Introduction
The polycytosine [poly(C)]-binding proteins (PCBPs)
are characterized by heterogeneous nuclear ribonucleoprotein
(hnRNP) K homology (KH) domains and high affinity sequence-specific
interactions with polycytosine [poly(C)] nucleic acid sequences. In
mammalian cells, five evolutionarily related PCBPs have been
identified: PCBP1–4 and hnRNP K (1). These PCBPs belong to one of two
subgroups: hnRNP K or the α-complex proteins (α-CPs or PCBP1–4)
(2). Each PCBP has three KH
domains. hnRNP K, PCBP1 (α-CP1 or hnRNPE1), and PCBP2 (α-CP2 or
hnRNPE2) have been extensively investigated (3,4).
Two other members of the α-CP family have also been discovered:
PCBP3 (α-CP3) and PCBP4 (α-CP4) (5).
All members of the PCBP family are related
evolutionarily. The common feature of all PCBPs is the presence of
three hnRNP KH domains (6). These
are RNA-binding modules that are approximately 70 amino acids in
length. The KH domain of PCBPs consists of three α-helices and
β-strands arranged as follows: β1-α1-α2-β2-β3-α3 (7). The Gly-X-X-Gly loop is located
between α1 and α2, and the variable loop is located between β2 and
β3. These KH domain sequences are conserved in PCBPs (7,8).
PCBP1 and PCBP2 share the highest level of amino acid sequence
similarity (89%). PCBP3 is more divergent, and PCBP4 is the most
distantly related (52% divergence from α-CP2) (5,9).
The function of PCBPs is dependent on their
localization to either the cytoplasm (mRNA stability and
translational regulation) or nucleus (transcription and splicing).
PCBP1 and PCBP2 are primarily localized in the nucleus and nuclear
speckles. By contrast, PCBP3 and PCBP4 are primarily localized in
the cytoplasm (9). The
signal-dependent post-translational modifications of PCBPs can
regulate their ability to bind nucleic acids. For example, the
phosphorylation of PCBP1 and PCBP2 markedly decreases their
RNA-binding activity (3), and the
phosphorylation of PCBP1 increases its DNA-binding activity
(10). Another important
determinant of the different functions of PCBPs is their
subcellular localization (9,10).
PCBP1 has been shown to function as a cytosolic iron chaperone
during the delivery of iron to ferritin. Such iron binding to PCBP1
may significantly alter its nucleic acid binding activity (11). PCBP2 can participate in
protein-protein interactions (12) and has been linked to the
regulation of poliovirus replication (13); it also plays a role in innate
immunity (14). PCBP4 (MCG10) can
induce apoptosis (15) and may
function as a lung tumor suppressor and its expression can inhibit
the proliferation and tumorigenesis of lung cancer cells, both
in vivo and in vitro, by delaying the progression of
the cell cycle (16,17). Members of this family perform
multiple functions by binding to poly(C) sequences, including mRNA
stabilization (18–20), translational silencing (21,22) and translational enhancement
(19,23).
In this study, we report the purification, refolding
and characterization of an α-CP2 protein that binds to
single-stranded DNA and RNA poly(C) sequences. We purified
recombinant α-CP2 using affinity column chromatography and
confirmed its identity using mass spectrometry. This study
demonstrates a dual binding function for the α-CP2 protein via
specific interactions with single-stranded DNA and RNA poly(C)
sequences. To our knowledge, we also demonstrate for the first time
that α-CP2 functions as a transcriptional activator by binding to
single-stranded poly(C) sequences.
Materials and methods
Plasmid construction
The single-strand forming construct, pGL-SS, was
generated by ligating an annealed double-stranded oligonucleotide
into the SacI and HindIII sites of pGL3-basic
(Promega, Madison, WI, USA) using the following oligonucleotide
sequences: 5′-ATTGAGCTCACAATCCACTCCTTCTCTCTCCTCCCTCCCCTCTAGCCTCTGAAGCTTTTC-3′)
(sense) containing a SacI site (underlined) and
5′-GAAAAGCTTCAGAGGCTAGAGGGGAGGGAGGAGAGAGAAGGAGTGGATTGTGAGCTCAAT-3′
(antisense) containing a HindIII site (underlined). To clone
the α-CP2 gene, total RNA was isolated from mouse NS20Y cells. RNA
was treated with RNase-free DNase (Promega) according to the
manufacturer's instructions. RT-PCR was performed using the OneStep
RT-PCR kit (Qiagen, Valencia, CA, USA). PCR was performed with
primers that were designed using the gene sequence information for
each protein: α-CP2 (Gene ID 6997238) 5′-AACTGCTAGACATGGACACCG-3′
(sense) and 5′-AGGTGGCATGGGTAGCAGCTAG-3′ (antisense). The PCR
conditions were as follows: 94°C for 3 min; 35 cycles of 94°C for 1
min, 55°C for 1 min, and 72°C for 1 min; and 72°C for 10 min. The
RT-PCR products were excised from a 1% agarose gel, purified using
a QIAquick gel extraction kit (Qiagen), and cloned into a
pCRII-TOPO vector (Invitrogen, Carlsbad, CA, USA). The candidate
plasmids containing inserts of the correct size were confirmed
using restriction enzyme digestion and DNA sequencing on an ABI
3100 sequencer (Applied Biosystems, Cambridge, MA, USA). For the
transient expression studies, the α-CP2 gene was cloned by
digesting the above pCRII-TOPO PCBP2 clone with 5′-HindIII
and 3′-XhoI into the same sites of a pcDNA4 vector
(Invitrogen), generating a pcDNA4-α-CP2 plasmid. The DNA sequences
of all constructs were confirmed by DNA sequencing. For the protein
expression experiments with Escherichia coli (E.
coli), the α-CP2 gene was cloned by digesting the above
pcDNA4-α-CP2 plasmid with 5′-HindIII and 3′-XhoI into
the same sites of a pET21b vector, generating a pET21b-α-CP2
plasmid. The DNA sequences of all constructs were confirmed by DNA
sequencing.
α-CP2 protein expression
Protein was expressed in LB medium containing
ampicillin (50 μg/ml). To obtain the protein, several cell growth
conditions were generated by varying the temperature and
isopropylthio-β-galactoside (IPTG) concentration. Typically, 2 ml
of an overnight culture were added to 100 ml of medium and
incubated with vigorous shaking at a temperature in the range of
37°C. When the culture reached OD600=0.5, protein
expression was induced with 1 mM IPTG. The samples were further
incubated for 4 h after induction. The cells were harvested by
centrifugation at 4,000 × g for 10 min at 4°C, washed with TE
buffer (10 mM Tris-HCl, 1 mM EDTA, pH 8.0) and stored at −80°C.
Preparation of inclusion bodies and
purification of recombinant α-CP2 protein
The cell pellet was resuspended in 30 ml of buffer A
(20 mM Tris-HCl, 100 mM NaCl, 1 mM PMSF, pH 7.0, containing 10 μl
of 1 mg/ml DNase I) and sonicated at 4°C with 5 cycles. The lysate
was centrifuged at 10,000 × g for 15 min at 4°C. The pellet was
resuspended in 5 volumes of buffer A, stirred at room temperature
for 5 min and centrifuged at 10,000 × g for 15 min at 4°C. The
inclusion bodies were then washed three times with 10 volumes of 20
mM Tris-HCl containing 100 mM NaCl at pH 7.0. The inclusion body
pellet was resuspended in 30 ml of buffer B (50 mM
NaH2PO4, 300 mM NaCl, pH 8.0, 8 M urea) to
solubilize the inclusion bodies. Sonication was necessary to
suspend the pellet. The suspension was then centrifuged at 10,000 ×
g for 20 min, and the supernatant was transferred to a clean tube.
The supernatant was then added to an equilibrated Ni-NTA column and
allowed to drain via gravity flow. The column was washed with
buffer B, and the His-tagged α-CP2 was eluted using an elution
buffer (50 mM NaH2PO4, 300 mM NaCl, 250 mM
imidazole, pH 8.0, 8 M urea). To determine which fractions contain
the His-tagged α-CP2, we analyzed an aliquot of each sample using
10% SDS-PAGE.
Folding of the α-CP2 protein
The washed inclusion bodies were resuspended in 5
volumes of buffer C (20 mM Tris-HCl, 1 mM EDTA, 10 mM DTT, 8 M
Urea, pH 7.0), stirred at room temperature for 60 min and
centrifuged at 10,000 × g for 15 min at room temperature. The
pellet was discarded and the supernatant (5–10 mg/ml) was collected
in a new tube. The refolding experiments were performed using
protein-folding spin-columns following the manufacturer's
recommendations (ProFoldin, Hudson, MA, USA).
SDS-PAGE, in-gel tryptic digestion and
matrix-assisted laser desorption/ionization time-of-flight
(MALDI-TOF) mass spectrometric analysis of α-CP2
The purified α-CP2 protein was resolved on a 10%
SDS-PAGE gel. The Coomassie blue-stained gel was destained, and a
gel slice containing the band of interest was subjected to in-gel
tryptic digestion as previously described (24). The tryptic peptides were extracted
with 5% acetic acid followed by 5% acetic acid and 50%
acetonitrile. The samples were dissolved in 5% acetic acid and
desalted using ZipTip™ C18 reverse-phase desalting Eppendorf tips
(Millipore, Billerica, CA, USA). The peptides were eluted with 2%
acetonitrile containing 0.1% trifluoroacetic acid (TFA) in a volume
of 20 μl. The samples were analyzed using a MALDI-TOF mass
spectrometer (Applied Biosystems). The masses of the monoisotopic
peaks were compared to a theoretical digestion of the protein by
trypsin. The Mascot database searching software (Matrix Science,
http://www.matrixscience.com) was used
to identify the binding proteins.
RNA electrophoretic mobility shift assay
(EMSA)
EMSA was performed as previously described (25). The single-stranded RNA probe
(5′-CUCUCCUCCCUCCCCUCUAGCCUC-3′) was end-labeled with
[γ-32P] dATP. The free nucleotides were separated by
centrifugation through a Sephadex G-25 column (Roche Diagnostics,
Indianapolis, IN, USA). The end-labeled single-stranded RNA probe
was incubated with recombinant α-CP2 (0.5 μg) in a final volume of
20 μl of RNA EMSA buffer [10 mM Tris (pH 7.8), 10% glycerol, 0.5 mM
EDTA, 1 mM MgCl2, 0.1 mg/ml bovine serum albumin, 0.5
mg/ml yeast tRNA and 5 units of RNAsin] at room temperature for 20
min. For the oligonucleotide competition analyses, a 100-fold molar
excess of a cold competitor RNA oligonucleotide was added to the
mixture prior to adding the probe. The reactions were then
incubated at 4°C for 30 min. The reaction mixtures were
electrophoresed on a non-denaturing 4% polyacrylamide gel in 0.5X
TBE (45 mM Tris-borate and 1 mM EDTA) at 4°C and visualized by
autoradiography.
DNA EMSA
EMSA was performed as described in a previous study
(26). The single-stranded probe
(5′-CAATCCACTCCTTCTCTCTCCTCCCTCCCCTCTAGCCTCTG-3′) was end-labeled
with [γ-32P] dATP. The free nucleotides were separated
by centrifugation through a Sephadex G-25 column (Roche
Diagnostics). The end-labeled single-stranded DNA probes were
incubated with recombinant α-CP2 (0.5 μg) in a final volume of 20
μl of EMSA buffer [10 mM Tris (pH 7.5), 5% glycerol, 1 mM EDTA, 50
mM NaCl, 1 mM DTT, 0.1 mg/ml poly(dI-dC)] at room temperature for
20 min. For the oligonucleotide competition analyses, a 100-fold
molar excess of a cold competitor oligonucleotide was added to the
mixture prior to adding the probe. The reactions were then
incubated at 4°C for 30 min. The reaction mixtures were
electrophoresed on a non-denaturing 4% polyacrylamide gel in 0.5X
TBE at 4°C and visualized by autoradiography.
S1 nuclease sensitivity assay
The pGL-SS plasmid was digested with various amounts
of S1 nuclease (Promega) in S1 nuclease buffer for 15 min at 37°C.
The digestion was terminated by phenol/chloroform extraction and
the plasmids were recovered by precipitation. The resulting
S1-treated plasmids were then digested further using XbaI,
and the products were resolved by electrophoresis on a 1% agarose
gel.
Transient transfection and reporter gene
assays
Mouse NS20Y neuroblastoma cells were grown in
Dulbecco's minimum essential medium supplemented with 10%
heat-inactivated fetal bovine serum at 37°C in a humidified
atmosphere of 5% CO2. The NS20Y cells were plated in
6-well dishes at a concentration of 0.5×106 cells/well
and cultured overnight prior to transfection. Equimolar
concentrations of various plasmids were transfected using the
Effectene transfection reagent (Qiagen) as previously described
(27). Briefly, for the
luciferase analysis of the pGL-SS promoter, 0.5 μg of the reporter
plasmids was mixed with the Effectene transfection reagent for 10
min before being added to the NS20Y cells. Forty-eight hours after
transfection, the cells grown to confluence were washed once with
phosphate-buffered saline and lysed with lysis buffer (Promega). To
correct for differences in transfection efficiency, a one-fifth
molar ratio of pCH110 (Amersham Biosciences, Piscataway, NJ, USA)
containing the β-galactosidase gene under the SV40 promoter was
included in each transfection for normalization. The luciferase and
β-galactosidase activities of each lysate were determined according
to the manufacturer's recommendations (Promega and Tropics,
respectively).
Western blot analysis
The proteins isolated from the NS20Y cells
transfected with the α-CP2 gene were incubated with treatment
buffer [62.5 mM Tris-HCl (pH 6.8), 2% SDS, 10% glycerol, 5%
2-mercaptoethanol] and boiled for 5 min. The treated extracts were
resolved by SDS-PAGE using a 12% polyacrylamide gel. The gels were
electroblotted onto polyvinylidene difluoride membranes (Amersham
Biosciences) in a transfer buffer (48 mM Tris-HCl, 39 mM glycine,
20% methanol). The membranes were blocked in a blocking solution
(10% dry milk and 0.1% Tween-20 in Tris-buffered saline) overnight
at 4°C. Western blot analysis with anti-Myc (Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA) and anti-β-actin
antibodies (Cell Signaling Technology, Beverly, MA, USA) was
performed according to the manufacturer's instructions (Amersham
Biosciences). The signals were detected using a Storm 840
PhosphorImager system (Amersham Biosciences).
Results
Expression and purification of α-CP2
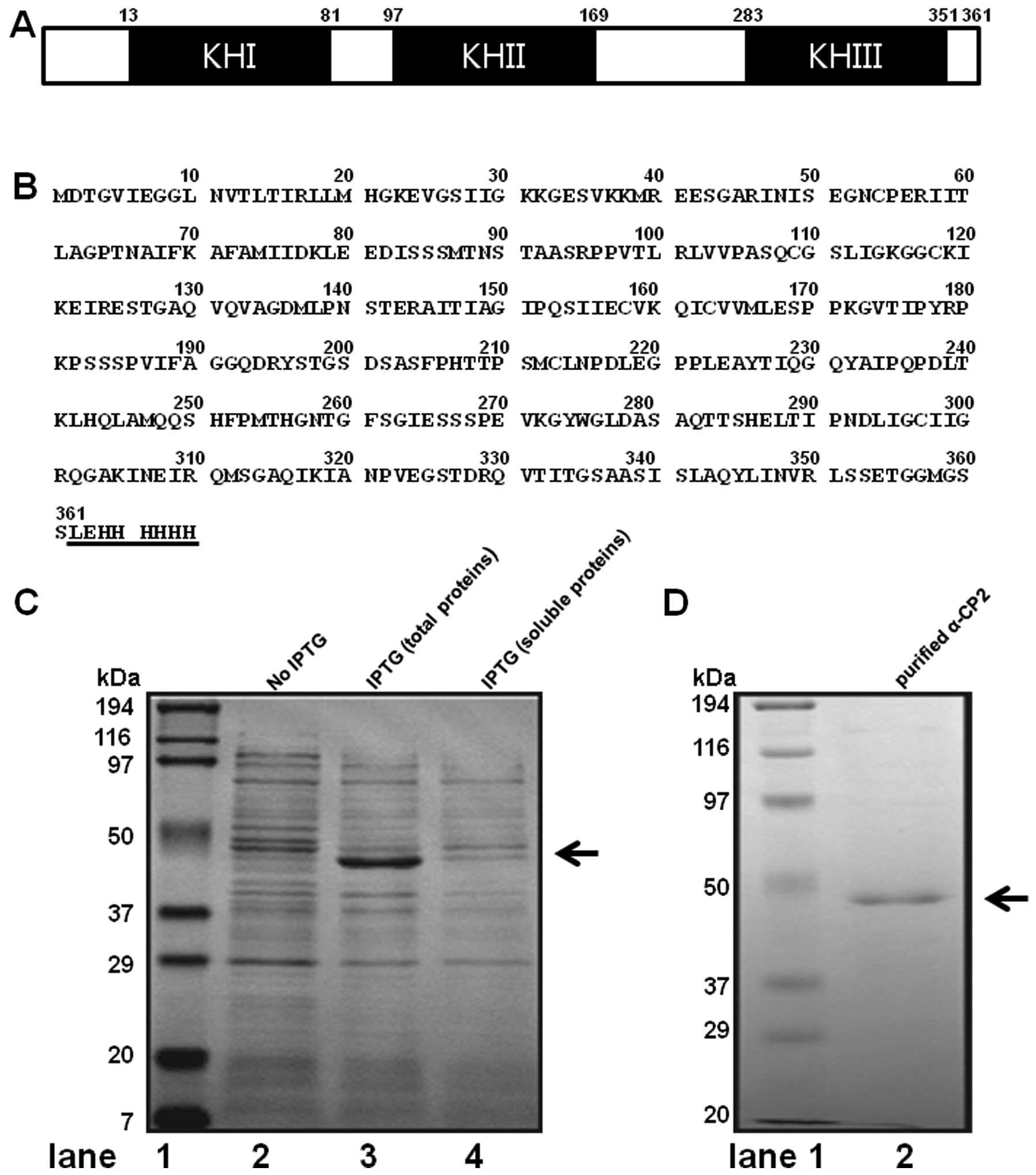
α-CP2 contains three hnRNPK homology (KH) domains,
two consecutive KH domains at the amino terminus and a third KH
domain at the carboxyl terminus, separated by an intervening
sequence. The mouse α-CP2 gene encodes a 361 amino acid protein
with a calculated molecular mass of 38,150 Da and a pI of 6.61
(Fig. 1A and B). The mouse α-CP2
gene was cloned into the pET21b vector, resulting in the expression
of a recombinant α-CP2 with a 6xHis-tag at the C-terminus
(α-CP2-His). The conditions for expressing the soluble protein of
α-CP2 in the E. coli strain BL21(DE3) were extensively
tested, including the temperatures for cell growth, cell culture
mediums (LB and 2xYT), and induction at different stages of growth.
However, almost all the conditions produced the inclusion body of
the α-CP2 protein. To obtain the maximum amount of insoluble
α-CP2-His protein, the expression conditions were optimized by a
series of trials. The highest percentage of insoluble protein was
obtained when the expression of the α-CP2-His protein was induced
by 1 mM IPTG at 37°C for 4 h. Under the optimal condition,
approximately 30% of the α-CP2-His was present in the insoluble
fraction, as analyzed by SDS-PAGE with Coomassie brilliant blue
staining (Fig. 1C). His-tags are
excellent tools for purifying recombinant proteins from crude E.
coli extracts, and immobilized metal affinity chromatography is
the most commonly used method for purifying recombinant proteins
containing a short 6xHis-tag. Thus, Ni-NTA His-binding resin
affinity chromatography was employed to purify the insoluble
recombinant α-CP2 under 8 M Urea. After washing with washing
buffer, the protein was eluted with 250 mM imidazole. The SDS-PAGE
results (Fig. 1D, lane 2)
revealed that the α-CP2-His protein was a single band. The
molecular weight was estimated to be 45 kDa. To confirm that we had
isolated the α-CP2 protein, we analyzed the purified band using
MALDI-TOF mass spectrometry and bioinformatics. Based on its high
score (score of 155) on the Mascot search results (Fig. 1E), the protein was identified as
mouse α-CP2.
Folding of α-CP2
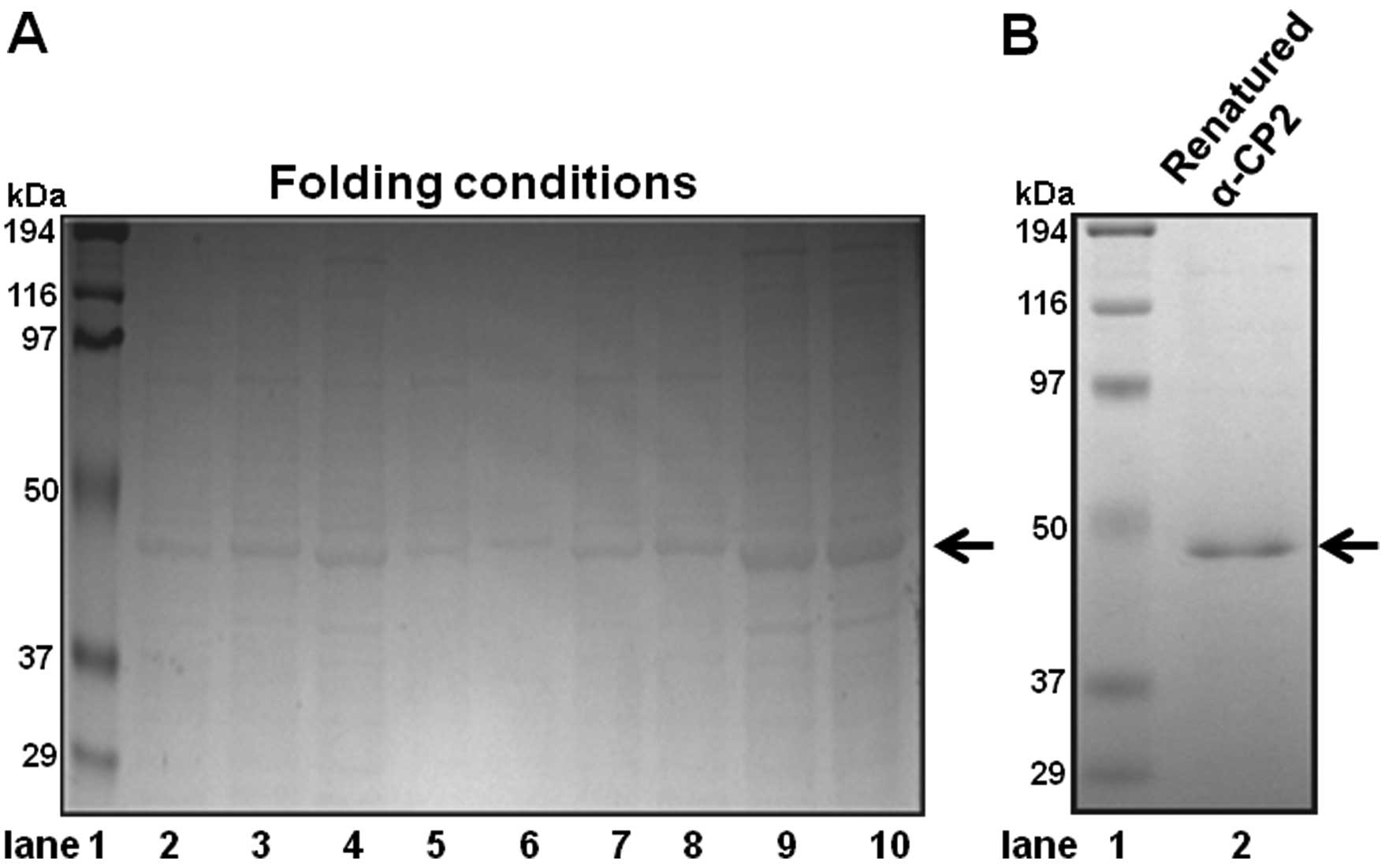
The folding conditions were extensively tested to
obtain the most active α-CP2 protein. To optimize the α-CP2 protein
folding conditions, we used the Spin-Column Protein Folding Screen
kit (ProFoldin Protein preparation and assay technologies, Hudson,
MA, USA). The ProFoldin Spin-Column was considered the most
effective. We tested several refolding methods, including ProFoldin
Spin-Columns, as well as others. Finally, we selected the ProFoldin
Spin-Columns due to its simple folding conditions and technical
simplicity. The screen kit includes nine different protein folding
spin-columns (column nos. 1–9). The nine different columns
represent the nine most promising folding conditions. Using the
Spin-Column Protein Folding Screen kit, we identified column no. 8
as the one with the optimal protein folding conditions (Fig. 2A, lane 9). Ni-NTA His-binding
resin affinity chromatography was employed to purify the insoluble
recombinant α-CP2 under 8 M urea. The denatured, purified α-CP2
protein was folded using the Spin-Column Protein Folding Screen kit
(column no. 8). The SDS-PAGE results revealed that the α-CP2
protein was folded and purified (Fig.
2B, lane 2).
RNA binding property of α-CP2
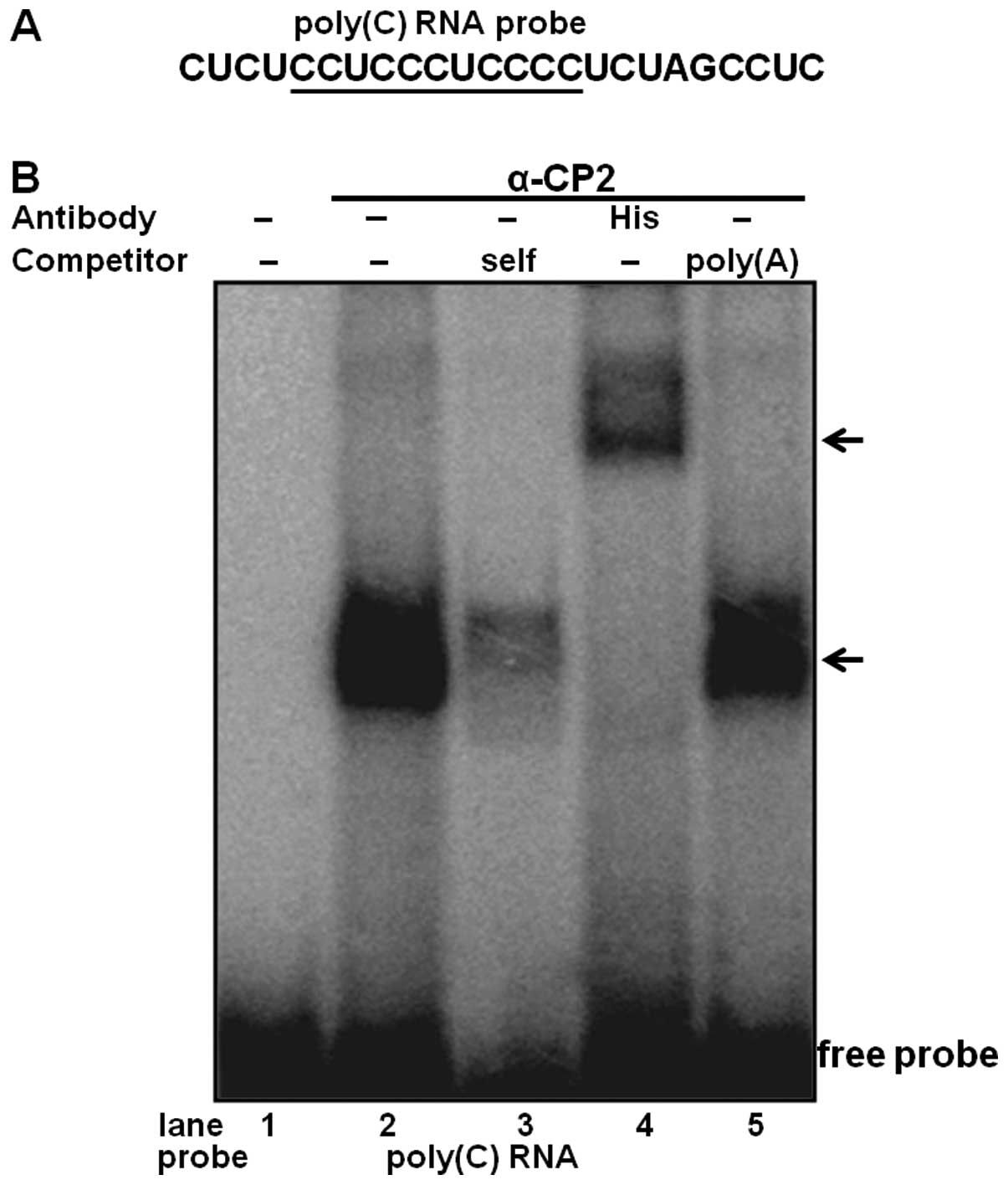
To determine the physical interaction of purified
α-CP2 with the RNA poly(C) sequence, an RNA EMSA was performed
using purified α-CP2 and 32P-labeled RNA (Fig. 3A). The purified α-CP2 protein was
able to shift the target RNA probe (Fig. 3B, lane 4). The specificity of this
RNA-protein interaction was verified by competitive inhibition in
the presence of a 100-fold excess of an unlabeled self-competitor
(Fig. 3B, lane 3) and a poly(A)
sequence of the same length as the competitor (Fig. 3B, lane 5). We also used an
anti-His antibody with the purified α-CP2 protein, which was
His-tagged from the pET21b-α-CP2 plasmid. The formation of the
α-CP2-RNA complex was abolished by the addition of the anti-His
antibody and supershifted (Fig.
3B, lane 4), indicating a specific interaction between α-CP2
and the RNA poly(C) sequence.
Single-stranded DNA binding property of
purified α-CP2
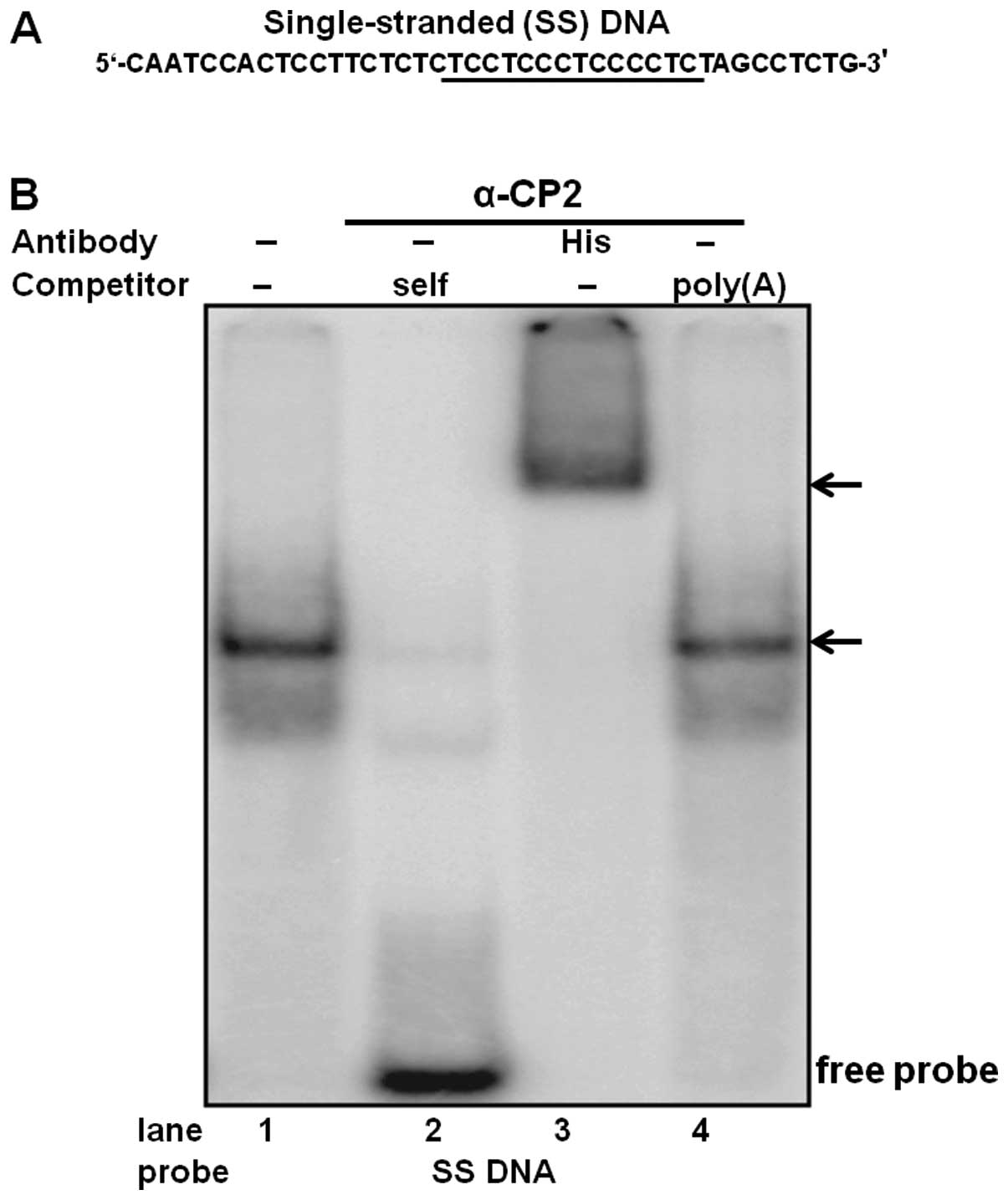
To determine the physical interaction of purified
α-CP2 with the single-stranded DNA C-rich sequence, DNA EMSA was
performed using purified α-CP2 protein and a 32P-labeled
single-stranded DNA oligonucleotide (Fig. 4A). The specificity of this
DNA-protein interaction was verified by competitive inhibition in
the presence of a 100-fold excess of an unlabeled self-competitor
(Fig. 4B, lane 2) and a poly(A)
sequence of the same length as the competitor (Fig. 4B, lane 4). We also used an
anti-His antibody with the purified α-CP2 protein, which was
His-tagged from the pET21b-α-CP2 plasmid. The formation of the
α-CP2-DNA complex was abolished by the addition of the anti-His
antibody and supershifted (Fig.
4B, lane 3), indicating a specific interaction between α-CP2
and the single-stranded DNA C-rich sequence.
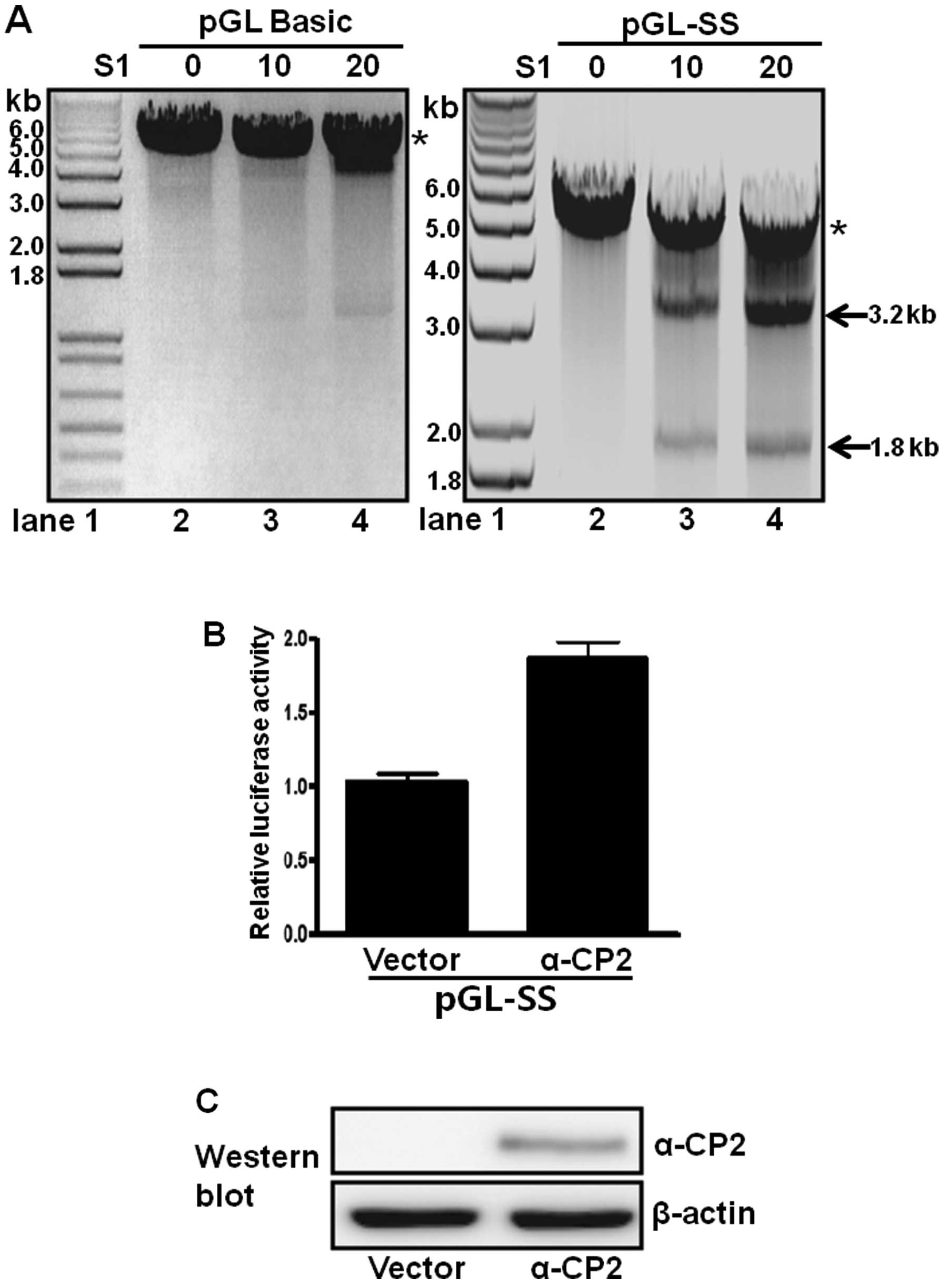
S1 nuclease sensitivity of promoter DNA
containing single-stranded poly(C) sequences
Single-stranded regions resulting from the non-B DNA
form, such as melting DNA or an intramolecular triplex structure,
are accessible to single-stranded-sensitive nucleases (e.g., S1
nuclease) at low concentrations (28). Accordingly, a pGL-SS plasmid
containing a poly(C) sequence inserted into the promoterless
pGL3-basic plasmid has promoter activity and was examined for its
S1 nuclease sensitivity. The plasmid was treated with or without S1
nuclease and then digested with XbaI. In the absence of S1
nuclease treatment, only a 5-kb XbaI-linearized DNA band was
observed in the digested pGL-SS sample (Fig. 5A, lane 2 in right panel). However,
treatment with both the S1 nuclease and XbaI produced two
DNA fragments of 1.8 and 3.2 kb, and the intensity of both bands
increased with increasing amounts of S1 nuclease (Fig. 5A, lanes 3 and 4, right panel).
These results suggest the presence of a single-stranded poly(C)
sequence located approximately 1.8 kb from the XbaI site
(Fig. 5A, right panel). When the
promoterless pGL3-basic plasmid was examined using the same
digestion procedures (Fig. 5A,
left panel), only the 5-kb linearized band was observed, with or
without S1 nuclease treatment. These results confirmed that the
poly(C) sequence of pGL-SS is present as a single-stranded DNA
(Fig. 5A, right panel).
α-CP2 transcriptionally regulates a
single-stranded poly(C)-containing promoter
The role of α-CP2 binding to the poly(C)-containing
promoter was examined by fusing the single-stranded promoter with a
luciferase reporter and co-transfecting these constructs with an
α-CP2 expression plasmid (pcDNA4Myc-HisA-α-CP2) into neuronal NS20Y
cells. α-CP2 activated approximately 80% of the activity of the
single-stranded promoter (Fig.
5B) compared with the cells transfected with the pcDNA4 vector
alone. Immunoblot analyses with anti-Myc were performed following
plasmid transfection to confirm the overexpression of the α-CP2
protein. β-actin was used as an internal control. The protein
levels of α-CP2 were increased, whereas the protein levels of α-CP2
were not detectable in the cells transfected with the pcDNA4 vector
alone (negative control) (Fig.
5C). These results indicate that α-CP2 acts as a
transcriptional activator of promoters containing single-stranded
poly(C) sequences.
Discussion
α-CP2 belongs to a family of KH domain-containing
proteins that specifically interact with poly(C) DNA/RNA sequences
and require three C-rich motifs (underlined) with a few intervening
nucleotides (29), such as
α-globin mRNA, 5′-CCCAACGGGCCCU CCUCCC-3′; folate receptor mRNA,
5′-CUCCAUUCCC ACUCCCU-3′; 15-LOX mRNA
CCCCACCCUCUUCCCC AAG-3′ (30–32). α-CP1 (PCBP1) has been shown to
bind specifically to the single-stranded DNA element of the
proximal promoter region in the mouse mu opioid receptor gene and
activate the gene (28). As
previously demonstrated, hnRNP K binds specifically to the
double-stranded DNA element of TC1 and TC2 and induces a
single-stranded conformation, in addition to binding to the
single-stranded TC3 sequence of the SRC1A gene promoter (33). Moreover, in a previous study, we
demonstrated that hnRNP K and α-CP3 (PCBP3) can specifically bind
to single-stranded and double-stranded DNA elements and may thus
act as a transcriptional regulator (34).
A previous study demonstrated that the soluble form
of α-CP2 can be produced in E. coli JM109 cells grown at
30°C overnight with IPTG (35).
However, the same culture and induction conditions produced
inclusion bodies of the α-CP2 protein. To overcome this problem,
α-CP2 was affinity-purified under denatured conditions and refolded
using the protein-folding spin-columns (34). To our knowledge, this is the first
study to demonstrate the purification, solubilization and folding
of α-CP2, and the production of a functionally active form for
RNA/DNA EMSA analysis using the E. coli protein expression
system.
In this study, we investigated the RNA/DNA binding
properties of α-CP2. α-CP2, a member of the PCBP family, binds to
the RNA/DNA poly(C) elements. Specific interactions between α-CP2
and RNA/DNA poly(C) sequences were observed using RNA/DNA EMSA
assays. The EMSA results demonstrated a sequence-specific
interaction between solubilized α-CP2 and the poly(C) rich RNA/DNA
sequences. The poly(C) rich sequence is enough for the adaption of
a single-stranded DNA conformation of the promoter region in the
mouse mu opioid receptor gene (28). The single-stranded DNA
conformation was sensitive to S1 nuclease digestion. An equilibrium
between the double-stranded DNA and the single-stranded DNA (melted
DNA structure) may exist, such that a small portion of the
single-stranded DNA structure can be digested by S1 nuclease
(28). When the single-stranded
DNA construct (pGL-SS) containing a poly(C) sequence and a plasmid
expressing α-CP2 were co-transfected, α-CP2 activated the
expression of a luciferase reporter. To our knowledge, this study
demonstrates for the first time that α-CP2 acts as a
transcriptional activator by binding to a single-stranded poly(C)
sequence.
Acknowledgements
The present study was supported by the RP-Grant 2011
from Ewha Womans University, the National Research Foundation of
Korea (20110006924 to H.S.C.) and a grant from the Korean Health
Technology R&D Project, Ministry of Health and Welfare,
Republic of Korea (A120071 to D.H.K.).
References
|
1
|
Du Z, Lee JK, Fenn S, Tjhen R, Stroud RM
and James TL: X-ray crystallographic and NMR studies of
protein-protein and protein-nucleic acid interactions involving the
KH domains from human poly(C)-binding protein-2. RNA. 13:1043–1051.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Makeyev AV and Liebhaber SA: The
poly(C)-binding proteins: a multiplicity of functions and a search
for mechanisms. RNA. 8:265–278. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Leffers H, Dejgaard K and Celis JE:
Characterisation of two major cellular poly(rC)-binding human
proteins, each containing three K-homologous (KH) domains. Eur J
Biochem. 230:447–453. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kiledjian M, Wang X and Liebhaber SA:
Identification of two KH domain proteins in the alpha-globin mRNP
stability complex. Embo J. 14:4357–4364. 1995.PubMed/NCBI
|
|
5
|
Makeyev AV and Liebhaber SA:
Identification of two novel mammalian genes establishes a subfamily
of KH-domain RNA-binding proteins. Genomics. 67:301–316. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Makeyev AV, Eastmond DL and Liebhaber SA:
Targeting a KH-domain protein with RNA decoys. RNA. 8:1160–1173.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Du Z, Lee JK, Tjhen R, et al: Crystal
structure of the first KH domain of human poly(C)-binding protein-2
in complex with a C-rich strand of human telomeric DNA at 1.7 A. J
Biol Chem. 280:38823–38830. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sidiqi M, Wilce JA, Vivian JP, et al:
Structure and RNA binding of the third KH domain of poly(C)-binding
protein 1. Nucleic Acids Res. 33:1213–1221. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chkheidze AN and Liebhaber SA: A novel set
of nuclear localization signals determine distributions of the
alphaCP RNA-binding proteins. Mol Cell Biol. 23:8405–8415. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Meng Q, Rayala SK, Gururaj AE, Talukder
AH, O'Malley BW and Kumar R: Signaling-dependent and coordinated
regulation of transcription, splicing, and translation resides in a
single coregulator, PCBP1. Proc Natl Acad Sci USA. 104:5866–5871.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shi H, Bencze KZ, Stemmler TL and Philpott
CC: A cytosolic iron chaperone that delivers iron to ferritin.
Science. 320:1207–1210. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Funke B, Zuleger B, Benavente R, et al:
The mouse poly(C)-binding protein exists in multiple isoforms and
interacts with several RNA-binding proteins. Nucleic Acids Res.
24:3821–3828. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Blyn LB, Swiderek KM, Richards O, Stahl
DC, Semler BL and Ehrenfeld E: Poly(rC) binding protein 2 binds to
stem-loop IV of the poliovirus RNA 5′ noncoding region:
identification by automated liquid chromatography-tandem mass
spectrometry. Proc Natl Acad Sci USA. 93:11115–11120. 1996.
|
|
14
|
You F, Sun H, Zhou X, et al: PCBP2
mediates degradation of the adaptor MAVS via the HECT ubiquitin
ligase AIP4. Nat Immunol. 10:1300–1308. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhu J and Chen X: MCG10, a novel p53
target gene that encodes a KH domain RNA-binding protein, is
capable of inducing apoptosis and cell cycle arrest in G(2)-M. Mol
Cell Biol. 20:5602–5618. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Castaño Z, Vergara-Irigaray N, Pajares MJ,
Montuenga LM and Pio R: Expression of alpha CP-4 inhibits cell
cycle progression and suppresses tumorigenicity of lung cancer
cells. Int J Cancer. 122:1512–1520. 2008.PubMed/NCBI
|
|
17
|
Pio R, Zudaire I, Pino I, et al: Alpha
CP-4, encoded by a putative tumor suppressor gene at 3p21, but not
its alternative splice variant alpha CP-4a, is underexpressed in
lung cancer. Cancer Res. 64:4171–4179. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Weiss IM and Liebhaber SA: Erythroid
cell-specific mRNA stability elements in the alpha 2-globin 3′
nontranslated region. Mol Cell Biol. 15:2457–2465. 1995.
|
|
19
|
Blyn LB, Towner JS, Semler BL and
Ehrenfeld E: Requirement of poly(rC) binding protein 2 for
translation of poliovirus RNA. J Virol. 71:6243–6246.
1997.PubMed/NCBI
|
|
20
|
Gamarnik AV and Andino R: Two functional
complexes formed by KH domain containing proteins with the 5′
noncoding region of poliovirus RNA. RNA. 3:882–892. 1997.PubMed/NCBI
|
|
21
|
Collier B, Goobar-Larsson L, Sokolowski M
and Schwartz S: Translational inhibition in vitro of human
papillomavirus type 16 L2 mRNA mediated through interaction with
heterogenous ribonucleoprotein K and poly(rC)-binding proteins 1
and 2. J Biol Chem. 273:22648–22656. 1998. View Article : Google Scholar
|
|
22
|
Ostareck DH, Ostareck-Lederer A, Shatsky
IN and Hentze MW: Lipoxygenase mRNA silencing in erythroid
differentiation: The 3′UTR regulatory complex controls 60S
ribosomal subunit joining. Cell. 104:281–290. 2001.PubMed/NCBI
|
|
23
|
Andino R, Boddeker N, Silvera D and
Gamarnik AV: Intracellular determinants of picornavirus
replication. Trends Microbiol. 7:76–82. 1999. View Article : Google Scholar
|
|
24
|
Patterson SD and Aebersold R: Mass
spectrometric approaches for the identification of gel-separated
proteins. Electrophoresis. 16:1791–1814. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kim CS, Hwang CK, Song KY, et al: Novel
function of neuron-restrictive silencer factor (NRSF) for
posttranscriptional regulation. Biochim Biophys Acta.
1783:1835–1846. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hwang CK, Wu X, Wang G, Kim CS and Loh HH:
Mouse mu opioid receptor distal promoter transcriptional regulation
by SOX proteins. J Biol Chem. 278:3742–3750. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Choi HS, Hwang CK, Kim CS, et al:
Transcriptional regulation of mouse mu opioid receptor gene: Sp3
isoforms (M1, M2) function as repressors in neuronal cells to
regulate the mu opioid receptor gene. Mol Pharmacol. 67:1674–1683.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ko JL and Loh HH: Single-stranded
DNA-binding complex involved in transcriptional regulation of mouse
mu-opioid receptor gene. J Biol Chem. 276:788–795. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Du Z, Fenn S, Tjhen R and James TL:
Structure of a construct of a human poly(C)-binding protein
containing the first and second KH domains reveals insights into
its regulatory mechanisms. J Biol Chem. 283:28757–28766. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Waggoner SA and Liebhaber SA: Regulation
of alpha-globin mRNA stability. Exp Biol Med (Maywood).
228:387–395. 2003.PubMed/NCBI
|
|
31
|
Ostareck-Lederer A, Ostareck DH, Standart
N and Thiele BJ: Translation of 15-lipoxygenase mRNA is inhibited
by a protein that binds to a repeated sequence in the 3′
untranslated region. EMBO J. 13:1476–1481. 1994.PubMed/NCBI
|
|
32
|
Xiao X, Tang YS, Mackins JY, et al:
Isolation and characterization of a folate receptor mRNA-binding
trans-factor from human placenta. Evidence favoring identity with
heterogeneous nuclear ribonucleoprotein E1. J Biol Chem.
276:41510–41517. 2001. View Article : Google Scholar
|
|
33
|
Ritchie SA, Pasha MK, Batten DJ, et al:
Identification of the SRC pyrimidine-binding protein (SPy) as hnRNP
K: implications in the regulation of SRC1A transcription. Nucleic
Acids Res. 31:1502–1513. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kang DH, Song KY, Choi HS, Law PY, Wei LN
and Loh HH: Novel dual-binding function of a poly (C)-binding
protein 3, transcriptional factor which binds the double-strand and
single-stranded DNA sequence. Gene. 501:33–38. 2012. View Article : Google Scholar
|
|
35
|
Bedard KM, Walter BL and Semler BL:
Multimerization of poly(rC) binding protein 2 is required for
translation initiation mediated by a viral IRES. RNA. 10:1266–1276.
2004. View Article : Google Scholar : PubMed/NCBI
|