1. Ginsenosides: the ginseng saponins
Ginseng (Panax ginseng C.A. Meyer) is widely
used as a component of oriental medicine in different countries
worldwide, including South Korea and China. The medicinal use of
ginseng dates back 2,000 years. Scientific approaches for the
chemical characterization of ginseng began when Garriques isolated
a type of saponin mixture, and named it panaquilon in 1854. At the
dawn of the 20th century, a group of scholars took the initiative
to isolate saponin components from the mixture. It was the Russian
pharmacologist, Brekhman, who first demonstrated that saponins may
be the active ingredients which exert the pharmacological effects
of ginseng (1). Brekhman’s report
was further confirmed when a wide variety of biologically active
ginseng saponins with variable molecular weights ranging between
800–1,200 were isolated in subsequent years (2).
Saponins are a group of compounds consisting of a
sugar moiety (glycone) and a non-sugar component (aglycone)
connected by ether linkage. Based on the structures of the
aglycone, saponins are classified as triterpenoid saponins and
steroid saponins. Triterpenoid saponins obtained from ginseng can
be further classified as tetracyclic dammarane-type saponins and
pentacyclic oleane-type saponins. Depending on the number of
hydroxyl groups present in the aglycone component, dammarane
saponins can again be classified into two types: protopanaxadiol
and protopanaxatriol saponins. According to the manufacturing
process, ginseng can be grouped as red or white ginseng. Amazingly,
there are apparent differences in the composition of ginseng
saponins between the red and white varieties. The red
ginseng-specific saponins are produced by heat processing of the
Korean ginseng source (3,4). The isolation and quality assessment
of ginseng saponins are usually carried out using thin layer
chromatography (TLC). The Rf values, the ratio of the distance
travelled by saponins to that of the solvent, in the TLC
chromatogram is used to identify an individual saponin. The
nomenclature of ginseng saponins is given by a general term
‘ginsenoside-Rx’, where ‘R’ stands for the root or radix, and x is
replaced with an alphabet from a to h, which specifies Rf values in
an ascending order.
Since the structure of ginsenoside Rg1 from the
mixture of ginseng saponins has been reported in 1971, >30
ginsenosides have been found in the roots and other parts of
Panax ginseng, and a total of >60 ginsenosides have been
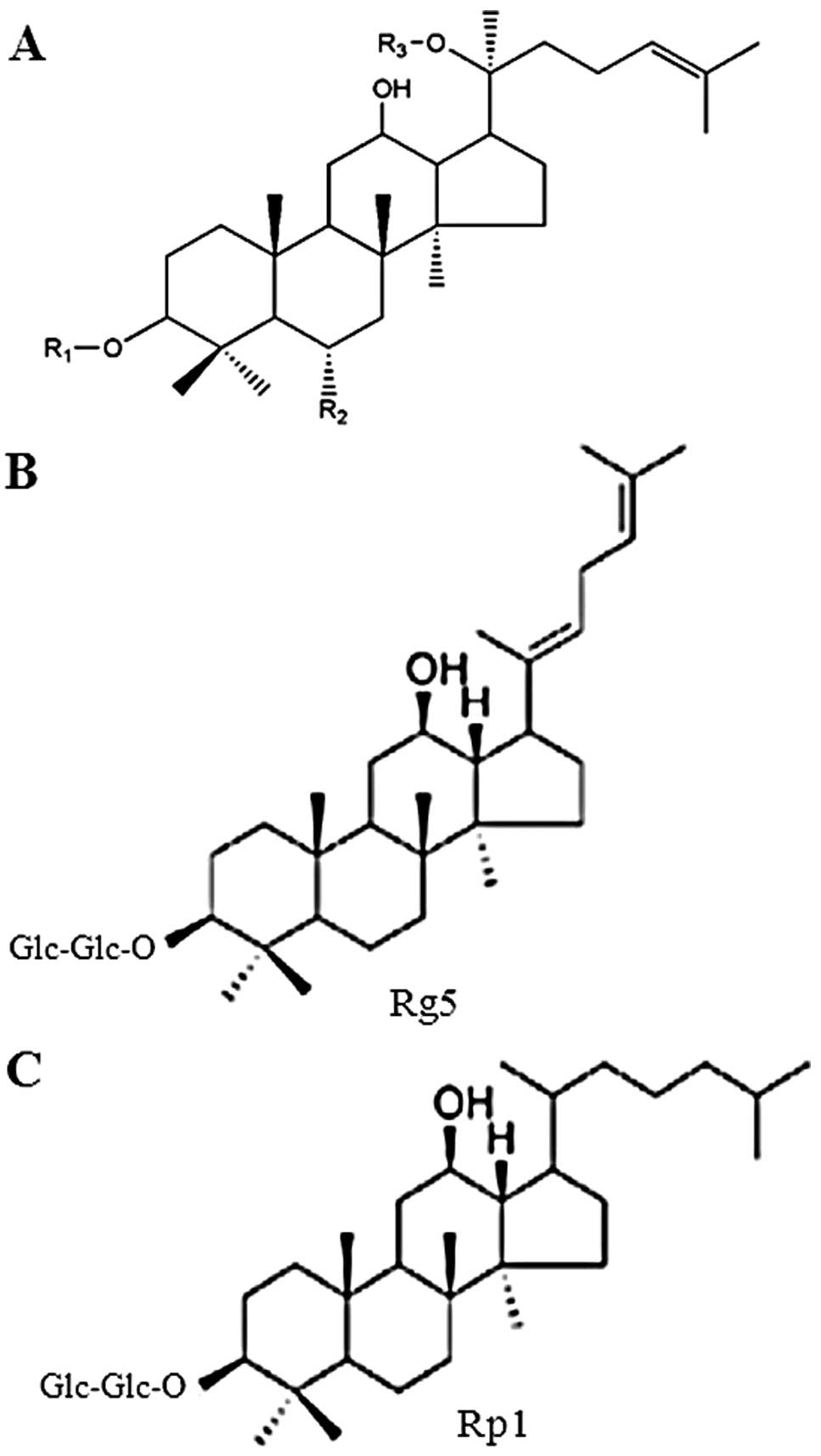
isolated from members of the Panax genus. Chemical
structures of several common ginsenosides are presented in Fig. 1. The substituents (R1, R2 and R3)
in Fig. 1A for selected
ginsenosides are listed in Table
I. In general, the ginseng saponins can be divided according to
the structure of the aglycone component of the molecule: i) the
oleanolic acid type, such as ginsenoside R0; ii) the
20(S)-protopanaxadiol type, such as ginsenosides Ra, Rb, Rc, Rd,
Rg3, Rh2 and Rs; and iii) the 20(S)-protopanaxatriol type, such as
ginsenosides Re, Rf, Rg1, Rg2 and Rh1 (2,5,6).
The detailed chemistry and the structure-activity relationship of
diverse classes of ginsenosides have been reviewed elsewhere
(6–8) and is beyond the scope this article.
The aim of this review was limited to the biochemistry behind the
anticancer activity of ginsenosides.
 | Table IList of substituents (R1, R2 and R3)
shown in Fig. 1A for selected
ginsenosides. |
Table I
List of substituents (R1, R2 and R3)
shown in Fig. 1A for selected
ginsenosides.
| Ginsenosides | R1 | R2 | R3 |
|---|
| Ra1 | Glc-Glc- | H |
Xyl-Ara(f)-Glc- |
| Ra2 | Glc-Glc- | H |
Xyl-Ara(f)-Glc- |
| Rb1 | Glc-Glc- | H | Glc-Glc- |
| Rb2 | Glc-Glc- | H | Ara(p)-Glc- |
| Rc | Glc-Glc- | H | Ara(f)-Glc- |
| Rd | Glc-Glc- | H | Glc- |
| Re | H | Rha-Glc-O- | Glc- |
| Rf | H | Glc-Glc-O- | H |
| Rg1 | H | Glc-O- | Glc- |
| Rg2 | H | Rha-Glc-O- | H |
| Rg3 | Glc-Glc- | H | H |
| Rh1 | H | Glc-O- | H |
| Rh2 | Glc- | H | H |
| Rp1 | Glc-Glc- | H | H |
| Rs3 | AcGlu-Glu- | H | H |
| Compound K | H | H | Glc- |
2. Ginsenosides in the prevention and
therapy of cancer
The anticancer properties of ginsenosides have been
extensively investigated over the past several decades.
Ginsenosides have been shown to elicit chemopreventive and
chemotherapeutic effects in a wide range of animal models of
experimental carcinogenesis (5,8–10)
(Table II). For instance, the
topical application of Rg3 on to the shaven backs of female ICR
mice has been shown to inhibit
12-O-tetradecanoylphorbol-13-acetate (TPA)-induced ornithine
decarboxylase activity and 7,12-dimethylbenz[a]anthracene
(DMBA)-initiated papilloma formation (11). Likewise, the oral administration
of ginsenoside Rp1 at the pre-, peri- and post-initiation stage,
has been shown to significantly reduce the incidence and the
multiplicity of chemically-induced mouse skin papillomas (12). As previously demonstrated, the
water extract of Korean red ginseng attenuated the development of
testosterone-induced benign prostate hyperplasia, a pre-neoplastic
condition that progresses to malignant prostate cancer (13).
 | Table IIInhibitory effects of ginsenosides on
the development and growth of tumors in vivo. |
Table II
Inhibitory effects of ginsenosides on
the development and growth of tumors in vivo.
| Ginsenosides | Animal model |
Mechanisms/effects | Refs. |
|---|
| Rp1 | DMBA-initiated and
croton oil-promoted skin papillomagenesis in Swiss albino mice | ↓ Tumor incidence
and multiplicity
↓ Lipid peroxidation
↑ Activity of microsomal enzymes
↑ Activity of SOD, GPx, GR and GST
↑ Level of reduced glutathione | (12) |
| Rg3 | DMBA-initiated and
TPA-promoted skin papillomagenesis in female ICR mice | ↓ Average number of
skin tumors
↓ Orinithine decarboxylase activity
↓ Expression of COX-2
↓ Activation of NF-κB and AP-1 | (11) |
| Rg3 | HCT-116 cells
xenograft tumor in nude mice | ↓ Xenograft tumor
growth
↓ Expression of PCNA
↓ Nuclear staining of β-catenin
↓ β-catenin/TCF-mediated transcriptional activity | (14) |
| Rg3 alone or with
gemcitabine | Lewis lung cancer
xenografts in syngenic C57BL/6 mice | ↓ Tumor
growth
↓ VEGF expression
↑ Survival of tumor-bearing mice | (15) |
| Rg3 | Administration of
SKOV-3 cells via lateral tail vein and intraperitoneal
administration of Rg3 daily for 20 days from the day of tumor
inoculation. | ↓ Lung metastasis
of SKOV-3 cells | (80) |
| Intradermal
injection of SKOV-3 cells to mice and treatment with Rg3 daily for
five days after cell xenograft tumors tumor inoculation | ↓ Tumor
angiogenesis in SKOV-3 | |
| Rg3 alone or with
cyclophosphamide | Human ovarian
cancer cell xenografts in nude mice | ↓ Tumor
growth
↓ Expression of PCNA and VEGF
↓ Microvessel density | (73) |
| Water extract
containing 20(s)-Rg3 |
Testosterone-induced benign prostate
hyperplasia in rats and prostate cell growth | ↓ Thickening of
prostate epithelium
↓ AR activity
↓ Proliferation of prostate cells | (13) |
| Rb2 | B16-BL6 melanoma
xenograft tumor in nude mice (intratumoral or oral administration
of Rb2) | ↓ Tumor
angiogenesis and growth | (76) |
| Rh2 | MDA-MB-231 cell
xenografts in nude mice (Rh2 given by oral gavage) | ↓ Tumor
growth,
↑ Intratumoral apoptotic bodies
↓ Proliferation index | (46) |
| M1 | Lewis lung
carcinoma in syngenic C57BL/6 mice | ↓ Lung
metastasis | (88) |
| Compound K | Gastric cancer
(SGC7901) cell tumor xenografts in athymic nude mice | ↓ Tumor volume and
weight
↓ Expression of Bid and MMP-9 in xenograft tumors and in liver | (17) |
The inhibitory effects of various ginsenosides on
the growth of different cancer cell xenograft tumors have been
reported. For example, treatment with Rg3 has been shown to inhibit
the growth of human colon cancer (HCT-116) cell xenograft tumors in
nude mice (14). The
administration of Rg3 alone or in combination with gemcitabine, has
also significantly inhibited the growth of Lewis lung carcinoma
cells implanted into C57BL/6 mice by suppressing tumor angiogenesis
(15). The oral administration of
Rh2 has been shown to reduce human prostate cancer (PC3) cell tumor
growth in nude mice (16).
Likewise, as previously demonstrated, the growth of human gastric
cancer (SGC7901) cells in nude mice was diminished by compound K,
an intestinal metabolite of ginsenoside Rb1, Rb2 and Rc (17). A previous study revealed that
25-methoxy-protopanaxadoil reduced the growth of human lung cancer
(A549) cell tumor xenografts in nude mice (18). Similarly,
25-methoxy-protopanaxadiol and 25-hydroxy-protopanaxadiol have been
shown to inhibit pancreatic cancer cell xenograft tumor growth
in vivo and to reduce the proliferative abitlity of these
cells in vitro (19).
Treatment with 20(S)-25-methoxyl-dammarane-3β, 12β, 20-triol, a
newly identified ginsenoside from Panax notoginseng, has
been shown to decrease the growth of human breast cancer (MCF-7 and
MDA-MB-231) cell tumor xenografts in nude mice (20). As previously shown,
25-hydroxy-protopanaxadiol, but not 25-hydroxy-protopanaxatriol,
inhibited PC3 cell xenograft tumor growth (21).
Over the past several decades, enormous efforts have
been made to elucidate the biochemistry behind the anticancer
properties of various ginsenosides. The major mechanisms of the
anticarcinogenic effects of ginsenosides include: i) the inhibition
of oxidative damage and inflammation; ii) the inhibition of tumor
cell proliferation; iii) the induction of cancer cell apoptosis;
iv) anti-angiogenic activity; v) inhibition of the invasion and
metastasis of tumor cells; and vi) the enhancement of the
sensitivity of resistant cells to chemotherapeutic agents (Fig. 2).
3. Biochemical basis of anticancer
properties of selected ginsenosides
Antioxidant activity
The oxidative stress-induced damage of cellular
macromolecules, such as proteins, nucleic acids and lipids
contributes to the neoplastic transformation of cells. Whereas
oxidative stress-induced tissue damage precipitates a state of
local tissue inflammation, persistent inflammation triggers the
generation of reactive oxygen species (ROS) and reactive nitrogen
species. A wide variety of antioxidant and anti-inflammatory
phytochemicals have been reported to possess anticancer properties.
Although it is generally accepted that the bioactivity of
ginsenosides is due to their ability to reduce oxidative stress and
inflammation, there have been limited studies unraveling the
molecular mechanisms of the antioxidant and anti-inflammatory
activity of individual ginsenosides. In general, ginsenosides
scavenge ROS and induce the expression and activity of various
antioxidant and/or detoxification enzymes. For instance, Rb1 has
been reported to protect plasmid DNA from damage by the direct
scavenging of •OH, which can bind to the double bond on
the side chain of Rb1, as well as hydrogen atoms adjacent to the
−OH group, including those of sugar moieties. Rb1 has
also elicited high reactivity to hypochlorous acid (HOCl), a
neutrophilic oxidant with mutagenic potential, effectively
inhibiting HOCl-induced tyrosine chlorination in a cell free system
(22). Ginsenosides Rg3 and Rh2
have been shown to reduce the ethanol-induced generation of ROS in
mouse hepatocyte cells (23).
Ginsenosides have been reported to activate several
antioxidant enzymes, which are transcriptionally regulated to a
great extent by factors, such as nuclear factor erythroid-derived
2-like 2 (Nrf2), nuclear factor-κB (NF-κB), or activator protein
(AP)-1 or -2. As peviously demonstrated, 20(s)-protopanaxadiol, but
not total saponins or 20(s)-protopanaxatriol, induced the
expression of superoxide dismutase (SOD), possibly by increasing
the AP2 transcription factor activity (24). Among the protopanaxadiols, Rb2 has
been shown to significantly induce the expression of genes encoding
antioxidant enzymes, such as SOD and catalase in vitro
(25). Ginsenoside Rd has been
shown to elevate intracellular glutathione levels by increasing the
activation of γ-glutamyl cysteine ligase (GCL) in rat hepatocyte
(H4IIE) cells through the induction of NF-κB DNA binding, but not
altering Nrf2 DNA binding (26).
As previously demonstrated, the induction of heme oxygenase-1
(HO-1) by ginsenoside 20(s)-protopanaxadiol inhibited the
activation of inflammatory signaling molecules in murine Raw 264.7
macrophages stimulated with lipopolysaccharide (LPS) (27). The co-treatment of macrophages
with HO-1 inhibitor abolished the inhibitory effects of this
ginsenoside on the expression of inducible nitric oxide (NO)
synthase (iNOS) and the activation of NF-κB (27). On the other hand, the treatment of
HepG2 cells with Rb1, Rg1 or 20(s)-protopanaxatriol, or with a
combination of 20(s)-protopanaxatriol with Rb1 or Rg1 has been
shown to enhance the binding of Nrf2 to antioxidant response
element (ARE), thereby increasing the total antioxidant activity
(28). A previous study revealed
that the treatment of Swiss albino mice with ginsenoside Rp1 for
seven days by gavage significantly elevated the levels of skin
microsomal cytochrome p-450 and cytochrome b5 and
glutathione-S-transferase (GST), and reduced glutathione
(GSH), glutathione peroxidase (GPx), glutathione reductase (GR),
DT-diaphorase, SOD and catalase levels, resulting in the enhanced
detoxification of carcinogens and the reduction of oxidative stress
(12).
Anti-inflammatory effects
Since chronic inflammation contributes to the
development and progression of tumors, the suppression of
inflammatory signaling is a rational approach for cancer prevention
and therapy. The anticancer activities of many phytochemicals have
been linked to their anti-inflammatory effects. Several
ginsenosides have been reported to interfere with inflammatory
signaling, thereby inhibiting tumor promotion and progression
(8,9). The topical application of
ginsenoside Rg3 has been shown to attenuate the TPA-induced
activation of NF-κB and AP-1, and the expression of the
pro-inflammatory enzyme, cyclooxygenase-2 (COX-2), accounting for
its antitumor promoting effects in chemically-induced mouse skin
carcinogenesis (11). As
previously demonstrated, 20(S)-Rg3, a predominant stereospecific
form of Rg3, inhibited the generation of ROS, but not that of NO,
and decreased the production of cytokines, such as tumor necrosis
factor-α (TNF-α), interleukin (IL)-1β, IL-6, and that of
prostaglandin E2 (PGE2) in LPS-stimulated Raw
264.7 murine macrophages (29).
Treatment with Rg3 also attenuated the LPS-induced expression of
COX-2 in macrophages and reduced TNF-α-induced matrix
metalloproteinase (MMP)-9 activity in human keratinocyte (HaCaT)
cells (29). Ginsenoside Rg5 has
also been shown to attenuate LPS-induced lung inflammation in mice
through the downregulation of NF-κB activity. Rg5 reduced the
expression of pro-inflammatory mediators, such as COX-2, iNOS,
IL-1β and TNF-α in LPS-stimulated alveolar macrophages by blocking
the activities of IL-1 receptor-associated kinases (IRAKs) and IκB
kinase-β (IKKβ), and subsequently inhibiting the phosphorylation
and nuclear accumulation of NF-κB (30). Ginsenoside Rb1 and its intestinal
metabolite, compound K, also function through a similar mechanism
in LPS-induced cytokine production in murine peritoneal
macrophages. These ginseng saponins have been shown to inactivate
IRAK1, IKKβ, NF-κB and mitogen-activated protein (MAP) kinases
(31). Moreover, the
administration of Rb1 or compound K by gavage attenuated
2,4,6-trinitrobenzenesulfonic (TNBS) acid-induced colitis in mice
by inhibiting colonic myeloperoxidase activity, decreasing the
expression of COX-2, iNOS, TNF-α, IL-1β and IL-6 through the
inactivation of NF-κB (31).
Likewise, the oral administration of ginsenoside Rd to rats
attenuated TNBS-induced colitis by inhibiting myeloperoxidase
activity and preventing the production of cytokines, such as TNF-α,
IL-1β and IL-6 through the blockade of p38 MAP kinase and
c-Jun-N-terminal kinase (JNK) (32).
In previous studies, the treatment of Raw 264.7
macrophages with either Rb1 (33)
or the ginseng metabolites, compound K (33) or 20(s)protopanaxatriol (34), diminished the LPS-induced
expression of COX-2 and iNOS by blocking the activation of NF-κB.
Of note, the mutation of either NF-κB- or CRE-binding sequences in
the COX-2 promoter did not alter the inhibitory effects of Rb1 on
TPA-induced COX-2 promoter activity; however, the effects were
abrogated upon the mutation of NF-IL-6 binding sites. These
findings suggest that NF-IL-6, but not NF-κB, is a target of Rb1 in
suppressing COX-2 expression. In a recent study, Huang et al
(35) demonstrated that Rg1
blocked the activation of transient receptor potential vanilloid-1
(TRPV1) and inhibited the transcriptional activity of NF-κB,
thereby suppressing the expression of COX-2, the production of
PGE2 and the secretion of IL-8 in capsaicin-stimulated
HaCaT keratinocytes, as well as in TRPV1-overexpressing human
embryonic kidney (HEK)-293 cells (35). Likewise, co-treatment with
ginsenoside Rb1 has been shown to diminish capsaicin-induced NF-κB
transcriptional activity and reduce the release of IL-8 and
PGE2 in HaCaT keratinocytes by blocking the activation
of TRPV1 (36).
Inhibition of proliferation and induction
of apoptosis in cancer cells
The anti-proliferative and apoptotic effects of a
wide variety of ginsenosides have been extensively investigated.
The mechanisms by which selected ginsenosides inhibit proliferation
and induce apoptosis in various cancer cells are discussed in this
section.
Rg3
Several ginsenosides of the Rg series have been
reported to inhibit proliferation and induce apoptosis in various
cancer cells in culture. In a previous study, the treatment of
human prostate cancer (LNCaP) cells with ginsenoside Rg3 reduced
cell proliferation through G1 phase cell cycle arrest, which was
associated with the decreased expression of the cyclin-dependent
kinase (Cdk) inhibitors, p21 and p27 (37). The authors demonstrated that Rg3
also attenuated the expression of prostate-specific antigen (PSA),
androgen receptor (AR), 5α-reductase and proliferating cell nuclear
antigen (PCNA). Moreover, Rg3 induced apoptosis in these cells by
inhibiting Bcl-2 expression and inducing caspase-3 activity
(37). Bae et al (13) recently demonstrated that Rg3
decreased the AR activity through the proteasomal degradation of AR
and inhibited androgen-induced benign prostate hyperplasia in rats.
Rg3 has also been shown to inhibit the expression of Bcl-2 and
Bcl-xL, and induce the expression of Bax, resulting in reduced
mitochondrial membrane potential, cytochrome c release and
the activation of caspase-3, thereby inducing apoptosis in human
hepatocellular carcinoma cells (38–40) and human colon cancer (HT-29) cells
(41). A recent study
demonstrated that Rg3 sensitized several liver cancer (HepG2,
SK-Hep1, Huh-7 and Hep3B) cells to TNF-related apoptosis-inducing
ligand (TRAIL)-induced apoptosis, but did not affect the survival
of normal hepatocytes. The enhancement of TRAIL-sensitivity and the
subsequent induction of cell death was mediated through the
upregulation of death receptor-5 (DR5) through the activation of an
endoplasmic reticulum (ER)-stress protein CCAAT/enhancer binding
protein (C/EBP) homology protein (CHOP), which is also known as
growth arrest- and DNA damage-inducible gene 153 (GADD153)
(42). The Rg3-induced activation
of cyclic-AMP-activated protein kinase (AMPK) accounted for its
apoptotic effects in HT-29 cells, as the co-treatment of cells with
Rg3 in the presence of the AMPK inhibitor, compound C, or in cells
transfected with AMPK-siRNA, abrogated Rg3-induced apoptosis
(41). According to a recent
study, Rg3 inhibited the growth and survival of human gastric
cancer (AGS) cells by blocking the activity of the transient
receptor potential melastatin-7 (TRPM7) channel. Rg3-mediated
sub-G1 arrest, the cleavage of poly(ADP-ribose) polymerase (PARP)
and the activation of caspases were abolished when AGS cells were
co-treated with chemical inhibitors of TRPM7 (43). Chen et al (44) reported that Rg3 inhibited cell
proliferation by blocking cell cycle progression and inducing
apoptosis in B16 melanoma cells. Rg3 attenuated the proliferation
of HCT116 cells in culture, at least in part, by decreasing the
nuclear localization of β-catenin and downregulating β-catenin/T
cell factor (TCF)-mediated transcriptional activity (14).
Rh2
Ginsenoside Rh2 has exhibited significantly more
potent cell death activity than ginsenoside Rg3 in HCT-116 and
SW-480 colorectal cancer cells through the generation of ROS, the
activation of p53, the increased expression of Bax and the reduced
expression of Bcl-2 (45).
Likewise, in a previous study, the Rh2-induced apoptosis in MCF-7
and MDA-MB-231 cells was accompanied by the downregulation of the
anti-apoptotic proteins, Bcl-2, Bcl-xL, Mcl-1, and the upregulation
of the pro-apoptotic proteins, Bak, Bax and Bim, thereby enhancing
the mitochondrial translocation of Bax and the activation of
caspases (46). The authors also
demonstrated that the administration of Rh2 by gavage significantly
attenuated the growth of MDA-MB-231 cell tumor xenografts in nude
mice (46). By contrast, Rh2 was
also found to induce apoptosis in rat glioma cells independent of
Bcl-2, as Rh2 did not induce apoptosis in Bcl-2-overexpressing
glioma cells (47,48). Likewise, a Bcl-2-insensitive
activation of caspase-3 was associated with the Rh2-mediated
apoptosis of human hepatoma SK-HEP1 cells (49). Whereas Rh2 significantly inhibited
the proliferation of intestinal cancer (Int-407 and Caco-2) cells
through sub-G1 arrest, the structurally related ginsenoside Rh1
failed to inhibit cell proliferation (50). Oh et al (51) demonstrated that Rh2 induced G1
phase arrest and reduced the proliferation of MCF-7 cells, partly
by decreasing the phosphorylation of retinoblastoma protein and
increasing the binding of retinoblastoma protein with E2F1 by
downregulating the activities of Cdk-2 and cyclin E, and the
induction of Cdk inhibitor p21. Likewise, the Rh2-mediated G1
arrest in A549 cells has been shown to be associated with the
reduced expression and the activity of cyclin D1, cyclin E and
Cdk-6, and increased the expression of Rb (52). The authors demonstrated that Rh2
induced apoptosis by increasing the expression of DR4 and the
subsequent activation of caspases-2, -3 and -8 (52). The treatment of human
neuroblastoma (SK-N-BE) cells with Rh2 has been shown to increase
cell death through the induction of caspase-1 and -3 activity, the
expression of p53 and Bax and the cleavage of PARP (10). In a previous study, the
pro-apoptotic effects of Rh2 in SK-HEP1 cells were mediated by the
caspase-3-dependent activation of protein kinase C-δ (PKC-δ). The
treatment of cells with caspase-3 inhibitor attenuated Rh2-induced
proteolytic cleavage and the activation of PKC-δ. The blockade of
caspase-3 activity or the pharmacological inhibition of PKCδ
abrogated Rh2-induced apoptosis in these cells (53). Moreover, the generation of ROS,
the activation of JNK1, the depolarization of mitochondrial
membrane potential and the subsequent activation of caspase-3 have
been shown to be associated with Rh2-induced apoptosis in HeLa
cells, which were abrogated by the overexpression of catalase or
treatment with N-acetyl cysteine (NAC) (54).
Another mechanism of Rh2-induced apoptosis in cancer
cells involves the disruption of membrane lipid rafts, thereby
resulting in the inactivation of Akt signaling and the subsequent
inhibition of Bad phosphorylation and the increased expression of
the pro-apoptotic proteins, Bim and Bax, in MDA-MB-231 cells
(55). The treatment of HeLa
cells with Rh2 has been shown to induce apoptosis through the
disruption of physical and chemical properties of membrane lipid
rafts through the internalization of caveolin-1 and GM1, followed
by the ligand-independent oligomerization of FAS, which was
abrogated by cholesterol overloading or transfection with FAS siRNA
(56). Recent studies have
demonstrated that Rh2 inhibits cancer cell proliferation and
induces apoptosis by modulating the function of certain micro-RNAs
(miRs) (57,58). For example, the miR array analysis
of Rh2-treated human glioma (U251) cells revealed the upregulation
of 14 miRs and the downregulation of 12 miRs. Transfection with
miR-128 inhibitor prevented the overexpression of miR-128 in
Rh2-treated U251 cells and blunted Rh2-induced apoptosis by
blocking the activation of caspase-3 and the transcriptional
activation of the miR-128 target gene, E2F3a (58).
Rp1
Rp1 is semi-synthesized from crude ginsenosides
(59). The incubation of human
breast cancer cells with ginsenoside Rp1 has been shown to
attenuate anchorage-dependent and -independent colony formation,
which was associated with the reduced stability of insulin-like
growth factor receptor (60). In
a previous study, Rp1 induced G1 phase arrest in HeLa cells by
inhibiting the expression of cyclin D1, E and A, and increasing the
expression of p21 without affecting the levels of p53 or serine-15
phosphorylated p53. In addition, prolonged incubation with Rp1
activated caspase-3, -8 and -9 by stimulating the mitochondrial
release of cytochrome c through the activation of Bax and
Bid, and the subsequent loss of mitochondrial membrane potential.
This led to the Rp1-induced cleavage of PARP and the induction of
apoptosis in HeLa cells (61).
Rk1
Ginsenoside Rk1 is a major bioactive component of
heat-processed ginseng. The concentration- and time-dependent
cytotoxic effects of Rk1 in human melanoma (SK-MEL2) cells have
been shown to be associated with the upregulation of Fas, FasL and
Bax protein and the downregulation of procaspase-8 and -3, mutant
p53 and Bcl-2 protein expression (62). Likewise, the activation of
caspase-3 and -8 by Rk1 induced apoptosis in HepG2 cells (63).
Others
Kim et al (64) reported that a diol-type ginseng
saponin Rs3 induced cell cycle arrest at the G1/S boundary in
SK-HEP-1 cells by increasing the expression p53 and p21 without
affecting the expression of cyclin E and cyclin A, p27 and PCNA.
However, Rs3 was found to inhibit the activities of cyclin E- and
cyclin A-associated kinases in these cells (64). The G1 phase cell cycle arrest was
also evident in human lung cancer (A549, H358 and H838) cells
treated with 25-methoxy-protopanaxadiol, which did not affect the
viability of normal lung epithelial (BAES-2B) cells (18). In a previous study,
25-hydroxy-protopanaxadiol, but not 25-hydroxy-protopanaxatriol
induced cell cycle arrest, as well as apoptosis in PC3 and LNCaP
cells by increasing the expression of p21, p27 and Bax, and
activating caspases and inducing the cleavage of PARP. Moreover,
25-hydroxy-protopanaxadiol has been shown to reduce the expression
of mouse double minute 2 (MDM2), E2F1, Bcl-2, Cdk-2/4/6 and cyclin
D1 as the underlying molecular mechanisms of the anti-proliferative
effects of this ginsenoside (65). Similarly,
25-OCH3-protopanaxadiol has been shown to decrease the
proliferation of human breast cancer cells by inducing G1 phase
cell cycle arrest and diminishing MDM2 expression (20).
Compound K, the intestinal metabolite of ginsenoside
Rb1, has been shown to decrease cell viability by inducing G2 phase
arrest and apoptosis in human gastric carcinoma (BGC823 and
SGC7901) cells through the upregulation of p21 and the
downregulation of cdc2 and cyclin B1 (17). Incubation with compound K induced
sub-G1 arrest in human multiple myeloma (U266) cells by blocking
the phosphorylation of signal transducer and activator of
transcription (STAT)3 and upstream janus-activated kinase (JAK)1,
but not JAK2, and diminishing the expression of STAT3 target genes,
such as Bcl-2, Bcl-xL and cyclin D1 (66). The authors demonstrated that
compound K induced the expression of protein phosphatase (PTP) and
that treatment with pervanadate, a PTP inhibitor, abrogated the
inhibitory effects of compound K on STAT3 phosphorylation and its
target gene expression (66).
Recent molecular docking studies with several
ginsenosides have revealed that Rf, Rg1, Rg3 and Rh2 have more
binding affinity with Bcl-2, Bcl-xL and Mcl-1 than other
ginsenosides, which have relatively lower binding affinity to
anti-apoptotic proteins. Therefore, ginsenosides represent a novel
class of potent inhibitors of anti-apoptotic proteins and may be
used for cancer chemotherapy (67). The antiproliferative and apoptotic
effects of ginsenosides are linked to their structural features. It
has been reported that ginsenoside 20(S)-Rh2, but not its
stereoisomer ginsenoside 20(R)-Rh2 inhibited the proliferation of
androgen-dependent and -independent prostate cancer cells in
vitro, suggesting that the stereochemistry of the hydroxyl
group at C-20 may play an important role in the antitumor activity
of ginsenoside Rh2 (68). In
vitro structure-related antitumor activity analysis revealed
that the induction of apoptosis in various cancer cells was in the
order of protopanaxadiol >20(S)-Rh2 >20(R)-Rh2 ≈ 20(R)-Rg3 ≈
20(S)-Rg3. These results suggest that ginsenosides with less polar
chemical structures possesses higher cytotoxic activity in cancer
cells. The cytotoxic potential of protopanaxadiol was further
increased by products obtained through hydrogenation and the
dehydration of protopanaxadiol (69).
Anti-angiogenic activity
One of the hallmarks of cancer is aberrant tumor
angiogenesis. As peviously demonstrated, the treatment of human
esophageal carcinoma cells with ginsenoside Rg3 in presence of
hypoxia resulted in reduced mRNA expression and the production of
vascular endothelial growth factor (VEGF), which was associated
with the decreased expression of hypoxia inducible factor 1α
(HIF1α) and COX-2 and the diminished NF-κB activity. Moreover, Rg3
attenuated the hypoxia-induced phosphorylation of extracellular
signal-regulated kinase (ERK1/2), JNK and STAT3 in these cells
(70). Treatment with Rg3 dose
dependently suppressed the VEGF-induced capillary tube formation of
human umbilical vein endothelial cells (HUVECs) on Matrigel and
ex vivo microvascular sprouting in rat aortic ring assay
through the inhibition of MMP-2 and MMP-9 activity (71). When administered in combination
with gemcitabine, Rg3 has been shown to significantly reduce the
growth of Lewis lung carcinoma cells transplanted in C57BL/6 mice
with a marked decrease in microvessel density and the reduced
expression of VEGF, thereby preventing tumor angiogenesis (15). Likewise, the combination of Rg3
with capecitabine (72) or
cyclophosphamide (73) augmented
the angiosuppressive activity of these chemotherapeutic agents in
human breast and ovarian cancer, respectively. According to the
latter study, Rg3 alone or in combination with cyclophosphamide
attenuated the growth of human ovarian cancer (SKOV-3) cell
xenografts in nude mice and induced a significant decrease in the
expression of PCNA and VEGF (73). Zhang et al (74) also demonstrated that in the
presence of Rg3, a low dose of cyclophosphamide was effective in
suppressing the growth of Lewis lung carcinoma cells by inhibiting
angiogenesis.
Endothelial progenitor cells (EPCs) appear to play a
key role in the growth of early tumors. In a recent study, Kim
et al (75) demonstrated
that the treatment of ex vivo cultured endothelial cells, a
type of EPCs, with Rg3 inhibited proliferation, migration and
tubular formation by blocking the VEGF-dependent activation of ERK
and p38 MAP kinases and inhibiting the mobilization of EPCs from
the bone marrow microenvironment to the peripheral circulation. The
intratumoral or oral administration of another ginsenoside Rb2 has
been shown to inhibit tumor angiogenesis in a model of B16-BL6
melanoma cell xenografts in nude mice. However, the intravenous
administration of Rb2 failed to inhibit the growth of xenograft
tumors. Moreover, Rb2 did not affect the growth of rat lung
endothelial cells, B16-BL6 melanoma cells or various types of
murine normal cells in vitro (76). Compound K significantly inhibited
the migration and tube formation in basic fibroblast growth factor
(bFGF)-treated HUVECs and reduced the secretion of VEGF and
increased the production of pigment epithelium-derived factor.
Moreover, compound K attenuated the phosphorylation of p38 MAP
kinase and Akt in bFGF-stimulated HUVECs, and inhibited
neovascularization in the Matrigel plugs excised from mice in
vivo (77).
Attenuation of invasion and
metastasis
Incubation with Rg3 has been shown to reduce the
migration of highly metastatic prostate cancer (PC3M) cells by
downregulating the expression of aquaporin-1 (AQP1), a water
channel protein that has been implicated in cell migration, through
the blockade of p38 MAP kinase phosphorylation. The inhibitory
effects of Rg3 on the migration of these cells were annulled either
by the overexpression of AQP1 or by the pharmacological inhibition
of p38 MAP kinase (78).
Ginsenosides Rg3 and Rb2 have been shown to significantly inhibit
the adhesion of B16-BL6 melanoma cells to fibronectin and laminin,
and attenuate the invasion of these cells into the reconstituted
basement membrane in a dose-dependent manner. Moreover, the lung
metastasis of B16-BL6 or colon 26-M3.1 cells in nude mice was
significantly inhibited by the intravenous or oral administration
of Rg3 or Rb2 (79). Likewise,
treatment with Rg3 has been shown to markedly reduce the expression
of MMP-9 and decrease the invasive and metastatic ability
(metastasis to lungs) of SKOV-3 cells in mice (80). Moreover, a previous study
demonstrated that the intraperitoneal administration of Rg3
prevented the metastasis (to the lungs) of B16 melanoma cells
(44).
In another study, treatment with Rg3 elicited a
decreased expression of chemokine receptor CXCR4 in MDA-MB-231
cells and reduced chemokine CXCL12-induced migration of these cells
(81). Although Rg3 failed to
affect bombesin-induced intestinal carcinogenesis, the ginsenoside
significantly decreased the peritoneal metastasis of intestinal
adenocarcinomas (82). Rg3 has
also been shown to inhibit the invasion of rat ascites hepatoma
cells (MM1), B16FE7 melanoma cells, human small cell lung carcinoma
and human pancreatic adenocarcinoma cells in a cell monolayer
invasion assay (83). Moreover,
Rg3 attenuated experimental pulmonary metastasis by highly
metastatic mouse melanoma (B16FE7) cells. The authors demonstrated
that Rg2 and Rb2 mildly inhibited invasion, but Rh1, Rh2, Rb1, Rc
or Re had no effect the invasive ability of these cells (83). By contrast, ginsenoside Rh1
(84) and Rd (85) suppressed the invasion and
metastasis of human hepatocellular carcinoma HepG2 cells. According
to the former study, Rh1 inhibited the mRNA expression and the
promoter activity of MMP-1 by blocking the activation of MAP
kinases, such as ERK, p38 MAP kinase and JNK. In addition, Rh1
diminished the phorbol ester-induced expression of c-Jun and c-Fos,
but failed to affect the DNA binding of AP-1 in HepG2 cells
(84). Treatment with ginsenoside
Rd has been shown to prevent the invasion and migration of HepG2
cells by downregulating the expression of MMP-1, MMP-2 and MMP-7,
by blocking the phosphorylation of ERK and p38 MAP kinase and by
inhibiting the activation of AP-1 (85). Likewise, ginsenoside Rh2 has been
shown to inhibit phorbol ester-induced MMP-9 mRNA expression and
diminish the invasion of astroglioma cells by blocking the
activation of NF-κB and AP-1 through the inhibition of p38 MAP
kinase, ERK and JNK phosphorylation (86). Rp1 markedly inhibited the
metastatic lung transfer of B16–F10 melanoma cells, presumably by
inhibiting the expression of β1-integrin, a cell surface receptor
that plays a key role in cell-matrix interaction (59).
Ginseng saponins are metabolized by intestinal
bacteria and produce a variety of metabolites, which also exhibit
anti-invasive and antimetastatic properties. For instance, compound
K markedly attenuated the colony formation, adhesion and invasion
of hepatocellular carcinoma (HCC) cells in vitro and
markedly inhibited spontaneous HCC cell metastatic growth in
vivo. This study also demonstrated that treatment with compound
K diminished the nuclear accumulation of p65 and significantly
reduced the expression of MMP-2 and MMP-9 (87). The intestinal bacterial metabolite
of Rb1 is 20-O-β-D-glucopyranosyl-20(S)-protopanaxadiol
(M1). The inhibition of spontaneous metastasis produced by the
subcutaneous administration of Lewis lung carcinoma cells in
syngenic C57BL/6 mice by Rb1 has been reported to be the effect of
its metabolite M1 (88). The
anti-metastatic effect of this ginsenoside metabolite was
comparable to that of the chemotherapeutic drug, 5-fluorouracil.
Treatment with M1 inhibited the TNF-α-induced activation of NF-κB
and reduced the expression of MMP-9, thereby suppressing the
invasion and migration of colon adenocarcinoma cells. Moreover, the
TNF-α-induced increase in lung and liver metastasis of colon
carcinoma was also abrogated by treatment with M1 (89).
Chemosensitizing and radiosensitizing
effects
Resistance to chemotherapy and radiotherapy is an
emerging challenge in reducing the global burden of cancer.
Attention has recently been paid to a wide variety of natural
compounds which enhance the sensitivity of various resistant cancer
cells to conventional chemotherapy and radiotherapy. Several
ginsenosides and their intestinal bacterial metabolites have been
reported to increase the chemosensitivity and radiosensitivity of
cancer cells. Kim et al (90) investigated the effects of a
combination of ginsenoside Rg3 with chemotherapeutic agents, such
as docetaxel, paclitaxel, cisplatin and doxorubicin on the growth
of colon cancer (SW480 and HCT-116) cells. Compared with treatment
with individual chemotherapeutic agents or Rg3, the combination of
each of the anticancer drugs with Rg3 elicited more pronounced
anti-proliferative effects on these cells. Moreover, treatment with
a combination of Rg3 and docetaxel induced apoptosis in these colon
cancer cells by inhibiting the activity of NF-κB, inducing the
expression of pro-apoptotic proteins (Bax, caspase-3 and -9) and
decreasing the expression of anti-apoptotic proteins [Bcl-2,
X-linked inhibitor of apoptosis (XIAP), inhibitor of apoptosis
(IAP)1, COX-2 and cyclin D1], compared with the effects observed by
treatment with docetaxel alone (90). The authors also demonstrated that
Rg3 increased the susceptibility of human prostate cancer (LNCaP,
PC3 and DU145) cells to docetaxel, cisplatin and doxorubicin.
Compared to treatment with Rg3 or docetaxel alone, the combination
treatment of Rg3 with docetaxel was more effective in the
inhibition of prostate cancer cell growth and the induction of
apoptosis, as well as G0/G1 cell cycle arrest accompanied by the
significant inhibition of NF-κB activity (91). Likewise, in another study, the
effects of combinations of Rh2 or its aglycon with docetaxel were
predominantly additive or synergistic in PC3, DU145 and C4-2
prostate cancer cells. Combinations of Rh2 or its aglycon with
docetaxel induced the regression of established PC3 tumors from
their initial size by 15 and 27%, respectively, and reduced the
expression of cell proliferation marker Ki-67 as compared with
animals treated with docetaxel alone (92).
In a previous study, a combination of Rg3 and
gemcitabine enhanced the growth-suppressive effect of gemcitabine
in C57BL/6 mice bearing Lewis lung carcinoma cells and prolonged
the survival of these tumor-bearing mice (15). Rg3 has been reported to enhance
the penetration of paclitaxel into Caco-2 monolayer cells by
downregulating the expression of p-glycoprotein (P-gp), also known
as multidrug resistance protein-1 (MDR1), increasing the oral
bioavailability of paclitaxel in rats. The enhancement of the oral
bioavailability of paclitaxel by Rg3 was further established by the
finding that the oral administration of a combination of paclitaxel
and Rg3 attenuated the growth of MCF-7 breast cancer cell
xenografts in nude mice more effectively than treatment with
paclitaxel alone (93).
Resistance to chemotherapy is associated with the
overexpression of the MDR1 gene. Ginsenosides Rd, Re, Rb1
and Rg1 have been shown to reduce the expression of MDR1 in
chemoresistant breast cancer (MCF7/ADR) cells. Further
investigation with Rd ginsenoside revealed that Rd failed to alter
the transcription of MDR1 but induced the ubiquitin-dependent
proteasomal degradation of MDR1 and reversed the doxorubicin
resistance of these cells (94).
In another study, Rg3 potentiated the cytotoxic effects of
cisplatin in CT-26 colon cancer cells by blocking the
cisplatin-induced activation of Nrf2 and the expression of
antioxidant enzymes HO-1 and NADPH:quinone oxidoreductase 1 (NQO1).
In addition, the combination with Rg3 reduced the cytotoxic effects
of cisplatin in normal LLC-RK1 kidney and NCTC1469 liver cells,
suggesting that Rg3 is effective in relieving the renal and hepatic
toxicity of cisplatin (95).
A synergistic inhibitory effect of ginsenoside Rh2
and cisplatin on the growth of a cell line (HRA) derived from
ascites of a patient with serous cystadenocarcinoma of the ovary
has been reported (96).
According to the authors, Rh2 alone did not exhibit any significant
growth inhibition of HRA cell xenografts in nude mice, but a
combination of Rh2 with cisplatin induced a more pronounced
inhibition of xenograft tumor growth, compared with treatment with
cisplatin alone (96). Similarly,
the treatment of LNCaP cell xenograft tumors with Rh2 plus
paclitaxel has been shown to induce a significant decrease in tumor
growth and serum PSA levels, suggesting a synergistic effect of Rh2
and paclitaxel (97). In another
study, Rh2 induced apoptosis in multidrug-resistant human breast
cancer cells by hypersensitizing these cells to paclitaxel. The
apoptosis induced by Rh2 in combination with paclitaxel was
mediated through the activation of glucocorticoid receptor, but not
the expression of p53 or caspase-3 (98). Chae et al (99) reported that the treatment of
NCI-H460 human lung cancer cells with compound K and simultaneous
exposure to gamma-radiation resulted in the increased apoptosis of
these cells through the generation of ROS and the activation of
caspase-3. Moreover, the combination of compound K and gamma-ray
led to the enhanced regression of NCI-H460 tumor xenografts in nude
mice (99).
4. Future perspectives
The molecular target-based prevention of cancer has
been widely accepted as a rational and practical strategy to reduce
the global burden of cancer. A number of dietary phytochemicals
have been reported to prevent cancer by targeting diverse
intracellular signaling molecules implicated in the carcinogenic
process. Extensively investigated ginseng and its vast array of
saponins are attractive candidates for the development of
chemopreventive and/or chemotherapeutic agents. As discussed in
this review, studies on individual ginsenosides have revealed that
many of them function through similar mechanisms by inhibiting
growth or inducing apoptosis in cancer cells. Thus, it would be
worthy to formulate a combination of ginsenosides, which would be
multitargeting and may be more portent in preventing tumor growth.
Such a formulation is KG-135, which is a mixture of ginsenosides
Rk1, Rg3 and Rg5. The incubation of human mammary epithelial cells
with KG-135 has been shown to inhibit the TPA-induced expression of
COX-2 by blocking the activation of NF-κB and AP-1 (100). In another study, KG-135
diminished the proliferative ability of human prostate cancer
(DU-145 and PC3) cells and reduced the growth of PC3 cell tumor
xenografts in nude mice by inducing cell cycle arrest through the
downregulation of cyclin D1 and Cdk expression (101). Moreover, KG-135 has been shown
to enhance etoposide-induced apoptosis in HeLa cells by activating
p53 and inducing the expression of Bax and p21 (102). These findings indicate that
there is ample scope to develop novel ginsenoside formulations by
mixing different types of ginsenosides. However, caution should be
exercised before such attempts, as there have been reports that
certain ginsenosides can promote tumor progression. For example,
ginsenoside Rh2 has been shown to enhance the metastatic potential
of BALB/c 3T3 cells in mice (103). Whereas the majority of
ginsenosides inhibit inflammation, ginsenoside Rd has been shown to
induce the expression of COX-2 and increase the production of
PGE2 in Raw 264.7 macrophages by enhancing the DNA
binding of C/EBP and cyclic-AMP response element binding protein
(CREB) (104). Therefore, the
combination of ginsenosides in the development of novel formulas
for chemoprevention and/or chemotherapy must be carried out
judiciously so that the overall therapeutic benefit may be best
achieved.
A few studies have demonstrated the prolongation of
survival and improvement of quality of life in cancer patients
receiving ginseng or its component, ginsenoside Rg3 (105,106). Kim et al (105) reported that a 12-week therapy
with ginseng improved the mental and physical health of patients
diagnosed with gynecological cancer and hepatobiliary cancer. The
administration of ginsenoside Rg3 together with chemotherapy has
also been shown to increase the post-surgical life span of patients
suffering from non-small cell lung cancer (106). These limited clinical
experiences and extensive preclinical study outcomes indicate the
potential of ginsenosides or their formulations in cancer
prevention and therapy. Further clinical trials with ginsenosides
or their combinations, and extensive pharmacokinetic studies are
required to perform dosage adjustment and to achieve bioavailable
doses of ginsenosides. It should also be taken into consideration
that ginsenosides, when administered by gavage, can undergo
intestinal metabolism, which often produces active metabolites.
Thus, more rigorous studies in terms of bioavailability, metabolism
and dose-response relationships would make this age-old oriental
medicine exploitable for cancer chemoprevention and/or
chemotherapy.
Acknowledgements
This study was supported by the Settlement Research
Grant-2012-0195 of Keimyung University allocated to Joydeb Kumar
Kundu.
References
|
1
|
Choi KT: Botanical characteristics,
pharmacological effects and medicinal components of Korean Panax
ginseng C A Meyer. Acta Pharmacol Sin. 29:1109–1118. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shibata S: Chemistry and cancer preventing
activities of ginseng saponins and some related triterpenoid
compounds. J Korean Med Sci. 16(Suppl): S28–S37. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kitagawa I, Taniyama T, Shibuya H, Noda T
and Yoshikawa M: Chemical studies on crude drug processing. V. On
the constituents of ginseng radix rubra (2): Comparison of the
constituents of white ginseng and red ginseng prepared from the
same Panax ginseng root. Yakugaku Zasshi. 107:495–505.
1987.(In Japanese).
|
|
4
|
Kitagawa I, Yoshikawa M, Yoshihara M,
Hayashi T and Taniyama T: Chemical studies of crude drugs (1).
Constituents of Ginseng radix rubra. Yakugaku Zasshi. 103:612–622.
1983.(In Japanese).
|
|
5
|
Shin HR, Kim JY, Yun TK, Morgan G and
Vainio H: The cancer-preventive potential of Panax ginseng:
a review of human and experimental evidence. Cancer Causes Control.
11:565–576. 2000. View Article : Google Scholar
|
|
6
|
Yun TK, Lee YS, Lee YH, Kim SI and Yun HY:
Anticarcinogenic effect of Panax ginseng C.A. Meyer and
identification of active compounds. J Korean Med Sci. 16(Suppl):
S6–S18. 2001.
|
|
7
|
Nag SA, Qin JJ, Wang W, Wang MH, Wang H
and Zhang R: Ginsenosides as anticancer agents: In vitro and in
vivo activities, structure-activity relationships, and molecular
mechanisms of action. Front Pharmacol. 3:252012.PubMed/NCBI
|
|
8
|
Qi LW, Wang CZ and Yuan CS: Ginsenosides
from American ginseng: chemical and pharmacological diversity.
Phytochemistry. 72:689–699. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hofseth LJ and Wargovich MJ: Inflammation,
cancer, and targets of ginseng. J Nutr. 137(Suppl 1): 183S–185S.
2007.PubMed/NCBI
|
|
10
|
Kim YS and Jin SH: Ginsenoside Rh2 induces
apoptosis via activation of caspase-1 and -3 and up-regulation of
Bax in human neuroblastoma. Arch Pharm Res. 27:834–839. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Keum YS, Han SS, Chun KS, et al:
Inhibitory effects of the ginsenoside Rg3 on phorbol ester-induced
cyclooxygenase-2 expression, NF-kappaB activation and tumor
promotion. Mutat Res. 523–524:75–85. 2003.PubMed/NCBI
|
|
12
|
Kumar A, Kumar M, Panwar M, et al:
Evaluation of chemopreventive action of Ginsenoside Rp1.
Biofactors. 26:29–43. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bae JS, Park HS, Park JW, Li SH and Chun
YS: Red ginseng and 20(S)-Rg3 control testosterone-induced prostate
hyperplasia by deregulating androgen receptor signaling. J Nat Med.
66:476–485. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
He BC, Gao JL, Luo X, et al: Ginsenoside
Rg3 inhibits colorectal tumor growth through the down-regulation of
Wnt/β-catenin signaling. Int J Oncol. 38:437–445. 2011.PubMed/NCBI
|
|
15
|
Liu TG, Huang Y, Cui DD, et al: Inhibitory
effect of ginsenoside Rg3 combined with gemcitabine on angiogenesis
and growth of lung cancer in mice. BMC Cancer. 9:2502009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Musende AG, Eberding A, Wood C, et al:
Pre-clinical evaluation of Rh2 in PC-3 human xenograft model for
prostate cancer in vivo: formulation, pharmacokinetics,
biodistribution and efficacy. Cancer Chemother Pharmacol.
64:1085–1095. 2009. View Article : Google Scholar
|
|
17
|
Hu C, Song G, Zhang B, et al: Intestinal
metabolite compound K of panaxoside inhibits the growth of gastric
carcinoma by augmenting apoptosis via Bid-mediated mitochondrial
pathway. J Cell Mol Med. 16:96–106. 2012. View Article : Google Scholar
|
|
18
|
Wang W, Rayburn ER, Hang J, Zhao Y, Wang H
and Zhang R: Anti-lung cancer effects of novel ginsenoside
25-OCH(3)-PPD. Lung Cancer. 65:306–311. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang W, Rayburn ER, Zhao Y, Wang H and
Zhang R: Novel ginsenosides 25-OH-PPD and 25-OCH3-PPD as
experimental therapy for pancreatic cancer: anticancer activity and
mechanisms of action. Cancer Lett. 278:241–248. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang W, Zhang X, Qin JJ, et al: Natural
product ginsenoside 25-OCH3-PPD inhibits breast cancer
growth and metastasis through down-regulating MDM2. PLoS One.
7:e415862012.PubMed/NCBI
|
|
21
|
Wang W, Rayburn ER, Hao M, et al:
Experimental therapy of prostate cancer with novel natural product
anti-cancer ginsenosides. Prostate. 68:809–819. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lü JM, Weakley SM, Yang Z, Hu M, Yao Q and
Chen C: Ginsenoside rb1 directly scavenges hydroxyl radical and
hypochlorous acid. Curr Pharm Des. 18:6339–6347. 2012.PubMed/NCBI
|
|
23
|
Park HM, Kim SJ, Mun AR, et al: Korean red
ginseng and its primary ginsenosides inhibit ethanol-induced
oxidative injury by suppression of the MAPK pathway in TIB-73
cells. J Ethnopharmacol. 141:1071–1076. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kim YH, Park KH and Rho HM:
Transcriptional activation of the Cu,Zn-superoxide dismutase gene
through the AP2 site by ginsenoside Rb2 extracted from a medicinal
plant, Panax ginseng. J Biol Chem. 271:24539–24543. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chang MS, Lee SG and Rho HM:
Transcriptional activation of Cu/Zn superoxide dismutase and
catalase genes by panaxadiol ginsenosides extracted from Panax
ginseng. Phytother Res. 13:641–644. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kim ND, Pokharel YR and Kang KW:
Ginsenoside Rd enhances glutathione levels in H4IIE cells via
NF-kappaB-dependent gamma-glutamylcysteine ligase induction.
Pharmazie. 62:933–936. 2007.PubMed/NCBI
|
|
27
|
Lee SH, Seo GS, Ko G, Kim JB and Sohn DH:
Anti-inflammatory activity of 20(S)-protopanaxadiol: enhanced heme
oxygenase 1 expression in RAW 264.7 cells. Planta Med.
71:1167–1170. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Saw CL, Yang AY, Cheng DC, et al:
Pharmacodynamics of ginsenosides: antioxidant activities,
activation of Nrf2, and potential synergistic effects of
combinations. Chem Res Toxicol. 25:1574–1580. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shin YM, Jung HJ, Choi WY and Lim CJ:
Antioxidative, anti-inflammatory, and matrix metalloproteinase
inhibitory activities of 20(S)-ginsenoside Rg3 in cultured
mammalian cell lines. Mol Biol Rep. 40:269–279. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kim TW, Joh EH, Kim B and Kim DH:
Ginsenoside Rg5 ameliorates lung inflammation in mice by inhibiting
the binding of LPS to toll-like receptor-4 on macrophages. Int
Immunopharmacol. 12:110–116. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Joh EH, Lee IA, Jung IH and Kim DH:
Ginsenoside Rb1 and its metabolite compound K inhibit IRAK-1
activation - the key step of inflammation. Biochem Pharmacol.
82:278–286. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yang XL, Guo TK, Wang YH, et al:
Ginsenoside Rd attenuates the inflammatory response via modulating
p38 and JNK signaling pathways in rats with TNBS-induced relapsing
colitis. Int Immunopharmacol. 12:408–414. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Park EK, Shin YW, Lee HU, et al:
Inhibitory effect of ginsenoside Rb1 and compound K on NO and
prostaglandin E2 biosyntheses of RAW264.7 cells induced by
lipopolysaccharide. Biol Pharm Bull. 28:652–656. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Oh GS, Pae HO, Choi BM, et al:
20(S)-Protopanaxatriol, one of ginsenoside metabolites, inhibits
inducible nitric oxide synthase and cyclooxygenase-2 expressions
through inactivation of nuclear factor-kappaB in RAW 264.7
macrophages stimulated with lipopolysaccharide. Cancer Lett.
205:23–29. 2004. View Article : Google Scholar
|
|
35
|
Huang J, Ding L, Shi D, et al: Transient
receptor potential vanilloid-1 participates in the inhibitory
effect of ginsenoside Rg1 on capsaicin-induced interleukin-8 and
prostaglandin E2 production in HaCaT cells. J Pharm Pharmacol.
64:252–258. 2012. View Article : Google Scholar
|
|
36
|
Huang J, Qiu L, Ding L, et al: Ginsenoside
Rb1 and paeoniflorin inhibit transient receptor potential
vanilloid-1-activated IL-8 and PGE2 production in a
human keratinocyte cell line HaCaT. Int Immunopharmacol.
10:1279–1283. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liu WK, Xu SX and Che CT:
Anti-proliferative effect of ginseng saponins on human prostate
cancer cell line. Life Sci. 67:1297–1306. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Jiang JW, Chen XM, Chen XH and Zheng SS:
Ginsenoside Rg3 inhibit hepatocellular carcinoma growth via
intrinsic apoptotic pathway. World J Gastroenterol. 17:3605–3613.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Park HM, Kim SJ, Kim JS and Kang HS:
Reactive oxygen species mediated ginsenoside Rg3- and Rh2-induced
apoptosis in hepatoma cells through mitochondrial signaling
pathways. Food Chem Toxicol. 50:2736–2741. 2012. View Article : Google Scholar
|
|
40
|
Zhang C, Liu L, Yu Y, Chen B, Tang C and
Li X: Antitumor effects of ginsenoside Rg3 on human hepatocellular
carcinoma cells. Mol Med Rep. 5:1295–1298. 2012.PubMed/NCBI
|
|
41
|
Yuan HD, Quan HY, Zhang Y, Kim SH and
Chung SH: 20(S)-Ginsenoside Rg3-induced apoptosis in HT-29 colon
cancer cells is associated with AMPK signaling pathway. Mol Med
Rep. 3:825–831. 2010.PubMed/NCBI
|
|
42
|
Lee JY, Jung KH, Morgan MJ, et al:
Sensitization of TRAIL-induced cell death by 20S-Ginsenoside Rg3
via CHOP-mediated DR5 upregulation in human hepatocellular
carcinoma cells. Mol Cancer Ther. 12:274–285. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kim BJ, Nah SY, Jeon JH, So I and Kim SJ:
Transient receptor potential melastatin 7 channels are involved in
ginsenoside Rg3-induced apoptosis in gastric cancer cells. Basic
Clin Pharmacol Toxicol. 109:233–239. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Chen J, Peng H, Ou-Yang X and He X:
Research on the antitumor effect of ginsenoside Rg3 in B16 melanoma
cells. Melanoma Res. 18:322–329. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Li B, Zhao J, Wang CZ, et al: Ginsenoside
Rh2 induces apoptosis and paraptosis-like cell death in colorectal
cancer cells through activation of p53. Cancer Lett. 301:185–192.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Choi S, Oh JY and Kim SJ: Ginsenoside Rh2
induces Bcl-2 family proteins-mediated apoptosis in vitro and in
xenografts in vivo models. J Cell Biochem. 112:330–340. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kim HE, Oh JH, Lee SK and Oh YJ:
Ginsenoside RH-2 induces apoptotic cell death in rat C6 glioma via
a reactive oxygen- and caspase-dependent but Bcl-X(L)-independent
pathway. Life Sci. 65:PL33–PL40. 1999.PubMed/NCBI
|
|
48
|
Kim YS, Jin SH, Lee YH, Kim SI and Park
JD: Ginsenoside Rh2 induces apoptosis independently of Bcl-2,
Bcl-xL, or Bax in C6Bu-1 cells. Arch Pharm Res. 22:448–453. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Park JA, Lee KY, Oh YJ, Kim KW and Lee SK:
Activation of caspase-3 protease via a Bcl-2-insensitive pathway
during the process of ginsenoside Rh2-induced apoptosis. Cancer
Lett. 121:73–81. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Popovich DG and Kitts DD: Ginsenosides
20(S)-protopanaxadiol and Rh2 reduce cell proliferation and
increase sub-G1 cells in two cultured intestinal cell lines,
Int-407 and Caco-2. Can J Physiol Pharmacol. 82:183–190. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Oh M, Choi YH, Choi S, et al:
Anti-proliferating effects of ginsenoside Rh2 on MCF-7 human breast
cancer cells. Int J Oncol. 14:869–875. 1999.PubMed/NCBI
|
|
52
|
Cheng CC, Yang SM, Huang CY, Chen JC,
Chang WM and Hsu SL: Molecular mechanisms of ginsenoside
Rh2-mediated G1 growth arrest and apoptosis in human lung
adenocarcinoma A549 cells. Cancer Chemother Pharmacol. 55:531–540.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Oh JI, Chun KH, Joo SH, Oh YT and Lee SK:
Caspase-3-dependent protein kinase C delta activity is required for
the progression of Ginsenoside-Rh2-induced apoptosis in SK-HEP-1
cells. Cancer Lett. 230:228–238. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Ham YM, Lim JH, Na HK, et al:
Ginsenoside-Rh2-induced mitochondrial depolarization and apoptosis
are associated with reactive oxygen species- and
Ca2+-mediated c-Jun NH2-terminal kinase 1 activation in
HeLa cells. J Pharmacol Exp Ther. 319:1276–1285. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Park EK, Lee EJ, Lee SH, et al: Induction
of apoptosis by the ginsenoside Rh2 by internalization of lipid
rafts and caveolae and inactivation of Akt. Br J Pharmacol.
160:1212–1223. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Yi JS, Choo HJ, Cho BR, et al: Ginsenoside
Rh2 induces ligand-independent Fas activation via lipid raft
disruption. Biochem Biophys Res Commun. 385:154–159. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
An IS, An S, Kwon KJ, Kim YJ and Bae S:
Ginsenoside Rh2 mediates changes in the microRNA expression profile
of human non-small cell lung cancer A549 cells. Oncol Rep.
29:523–528. 2013.PubMed/NCBI
|
|
58
|
Wu N, Wu GC, Hu R, Li M and Feng H:
Ginsenoside Rh2 inhibits glioma cell proliferation by targeting
microRNA-128. Acta Pharmacol Sin. 32:345–353. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Park TY, Park MH, Shin WC, et al:
Anti-metastatic potential of ginsenoside Rp1, a novel ginsenoside
derivative. Biol Pharm Bull. 31:1802–1805. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Kang JH, Song KH, Woo JK, et al:
Ginsenoside Rp1 from Panax ginseng exhibits anti-cancer
activity by down-regulation of the IGF-1R/Akt pathway in breast
cancer cells. Plant Foods Hum Nutr. 66:298–305. 2011.
|
|
61
|
Kumar A, Kumar M, Park TY, et al:
Molecular mechanisms of ginsenoside Rp1-mediated growth arrest and
apoptosis. Int J Mol Med. 24:381–386. 2009.PubMed/NCBI
|
|
62
|
Kim JS, Joo EJ, Chun J, et al: Induction
of apoptosis by ginsenoside Rk1 in SK-MEL-2-human melanoma. Arch
Pharm Res. 35:717–722. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Kim YJ, Kwon HC, Ko H, et al: Anti-tumor
activity of the ginsenoside Rk1 in human hepatocellular carcinoma
cells through inhibition of telomerase activity and induction of
apoptosis. Biol Pharm Bull. 31:826–830. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Kim SE, Lee YH, Park JH and Lee SK:
Ginsenoside-Rs3, a new diol-type ginseng saponin, selectively
elevates protein levels of p53 and p21WAF1 leading to induction of
apoptosis in SK-HEP-1 cells. Anticancer Res. 19:487–491.
1999.PubMed/NCBI
|
|
65
|
Wang W, Wang H, Rayburn ER, Zhao Y, Hill
DL and Zhang R: 20(S)-25-methoxyl-dammarane-3beta, 12beta,
20-triol, a novel natural product for prostate cancer therapy:
activity in vitro and in vivo and mechanisms of action. Br J
Cancer. 98:792–802. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Park S, Lee HJ, Jeong SJ, et al:
Inhibition of JAK1/STAT3 signaling mediates compound K-induced
apoptosis in human multiple myeloma U266 cells. Food Chem Toxicol.
49:1367–1372. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Sathishkumar N, Sathiyamoorthy S, Ramya M,
Yang DU, Lee HN and Yang DC: Molecular docking studies of
anti-apoptotic BCL-2, BCL-XL, and MCL-1 proteins with ginsenosides
from Panax ginseng. J Enzyme Inhib Med Chem. 27:685–692.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Liu J, Shimizu K, Yu H, Zhang C, Jin F and
Kondo R: Stereospecificity of hydroxyl group at C-20 in
antiproliferative action of ginsenoside Rh2 on prostate cancer
cells. Fitoterapia. 81:902–905. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Dong H, Bai LP, Wong VK, et al: The in
vitro structure-related anti-cancer activity of ginsenosides and
their derivatives. Molecules. 16:10619–10630. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Chen QJ, Zhang MZ and Wang LX: Gensenoside
Rg3 inhibits hypoxia-induced VEGF expression in human cancer cells.
Cell Physiol Biochem. 26:849–858. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Yue PY, Wong DY, Wu PK, et al: The
angiosuppressive effects of 20(R)- ginsenoside Rg3. Biochem
Pharmacol. 72:437–445. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Zhang Q, Kang X, Yang B, Wang J and Yang
F: Antiangiogenic effect of capecitabine combined with ginsenoside
Rg3 on breast cancer in mice. Cancer Biother Radiopharm.
23:647–653. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Xu TM, Xin Y, Cui MH, Jiang X and Gu LP:
Inhibitory effect of ginsenoside Rg3 combined with cyclophosphamide
on growth and angiogenesis of ovarian cancer. Chin Med J (Engl).
120:584–588. 2007.PubMed/NCBI
|
|
74
|
Zhang Q, Kang X and Zhao W: Antiangiogenic
effect of low-dose cyclophosphamide combined with ginsenoside Rg3
on Lewis lung carcinoma. Biochem Biophys Res Commun. 342:824–828.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Kim JW, Jung SY, Kwon YH, et al:
Ginsenoside Rg3 attenuates tumor angiogenesis via inhibiting
bioactivities of endothelial progenitor cells. Cancer Biol Ther.
13:504–515. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Sato K, Mochizuki M, Saiki I, Yoo YC,
Samukawa K and Azuma I: Inhibition of tumor angiogenesis and
metastasis by a saponin of Panax ginseng, ginsenoside-Rb2.
Biol Pharm Bull. 17:635–639. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Jeong A, Lee HJ, Jeong SJ, Lee EO, Bae H
and Kim SH: Compound K inhibits basic fibroblast growth
factor-induced angiogenesis via regulation of p38 mitogen activated
protein kinase and AKT in human umbilical vein endothelial cells.
Biol Pharm Bull. 33:945–950. 2010. View Article : Google Scholar
|
|
78
|
Pan XY, Guo H, Han J, et al: Ginsenoside
Rg3 attenuates cell migration via inhibition of aquaporin 1
expression in PC-3M prostate cancer cells. Eur J Pharmacol.
683:27–34. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Mochizuki M, Yoo YC, Matsuzawa K, et al:
Inhibitory effect of tumor metastasis in mice by saponins,
ginsenoside-Rb2, 20(R)- and 20(S)-ginsenoside-Rg3, of red ginseng.
Biol Pharm Bull. 18:1197–1202. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Xu TM, Cui MH, Xin Y, et al: Inhibitory
effect of ginsenoside Rg3 on ovarian cancer metastasis. Chin Med J
(Engl). 121:1394–1397. 2008.PubMed/NCBI
|
|
81
|
Chen XP, Qian LL, Jiang H and Chen JH:
Ginsenoside Rg3 inhibits CXCR4 expression and related migrations in
a breast cancer cell line. Int J Clin Oncol. 16:519–523. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Iishi H, Tatsuta M, Baba M, et al:
Inhibition by ginsenoside Rg3 of bombesin-enhanced peritoneal
metastasis of intestinal adenocarcinomas induced by azoxymethane in
Wistar rats. Clin Exp Metastasis. 15:603–611. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Shinkai K, Akedo H, Mukai M, et al:
Inhibition of in vitro tumor cell invasion by ginsenoside Rg3. Jpn
J Cancer Res. 87:357–362. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Yoon JH, Choi YJ and Lee SG: Ginsenoside
Rh1 suppresses matrix metalloproteinase-1 expression through
inhibition of activator protein-1 and mitogen-activated protein
kinase signaling pathway in human hepatocellular carcinoma cells.
Eur J Pharmacol. 679:24–33. 2012. View Article : Google Scholar
|
|
85
|
Yoon JH, Choi YJ, Cha SW and Lee SG:
Anti-metastatic effects of ginsenoside Rd via inactivation of MAPK
signaling and induction of focal adhesion formation. Phytomedicine.
19:284–292. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Kim SY, Kim DH, Han SJ, Hyun JW and Kim
HS: Repression of matrix metalloproteinase gene expression by
ginsenoside Rh2 in human astroglioma cells. Biochem Pharmacol.
74:1642–1651. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Ming Y, Chen Z, Chen L, et al: Ginsenoside
compound K attenuates metastatic growth of hepatocellular
carcinoma, which is associated with the translocation of nuclear
factor-kappaB p65 and reduction of matrix metalloproteinase-2/9.
Planta Med. 77:428–433. 2011. View Article : Google Scholar
|
|
88
|
Hasegawa H and Uchiyama M: Antimetastatic
efficacy of orally administered ginsenoside Rb1 in dependence on
intestinal bacterial hydrolyzing potential and significance of
treatment with an active bacterial metabolite. Planta Med.
64:696–700. 1998. View Article : Google Scholar
|
|
89
|
Choo MK, Sakurai H, Kim DH and Saiki I: A
ginseng saponin metabolite suppresses tumor necrosis
factor-α-promoted metastasis by suppressing nuclear factor-κB
signaling in murine colon cancer cells. Oncol Rep. 19:595–600.
2008.PubMed/NCBI
|
|
90
|
Kim SM, Lee SY, Yuk DY, et al: Inhibition
of NF-kappaB by ginsenoside Rg3 enhances the susceptibility of
colon cancer cells to docetaxel. Arch Pharm Res. 32:755–765. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Kim SM, Lee SY, Cho JS, et al: Combination
of ginsenoside Rg3 with docetaxel enhances the susceptibility of
prostate cancer cells via inhibition of NF-kappaB. Eur J Pharmacol.
631:1–9. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Musende AG, Eberding A, Jia W, Ramsay E,
Bally MB and Guns ET: Rh2 or its aglycone aPPD in combination with
docetaxel for treatment of prostate cancer. Prostate. 70:1437–1447.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Yang LQ, Wang B, Gan H, et al: Enhanced
oral bioavailability and anti-tumour effect of paclitaxel by
20(s)-ginsenoside Rg3 in vivo. Biopharm Drug Dispos.
33:425–436. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Pokharel YR, Kim ND, Han HK, Oh WK and
Kang KW: Increased ubiquitination of multidrug resistance 1 by
ginsenoside Rd. Nutr Cancer. 62:252–259. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Lee CK, Park KK, Chung AS and Chung WY:
Ginsenoside Rg3 enhances the chemosensitivity of tumors to
cisplatin by reducing the basal level of nuclear factor erythroid
2-related factor 2-mediated heme oxygenase-1/NAD(P)H quinone
oxidoreductase-1 and prevents normal tissue damage by scavenging
cisplatin-induced intracellular reactive oxygen species. Food Chem
Toxicol. 50:2565–2574. 2012.
|
|
96
|
Kikuchi Y, Sasa H, Kita T, Hirata J, Tode
T and Nagata I: Inhibition of human ovarian cancer cell
proliferation in vitro by ginsenoside Rh2 and adjuvant effects to
cisplatin in vivo. Anticancer Drugs. 2:63–67. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Xie X, Eberding A, Madera C, et al: Rh2
synergistically enhances paclitaxel or mitoxantrone in prostate
cancer models. J Urol. 175:1926–1931. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Jia WW, Bu X, Philips D, et al: Rh2, a
compound extracted from ginseng, hypersensitizes
multidrug-resistant tumor cells to chemotherapy. Can J Physiol
Pharmacol. 82:431–437. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Chae S, Kang KA, Chang WY, et al: Effect
of compound K, a metabolite of ginseng saponin, combined with
gamma-ray radiation in human lung cancer cells in vitro and in
vivo. J Agric Food Chem. 57:5777–5782. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Park SA, Kim EH, Na HK and Surh YJ: KG-135
inhibits COX-2 expression by blocking the activation of JNK and
AP-1 in phorbol ester-stimulated human breast epithelial cells. Ann
NY Acad Sci. 1095:545–553. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Yoo JH, Kwon HC, Kim YJ, Park JH and Yang
HO: KG-135, enriched with selected ginsenosides, inhibits the
proliferation of human prostate cancer cells in culture and
inhibits xenograft growth in athymic mice. Cancer Lett. 289:99–110.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Lee WH, Choi JS, Kim HY, et al:
Potentiation of etoposide-induced apoptosis in HeLa cells by
co-treatment with KG-135, a quality-controlled standardized
ginsenoside formulation. Cancer Lett. 294:74–81. 2010. View Article : Google Scholar
|
|
103
|
Tatsuka M, Maeda M and Ota T:
Anticarcinogenic effect and enhancement of metastatic potential of
BALB/c 3T3 cells by ginsenoside Rh(2). Jpn J Cancer Res.
92:1184–1189. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Jeong HG, Pokharel YR, Han EH and Kang KW:
Induction of cyclooxygenase-2 by ginsenoside Rd via activation of
CCAAT-enhancer binding proteins and cyclic AMP response binding
protein. Biochem Biophys Res Commun. 359:51–56. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Kim JH, Park CY and Lee SJ: Effects of sun
ginseng on subjective quality of life in cancer patients: a
double-blind, placebo-controlled pilot trial. J Clin Pharm Ther.
31:331–334. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Lu P, Su W, Miao ZH, Niu HR, Liu J and Hua
QL: Effect and mechanism of ginsenoside Rg3 on postoperative life
span of patients with non-small cell lung cancer. Chin J Integr
Med. 14:33–36. 2008. View Article : Google Scholar : PubMed/NCBI
|