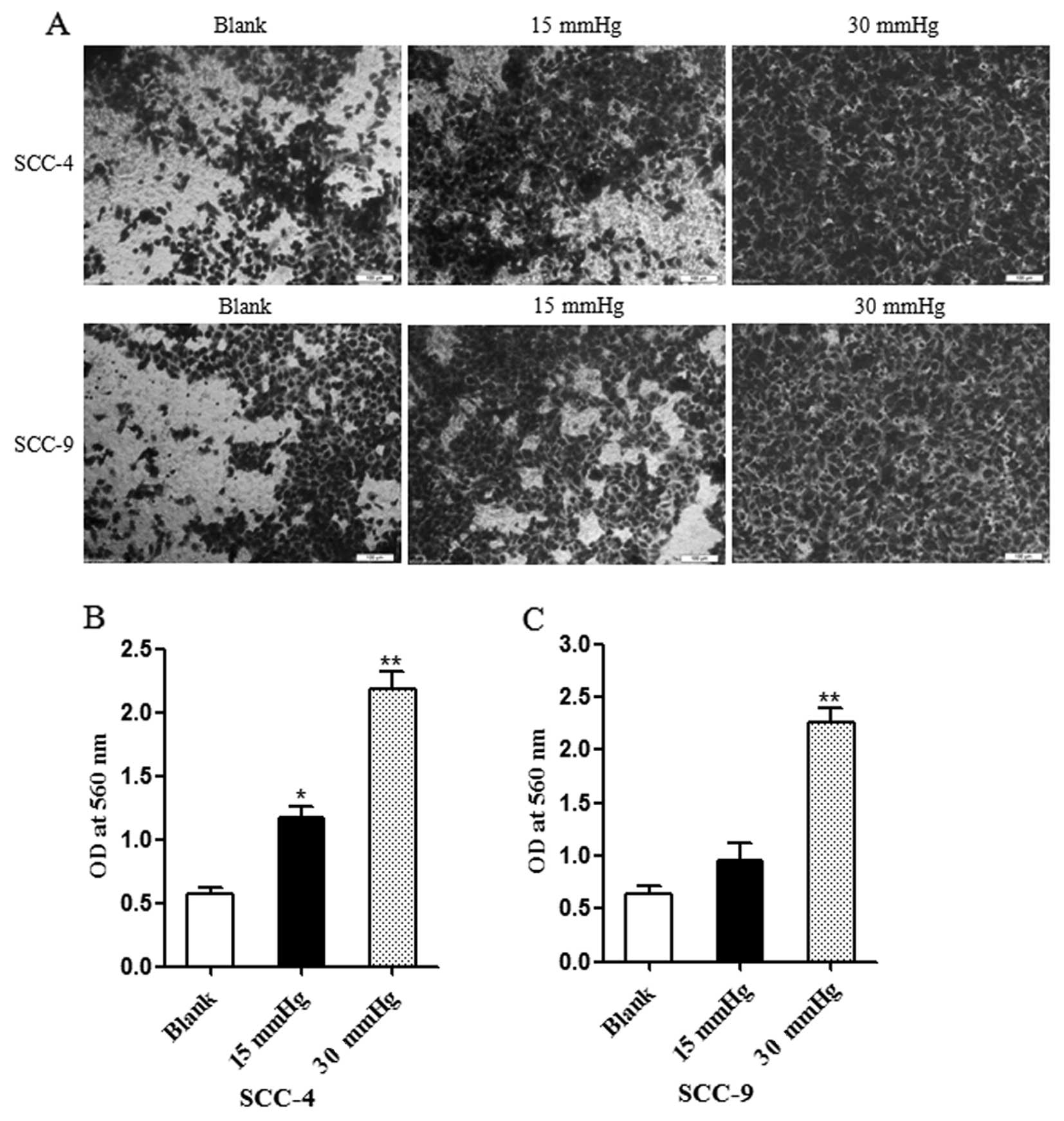
Increased extracellular pressure promotes
OSCC cell invasion in vitro
The proteolytic degradation of the ECM components is
critical for tumor cell invasion. Matrigel-coated Millicell
Chambers were used to determine the influence of extracellular
pressure on the invasion capacity of the cancer cells. Quantitative
analysis demonstrated that the invasion ability of the cells from
the group exposed to 30 mmHg pressure was significantly high,
compared with the blank group and the group exposed to 15 mmHg
pressure (P<0.01) (Fig. 3). In
addition, 15 mmHg extracellular pressure enhanced the invasion
ability of the SCC-4 cells, compared with the blank group
(P<0.05).
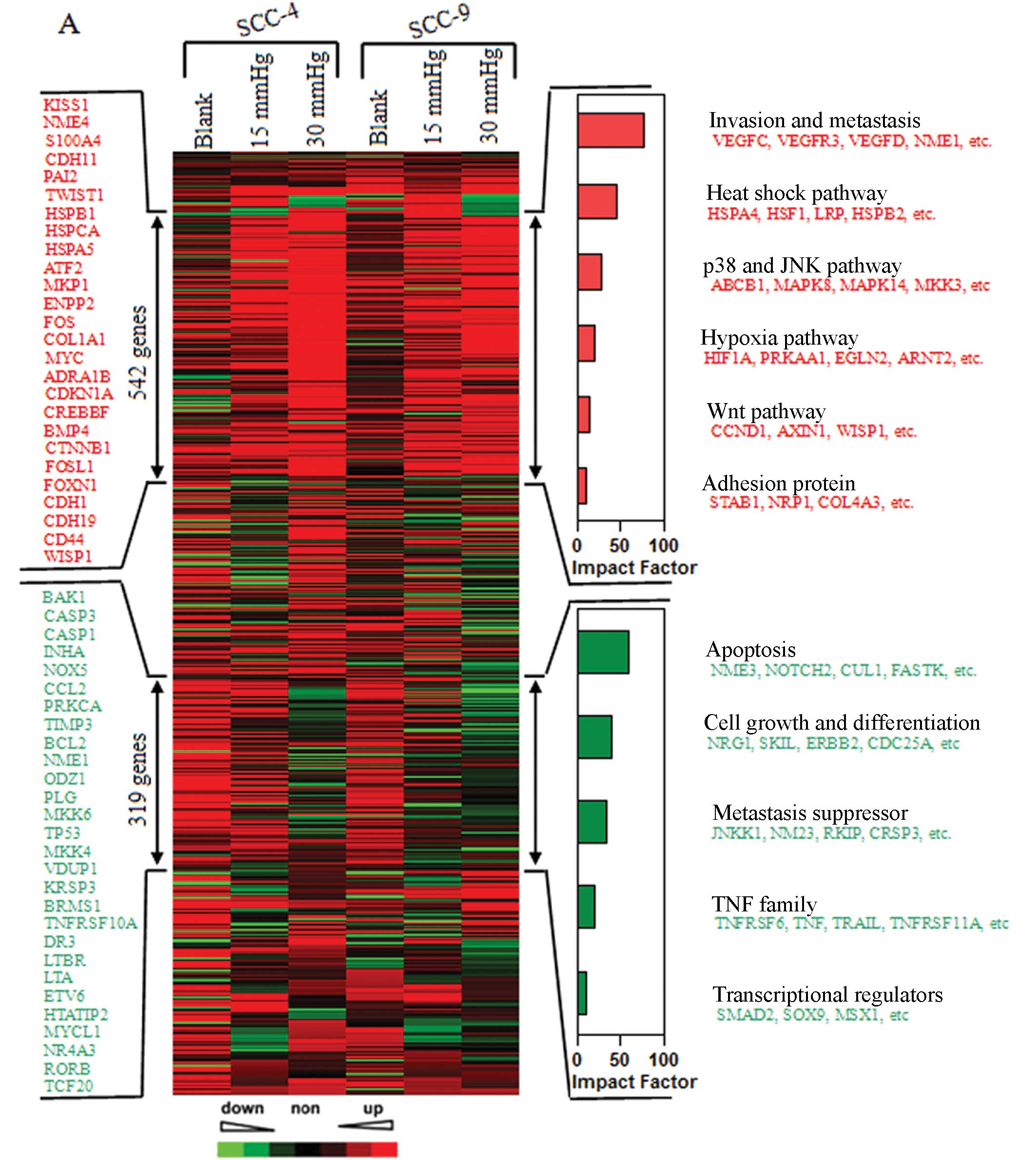
Gene expression profile analysis for OSCC
cells subjected to elevated extracellular pressure
To further investigate the effects of increased
extracellular pressure on downstream targets in OSCC cells, we
performed gene expression profiling analysis on the SCC-4 and SCC-9
cells exposed to 0, 15 and 30 mmHg pressure. With the increase in
extracellular pressure, unsupervised clustering analysis of 1,827
genes identified two groups of genes (trees 1 and 2) with
significantly altered expression levels (by >2-fold) in the
SCC-4 and SCC-9 cells. Tree 1 included 542 upregulated genes, and
tree 2 contained 319 downregulated genes. Functional profiling of
these genes revealed that the majority of these genes are involved
in invasion and metastasis, as well as the heat shock pathway, the
p38 and JNK signaling pathway, apoptosis and the cell growth and
differentiation signaling pathway (Fig. 4A).
qRT-PCR for tumor-related genes
We then used qRT-PCR to confirm the
pressure-dependent expression of over 20 genes identified in the
microarray analysis. Apart from the expression of the KISS1 gene in
SCC-4 cells, the expression levels of the other genes were
consistent with those from microarray analysis (Fig. 4B–C).
Discussion
Since Young et al 13) firstly identified that
IFP is significantly higher in tumors compared with normal tissues,
an enhanced IFP has been identified in many solid tumors, and it
has been used as an important prognostic factor. It has been shown
to be the single best indicator of survival for patients with
cancer of the cervix (5). Several
studies have demonstrated the correlation between IFP and the
malignant characteristics of tumors (14–16). van der Voort van Zyp et al
(16) identified that an
increased extracellular pressure of 15 mmHg can stimulate colon
cancer cell adhesion to surgical wounds, promoting tumor
recurrence. Roh et al (5)
found that the IFP increased with the grade (degree of
differentiation) of squamous cell carcinoma of the uterine cervix.
Although there is no doubt that the IFP is related to tumor size,
invasion and metastasis, we wished to determine whether the
increased IFP would in turn promote tumor cell proliferation and
metastasis. Therefore, in this study, to further investigate the
effect of IFP on OSCC cells, we investigated the effects of
increased IFP on SCC-4 and SCC-9 cells exposed to conditions
mimicking IFP (0, 15 and 30 mmHg above atmospheric pressure) in
vitro. Of note, the proliferation and invasion of SCC-4 cells
significantly increased in the group exposed to 30 mmHg pressure
in vitro. Furthermore, to our knowledge, this is the first
study to demonstrate the effect of a high IFP on OSCC cells. The
exposure of OSCC cells to extracellular pressure induces an
aggressive cancer phenotype that promotes cancer cell growth and
metastasis.
To identify the potential molecular mechanisms
responsible for the effects of elevated extracellular pressure on
tumor growth and invasion, we used microarray analysis to detect
the gene expression profiles in SCC-4 and SCC-9 cells treated with
different pressure values. A total of 1,827 genes with altered
expression levels (by >2-fold) was identified. A total of 542
genes were upregulated with the increase in pressure in the SCC-4
and SCC-9 cells; these genes were mainly involved in invasion and
metastasis, the heat shock pathway, the p38 and JNK signaling
pathway and the hypoxia signaling pathway. In addition, 319 genes
were found to be downregulated with the elevation in pressure
values in the SCC-4 and SCC-9 cells, which were mainly involved in
apoptosis, cell growth and differentiation and metastasis.
Subsequently, we used qRT-PCR to confirm the pressure-dependent
expression of over 20 genes identified in the microarray analysis.
Heat shock protein (HSP)A4, also known as HSP70, has been shown to
be significantly associated with low differentiation and poor
prognosis in a number of malignant tumors (17–19). CCND1, also known as cyclin D1, is
overexpressed in several human tumors; its expression is induced by
growth factors and occurs at multiple levels, including increased
transcription, translation and protein stability (20–22). Cyclin D1 plays an important role
in G1 phase transition, which is a kinase-independent function, by
sequestering cyclin-dependent kinase (CDK) inhibitors, such as
CDKN1A (p21) and CDKN1B (p27) for the efficient activation of
CDK2-containing complexes (23,24). Since hypoxia is clearly related to
radioresistance, it has been suggested that the abnormal
vascularization of tumors leads to increased hypoxia, whereas
reperfusion improves the radiosensitivity of tumors (25,26). It has been reported that the
hypoxic environment consists of rapidly growing cancer cells that
proliferate faster than the vasculature within the environment,
creating low oxygen tension in the central and intermediate regions
of the tumor mass. This activates the transcriptional factor,
hypoxia-inducible factor (HIF-1a), that is known to promote
angiogenesis for tumor cell survival and the formation of a central
hypoxic region and a normoxic peripheral region; this is possibly
regulated by IFP (27–29).
Taken together, to our knowledge, this study
demonstrates for the first time the important role of an elevated
IFP and the corresponding molecular mechanisms, particularly, the
effect of lymphatic metastasis-related gene activation on this
process, in promoting OSCC proliferation, as well as invasion and
metastasis. Our data suggest the important potential clinical
application of measuring IFP; measuring tumor IFP may be a generic
marker of prognosis and response to therapy.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant no. 30973345), the Doctoral Fund
of Ministry of Education of China (grant no. 20090181110082) and
the Fund of the Department of Health of Sichuan Province (grant no.
130231).
References
|
1
|
Cairns R, Papandreou I and Denko N:
Overcoming physiologic barriers to cancer treatment by molecularly
targeting the tumor microenvironment. Mol Cancer Res. 4:61–70.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lunt SJ, Fyles A, Hill RP and Milosevic M:
Interstitial fluid pressure in tumors: therapeutic barrier and
biomarker of angiogenesis. Future Oncol. 4:793–802. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gutmann R, Leunig M, Feyh J, et al:
Interstitial hypertension in head and neck tumors in patients:
correlation with tumor size. Cancer Res. 52:1993–1995.
1992.PubMed/NCBI
|
|
4
|
Fukumura D and Jain RK: Tumor
microenvironment abnormalities: causes, consequences, and
strategies to normalize. J Cell Biochem. 101:937–949. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Roh HD, Boucher Y, Kalnicki S, Buchsbaum
R, Bloomer WD and Jain RK: Interstitial hypertension in carcinoma
of uterine cervix in patients: possible correlation with tumor
oxygenation and radiation response. Cancer Res. 51:6695–6698.
1991.PubMed/NCBI
|
|
6
|
Milosevic M, Fyles A, Hedley D, et al:
Interstitial fluid pressure predicts survival in patients with
cervix cancer independent of clinical prognostic factors and tumor
oxygen measurements. Cancer Res. 61:6400–6405. 2001.
|
|
7
|
Rofstad EK, Tunheim SH, Mathiesen B, Graff
BA, Halsør EF, Nilsen K and Galappathi K: Pulmonary and lymph node
metastasis is associated with primary tumor interstitial fluid
pressure in human melanoma xenografts. Cancer Res. 62:661–664.
2002.PubMed/NCBI
|
|
8
|
Fyles A, Milosevic M, Pintilie M, Syed A,
Levin W, Manchul L and Hill RP: Long-term performance of interstial
fluid pressure and hypoxia as prognostic factors in cervix cancer.
Radiother Oncol. 80:132–137. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lunt SJ, Kalliomaki TM, Brown A, Yang VX,
Milosevic M and Hill RP: Interstitial fluid pressure, vascularity
and metastasis in ectopic, orthotopic and spontaneous tumours. BMC
Cancer. 8:22008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
de Aguiar A Jr, Kowalski LP and de Almeida
OP: Clinicopathological and immunohistochemical evaluation of oral
squamous cell carcinoma in patients with early local recurrence.
Oral Oncology. 43:593–601. 2007.PubMed/NCBI
|
|
11
|
Parker B and Sukumar S: Distant metastasis
in breast cancer: molecular mechanisms and therapeutic targets.
Cancer Biol Ther. 2:14–21. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Young JS, Lumsden CE and Stalker AL: The
significance of the tissue pressure of normal testicular and of
neoplastic (Brown-Pearce carcinoma) tissue in the rabbit. J Pathol
Bacteriol. 62:313–333. 1950. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Basson MD, Yu CF and Herden-Kirchoff O:
Effects of increased ambient pressure on colon cancer cell
adhesion. J Cell Biochem. 78:47–61. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Thamilselvan V and Basson MD: Pressure
activates colon cancer cell adhesion by inside-out focal adhesion
complex and actin cytoskeletal signaling. Gastroenterology.
126:8–18. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
van der Voort van Zyp J, Thamilselvan V,
Walsh M, Polin L and Basson MD: Extracellular pressure stimulates
colon cancer cell adhesion in vitro and to surgical wounds by Src
(sarcoma protein) activation. Am J Surg. 188:467–473.
2004.PubMed/NCBI
|
|
17
|
Lazaris AC, Theodoropoulos GE, Davaris PS,
Panoussopoulos P, Nakopoulos L, Kittas C and Golematis BC: Heat
shock protein 70 and HLA-DR molecules tissue expression. Prognostic
implication in colorectal cancer. Dis Colon Rectum. 38:739–745.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lazaris AC, Chatzigianni EB,
Panoussopoulos D, Tzimas GN, Davaris PS and Golematis BC:
Proliferating cell nuclear antigen and heat shock protein 70
immunolocalization in invasive ductal breast cancer not otherwise
specified. Breast Cancer Res Treat. 43:43–51. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bauer K, Nitsche U, Slotta-Huspenina J, et
al: High HSP27 and HSP70 expression levels are independent adverse
prognostic factors in primary resected colon cancer. Cell Oncol
(Dordr). 35:197–205. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Quintayo MA, Munro AF, Thomas J, et al:
GSK3β and cyclin D1 expression predicts outcome in early breast
cancer patients. Breast Cancer Res Treat. 136:161–168. 2012.
|
|
21
|
Shih LC, Tsai CW, Tsai MH, et al:
Association of cyclin D1 genotypes with nasopharyngeal carcinoma
risk. Anticancer Res. 32:1093–1098. 2012.PubMed/NCBI
|
|
22
|
Huang SF, Cheng SD, Chuang WY, et al:
Cyclin D1 overexpression and poor clinical outcomes in Taiwanese
oral cavity squamous cell carcinoma. World J Surg Oncol. 10:402012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Polyak K, Kato JY, Solomon MJ, et al:
p27Kip1, a cyclin-Cdk inhibitor, links transforming growth
factor-beta and contact inhibition to cell cycle arrest. Genes Dev.
8:9–22. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sherr CJ and Roberts JM: CDK inhibitors:
positive and negative regulators of G1-phase progression. Genes
Dev. 13:1501–1512. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Moeller BJ, Richardson RA and Dewhirst MW:
Hypoxia and radiotherapy: opportunities for improved outcomes in
cancer treatment. Cancer Metastasis Rev. 26:241–248. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cerniglia GJ, Pore N, Tsai JH, et al:
Epidermal growth factor receptor inhibition modulates the
microenvironment by vascular normalization to improve chemotherapy
and radiotherapy efficacy. PLoS One. 4:82009. View Article : Google Scholar
|
|
27
|
Liao D and Johnson RS: Hypoxia: a key
regulator of angiogenesis in cancer. Cancer Metastasis Rev.
26:281–290. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Carmeliet P: Angiogenesis in health and
disease. Nat Med. 9:653–660. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mizobuchi H, García-Castellano JM, Philip
S, et al: Hypoxia markers in human osteosarcoma: an exploratory
study. Clin Orthop Relat Res. 466:2052–2059. 2008. View Article : Google Scholar : PubMed/NCBI
|